Abstract
Unfractionated peripheral blood stem cell (PBSC) grafts contain measurable quantities of myeloma cells and are therefore a potential source of relapse posttransplantation. In contrast, fluorescence-activated cell sorting (FACS)-sorted CD34+Thy1+ Lin− peripheral blood cells are substantially enriched for stem cell activity, yet contain virtually no clonal myeloma cells. A study was performed in patients with symptomatic myeloma, who had received 12 months or less of preceding standard chemotherapy, to evaluate the feasibility of large scale purification of primitive hematopoietic stem cells in order to study engraftment kinetics posttransplantation and the degree of tumor cell contamination of this cell population, based on polymerase chain reaction (PCR) analysis for the patient-specific complementarity-determining region III (CDR III). PBSC were mobilized with high dose cyclophosphamide and granulocyte-macrophage colony-stimulating factor (GM-CSF). A combination of elutriation and chemical lysis was used to deplete PBSC collections of monocytes, granulocytes, erythrocytes, and platelets. Subsequently, CD34+ Thy1+ Lin−progenitor cells were purified with high speed cell sorting. Of the 10 evaluable patients, nine met the required minimum criteria of ≥7.2 × 105 cells/kg to support tandem transplants. After high dose melphalan (200 mg/m2) eight engrafted successfully, although granulocyte (absolute neutrophil count [ANC] >0.5 × 109/L, 16 days) and platelet recovery (platelets > 50 × 109/L, 39 days) was substantially delayed when compared with unmanipulated PBSC grafts; one patient required infusion of a reserve graft because of lack of evidence of engraftment by day +28. Three patients proceeded to a second graft with high dose melphalan and total body irradiation; two required infusion of a reserve graft and both died of infectious complications; one showed delayed, but complete, engraftment after this myeloablative regimen. Two of the nine evaluable patients attained a clinical complete remission (CR). The grafts from three patients were tested for tumor contamination and contained no detectable clonal myeloma cells. Larger quantities of purified cells may be required to resolve the problem of delayed engraftment.
BOTH A FRENCH RANDOMIZED study1 and a pair-mate analysis between our total therapy patients and comparable patients treated with standard therapy on protocols of the Southwest Oncology Group2 indicate that autotransplants not only effect higher complete remission (CR) rates, but also significantly extend event-free survival (EFS) and overall survival (OS). In newly diagnosed patients, tandem transplants can induce molecular remissions based on quantitative polymerase chain reaction (PCR) amplification assays of patient-specific complementarity-determining region III (CDR III) DNA sequences on bone marrow (BM) samples, able to detect one clonal B cell in a background of 105 normal cells.3 4
Using either sensitive immunofluorescence techniques or PCR for CDR III, myeloma cells have been detected in virtually all peripheral blood stem cell (PBSC) products.5-7 Most patients have between 0.1% and 1% clonal B cells in their PBSC collections,6resulting in the infusion of approximately 0.5 to 5 × 108 myeloma cells with each transplant. These quantities are probably sufficient to contribute to relapse after transplantation, as has been shown with autotransplants in acute myeloid leukemia8 and neuroblastoma.9 In an attempt to obtain tumor-free grafts, bone marrows of myeloma patients have been purged with 4-hydroperoxycyclophosphamide (4HC)10 or with monoclonal antibodies,11 but these manipulations have resulted in delayed engraftment. In contrast, purified CD34+ cells reconstitute hematopoiesis rapidly and reliably12-15 and the CD34 antigen is thought not to be expressed on myeloma cells.16 However, with the currently available techniques, the purity of the CD34+ selected cells varies between 30% and 90%,12-15 still leaving detectable levels of myeloma cells in these preparations.7,16 Further purification of these selected CD34+ cells by fluorescence-activated cell sorting (FACS) appears to completely eliminate all clonal B cells,16,17suggesting that residual myeloma cells in CD34+ selected grafts are due to contaminating CD34-negative cells. Other investigators have, however, reported that cells clonally related to the myeloma cells can be found within the highly purified CD34+ fraction.18 19
Based on these data, we decided to investigate whether a subpopulation of CD34 cells, CD34+ Thy1+Lin−, purified by FACS, would provide us with tumor-free grafts. CD34+ Thy1+Lin− are substantially enriched for stem cell activity20,21 and give rise to myeloid, B-, and T-cell lineages.20,22 Our preclinical studies have shown that purified CD34+ Thy1+Lin− stem cells do not contain clonal myeloma cells, as measured with PCR for CDR III.6 A clinical study was started using high dose cyclophosphamide (HD-CTX) plus granulocyte-macrophage colony-stimulating factor (GM-CSF) to mobilize PBSCs for selection of CD34+ Thy1+Lin− cells in support of tandem high dose chemotherapy/radiotherapy regimens. The aims of our study were to evaluate in how many patients adequate amounts of these CD34+ Thy1+ Lin−cells could be collected, what their purity and recovery rates were, whether large scale purified cells were tumor-free, and what the engraftment kinetics of these cells were posttransplantation.
MATERIALS AND METHODS
Patients.
Fifteen patients with symptomatic multiple myeloma (MM) and ≤12 months of preceding standard dose chemotherapy were enrolled in the study. Patients were between 15 and 70 years of age and their BM contained < 30% plasma cells (percent of all cells) before peripheral stem cell mobilization. The required Zubrod performance score was 0 or 1. Other requirements were: creatinine ≤2 mg/dL; forced vital capacity and diffusing capacity for carbon monoxide ≥50% of predicted; systolic ejection fraction ≥50%, direct bilirubin ≤1.5 mg/dL and transaminases ≤two times the upper limit of normal; absolute neutrophil count (ANC) ≥1.5 × 109/L and platelets ≥150 × 109/L; negative serology for human immunodeficiency virus (HIV) and hepatitis B surface antigen and no evidence of active infection. Patients with a second malignancy, which was active or had been diagnosed within 5 years before study entry, as well as patients with recent chemotherapy (≤4 weeks) or excessive prior local radiotherapy, which would not allow total body irradiation of 1,125 cGy, were excluded. Five patients had stem cells purified, but had not received a transplant with these selected stem cells at the time the study was terminated. However, four of these patients had adequate numbers of selected cells for two autotransplants, while one patient failed to meet the criteria to start the selection process (see below).
Therapy.
Mobilization of PBSCs was attained by administration of HD CTX 6 g/m2, followed by GM-CSF (250 mg/m2) until completion of the collections.23 Apheresis was started when the ANC count was >0.5 × 109/L and platelets approached or exceeded 50 × 109/L. A sample of the apheresis product was analyzed for cell count and CD34+ Thy1+ Lin−cell content. The apheresis product was sent immediately by overnight mail after each collection to Systemix, Inc, Palo Alto, CA, if the following criteria were met: (1) total number of WBC ≥109; (2) percentage of CD34+ Thy1+Lin− cells ≥1.5%; (3) percentage of viable cells ≥70%; and (4) the red blood cell (RBC) contamination of the undiluted sample ≤0.9 × 106/μL. Patients who were unable to meet these criteria during 3 consecutive days were considered failures. After obtaining adequate numbers of selected CD34+ Thy1+Lin− cells, defined as ≥3.6 × 105/kg for each transplant (see Discussion), additional unfractionated PBSC were collected as back-up in case of delayed engraftment with the selected cells (ANC count < 0.5 × 109/L by day +28 posttransplantation). A dose of ≥3 × 108/kg mononuclear cells/kg had to be available as a reserve graft. Nine patients proceeded with the first transplant after melphalan 100 mg/m2 daily for 2 days (days −3 and −2); the selected cells were given after 1 day of rest (day 0).2 Patients with sustained engraftment not requiring a reserve graft were eligible for a second autotransplant. The preparative regimen for the second transplant consisted of melphalan 140 mg/m2 (day −4) followed by fractionated total body irradiation (1,125 cGy) in nine fractions over 3 days on days −3, −2, and −1. Selected cells were infused the day after completion of the radiotherapy (day 0). All patients were treated in a high efficiency particulate air-filtered room with prophylactic antimicrobial, antifungal, antipneumocystis, and antiviral therapy. The second transplant was scheduled 4 to 6 months after the first.
Response criteria.
Patients were evaluated for response at 6 weeks and 3 months after their autotransplant. A partial remission (PR) required a tumor mass reduction of at least 75%, including a Bence-Jones proteinuria to less than 100 mg/day and BM aspirate and biopsy with no more than 5% plasma cells. A CR was defined by the absence of monoclonal gammopathy in serum and urine, using immunofixation analysis, and a normal BM aspirate and biopsy. With both PR and CR, these findings had to be present on at least two occasions with a minimum interval of 2 months. New lytic lesions (but not compression fractures), hypercalcemia, or other new evidence of disease constituted relapse or progressive disease. EFS and OS were both calculated from the time of the first transplant. Events included disease recurrence and death from any cause; in case of OS, deaths from disease or other causes were considered events.
Flow sorting of plasma cells and generation of the CDR III primer for allele-specific oligonucleotide (ASO)-PCR.
Ficoll-Hypaque-separated BM mononuclear cells (BMMCs) were stained for the CD38 and CD45 antigens (CD45 fluorescein isothiocyanate [FITC]; CD38-phycoerythrin [PE], Becton Dickinson [BD], Mountain View, CA). The CD38bright CD45low cells were sorted using a FACStar Plus cell sorter (BD) as described previously.6DNA extraction, generation of the CDR III primers, and radiolabeled ASO-PCR reaction were performed. Dilution curves with normal peripheral blood lymphocyte DNA between 10% and 0.001% myeloma cell DNA were generated for each patient. Autoradiograms were scanned densitometrically and quantitation of myeloma cells in PBSC collections was performed by log linear regression, as described before.
Stem cell purification.
On receipt at SyStemix, each day's collection was analyzed for cell number, viability, and stem cell phenotype and was processed using standard operating procedures in accordance with good manufacturing practices (GMP). Each incoming apheresis product was enriched for mononuclear cells by counterflow centrifugal elutriation using a Beckman model J6-MI centrifuge equipped with a standard rotor and large Sanderson elutriation chamber (Beckman Instruments, Palo Alto, CA). After mixing, the unfractionated apheresis samples were injected into the elutriator at a flow rate of 25 mL/minute. The cells were elutriated at 22°C with a rotor speed of 2,000 RPM in phosphate-buffered saline (PBS) containing 0.5% human serum albumin (HSA; Alpha Therapeutics, Los Angeles, CA), 0.1% dextrose, and 0.3 mmol/L EDTA. Cell fractions eluting at 48 to 70 mL/minute (Fr 48-70 cells) were pooled and subsequently treated with 5 mmol/L phenylalanine methyl ester hydrochloride (PME; Terumo Medical Corp, Elkton, MD) to remove residual monocytes and immature granulocytes.24 25The Fr 48-70 cells were resuspended to a concentration of 2 × 107 cells/mL in Minimal Essential Medium (MEM; Gibco, Grand Island, NY) and mixed with an equal volume of freshly prepared 10 mmol/L PME in MEM, plus 200 U/mL recombinant DNAse (Benzonase; Nycomed Pharma, Copenhagen, Denmark). The PME solution was adjusted to pH 7.4 with 0.5 mol/L NaOH before mixing with the cells. The cells were incubated for 40 minutes at room temperature, then 30 minutes at 37°C. The PME-treated cells were centrifuged through 20% Percoll (Pharmacia, Uppsala, Sweden) at 2,000g to remove debris. Residual erythrocytes were lysed with 150 mmol/L ammonium chloride lysis buffer, pH 7.5, for 5 minutes at 4°C and washed in PBS containing 1% HSA. Before staining, the cells were incubated with 0.1% human immunoglobulin (Gammimune; Bayer, Inc, Berkeley, CA) for 10 minutes at 4°C. The cells were stained at a concentration of 2 × 107 cells/mL for 20 minutes at 4°C with a panel of SyStemix-manufactured monoclonal antibodies that recognize CD34, Thy1, CD14, and CD15 antigens (2.5 μg/mL CD34-Sulforhodamine; 2.5 μg/mL Thy1-Biotin; 1 μg/mL CD14-FITC; 1 μg/mL CD15-FITC). Without washing, streptavidin (Societa Prodotti Antibiotici; Milano, Italy) was added to the cells at a final concentration of 0.15 mg/mL and incubated for an additional 20 minutes. Cells were washed twice in cold PBS with 1% HSA; 2 × 107/mL cells were resuspended and incubated for 20 minutes at 4°C with 5 μg/mL PE-biotin (SyStemix-manufactured). In preparation for sorting, the cells were washed once with cold PBS with 1% HSA and resuspended to 2 × 107 cells/mL in the same buffer solution plus 100 U/mL Benzonase. Cells were sorted using a SyStemix-manufactured dual laser high-speed fluorescence-activated cell sorter. FITC and PE were excited by an argon laser emitting 488 nm light and sulforhodamine (SRG) was excited by a rhodamine 6G dye laser emitting 590 nm light. Forward angle and side scatter sort windows were set up to exclude very small or very large cells and cell clusters. Selection criteria for CD34 (SRG) and the lineage markers CD14 and CD15 (Lin-FITC) were based on the fluorescence of unstained cells (autofluorescence), and the selection criteria for the Thy1(PE) were based on the background fluorescence of cells stained with anti–Thy1-biotin and biotin-PE with no streptavidin. Cells were sorted at 4°C at a rate of 15,000 to 20,000 cells per second. The CD34+ Thy1+Lin− cells were sorted directly into a culture tube containing a small volume of SyStemix-manufactured culture media.
Cryopreservation.
After sorting, the purified HSC suspension was washed free of sorting sheath fluid, counted with a hemacytometer, and resuspended in SyStemix-manufactured cryopreservation media containing 2% hetastarch, 4% HSA, and 7.5% dimethyl sulfoxide (DMSO) as cryoprotectants, and aliquoted into four cryovials (two per transplant). Cells were frozen using a programmable step-down freezer (Cryomed #110; Forma Scientific, Marietta, OH) with a freezing profile previously optimized for HSC survival. The step-down freezer is programmed to freeze at −1°C/minute until the sample reaches −45°C, then at −10°C/minute until the sample reaches −140°C. After the product temperature reached −140°C, the vials were transferred to the liquid phase of a liquid nitrogen cryostorage tank until they were shipped to the University of Arkansas for Medical Sciences in a liquid nitrogen dry shipper (CP65; Taylor-Wharton Scientific, Harrisburg, PA). The selected cells were stored at the University of Arkansas in the liquid phase of nitrogen until the time of transplantation, when the selected cells were rapidly thawed in a warm water bath, diluted in heparinized medium, and infused via a central venous line.
RESULTS
Mobilization phase.
Fifteen patients received HD CTX 6 g/m2 and GM-CSF 250 mg/m2 for stem cell mobilization with the intent to select CD34+ Thy1+ Lin−cells, but five patients never received a transplant with selected stem cells, due to early termination of the study (see Materials and Methods). Clinical data and outcome of the remaining 10 patients are summarized in Table 1. One patient failed to meet the minimum criteria on 3 consecutive days to proceed with the selection procedure (see Materials and Methods). This patient had received seven cycles of the M2 protocol [vincristine, 1,3-bis(2-chloroethyl)nitrosourea (BCNU), melphalan, cyclophosphamide, and prednisone] before the mobilization phase. He had a total of 5.37 × 106/kg CD34+ cells collected and proceeded with high dose melphalan supported by an unselected peripheral stem cell transplant. The median number of CD34+ cells collected in the remaining nine patients was 30.5 × 106/kg (range, 9.2 to 84.7), obtained during one apheresis procedure in one patient, two procedures in two patients, three in five patients, and four in one patient. The median number of CD34+Thy1+ Lin− cells collected was 10.5 × 106/kg (range, 8.5 to 19). The median recovery rate of CD34+ cells was 6% (range, 3% to 13%). The median purity of CD34+ Thy1+Lin− cells was 91%; this percentage is a conservative estimate, as the remaining events were almost entirely due to contaminating RBC and cell debris. The median cell viability was 98% after sorting and 86% after thawing before infusion (Table 2).
Clinical Data and Outcome
| . | Sex/Age . | Isotype . | Stage . | Duration Standard Therapy (months) . | CD34/kg Collected (×106) . | CD34+ Thy1+/kg per Transplant (×105) . | Viability Postthawing (%) . | Response . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M/54 | IgGλ | IIIB | 4 | 31.4 | 3.9 | 82 | NR | Died, MOF |
| 2 | M/52 | NS | IIIA | 7 | 24.4 | 3.7 | 79 | CR | Died, ARDS |
| 3 | M/55 | IgGκ | IIIA | 5 | 55.8 | 8.5 | 86 | NR | Alive, stable disease 20 months+ |
| 4 | M/42 | −κ | IIIB | 9 | 5.4 | — | — | PR | Failure to collect |
| 5 | M/58 | IgAκ | IIIA | 2 | 38.1 | 5.7 | 86 | NR | Alive, stable disease 19 months+ |
| 6 | M/57 | IgAλ | IIA | 2 | 27.6 | 4.6 | 75 | NR | Alive, stable disease 18 months+ |
| 7 | M/30 | NS | IIIA | 3 | 30.5 | 3.6 | 93 | CR | Alive, relapse 7 months |
| 8 | F/49 | IgGκ | IIA | 6 | 9.2 | 3.9 | 89 | NR | Alive, progression 10 months |
| 9 | M/47 | IgGκ | IIA | 3 | 84.7 | 3.6 | 88 | PR | Alive, progression 11 months |
| 10 | M/53 | IgGλ | IIA | 3 | 17.5 | 3.7 | 51 | NR | Alive, stable disease 16 months+ |
| . | Sex/Age . | Isotype . | Stage . | Duration Standard Therapy (months) . | CD34/kg Collected (×106) . | CD34+ Thy1+/kg per Transplant (×105) . | Viability Postthawing (%) . | Response . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M/54 | IgGλ | IIIB | 4 | 31.4 | 3.9 | 82 | NR | Died, MOF |
| 2 | M/52 | NS | IIIA | 7 | 24.4 | 3.7 | 79 | CR | Died, ARDS |
| 3 | M/55 | IgGκ | IIIA | 5 | 55.8 | 8.5 | 86 | NR | Alive, stable disease 20 months+ |
| 4 | M/42 | −κ | IIIB | 9 | 5.4 | — | — | PR | Failure to collect |
| 5 | M/58 | IgAκ | IIIA | 2 | 38.1 | 5.7 | 86 | NR | Alive, stable disease 19 months+ |
| 6 | M/57 | IgAλ | IIA | 2 | 27.6 | 4.6 | 75 | NR | Alive, stable disease 18 months+ |
| 7 | M/30 | NS | IIIA | 3 | 30.5 | 3.6 | 93 | CR | Alive, relapse 7 months |
| 8 | F/49 | IgGκ | IIA | 6 | 9.2 | 3.9 | 89 | NR | Alive, progression 10 months |
| 9 | M/47 | IgGκ | IIA | 3 | 84.7 | 3.6 | 88 | PR | Alive, progression 11 months |
| 10 | M/53 | IgGλ | IIA | 3 | 17.5 | 3.7 | 51 | NR | Alive, stable disease 16 months+ |
Abbreviations: NS, nonsecretory; MOF, multiorgan failure.
Details of the Collection Phase
| Median number CD34+ cells/kg collected (×106) | 24 (7 to 56) |
| Median number CD34+ cells/kg sorted (×106) | 1.1 (0.9 to 6.5) |
| Median number CD34+Thy+ cells sorted (×106) | 1.0 (0.85 to 1.9) |
| Median recovery rate of CD34+cells (%) | 6 (3 to 13) |
| Median purity of CD34+ cells (%) | 94 (89 to 96) |
| Median purity of CD34+ Thy+ (%) | 91 (82 to 95) |
| Median viability of sorted cells (%) | 96 (89 to 98) |
| Median viability of cell infused with Tx-1 (%) | 86 (51 to 93) |
| Median number CD34+ cells/kg collected (×106) | 24 (7 to 56) |
| Median number CD34+ cells/kg sorted (×106) | 1.1 (0.9 to 6.5) |
| Median number CD34+Thy+ cells sorted (×106) | 1.0 (0.85 to 1.9) |
| Median recovery rate of CD34+cells (%) | 6 (3 to 13) |
| Median purity of CD34+ cells (%) | 94 (89 to 96) |
| Median purity of CD34+ Thy+ (%) | 91 (82 to 95) |
| Median viability of sorted cells (%) | 96 (89 to 98) |
| Median viability of cell infused with Tx-1 (%) | 86 (51 to 93) |
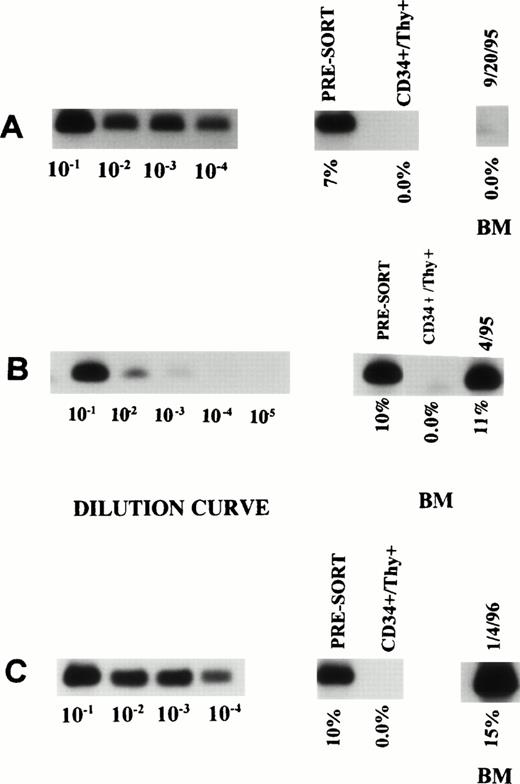
Tumor contamination of the selected stem cells.
The selected cells of three of the nine patients were analyzed for the presence of myeloma-related clonal B cells using PCR reaction for CDR III. With a sensitivity to detect one tumor cell in 100,000 nonclonal cells, no evidence of clonal B cells was detected in the FACS-sorted cells (Fig 1).
ASO-PCR on PBSC harvest, purified CD34+Thy1+ Lin− cells and posttransplant BM in three patients (A, B, and C). The first 4 or 5 lanes represent the dilution curves for each patient. The subsequent lanes represent DNA from unsorted as well as purified CD34+ Thy1+ Lin−cells. The last lane shows the degree of tumor contamination in the posttransplant BM.
ASO-PCR on PBSC harvest, purified CD34+Thy1+ Lin− cells and posttransplant BM in three patients (A, B, and C). The first 4 or 5 lanes represent the dilution curves for each patient. The subsequent lanes represent DNA from unsorted as well as purified CD34+ Thy1+ Lin−cells. The last lane shows the degree of tumor contamination in the posttransplant BM.
Engraftment kinetics, immunologic recovery, and toxicities.
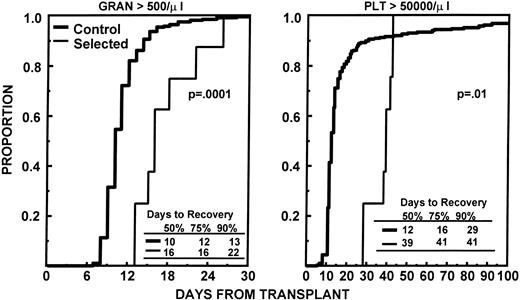
The nine patients with adequate quantities of CD34+Thy1+ Lin− cells received high dose melphalan at 200 mg/m2 as preparative regimen for their first transplant. The median number of sorted cells infused was 4.6 × 105/kg (range, 3.6 to 8.5). One patient failed to show signs of engraftment (ANC >0.5 × 109/L and platelets >20 × 109/L) by day 28 and received a nonselected PBSC graft on day 31. He reached an ANC of 0.5 × 109/L by day 37 and platelets > 20 × 109/L untransfused by day 44. The times to ANC and platelet recovery for the other eight patients are provided in Table 3. Figure 2 shows the engraftment kinetics of these eight patients, as well as those of a control group of patients, mobilized with an identical regimen and no more than 12 months of prior standard chemotherapy. For patients who received selected CD34+ Thy1+Lin− cells, the median times to ANC ≥0.5 × 109/L and ≥2.5 × 109/L were 16 and > 78 days, respectively; the median times to platelets ≥20, 50, and 100 × 109/L were 21, 39, and 43 days, respectively. Five patients required more than 100 days posttransplantation to recover their granulocyte count to ≥2.5 × 109/L and/or their platelets to ≥100 × 109/L. None of the eight evaluable patients had recovered an absolute CD4 count of more than 100/μL by day +100. Four patients received a delayed second transplant with unmanipulated stem cells 5, 6, 7, and 8 months, respectively, after their first transplant with CD34+Thy1+ Lin− cells; the CD4 count in these patients remained less than 100/μL up to the time of their second transplant. Toxicities and transfusion requirements were assessed on the nine patients who received selected cells. Seven patients developed fever > 101°F, but only one had a proven septicemia with streptococcus pneumoniae. Other moderate to severe toxicities observed were mucositis in four patients, diarrhea in seven patients, nausea and vomiting in eight patients, and esophagitis in one patient. The median numbers of RBC and platelet transfusions required after transplantation were four (range, 0 to 14) and 6 (range, 4 to 18), respectively.
Time to Engraftment (Days Posttransplantation)
| . | ANC (×109/L) . | Platelets (×109/L) . | |||
|---|---|---|---|---|---|
| 0.5 . | 2.5 . | 20 . | 50 . | 100 . | |
| 1 | 16 | >100 | 32 | 41 | >100 |
| 2 | 22 | 25 | 26 | 39 | 39 |
| 3 | 13 | >100 | 18 | 36 | 36 |
| 4 | 18 | 56 | 23 | 42 | 42 |
| 5 | 13 | 21 | 17 | 42 | >100 |
| 6 | 15 | >100 | 17 | 28 | 44 |
| 7 | 16 | 43 | 18 | 28 | 36 |
| 8 | 26 | >100 | 25 | 38 | 56 |
| Median | 16 (13-26) | >78 (21->100) | 21 (17-32) | 39 (28-42) | 43 (36->100) |
| . | ANC (×109/L) . | Platelets (×109/L) . | |||
|---|---|---|---|---|---|
| 0.5 . | 2.5 . | 20 . | 50 . | 100 . | |
| 1 | 16 | >100 | 32 | 41 | >100 |
| 2 | 22 | 25 | 26 | 39 | 39 |
| 3 | 13 | >100 | 18 | 36 | 36 |
| 4 | 18 | 56 | 23 | 42 | 42 |
| 5 | 13 | 21 | 17 | 42 | >100 |
| 6 | 15 | >100 | 17 | 28 | 44 |
| 7 | 16 | 43 | 18 | 28 | 36 |
| 8 | 26 | >100 | 25 | 38 | 56 |
| Median | 16 (13-26) | >78 (21->100) | 21 (17-32) | 39 (28-42) | 43 (36->100) |
Depicts the time to recover granulocytes to ≥0.5 × 109/L and platelets to ≥50 × 109/L for the eight patients who received CD34+Thy1+ Lin− cells compared with that of 128 control patients with ≤12 months of preceding standard chemotherapy who received unmanipulated PBSC mobilized with the same regimen.
Depicts the time to recover granulocytes to ≥0.5 × 109/L and platelets to ≥50 × 109/L for the eight patients who received CD34+Thy1+ Lin− cells compared with that of 128 control patients with ≤12 months of preceding standard chemotherapy who received unmanipulated PBSC mobilized with the same regimen.
The first three patients enrolled in this study received a second transplant after melphalan 140 mg/m2 and total body irradiation of 1,125 cGy. The number of sorted cells infused/kg were 1.6, 2.8, and 8.2 × 105, respectively; the viability of the infused cells was 78%, 56%, and 67%, respectively. Patient no. 1 developed a life-threatening enterocolitis with staphylococcus aureus and Clostridium difficile on day +14, requiring two surgical interventions. Because of these problems and the slow recovery after his first transplant, the patient received additional unselected PBSC on day +15. He recovered an ANC of 0.5 and 2.5 × 109/L on day +23 and 24, respectively. However, he developed multiorgan failure on day +22, requiring mechanical ventilation. He eventually died on day +91 of overwhelming septicemia. Patient no. 2 required infusion of nonselected PBSC on day +34 because of failure to engraft. On day +36, he developed a right upper lobe infiltrate, treated with broad-spectrum antibiotics. He recovered an ANC of 0.5 and 2.5 × 109/L on day 42 and 44, respectively. Because of progressive pneumonia, requiring mechanical ventilation on day +45, an open lung biopsy was performed on day +46, showing diffuse alveolar damage with no causative infectious agent. He eventually died on day +56 of adult respiratory distress syndrome (ARDS) and pulmonary hemorrhage. The third patient showed slow recovery after his second transplant with selected cells, but did not require infusion of nonselected mobilized PBSC. The times to ANC > 0.5 and > 2.5 × 109/L were 14 and 30 days, respectively; the times to platelets > 20, > 50 and > 100 × 109/L untransfused were 39, 39, and 62 days, respectively. This patient is alive 18+ months after his second transplant. Because of the difficulties encountered with these second transplants, the study was discontinued. After the first transplant with selected cells, two patients achieved a complete and two a partial remission. Three patients progressed at 7, 10, and 11 months, respectively, including one CR and one PR patient. Two patients died after their second transplant, as described above. The projected EFS and OS at 24 months is 60% and 80%, respectively.
DISCUSSION
The minimum quantities of highly purified and viable FACS-sorted hematopoietic cells (7.2 × 105/kg) required to support tandem transplant were obtained in nine of 10 patients who had received only limited standard chemotherapy before stem cell mobilization (≥12 months). The purity and viability of the selected cells before freezing was excellent (>90%). However, the recovery of CD34+ cells was low. Although only one patient required infusion of unselected mobilized PBSC as back-up after the first transplant with high dose melphalan, the median times to granulocyte recovery to > 0.5 × 109/L (16 days) and platelets > 50 × 109/L (39 days) were considerably longer than the 10 and 12 days, respectively, observed in our historical control group of patients with ≥12 months of prior chemotherapy receiving unselected cells (Fig 2). The delayed engraftment could theoretically be the result of the immature phenotype of the selected cells, requiring a longer time to provide sufficient quantities of granulocytes and platelets. Alternatively, it could be due to administration of inadequate amounts of hematopoietic stem cells. It remains unknown how many CD34+Thy1+ Lin− cells are required for full engraftment after transplantation. We and others have shown that after unselected PBSC transplants, prompt engraftment occurs with administration of ≥2 × 106/kg CD34+cells.23,26,27 The same number of CD34+ cells selected with an avidin-biotin immunoadsorption method also provides rapid hematologic recovery posttransplantation.12-14Thy1 expression has been detected on approximately 25% of adult BM CD34+ cells.28 Based on these data, we assumed that a dose of 0.5 × 106/kg FACS-sorted CD34+ Thy1+ Lin−cells would allow prompt engraftment. Taking into account an average 90% viability and a 90% purity of the selected cells, a cell dose of 3.6 × 105 pure and viable CD34+Thy1+ Lin− cells/kg should have been sufficient for a single transplant. Thy1expression, however, of mobilized CD34+ PBSC is higher than on BM CD34+ cells (median, 25% to 50%),29,30with the highest percentages of CD34+Thy1+ Lin− cells present during the first 2 days of collections.31,32 It appears much more plausible that the delayed engraftment observed in our study was due to infusion of inadequate quantities of purified stem cells based on the following circumstantial evidence. First, in a study by Schiller et al14, transplantation of < 2 × 106/kg selected CD34+ PBSC after high dose chemotherapy for advanced myeloma resulted in significantly prolonged neutropenia and thrombocytopenia with median times to ANC > 0.5 × 109/L and platelets > 20 × 109/L of 14 and 21 days versus 12 days for both parameters when ≥2 × 106/kg selected cells were infused. The engraftment times of patients with insufficient CD34+ cells in that study were comparable with our findings. Second, in lethally irradiated mice, rapid and sustained hematopoietic recovery was observed when > 200 purified SCA1+Thy1+ Lin− cells were transplanted and very little difference in engraftment kinetics was observed between mice receiving purified BM stem cells and those administered unmanipulated BM containing identical numbers of SCA1+ Thy1+Lin− cells.33 Even when purified stem cells were mixed with SCA1− BM cells in a competitive repopulation assay, most of the early myeloid cells were derived from the SCA1+Thy1+ Lin− cells, indicating their superiority over SCA1− cells, even in the early phases of myeloid reconstitution.33 Third, if delayed engraftment was caused by the immaturity of the purified stem cells, a swift increase in granulocyte and platelet counts to normal levels would have been expected, once engraftment had been established. This was not the case in our study.
Because of the delayed engraftment observed in our patients, the question arises whether the recovery in peripheral counts posttransplantation was effected by the infused progenitor cells or was the result of endogenous BM recovery, as melphalan 200 mg/m2 is probably not a myeloablative regimen. No data are available on recovery of BM function after administration of such a high dose of melphalan without stem cell rescue. However, when melphalan 140 mg/m2 was administered to 56 patients with GM-CSF support, but without a stem cell graft, the median time to ANC > 0.5 × 109/L was 23.5 days (range, 21 to 28),34 considerably longer than the 16 days (range, 13 to 26) observed in our study. No data on platelet recovery were available in this French study. In our patient no. 3, a day +15 BM showed 50% cellularity with all hematopoietic lineages well represented and with only 5% plasma cells. Such a high cellularity is not expected after high dose melphalan without stem cell rescue. This same patient showed full engraftment, although delayed, after his second autotransplant with selected cells when he received a truly myeloablative conditioning regimen with melphalan 140 mg/m2 and total body irradiation of 1,125 cGy. Therefore, it appears likely that the purified hematopoietic stem cells contributed to a large extent to hematopoietic recovery, although we cannot completely exclude that in some patients hematologic recovery might have been due at least in part to endogenous BM recovery.
Although the viability of the sorted cells was high before freezing and before administration of the first autotransplant, cell viability in two of the three patients receiving a second autotransplant was low (56% and 67%). It is unclear whether the decrease in viability with the second transplant is the consequence of increased fragility of the purified cells because of the long sorting process, or due to the storage of only small quantities of cells for several months.
Finally, the clinical impact of tumor-free grafts on the outcome of MM patients remains questionable at this time. An average unmanipulated PBSC graft contains approximately 108 myeloma cells.6 Only one third of all patients (untreated and treated, including refractory MM) referred to our center and scheduled to receive tandem transplants achieve a CR.7 The other patients not attaining a CR still have at least 109 myeloma cells left after tandem transplants.35 This is approximately 10 to 100 times more than the average amount of myeloma cells infused with each transplant.6 Unless our high dose chemotherapy can reduce the tumor load to < 106 myeloma cells, ie, a ≥7 log reduction of malignant cells from the time of diagnosis,35 stem cell selection is unlikely to be beneficial. Such a level of tumor reduction is unlikely to occur in > 15% of patients, ie, half of the patients who attain a CR. Moreover, stem cell selection processes deplete not only tumor cells, but also T cells, which will prolong immune reconstitution posttransplantation, as suggested by our study; this will be even more pronounced if highly immunosuppressive preparative regimens are used containing total body irradiation.36 It appears much more likely that posttransplantation immunologically-based strategies such as idiotype vaccination, dendritic cell infusions, or humanized monoclonal antibodies targeting the clonogenic myeloma cells, will ultimately improve the outcome in MM. These interventions should affect both myeloma cells remaining in the patient posttransplantation and myeloma cells reinfused with the graft.
ACKNOWLEDGMENT
The authors gratefully acknowledge the many physicians who referred patients for the study, the technical expertise of Sandy Mattox and Dwayne Bracy, the expert and compassionate care of the bone marrow transplant staff, and the diligent secretarial assistance provided by Conelia Williamson and Michele Mullins.
Supported in part by Grant No. CA 55819 from the National Cancer Institute, Bethesda, MD.
Address reprint requests to G. Tricot, MD, PhD, Bone Marrow/Stem Cell Program, University of Maryland Cancer Center, Room 922C, 22 S Greene St, Baltimore, MD 21201-1595.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal