Abstract
Ex vivo expansion of hematopoietic stem cell (HSC) is an attractive technology for its potency of a variety of clinical applications. Such a technology has been achieved to some extent with combinations of various cytokines or continuous perfusion cultures. However, much more improvement is required especially for expansion of primitive hematopoietic progenitors. We propose here a novel molecular approach that might have the potential to compensate the current expansion. We designed an adenovirus vector to transiently express human epidermal growth factor receptor (EGFR), which is known to transduce only a mitogenic, but not a differentiation signal to mouse bone marrow cells on human purified CD34+ peripheral blood (PB) cells, and tried to expand these cells with EGF ex vivo. Because we found that exposure of CD34+ PB cells to cytokines induced surface expression of adenovirus-internalization receptor and rendered these cells permissive to adenovirus infection, we infected these cells with the adenovirus vector carrying EGFR gene in the presence of cytokines. Two-color flow cytometric analysis demonstrated that 60.3% ± 22.4% of CD34+ cells expressed the adenovirus-mediated EGFR. Moreover, long-term culture-initiating cell assay showed that adenovirus vector could transduce more primitive progenitors. Subsequently, we tried to expand these cells in suspension culture with EGF for 5 days. Methylcellulose clonal assay showed that EGF induced 5.0- ± 2.4-fold proliferation of the colony-forming unit pool during 5 days of expansion. The simple procedure of efficient adenovirus gene delivery to immature hematopoietic cells proved promising, and this technique was potentially applicable for a novel strategy aiming at ex vivo expansion of hematopoietic progenitors.
A GREAT INTEREST has been focused on ex vivo expansion of hematopoietic stem cells (HSCs) in clinical hematology for a variety of clinical applications.1,2 It could complement current bone marrow (BM) or peripheral blood stem cell transplantation (PBSCT) technology. We would be able to use cord blood stem cells for allogeneic transplantation to adults, or much fewer PBSCs would be sufficient for PBSCT. Finally, as a target of hematological gene therapy, ex vivo expansion of HSCs is an indispensable technology. To date, many previous studies reported amplification of hematopoietic progenitors, and most of them used various combinations of cytokines3 or stroma cells.4 In particular, incubation of purified CD34+ cells with combinations of interleukin-3 (IL-3) and other cytokines has been the most commonly studied technique, and some groups reported successful clinical application of this technique.5-7 However, IL-3 acts on relatively late-stage progenitors and would differentiate these cells rather than proliferate.1 Therefore, a technique to expand primitive progenitors with keeping their immaturity is required.
To address these issues, we propose here a novel molecular approach that might have the potential to enhance the current expansion. We tried to express epidermal growth factor receptor (EGFR) transiently on human hematopoietic progenitors ex vivo and expand them with EGF as a self-renewal factor, because ectopic EGFR expressed on mouse BM cells was known to transduce only a mitogenic, but not a differentiation signal.8 Thus, it could be expected that hematopoietic immature cells expressing ectopic EGFR could be expanded by EGF without differentiation.
For this purpose, we used an adenovirus vector for gene delivery, because adenoviral DNA rarely integrate into host cell genome, which is favorable for our purpose in that no adverse effects would occur in patients after reinfusion of expanded progenitors. Furthermore, an adenovirus vector could transduce nonreplicating cells; thus, it has an advantage for transduction of quiescent HSCs.
Recently, gene transfer technique with an adenovirus vector has greatly advanced, and replication-deficient adenovirus vectors were used in some clinical trials for cystic fibrosis.9,10 Karlsson et al11 first succeeded in transducing an exogenous gene to hematopoietic cells using a recombinant adenovirus and suggested its potential use. However, only a few studies reported the application of this vector for hematopoietic systems.12 13
In the present study, we first examined the adenoviral gene transduction of hematopoietic progenitors. In particular, we analyzed the expression of adenovirus-internalization receptor on the surface of CD34+ cells. Second, we transduced exogenous EGFR to hematopoietic progenitors with a recombinant adenovirus vector and tried to expand them ex vivo to examine the feasibility of this novel molecular strategy.
MATERIALS AND METHODS
Cells and cell separation procedures.
Human peripheral blood (PB) mononuclear cells (MNCs) mobilized with cyclophosphamide and filgrastim were collected by apheresis from 7 patients undergoing therapy for nonhematologic malignancies after informed consent was obtained.
CD34+ cells were enriched using Isolex 50 Stem Cell Research Reagent Kit (Baxter Healthcare Corp, Deerfield, IL) according to the manufacturer's instructions. Final CD34+ cell fraction was stained with fluorescein isothiocyanate (FITC)-conjugated 8G12 monoclonal antibody (MoAb) to CD34 (anti–HPCA-2; Becton Dickinson, San Jose, CA) and fixed with paraformaldehyde, and the purity was quantitated with a FACSort flow cytometer (Becton Dickinson). More than 85% of the purified cells were verified to be CD34+ by flow cytometric analysis.
For the experiments of Table 1, Fig 3, and Fig 5, CD34+cells, CD34+ EGFR+ cells, and CD34+EGFR− cells were sorted with a flow cytometer (FACS Vantage; Becton Dickinson), respectively. The purity of each sorted population as verified by reanalysis was greater than 95%.
Colony Formation From CD34+EGFR+ and CD34+ EGFR− Cells in Clonal Culture
| . | No. of Colonies per 200 Cells . | ||||
|---|---|---|---|---|---|
| CFU-Mix . | BFU-E . | CFU-GM . | CFU-M . | Total . | |
| CD34+ EGFR+ | 2.3 ± 1.2 | 9.3 ± 1.5 | 43.0 ± 5.2 | 5.7 ± 0.6 | 60.3 ± 6.0 |
| CD34+ EGFR− | 1.0 ± 0.0 | 4.7 ± 2.5 | 53.3 ± 5.9 | 6.7 ± 1.2 | 65.7 ± 7.2 |
| . | No. of Colonies per 200 Cells . | ||||
|---|---|---|---|---|---|
| CFU-Mix . | BFU-E . | CFU-GM . | CFU-M . | Total . | |
| CD34+ EGFR+ | 2.3 ± 1.2 | 9.3 ± 1.5 | 43.0 ± 5.2 | 5.7 ± 0.6 | 60.3 ± 6.0 |
| CD34+ EGFR− | 1.0 ± 0.0 | 4.7 ± 2.5 | 53.3 ± 5.9 | 6.7 ± 1.2 | 65.7 ± 7.2 |
Enriched CD34+ cells were infected with the Ax/hEGFR virus and sorted into CD34+ EGFR+ versus CD34+ EGFR− cell populations. Two hundred cells of each population were cultured in methylcellulose culture with SCF, IL-6, IL-3, G-CSF, Tpo, and Epo, and colonies were scored on day 14. Results represent the mean number of colonies per 200 cells ± SD of triplicate cultures.
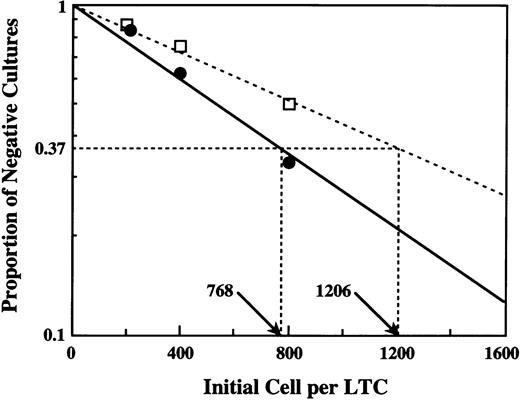
Quantitation of LTC-IC by limiting dilution analysis. Four dilutions of CD34+ EGFR+ cells (•) and CD34+ EGFR− cells (□) were cultured over irradiated stromal layers, and the number of clonogenic cells detectable after 7 weeks was determined. In this experiment, the frequency of LTC-IC was 1:768 cells in CD34+EGFR+ cells (—) and 1:1,206 in CD34+EGFR− cells (---).
Quantitation of LTC-IC by limiting dilution analysis. Four dilutions of CD34+ EGFR+ cells (•) and CD34+ EGFR− cells (□) were cultured over irradiated stromal layers, and the number of clonogenic cells detectable after 7 weeks was determined. In this experiment, the frequency of LTC-IC was 1:768 cells in CD34+EGFR+ cells (—) and 1:1,206 in CD34+EGFR− cells (---).
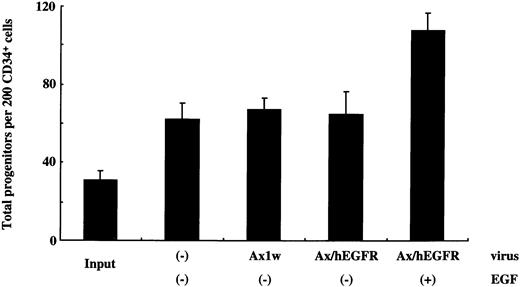
Generation of total progenitors after infection and liquid culture of sorted 200 CD34+ cells. One-milliliter cultures were initiated with 1.0 × 104 sorted CD34+ cells in the StemPro-34 SFM Complete Medium containing IL-6 and SCF in the presence or absence of EGF. These cells were incubated with the Ax1w or Ax/hEGFR virus, and control cells were cultured without adenovirus vectors. After 5 days of incubation, 1/50 of the cells of each fraction were subjected to methylcellulose clonal culture with SCF, IL-6, IL-3, and Epo in triplicate. The data presented are from a single patient, and the mean ± SD of results from three dishes is shown.
Generation of total progenitors after infection and liquid culture of sorted 200 CD34+ cells. One-milliliter cultures were initiated with 1.0 × 104 sorted CD34+ cells in the StemPro-34 SFM Complete Medium containing IL-6 and SCF in the presence or absence of EGF. These cells were incubated with the Ax1w or Ax/hEGFR virus, and control cells were cultured without adenovirus vectors. After 5 days of incubation, 1/50 of the cells of each fraction were subjected to methylcellulose clonal culture with SCF, IL-6, IL-3, and Epo in triplicate. The data presented are from a single patient, and the mean ± SD of results from three dishes is shown.
Determination of expression of integrin αvβ3 on CD34+ cell surface.
PB MNCs mobilized and collected by apheresis were separated by Lymphoprep (1.077 g/mL; Nycomed Pharma AS, Oslo, Norway). Cells were washed with phosphate-buffered saline (PBS) containing 1% nonimmune rabbit serum and incubated in the presence of 25 μg of anti-integrin αvβ3 MoAb, LM609 (Biogenesis, Poole, UK) per milliliter for 30 minutes at 4°C. Cells were washed twice and then incubated with rabbit antimouse Ig antibody conjugated to phycoerythrin (PE; DAKO AS, Glostrup, Denmark) for 30 minutes at 4°C. Control cell samples were incubated with the secondary antibody alone. Cells were washed twice and then incubated with FITC-8G12 MoAb to CD34 or isotype FITC control (Becton Dickinson). After additional washes, cells were analyzed by a FACSort flow cytometer with the Cell Quest program (Becton Dickinson).
For cell activation studies, CD34+ cells were purified as described above and cultured with IL-3 (5 ng/ml; Kirin Brewery Inc, Takasaki, Japan), IL-6 (20 ng/mL; Kirin Brewery Inc), or stem cell factor (SCF; 100 ng/mL; Kirin Brewery Inc) in various combinations in RPMI 1640 medium containing 10% fetal calf serum (FCS). After 48 hours of incubation, the cells were double-stained and analyzed as described above. CD34+ cells cultured in medium containing 10% FCS without cytokines were stained in the same way and served as a control. To set the CD34+ fraction, cells that were incubated with FITC-labeled isotype control (mouse Ig G1; Becton Dickinson) instead of antihuman CD34 MoAb were used as a negative control (data not shown), and gated cells were analyzed for the expression of integrin αvβ3.
Recombinant adenovirus vectors.
The recombinant, replication-deficient adenovirus vectors encoding human EGFR were generated as previously described.14Briefly, the human EGFR coding sequence was placed under the control of CAG promoter in the pAx1CAwt expression plasmid15containing almost whole genome of adenovirus without E1A, E1B, and E3 regions (pAxCAT-hEGFR). The replication-deficient recombinant adenovirus AxCAT-hEGFR (Ax/hEGFR) was obtained by in vivo homologous recombination after cotransfection into the 293 human embryonic kidney cell line with pAxCAT-hEGFR, and adenovirus DNA-terminal protein complex was digested with EcoT22I.14 Viruses were purified by ultracentrifugation through two cesium chloride gradients and stored in Ca2+-Mg2+–free PBS with 10% glycerol at −80°C.16 The titer of produced adenovirus vectors was determined by a limiting dilution assay using the 293 cells.16 The mock replication-deficient vector Ax1w14 was generously provided by Dr I. Saito (University of Tokyo, Tokyo, Japan) and used as a control.
Determination of surface expression of EGFR on CD34+ cells.
For determination of adenovirus-mediated transduction efficiency, enriched CD34+ cells were cultured in 2 mL of RPMI 1640 medium containing 1% human serum albumin (HSA) at 1 × 105 cells per well. The cells were incubated at the indicated MOI of the Ax1w or Ax/hEGFR virus with 5 ng of IL-3, 20 ng of IL-6, and 100 ng of SCF per milliliter.
After 60 hours of infection, cells were incubated with antihuman EGFR MoAb (Ab-1; Oncogene Science Inc, Cambridge, MA) at a concentration of 1 μg/mL. Control cell samples were incubated with the isotype control antibody (anti-keyhole limpet hemocyanin, IgG2a; Becton Dickinson) in the same way. After washed with PBS twice, the cells were incubated with rabbit antimouse Ig antibody conjugated to PE (DAKO AS) for 30 minutes at 4°C. The cells were washed twice and then incubated with FITC-8G12 MoAb to CD34 or FITC-labeled isotype control (mouse Ig G1). After additional washes, cells were analyzed by a FACSort flow cytometer with the Cell Quest program (Becton Dickinson). The cells incubated with the FITC-labeled isotype control were used to set the upper boundary of the negative cell autofluorescence and CD34+ fraction (data not shown).
Clonal culture of sorted CD34+EGFR+ and CD34+EGFR− cells.
CD34+ PB cells were enriched by immune beads and infected with Ax/hEGFR in the same way. After 60 hours of infection, the cells were sorted into CD34+ EGFR+ or CD34+ EGFR− fractions and were subsequently incubated in triplicate at concentrations of 200 cells/mL in methylcellulose culture, as previously reported.17,18 One milliliter of culture mixture containing cells, 0.9% methylcellulose (Shinetsu Chemical, Tokyo, Japan) -α minimum essential medium (αMEM), 30% fetal bovine serum (FBS; Hyclone Laboratories Inc, Logan, UT), 1% deionized fraction V bovine serum albumin (BSA; Sigma Chemical Co, St Louis, MO), 0.05 mmol/L 2-mercaptoethanol, 20 ng/mL IL-3, 100 ng/mL IL-6, 100 ng/mL SCF, 10 ng/mL granulocyte colony-stimulating factor (G-CSF; Kirin Brewery Inc), 10 ng/mL thrombopoietin (Tpo; Kirin Brewery Inc), and 2 U/mL erythropoietin (Epo; Kirin Brewery Inc) was plated into 35-mm Lux standard nontissue culture dishes (Nunc, Roskilde, Denmark) and incubated at 37°C in a humidified atmosphere flushed with 5% CO2 in air. All cultures were scored at day 14 according to criteria reported previously.17-19
A limiting dilution analysis of long-term culture-initiating cells (LTC-IC).
To prepare feeders, MNCs obtained from the adult BM donor were used as described.20-22 The MNCs were first cultured at a concentration of 2 × 106 cells/mL in LTC medium (MyeloCult H5100; Stem Cell Technologies Inc, Vancouver, British Columbia, Canada) supplemented with 10−6 mol/L hydrocortisone. After culturing for 3 weeks, greater than 80% of the confluent stromal layers were irradiated (15 Gy of 250-kV peak x-rays) and trypsinized. The cells were resuspended in LTC medium and seeded in 96-well flat-bottomed microwell plates at 3 × 104 cells per well for reestablishing the stromal feeder layer. The following day, the sorted CD34+ EGFR+ cells and CD34+ EGFR− cells were plated into each of the 96 wells at four different dilutions (range, 100 to 800 cells/well), with a total volume of 200 μL/well. At weekly intervals, half of the nonadherent cells were removed; at the same time, half of the medium was replaced. After 7 weeks, the nonadherent cells and the adherent cells suspended by repetitive pipetting were plated in clonal methylcellulose culture supplemented with SCF, IL-6, IL-3, G-CSF, and Epo to determine the total clonogenic cell content of each LTC.
Ex vivo expansion and colony-forming assay.
For expansion in suspension culture, purified 1.0 × 104CD34+ cells were cultured in 1 mL of RPMI 1640 medium containing 1% HSA, 20 ng of IL-6, and 100 ng of SCF per milliliter in the presence or absence of 2 ng of EGF per milliliter. The cells were incubated with the Ax1w or Ax/hEGFR virus at a multiplicity of infection (MOI) of 1,000 plaque-forming units (pfu)/cell and control cells were cultured without adenovirus vectors. After 5 days of incubation at 37°C in 5% CO2 and 5% O2, cells were washed with PBS and 1/50 cells were subjected to semisolid cultures in 1 mL of 1.2% methylcellulose-αMEM containing 30% FBS, 1% BSA, 0.05 mmol/L 2-mercaptoethanol, 5 ng/mL IL-3, 20 ng/mL IL-6, 100 ng/mL SCF, and 2 U/mL Epo in duplicate after removal of viral particles. After 14 days of incubation at 37°C in 5% CO2 and 5% O2, colony-forming unit–granulocyte-macrophage (CFU-GM) and burst-forming unit-erythroid (BFU-E) were scored using a dissecting microscope and standard criteria for their identification.17 The experiments were repeated five times using CD34+ cells from 5 patients, respectively. For the experiment of Fig 5, sorted CD34+ cells from 1 patient and specific serum-free medium (StemPro-34 SFM Complete Medium; Life Technologies, Rockville, MD) were used, and other procedures were performed in the same way.
RESULTS
Cytokine-induced expression of integrin αvβ3 on CD34+ cell surface.
To investigate the feasibility of adenoviral transduction to normal hematopoietic progenitor cells, we analyzed surface expression of integrin αvβ3, which is known to be one of the internalization receptors for adenovirus, on human CD34+ PB cells (Fig 1). A previous work demonstrated that the adenovirus attachment to the cell surface and the subsequent step of internalization occur through distinct receptors23; they showed that the vitronectin-binding integrins αvβ3 and αvβ5 promote internalization of adenovirus.
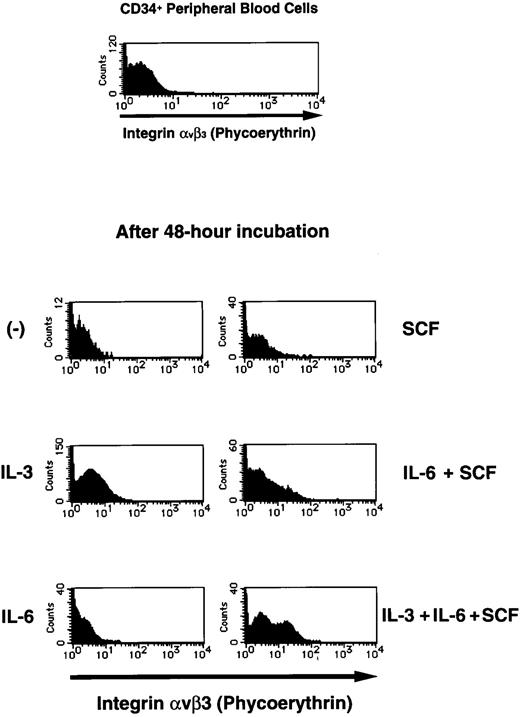
Flow cytometric analysis of integrin αvβ3 expression on human CD34+ PB cells. For cell activation study, purified human CD34+ PB cells were incubated in the absence or presence of IL-3 (5 ng/mL), IL-6 (20 ng/mL), or SCF (100 ng/mL) for 48 hours. Flow cytometric analysis was performed by staining for both CD34 and integrin αvβ3 simultaneously as described in the Materials and Methods. First, CD34+ PB cells were gated, and the expression of integrin αvβ3on gated CD34+ PB cells was shown.
Flow cytometric analysis of integrin αvβ3 expression on human CD34+ PB cells. For cell activation study, purified human CD34+ PB cells were incubated in the absence or presence of IL-3 (5 ng/mL), IL-6 (20 ng/mL), or SCF (100 ng/mL) for 48 hours. Flow cytometric analysis was performed by staining for both CD34 and integrin αvβ3 simultaneously as described in the Materials and Methods. First, CD34+ PB cells were gated, and the expression of integrin αvβ3on gated CD34+ PB cells was shown.
We found that a very small population (1.76% ± 1.12%) of CD34+ PB cells expressed integrin αvβ3 and that this was unchanged after these cells were cultured in the absence of cytokines for 48 hours. However, exposure of these cells to various cytokines induced cell surface expression of integrin αvβ3 on CD34+ PB cells (Fig 1). Among the cytokines examined, IL-3 raised the surface expression of integrin αvβ3 most strongly. Moreover, the various combinations of cytokines induced the expression more than a single cytokine. In particular, exposure of CD34+ cells to the combination of IL-3, IL-6, and SCF for 48 hours induced cell surface expression of integrin αvβ3 on 33.3% ± 26.5% of these cells (Fig 1). These results suggested that exposure of CD34+ PB cells to cytokines could confer susceptibility to adenovirus infection and increase adenoviral transduction efficiency.
Adenovirus transduction of human CD34+ PB cells.
Whereas adenoviral gene transfer into the adherent cells is achieved with quite a high efficiency, that into hematopoietic cells was reported to be lower in the previous study.24 Next, we evaluated efficiency of adenoviral gene transfer into human hematopoietic progenitors. We constructed a recombinant replication-deficient adenovirus vector expressing human EGFR (Ax/hEGFR) and used them to monitor viral transduction. The gene product is transmembrane protein; thus, we could detect expression of this gene in viable cells by the standard flow cytometric analysis.
We infected CD34+ cells purified from human PB MNCs (purity was >85% in each experiment) with the Ax/hEGFR virus at an MOI of 250 or 1,000 pfu/cell and analyzed surface expression of EGFR after 60 hours of incubation in the serum-free medium containing IL-3, IL-6, and SCF, because we found that the presence of FCS decreased the efficiency of adenoviral gene transfer using a human leukemic cell line (data not shown). Before the analysis, we confirmed that CD34+ PB cells expressed very little EGFR on their surface endogenously, and this was unchanged during ex vivo culture (data not shown). We also confirmed that these high MOIs had little influence on loss of viability of CD34+ cells by the Trypan Blue dye exclusion test under these conditions.
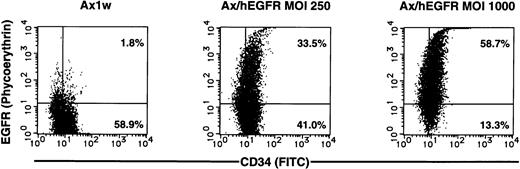
Two-color flow cytometric analysis showed that 60.3% ± 22.4% of the CD34+ cells expressed EGFR (representative result in Fig2). However, only 4.61% ± 1.55% of the CD34+ cells expressed EGFR in the medium containing FCS without cytokines (data not shown). Cells infected with the mock Ax1w virus were negative, indicating that the observed staining is not due to exposure of these cells to adenoviral particles. For the subsequent experiments of ex vivo expansion, we also analyzed surface expression of EGFR after incubation in IL-6 and SCF and observed that it was not significantly changed (data not shown).
Representative result of two-color flow cytometric analysis of enriched CD34+ PB cells after 60 hours of infection with the Ax1w (mock vector) or Ax/hEGFR virus at an MOI of 250 or 1,000 pfu/cell. Enriched CD34+ PB cells (purity, 86.2%) were cultured with each adenovirus vector in a serum-free medium in the presence of cytokines. After 60 hours of infection, cells were double-stained simultaneously and analyzed as described in the Materials and Methods.
Representative result of two-color flow cytometric analysis of enriched CD34+ PB cells after 60 hours of infection with the Ax1w (mock vector) or Ax/hEGFR virus at an MOI of 250 or 1,000 pfu/cell. Enriched CD34+ PB cells (purity, 86.2%) were cultured with each adenovirus vector in a serum-free medium in the presence of cytokines. After 60 hours of infection, cells were double-stained simultaneously and analyzed as described in the Materials and Methods.
Colony-forming ability of transduced CD34+ cells.
To show directly that adenoviral vectors were transducing functional progenitors, we sorted infected cells into CD34+EGFR+ cell versus CD34+ EGFR−cell populations and assayed for clonogenic cultures. As shown in Table1, CD34+ EGFR+cells clearly do have colony-forming ability at the almost same frequency as that of the CD34+ EGFR− cells. CD34+ EGFR+ cells could especially produce Mix colonies, suggesting that more immature progenitors could be transduced with adenovirus vectors. Thus, the recombinant adenovirus was shown to be a useful vehicle for readily efficient gene transduction of human hematopoietic progenitors.
Gene transduction of human LTC-ICs by adenovirus vectors.
Subsequently, we assayed the frequency of LTC-ICs, which are believed to reflect primitive hematopoietic progenitor cells, in each sorted population. Purified CD34+ PB cells by immune beads were infected during 60 hours of incubation in the same way and sorted into two populations, CD34+ EGFR+ cells versus CD34+ EGFR− cells. Each population was distributed into microwells containing pre-established irradiated adherent layer cells. For each evaluation, 4 cell concentrations were used with 8 replicates per concentration. The frequency of negative wells (no clonogenic progenitors detectable 7 weeks later) was then determined. The frequency of LTC-ICs in the starting population was calculated by Poisson statistics and the weighted mean method with iterative procedures to determine the best linear fit (Fig3). The frequency of LTC-ICs in a population of CD34+ EGFR+ cells was 1:768, whereas that in a population of CD34+ EGFR−cells was 1:1,206, indicating that the CD34+EGFR+ population included more primitive progenitor cells than the CD34+ EGFR− population.
Ex vivo expansion of hematopoietic progenitors using EGFR-expressing recombinant adenovirus.
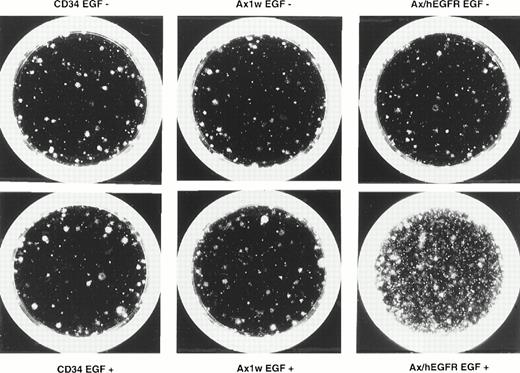
To examine the feasibility of ex vivo expansion of hematopoietic progenitors using EGFR-expressing recombinant adenovirus, we performed the methylcellulose clonal assay to evaluate the proliferation of colony-producing cells. In 5 experiments, we introduced the Ax1w (a mock adenovirus vector) or Ax/hEGFR virus to 1.0 × 104purified CD34+ PB cells at an MOI of 1,000 pfu/cell and cultured for 5 days in a serum-free medium containing IL-6 and SCF in the presence or absence of EGF. Under the same conditions, 1.0 × 104 uninfected CD34+ cells were cultured as a control. In this condition, we observed that transduction efficiency was equivalent to that in IL-3, IL-6, and SCF (data not shown). After 5 days of incubation, 1/50 of these cells were subjected to the semisolid culture assay in duplicate. As shown in Table2, the colony-forming unit pool from CD34+ PB cells incubated with the Ax/hEGFR virus and EGF was 5.0- ± 2.4-fold expanded, with a range of 2.6- to 8.2-fold during the 5 days of culture as compared with that of the uninfected CD34+ PB cells. However, colony-forming cells from CD34+ cells incubated with the Ax/hEGFR virus in the absence of EGF and with the Ax1w virus in the presence or absence of EGF showed no significant proliferation. No colony was formed from the suspension culture without cytokines in all conditions of CD34+ cells (data not shown). Not only the number of colonies, but also the EGF signal seemed to increase the size and cell density of colonies (Fig 4).Among proliferated colonies, BFU-E were predominantly expanded.
Effect of EGF Through Adenovirus-Mediated EGFR on the Expansion of Colony-Forming Cells
| Virus . | EGF . | No. of Colonies Derived From 200 CD34+ Cells . | |
|---|---|---|---|
| CFU-GM . | BFU-E . | ||
| (−) | (−) (+) | 29.4 ± 2.6 26.0 ± 9.3 | 2.6 ± 1.2 2.6 ± 1.5 |
| Ax1w | (−) (+) | 35.1 ± 11.5 38.6 ± 8.0 | 2.4 ± 1.5 2.8 ± 1.4 |
| Ax/hEGFR | (−) (+) | 36.4 ± 10.0 107.5 ± 38.7 | 2.6 ± 1.0 38.2 ± 27.5 |
| Virus . | EGF . | No. of Colonies Derived From 200 CD34+ Cells . | |
|---|---|---|---|
| CFU-GM . | BFU-E . | ||
| (−) | (−) (+) | 29.4 ± 2.6 26.0 ± 9.3 | 2.6 ± 1.2 2.6 ± 1.5 |
| Ax1w | (−) (+) | 35.1 ± 11.5 38.6 ± 8.0 | 2.4 ± 1.5 2.8 ± 1.4 |
| Ax/hEGFR | (−) (+) | 36.4 ± 10.0 107.5 ± 38.7 | 2.6 ± 1.0 38.2 ± 27.5 |
One-milliliter cultures were initiated with 1.0 × 104 enriched CD34+ cells in a medium containing IL-6 and SCF in the presence or absence of EGF. These cells were incubated with the Ax1w or Ax/hEGFR virus, and control cells were cultured without adenovirus vectors. After 5 days of incubation, 1/50 of the cells of each fraction were subjected to methylcellulose clonal culture with SCF, IL-6, IL-3, and Epo in duplicate. The experiments were repeated five times using CD34+ cells from 5 patients, respectively. The mean ± SD of five experiments is shown.
Photomicrograph of the effect of EGF through adenovirus-mediated EGFR on the expansion of colony-forming cells after 5 days of suspension culture. Purified CD34+ PB cells were expanded as described in the Materials and Methods in the presence or absence of EGF and plated in methylcellulose colony assay. This figure shows the representative results of day-28 colonies of the expanded cells from 2,000 CD34+ cells in each fraction. In the figure, CD34 denotes the results of the uninfected control fractions.
Photomicrograph of the effect of EGF through adenovirus-mediated EGFR on the expansion of colony-forming cells after 5 days of suspension culture. Purified CD34+ PB cells were expanded as described in the Materials and Methods in the presence or absence of EGF and plated in methylcellulose colony assay. This figure shows the representative results of day-28 colonies of the expanded cells from 2,000 CD34+ cells in each fraction. In the figure, CD34 denotes the results of the uninfected control fractions.
Using the cell sorter, we prepared more purified CD34+cells and repeated the same experiment to exclude the indirect effect of CD34− cells upon the experiment (Fig5). These results indicated that EGF could enhance proliferation of hematopoietic progenitors through the adenovirus-mediated EGFR specifically. Further analysis of CD34+ cells from BM showed similar results (data not shown) and suggested that the alternative sources of immature cells were also permissive for adenovirus infection and potentially effective for expansion.
DISCUSSION
To date, recombinant adenovirus vectors have been shown to be effective vehicles for transient expression of various genes in many systems.25 Indeed, in clinical trials for gene therapy to cystic fibrosis patients, replication-deficient adenovirus vectors were used to transfer the cystic fibrosis transmembrane conductance regulator gene to the airway epithelial surface.9 10 No significant toxicity was observed except for the elicited immune response in these studies. Therefore, the high efficiency, safety, and simplicity of handling in this vector system is very attractive.
However, as for hematopoietic system, only a few studies reported application of this vector.12 13 This might be due to two reasons. One is because transduction efficiency of the adenovirus vector to immature human hematopoietic cells has not been adequately evaluated. The other reason is because adenoviral gene transfer causes transient expression of the inserted gene, and the situation in which such transient expression is more favorable than stable expression has not been proposed.
Concerning adenoviral transduction to human leukemic cells, Wattel et al24 reported that adenovirus could not efficiently be used for direct gene transfer in leukemic cells. Recently, two studies using recombinant adenoviruses encoding lacZ or alkaline phosphatase reporter gene described the efficiency of adenoviral transduction to human CD34+ cells.26 27 They reported that 6.0% to 20.0% and 25% to 35% of CD34+ cells were infected, respectively.
In this study, we first analyzed the surface expression of integrin αvβ3 on human CD34+ PB cells. Previous works showed that human PB monocytes and T lymphocytes express a very small amount of integrin αvβ3, whereas exposure to cytokines or stimulation with mitogens induces its surface expression and renders these cells susceptible to adenovirus-mediated gene delivery.28 29 However, little is known about the expression of this molecule on human hematopoietic progenitors. Our findings showed that CD34+ PB cells did not natively express integrin αvβ3 on their surface, whereas exposure of these cells to cytokines induced cell surface expression of integrin αvβ3 (Fig1). Therefore, adenoviral gene transfer to the CD34+ PB cells would require two steps: induction of surface expression of adenovirus internalization receptor on CD34+ PB cells and adenovirus attachment and uptake into these cells. However, we observed that a small population of CD34+ PB cells cultured in medium without cytokines could also express adenovirus-mediated gene products (4.61% ± 1.55% of CD34+ PB cells; data not shown). This is probably because adenovirus vectors would be internalized via integrin αvβ5 known to function as another adenoviral receptor or other unidentified receptors.
Based on our findings described above, we introduced the Ax/hEGFR virus into the CD34+ PB cells and evaluated the efficiency of gene transduction. After 60 hours of incubation with the Ax/hEGFR virus at an MOI of 1,000 pfu/cell, 60.3% ± 22.4% of the CD34+cells expressed exogenous EGFR with no obvious cell toxicity (Fig 2). The transduction efficiency of our results was relatively higher than that of the previous report,26 possibly because we used cytokines to induce cell surface expression of adenovirus internalization receptors, in addition to the fact that we used the serum-free medium and the CAG promoter.30
To evaluate the transduction efficiency of immature progenitors with more accuracy, we sorted CD34+ PB cells expressing adenovirus-mediated EGFR after 60 hours of incubation and subjected these cells to semisolid clonal culture and LTC-IC assay. In clonogenic culture, the transduced CD34+ PB cells could produce various types of colonies, including CFU-Mix at the nearly same frequency as the nontransduced CD34+ cells (Table 1), indicating that immature progenitors such as CFU-Mix are permissive for adenovirus infection. Moreover, the result of LTC-IC assay showed that the transduced EGFR-expressing CD34+ cells contained a number of LTC-ICs (Fig 3). The frequency of LTC-IC in a population of the transduced CD34+ cells (1:768) was higher than that of the nontransduced CD34+ cells (1:1,206). This might suggest that immature progenitors would be more permissive to adenovirus infection, or LTC-IC expressing adenovirus-mediated EGFR could be expanded during culture with EGF contained in LTC medium. As a consequence of these results, adenovirus vectors were shown to be effective vehicles for gene transduction of human hematopoietic primitive cells.
Given the features of adenoviral transduction, ie, one is transient expression which is not subject to integration site-specific effects and another is unavoidable host immune reaction if used in vivo, we believed that the adenoviral gene delivery system would be suited for ex vivo expansion of hematopoietic immature cells. Thus, we tried to expand hematopoietic progenitors ex vivo through adenovirus-mediated EGFR.
We used EGF signal for expansion of hematopoietic progenitors with their immaturity, because EGFR expressed on mouse BM cells is reported to be able to transduce proliferative signal of EGF with no influence to differentiation.8 They introduced EGFR into primary mouse BM cells using retroviral gene transfer and showed that EGF acted on these cells synergistically with IL-3 in cell proliferation even under IL-3 saturation condition. In the avian hematopoietic system, c-erbB, the avian homologue of EGFR, has been shown to be present in very early avian stem cells and plays a physiological role in self-renewal.31,32 Using HL60 cells expressing EGFR introduced by retroviral gene transfer, Chen et al33 showed that exogenous EGFR confers a self-renewal signal that can shift the balance of RA-induced terminal differentiation toward self-renewal and results in a partial block of differentiation. These results previously reported suggested that EGF signal could have possibility to be used to amplify hematopoietic primitive cells without differentiation as neuronal cells, although we did not demonstrate it directly in this report.
We tried to expand colony-forming cells expressing adenovirus-mediated EGFR in suspension culture with EGF. As shown in Table 2, EGF could produce a 2.6- to 8.2-fold increase in colony-forming cells from CD34+ PB cells expressing adenovirus-mediated EGFR over 5 days of culture, whereas no significant proliferation of colony-forming cells from CD34+ PB cells incubated in other conditions was observed. It was also shown that the toxicity of adenovirus vectors under this condition was negligible, because we observed no significant difference between the colony-forming unit pools from the CD34+ PB cells uninfected and infected with the mock Ax1w adenovirus vector. A similar result was obtained from more purified CD34+ PB cells (Fig 5).
Interestingly, BFU-E showed more dominant expansion over CFU-GM (Table2). c-erbB, the avian homologue of EGFR, is known to transmit the self-renewal signal in avian erythroid progenitors.31,32 In mammalian hematopoiesis, a recent study of targeted disruption of mouse EGFR clearly showed that EGFR is not involved directly.34 However, our results might suggest that an unidentified signal would control proliferation of human erythroid progenitors as in avian erythropoiesis, and its intracellular downstream would be shared with that of EGFR. Further studies are required to clarify the presence and the characteristics of the self-renewal signal involved in human erythropoiesis.
Most approaches to ex vivo expansion of hematopoietic immature cells thus far use IL-3, leaving some unresolved issues. Especially, ex vivo expansion of more primitive progenitors has not been achieved, because these cells do not express the IL-3 receptor and IL-3 acts on relatively late-stage progenitors.35 To address this issue, Sui et al36 used IL-6 and SCF signal and succeeded in expansion of immature population of hematopoietic cells. In this study, we used those early-acting cytokines for minimal cytokine-induced differentiation and showed that EGF enhanced the expansion of colony-forming cells with those cytokines through the ectopic expression of EGFR. However, it is not clarified directly yet that our approach could proliferate primitive cells such as LTC-ICs. Indeed, there is a possibility that our results of expansion were caused from induced differentiation of more primitive progenitors by our manipulations. Thus, direct evaluation of expansion of more primitive progenitor populations is required.
Incidentally, the high efficiency and simplicity of adenoviral gene delivery to human primitive hematopoietic cells is very promising. Other genes coding for signaling molecules such as transcription factors or cytokine-dependent kinases could be candidates to be transferred with adenovirus vectors and promote the proliferation of hematopoietic progenitors. Moreover, this approach could be applied to proliferation of other kinds of primary cells derived from various tissues, such as neuronal or epithelial cells. For the future clinical application, determination of more effective signaling molecules, especially capable of expanding the self-renewal HSCs as well as examination of the safety of adenovirus vectors should be required.
ACKNOWLEDGMENT
The authors appreciate Izumu Saito and Yumi Kanegae for instruction of an efficient method of constructing recombinant adenoviruses. We thank Yoichi Shibata and Jyun-ichi Miyazaki for providing clinical samples and CAG promoter. We appreciate Kirin Brewery Inc and Baxter Healthcare Corp for generous donation of cytokines and Isolex 50.
Supported by grants from the Ministry of Education, Science, and Culture and the Ministry of Health and Welfare, Japan.
Address reprint requests to Hisamaru Hirai, MD, PhD, Associate Professor, The Third Department of Internal Medicine, Faculty of Medicine, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113, Japan; e-mail: hhirai-tky@umin.u-tokyo.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal