Abstract
Recombinant adeno-associated virus vectors (AAV) were prepared in high titer (1012 to 1013 particles/mL) for the expression of human factor IX after in vivo transduction of murine hepatocytes. Injection of AAV-CMV-F.IX (expression from the human cytomegalovirus IE enhancer/promoter) into the portal vein of adult mice resulted in no detectable human factor IX in plasma, but in mice injected intravenously as newborns with the same vector, expression was initially 55 to 110 ng/mL. The expression in the liver was mostly transient, and plasma levels decreased to undetectable levels within 5 weeks. However, long-term expression of human F.IX was detected by immunofluorescence staining in 0.25% of hepatocytes 8 to 10 months postinjection. The loss of expression was likely caused by suppression of the CMV promoter, because polymerase chain reaction data showed no substantial loss of vector DNA in mouse liver. A second vector in which F.IX expression was controlled by the human EF1α promoter was constructed and injected into the portal vein of adult C57BL/6 mice at a dose of 6.3 × 1010 particles. This resulted in therapeutic plasma levels (200 to 320 ng/mL) for a period of at least 6 months, whereas no human F.IX was detected in plasma of mice injected with AAV-CMV-F.IX. Doses of AAV-EF1α-F.IX of 2.7 × 1011particles resulted in plasma levels of 700 to 3,200 ng/mL. Liver-derived expression of human F.IX from the AAV-EF1α-F.IX vector was confirmed by immunofluorescence staining. We conclude that recombinant AAV can efficiently transduce hepatocytes and direct stable expression of an F.IX transgene in mouse liver, but sustained expression is critically dependent on the choice of promoter.
HEMOPHILIA B is the X-linked bleeding diathesis that results from a deficiency of functional factor IX (F.IX) in the circulation. Current treatment of hemophilia B consists of intravenous infusion of plasma-derived or recombinant F.IX in response to bleeds, but the expense and inconvenience of this approach have fueled efforts to establish an experimental basis for gene therapy of hemophilia B. F.IX is normally synthesized in the liver, and although biologically active F.IX can be synthesized in other tissues as well, the liver has been a favored target for gene therapy of hemophilia.1-4 Retroviral vectors have been used successfully to direct long-term expression of F.IX in livers of experimental animals, but the levels achieved have been 1 to 2 logs below that required for therapeutic efficacy.5 In addition, the requirement for dividing target cells has meant that vector infusion must be preceded by partial hepatectomy, an unappealing strategy for human trials. Adenoviral vectors, which do not require a dividing target cell, efficiently transduce liver and can direct high-level expression of F.IX in experimental animals after intravenous injection,6,7 but expression is short-lived because of a host immune response that eliminates vector-transduced cells.8 9
We have recently begun to explore the use of an adeno-associated viral vector (AAV) as a gene delivery vehicle for F.IX. AAV offers several advantages as a gene delivery vehicle: the parental wild-type AAV, a single-stranded DNA virus of 4,680 bases, it is nonpathogenic, and the vector is devoid of any viral coding sequences, reducing the risk of host immune response due to ongoing viral gene expression. Cells transduced by AAV vectors in vivo are apparently not targeted by a cellular immune response.10-12 The vector can accommodate inserts of up to 4.7 kb, can be prepared in titers exceeding 1012 particles/mL, and can transduce both dividing and nondividing cells. The biology of recombinant AAV (rAAV) transduction is still incompletely understood. In vitro studies have shown that rAAV transduction is enhanced by the presence of adenovirus; Ferrari et al13 and Fisher et al14 have used adenoviral deletion mutants to map this function to a specific adenoviral gene product (Ad E4 orf6), which acts to facilitate second-strand synthesis from an AAV template. Exposure to genotoxic reagents can exert a similar effect, presumably because these agents also trigger events that facilitate efficient production of duplex AAV.15,16 In vivo experiments with rAAV have given mixed results. Both muscles and central nervous system are stably and efficiently transduced with rAAV.10,11,17 Despite efficient delivery of the rAAV genome to liver, the number of hepatocytes expressing the transgene has been disappointingly low, unless AAV vector was injected simultaneously with adenovirus or the liver was irradiated before vector administration.14,18 However, a recent report shows that high levels of liver-derived expression from an AAV vector can be achieved without these modifications.19 In this work we demonstrate that (1) the cellular milieu of newborn mouse liver allows efficient transduction with an AAV vector; (2) stable, efficient gene transfer to hepatocytes is achieved with an AAV-CMV-F.IX vector, but expression decreases to undetectable levels over a period of 5 to 8 weeks; and (3) gene transfer to adult liver results in stable therapeutic plasma levels of F.IX when transgene expression is controlled by the human EF1α promoter.
MATERIALS AND METHODS
Viral vectors.
Plasmid pAAV-CMV-F.IX contains the AAV2 inverted terminal repeats (nucleotides [nt] 1-145 at both ends) flanking a human F.IX (hF.IX) minigene cassette. The hF.IX cassette was created as follows: exon 1 and a portion of intron I (to the PvuII site at nt 1098), and intron I (beginning at the PvuII site at nt 5882) and exon 2 (up to the Eae I site) were amplified from genomic DNA and ligated at the PvuII site.20 The remainder of the F.IX cDNA including 1.3 kb of the 3′ untranslated sequence (UT) was excised from an F.IX cDNA-containing plasmid as an Eae I-HindIII fragment, and ligated to the exon 1-intron I-exon 2 fragment at the Eae I site. This construct containing the hF.IX cDNA interrupted by a fragment of intron I was cloned into pUC 19, then excised at Kpn I and Apa I sites (eliminating all but 228 nt of the 3′ UT). The ApaI site was filled in and the insert cloned into the expression plasmid pCEP4 (Invitrogen, San Diego, CA).
Sal I sites were used to excise from the expression plasmid a cassette containing the CMV enhancer/promoter, the hF.IX sequences, and the SV40 poly A sequence. This hF.IX minigene cassette was cloned into psub 201, a plasmid containing AAV ITRs.21 Recombinant AAV-CMV-F.IX was produced and purified as outlined elsewhere.14,22,23 The genome titer of rAAV-F.IX was determined by slot-blot hybridization using a CMV-specific probe; serial dilutions of the pAAV-CMV-F.IX plasmid served as a standard. The titer of infectious units was determined by transduction of HEK 293 cell line 84-31 (stably transfected with adenovirus gene E4)14 with serial dilutions of rAAV followed by immunofluorescence staining for F.IX 24 hours posttransduction. Purified AAV-CMV-F.IX routinely lacked detectable amounts of contaminating adenovirus when analyzed by transduction of 293 cells followed by staining for β-galactosidase as described by Fisher et al.14 Wild-type AAV was detected with <1 infectious unit per 109 vector genomes using the method described by Fisher et al.10
A second vector was constructed based on plasmid pV4.1e-hF.IX. The eukaryotic expression vector p4.1e contains the enhancer/promoter sequence of human elongation factor 1α (EF1α), a 2.4-kb sequence,24 and the human growth hormone polyA signal. A 1.6-kb EcoRI-Apa I fragment representing the entire open reading frame of the hF.IX cDNA (with optimized Kozak consensus sequence) and 0.2 kb of the 3′ untranslated region was blunt ended and ligated into the HindII site (a unique site located between promoter and polyA sequences) of p4.1e creating p4.1e-hF.IX. The entire expression cassette of this plasmid was then excised as a 4,673-bp Not I fragment and inserted into the uniqueNot I site, between the two inverted terminal repeat (ITR) sequences, of plasmid pV to create pV4.1e-hF.IX. This plasmid was used for vector encapsidation using a newly developed adenovirus-free system resulting in vector AAV-EF1α-F.IX.25 Vector encapsidation was performed as described elsewhere,26 except that an adenovirus helper plasmid was used instead of adenovirus particles to supply E2a, E4, and VA. The physical vector titer was determined by a quantitative dot-blot assay.26
Transduction of cells in vitro.
In vitro transductions of HeLa cells were performed to establish that the vector AAV-CMV-F.IX can direct expression of hF.IX. HeLa cells were seeded at 20% confluency in a 24-well microtiter plate. Six hours later, 5 × 109 vector genomes of AAV-CMV-F.IX were added to the wells, and conditioned medium was assayed for hF.IX by enzyme-linked immunosorbent assay (ELISA) 36 hours later. Similarly, HepG2 cells were seeded in four-chamber slides (Nunc, Roskilde, Denmark) at 20% confluency (105 cells per chamber), and infected with AAV-CMV-F.IX 6 hours later (2 × 108 vector genomes per chamber). Medium (Dulbecco's modified Eagles's medium, including 2% fetal bovine serum) was changed every 24 hours and 100-μL aliquots were assayed for hF.IX by ELISA.7 Cells were fixed for immunofluorescence staining 96 hours after infection. For in vitro transduction experiments with AAV-EF1α-F.IX, 293 cells were seeded in a 12-well plate at a density of 1 × 105 cells/well and, 24 hours later, 6 × 1010 vector genomes were added to the wells. Twenty-four–hour conditioned media were collected on day 4 postinfection and assayed for F.IX using the Asserachrom IX:Ag ELISA kit (American Bioproducts, Parsippany, NJ).
Animal procedures.
CD-1 mice [Strain Crl: CD-1 (ICR) BR; Charles River Breeding Laboratories, Wilmington, MA] are an outbred strain. In adult mice (4 weeks old), AAV-CMV-F.IX vector was introduced into the portal circulation through injection beneath the splenic capsule as described by Fisher et al.14 Newborn mice were injected on day 1 of life via a superficial temporal vein. Total volumes used for injection were < 50 μL (1 to 2 × 1011 vector genomes of AAV-CMV-F.IX in HEPES-buffered saline, pH 7.8). To obtain enough plasma to measure hF.IX levels, some animals were killed during the first 2 weeks of life. Starting from week 5 after injection, the (now) adult mice were bled from the retro-orbital plexus as described by Walter et al.7 AAV vector (AAV-CMV-F.IX or AAV-EF1α-F.IX) was injected directly into the portal vein of adult C57BL/6 mice (5 weeks old). Briefly, animals were anesthetized with an intraperitoneal injection of ketamine and xylazine, and the portal vein was exposed through a ventral midline incision followed by displacing the intestinal duct. Fifty microliters of AAV vector solution was slowly injected into the portal vein with a Hamilton syringe (Hamilton Company, Reno, NV). The peritoneal cavity was sutured with 4-0 silk (Ethicon, Sommerville, NJ), and the skin was closed with 4-0 Vicryl (Ethicon).
Measurement of human F.IX and anti-hF.IX in mouse plasma.
ELISA for detection of hF.IX in mouse plasma was performed as outlined previously.7 Western blots to demonstrate the presence of anti-hF.IX were performed as described by Dai et al,27except that a horseradish peroxidase–conjugated anti-mouse IgG antibody was used to detect antigen-antibody complexes with enhanced chemiluminescence (ECL) reagent (Amersham, Arlington Heights, IL). Mouse plasma samples were diluted 1:200 or 1:500.
Immunofluorescence staining.
Liver and other tissues were excised from experimental animals and frozen in OTC embedding compound on a dry ice/methyl butane bath. Cryosections of tissue as well as cells grown on chamber slides were fixed with 3% paraformaldehyde in phosphate-buffered saline, pH 7.4, and subsequently stained for hF.IX as described.22 Fluorescence microscopy was performed with a Nikon FXA microscope (Nikon Inc, Melville, NY). Percentage of positive cells (six random fields per section at an appropriate magnification, at least six slides of liver cross section per animal) was determined with the Leica Q500MC Image Processing and Analysis System (Leica, Deerfield, IL).
DNA analysis.
Genomic DNA was isolated from liver and other tissues as described by Sambrook et al.28 For comparison of copy number of the hF.IX gene at 5 days and 240 days posttransduction, a 437-bp fragment of the hF.IX cDNA was amplified using an upper primer F.IX3 (5′-ACATCACTCAAAGCACCCAATCAT-3′) based on sequence in exon 6 encoding the activation peptide and a lower primer F.IX4 (5′-TCTTCCCCAGCCACTTACATAGC-3′) derived from sequence in exon 8. The upper primer shows little sequence similarity with mouse F.IX sequences. Fifty nanograms of total genomic liver DNA was used per reaction in a 50-μL volume (AmpliTaq PCR kit from Perkin-Elmer [Norwalk, CT]; 1.5 mmol/L MgCl2). After initial denaturation at 94°C for 4 minutes, 32 cycles were performed as follows: 94°C for 1 minute, 52°C for 1 minute, 72°C for 1 minute. After a final incubation step at 72°C for 10 minutes, 10 μL of the polymerase chain reaction (PCR) product was mixed with an equal volume of the reaction product which was obtained by applying the same PCR conditions and samples, except that primers for the amplification of a 162-bp fragment of the mouse alkaline phosphatase gene18 were used in place of F.IX3 and F.IX4. PCR products were separated on a 1.5% agarose gel, transferred to a nylon membrane, and sequentially hybridized using radioactively labeled probes specific to the 162-bp mouse AP fragment and the 437-bp hF.IX fragment. Random primed labeling reactions were performed with the Prime-it II kit (Stratagene, La Jolla, CA). After each hybridization, the membrane was placed on an x-ray film for 8 hours or analyzed with a phosphorimaging system. Control reactions using pAAV-CMV-F.IX mixed with 50 ng of mouse genomic DNA showed a linear response in signal strength ranging from 0.03 to 3 plasmid molecules/diploid mouse genome.
RESULTS
AAV-F.IX vectors direct expression of human F.IX in cultured cells.
Titers of recombinant AAV were routinely in the range of 1012 to 1013 vector genomes/mL and 109 to1010 infectious units/mL. Human hepatoma (HepG2) cells were transduced with AAV-CMV-F.IX (Fig 1A) while growing on chamber slides. This liver-derived cell line expresses several clotting factors, but not F.IX.29 F.IX was detected in the cytoplasm of transduced cells by immunofluorescence staining 96 hours postinfection and in the 24-hour conditioned medium by ELISA (8.5 pg F.IX/cell/24 h). As previously reported for growing HeLa cells,14 we observed that a ratio of approximately 104 vector particles/cell was required for high-efficiency transduction. No F.IX was detected in cells not transduced with vector (data not shown). Human embryonic kidney (293) cells transduced with AAV-EF1α-F.IX (Fig1B) secreted F.IX in the media at 0.3 pg/cell/ 24 h when analyzed as described in Materials and Methods.
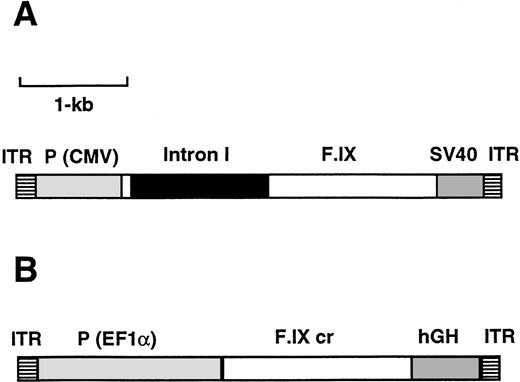
Map of the AAV vectors AAV-CMV-F.IX (A) and AAV-EF1α-F.IX (B). ITR, AAV inverted terminal repeat; P (CMV), cytomegalovirus immediate early enhancer/promoter; F.IX, intron I, the coding region of the F.IX cDNA including a portion of intron I (see Materials and Methods); SV40, the simian virus 40 polyadenylation signal; F.IX cr, coding region of the human F.IX cDNA; P (EF1α), promoter of the human polypeptide elongation factor EF1α gene; hGH, polyadenylation signal of the human growth hormone gene.
Map of the AAV vectors AAV-CMV-F.IX (A) and AAV-EF1α-F.IX (B). ITR, AAV inverted terminal repeat; P (CMV), cytomegalovirus immediate early enhancer/promoter; F.IX, intron I, the coding region of the F.IX cDNA including a portion of intron I (see Materials and Methods); SV40, the simian virus 40 polyadenylation signal; F.IX cr, coding region of the human F.IX cDNA; P (EF1α), promoter of the human polypeptide elongation factor EF1α gene; hGH, polyadenylation signal of the human growth hormone gene.
Expression of human F.IX is readily achieved in hepatocytes in vivo after intravenous injection of newborn mice with AAV-CMV-F.IX, but expression at detectable levels in plasma is transient.
Intravenous or portal vein injection of rAAV results in expression primarily in liver.14,18 A previous report had documented that levels of expression of β-galactosidase from an AAV vector in livers of adult mice are improved by several orders of magnitude when adenovirus containing intact Ad E1 and E4 is added to the rAAV preparation before injection.14 We first determined whether a similar finding was observed with AAV-CMV-F.IX. In a preliminary experiment, adult CD-1 mice were injected with 1.5 × 1010 vector genomes of AAV-CMV-F.IX alone or combined with 5 × 1010 particles of E2a-deleted adenovirus dl 802 (which does not include a transgene). Serial blood samples were obtained after injection and assayed for human F.IX by ELISA. Injection of AAV-CMV-F.IX in combination with adenovirus resulted in transient expression of hF.IX at therapeutic levels (200 to 500 ng/mL at day 3 postinjection, 0 ng/mL at day 7, n = 2). AAV-CMV-F.IX alone did not produce detectable levels of hF.IX in the plasma, even when higher doses (1011 vector genomes) or different routes of administration (portal vein v tail vein injection) were used (data not shown). The ELISA used for these measurements has a lower limit of detection of approximately 3 ng hF.IX/mL. The lack of expression was not the result of antibody formation against hF.IX, based on an ELISA specific for antibodies to hF.IX (data not shown).22
Based on the observation made by others that actively dividing cells, eg, cells that undergo DNA synthesis, are more readily transduced by AAV,30 we wished to determine whether newborn liver was a better target for transduction with AAV-CMV-F.IX. A total of 14 CD-1 mice were injected intravenously with AAV-CMV-F.IX at day 1 of life in two independent experiments. Only one animal was lost during this procedure. Mice killed at day 5 postinjection displayed 55 to 110 ng of hF.IX per mL of mouse plasma. Expression levels decreased subsequently, and hF.IX was still detectable in plasma of some animals 5 weeks after injection, but not thereafter (Table 1). Immunofluorescence staining showed a clear correlation between the proportion of liver cells expressing human F.IX and the concentration of hF.IX in the plasma (Table 1 and Fig 2). A large proportion of those hepatocytes that were positive for hF.IX gave only weak fluorescence signals at day 5 with a further decrease in signal by day 14 (Fig 2B and data not shown). The data given in Table 1indicate that up to 90% of the initial expression was transient. No gene transfer was observed in other organs such as spleen, heart, or lung by PCR or immunofluorescence staining on samples obtained 240 days postinjection (data not shown).
Expression of Human F.IX in CD-1 Mice After Intravenous Injection of AAV-CMV-F.IX at Day 1 of Life
| Days Postinjection . | Expression of hF.IX in Plasma (ng/mL)-150 . | % Hepatocytes Expressing hF.IX-151 . |
|---|---|---|
| 5 | 77 (±24) | 3 (±0.6) |
| 14 | 15 (±6) | 1.3 (±0.5) |
| 35 | 2 (±3.5) | ND |
| 60 | 0 | ND |
| 240-300 | 0 | 0.25 (±0.1) |
| Days Postinjection . | Expression of hF.IX in Plasma (ng/mL)-150 . | % Hepatocytes Expressing hF.IX-151 . |
|---|---|---|
| 5 | 77 (±24) | 3 (±0.6) |
| 14 | 15 (±6) | 1.3 (±0.5) |
| 35 | 2 (±3.5) | ND |
| 60 | 0 | ND |
| 240-300 | 0 | 0.25 (±0.1) |
Animals were killed at day 5 (n = 3), day 14 (n = 2), and day 240-300 (n = 8).
Abbreviation: ND, not determined.
As determined by ELISA.
As determined by image analysis of immunofluorescent staining of tissue sections.
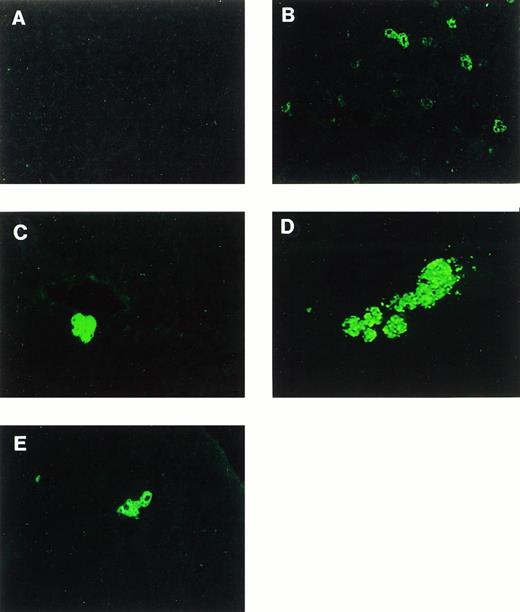
Immunofluorescence staining of human F.IX in mouse liver. (A) Uninjected animal. (B through E) Mice intravenously injected with 2 × 1011 particles of AAV-CMV-F.IX at day 1 of life. (B) Day 5 postinjection. (C and D) Day 240 postinjection. (E) Day 300 postinjection. Original magnification × 200 (×400 in E).
Immunofluorescence staining of human F.IX in mouse liver. (A) Uninjected animal. (B through E) Mice intravenously injected with 2 × 1011 particles of AAV-CMV-F.IX at day 1 of life. (B) Day 5 postinjection. (C and D) Day 240 postinjection. (E) Day 300 postinjection. Original magnification × 200 (×400 in E).
AAV-CMV-F.IX mediates long-term gene transfer in hepatocytes after injection in the newborn period.
Plasma samples of the eight remaining animals were tested for the presence of antibodies to hF.IX 8 months postinjection. Figure 3 shows that two mice had developed circulating antibody. Aware of the possibility that loss of detectable levels of hF.IX in plasma might be due to antibody formation, we wished to determine whether there was evidence for stable AAV-mediated gene transfer and expression at the cellular level. Immunofluorescence staining showed that approximately 0.25% of hepatocytes were still expressing hF.IX 8 to 10 months after injection. Interestingly, at these late time points, hepatocytes expressing hF.IX often appeared in clusters of 2 to 8 cells (Fig 2C through E).
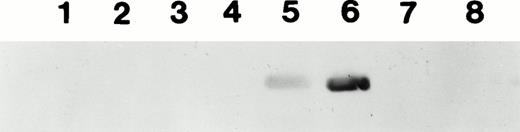
Western blot to detect the formation of circulating antibody in the plasma of mice injected with 1 to 2 × 1011 particles of AAV-CMV-F.IX at day 1 of life. Lanes 1 through 8 show plasma samples from eight different animals taken at day 240 postinjection. Samples were diluted 1:500. Identical results were obtained from 1:200 dilutions (data not shown). Animals in lanes 5 and 6 had developed detectable levels of anti-hF.IX.
Western blot to detect the formation of circulating antibody in the plasma of mice injected with 1 to 2 × 1011 particles of AAV-CMV-F.IX at day 1 of life. Lanes 1 through 8 show plasma samples from eight different animals taken at day 240 postinjection. Samples were diluted 1:500. Identical results were obtained from 1:200 dilutions (data not shown). Animals in lanes 5 and 6 had developed detectable levels of anti-hF.IX.
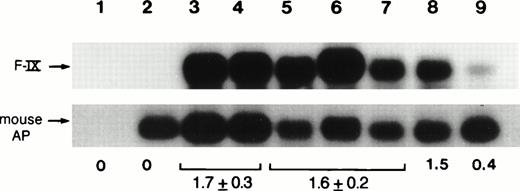
As additional evidence for gene transfer, DNA extracted from the livers of mice killed 5 days after injection showed the presence of the hF.IX transgene. PCR reactions were performed to amplify a 0.4-kb sequence of the introduced hF.IX vector (see Materials and Methods). Southern blot hybridization of PCR products showed amplification of a fragment of the expected size representing a 0.4-kb fragment of the hF.IX cDNA (Fig 4, lanes 3 and 4). No amplification product was obtained from DNA isolated from an uninjected mouse (Fig 4, lane 2). Amplification products representing a 162-bp fragment of the mouse embryonic alkaline phosphatase gene18 served as control to normalize band intensities of the amplification products. When the same analysis was performed on liver DNA from animals killed 240 to 300 days postinjection, the ratio of the band intensities for the donated hF.IX sequences compared with the endogenous mouse sequence was approximately the same (Fig 4, lanes 5 through 7), indicating that no substantial loss of vector DNA had taken place.
Analyses of genomic DNA isolated from livers after injection of CD-1 mice with 2 × 1011 particles of AAV-CMV-F.IX at day 1 of life. Southern blot hybridization of PCR products from liver DNA isolated from mice killed at day 5 (lanes 3 and 4) and day 240 (lanes 5 through 7) postinjection. Lane 1, no DNA added; lane 2, untransduced mouse DNA; lane 8, 0.3 copies of pAAV-CMV-F.IX per diploid mouse genome present in 50 ng of untransduced mouse DNA; lane 9, 0.06 copies of pAAV-CMV-F.IX per diploid mouse genome; mouse AP, 162-bp fragment from the mouse embryonic alkaline phosphatase gene; F-IX, 437-bp fragment from the human F.IX cDNA amplified with primer pair F.IX3/4. The average ratio of the two signals is indicated for day 5 as well as day 240 postinjection (as determined with the phosphorimaging system). Numbers in brackets are the standard deviation.
Analyses of genomic DNA isolated from livers after injection of CD-1 mice with 2 × 1011 particles of AAV-CMV-F.IX at day 1 of life. Southern blot hybridization of PCR products from liver DNA isolated from mice killed at day 5 (lanes 3 and 4) and day 240 (lanes 5 through 7) postinjection. Lane 1, no DNA added; lane 2, untransduced mouse DNA; lane 8, 0.3 copies of pAAV-CMV-F.IX per diploid mouse genome present in 50 ng of untransduced mouse DNA; lane 9, 0.06 copies of pAAV-CMV-F.IX per diploid mouse genome; mouse AP, 162-bp fragment from the mouse embryonic alkaline phosphatase gene; F-IX, 437-bp fragment from the human F.IX cDNA amplified with primer pair F.IX3/4. The average ratio of the two signals is indicated for day 5 as well as day 240 postinjection (as determined with the phosphorimaging system). Numbers in brackets are the standard deviation.
AAV-EF1α-F.IX mediates stable expression of therapeutic levels of human F.IX after injection into portal vein of adult mice.
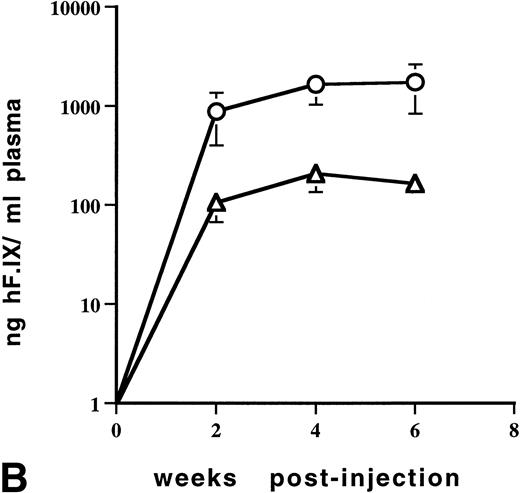
For subsequent experiments, C57BL/6 mice were chosen because this strain of immunocompetent mice is known not to produce antibodies against human F.IX after intravenous injection of viral vectors.19,31 Analogous to experiments with CD-1 mice described above, adult C57BL/6 mice (n = 2) injected with 1 × 1011 vector genomes of AAV-CMV-F.IX into the portal vein did not yield detectable plasma levels of hF.IX when plasma was analyzed weekly up to 7 weeks postinjection with a lower limit of detection of about 3 ng/mL (Fig 5A). Because of data suggesting that the CMV promoter is specifically inactivated in liver, a vector was constructed using the EF1α promoter.24 When 6.3 × 1010 vector genomes of AAV-EF1α-F.IX were injected into the portal vein of adult C57BL/6 mice (n = 2), expression of therapeutic plasma levels of hF.IX was evident. Initially low levels gradually increased, reaching a plateau of 200 to 320 ng/mL 5 weeks after injection. Therapeutic levels were stable for at least 6 months (Fig 5A). In a more detailed dose-response study, portal vein injections were carried out in adult C57BL/6 mice using 2.7 × 1011, 5.5 × 1010, or 1 × 1010 particles of AAV-EF1α-F.IX (n = 4 per dose). Mice with the lowest dose injected had hF.IX levels below 6 ng/mL plasma (data not shown). The intermediate dose resulted in 150 to 200 ng/mL at week 6 postinjection (Fig 5B) while mice injected with the highest dose expressed 700 to 3,200 ng/mL at that time point (Fig 5B).
Levels of human F.IX in plasma of adult C57BL/6 mice as a function of time after portal vein injection of rAAV vector. (A) Mice were injected with 1 × 1011 vector particles of AAV-CMV-F.IX (□) or 6.3 × 1010 particles of AAV-EF1α-F.IX (▵ and ○). Each line represents an individual animal. (B) Mice were injected with 5.5 × 1010 particles (▵, n = 4) or 2.7 × 1011 particles (○, n = 4) of AAV-EF1α-F.IX. Each line represents the average of four mice. Vertical bars are the standard deviation.
Levels of human F.IX in plasma of adult C57BL/6 mice as a function of time after portal vein injection of rAAV vector. (A) Mice were injected with 1 × 1011 vector particles of AAV-CMV-F.IX (□) or 6.3 × 1010 particles of AAV-EF1α-F.IX (▵ and ○). Each line represents an individual animal. (B) Mice were injected with 5.5 × 1010 particles (▵, n = 4) or 2.7 × 1011 particles (○, n = 4) of AAV-EF1α-F.IX. Each line represents the average of four mice. Vertical bars are the standard deviation.
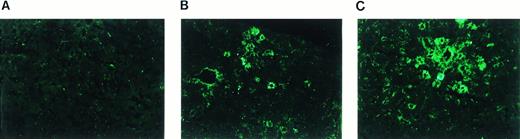
Immunofluorescence staining of mouse liver 22 weeks postinjection with 2.1 × 1010 particles of AAV-EF1α-F.IX demonstrates expression of hF.IX in hepatocytes, while liver sections of an animal injected with AAV-CMV-F.IX were mostly negative with rare individual positive cells (Fig 6A through C and data not shown). In the AAV-EF1α-F.IX–injected mouse, groups of positive hepatocytes were observed mostly in close proximity to portal triads with isolated positive cells scattered throughout the cross section.
Immunofluorescence staining of human F.IX expressed in hepatocytes 22 weeks after injection of AAV-CMV-F.IX (A) or AAV-EF1α-F.IX (B and C) into portal vein of adult C57BL/6 mice. The dose injected in the mouse used for liver sections (B) and (C) was 2.1 × 1010 vector genomes. Plasma levels of hF.IX were 120 ng/mL at the time this mouse was killed. Original magnification × 200.
Immunofluorescence staining of human F.IX expressed in hepatocytes 22 weeks after injection of AAV-CMV-F.IX (A) or AAV-EF1α-F.IX (B and C) into portal vein of adult C57BL/6 mice. The dose injected in the mouse used for liver sections (B) and (C) was 2.1 × 1010 vector genomes. Plasma levels of hF.IX were 120 ng/mL at the time this mouse was killed. Original magnification × 200.
DISCUSSION
In previous work we showed that transduction of mouse muscle with AAV-CMV-F.IX results in the expression of stable therapeutic levels of human F.IX.22 Similarly, high levels of expression of hF.IX from a CMV promoter have been achieved in neonatal and adult mouse liver using an adenovirus vector.7 Therefore, the vector construct outlined in Fig 1A was initially chosen for transduction of mouse hepatocytes. Transduction of a liver-derived cell line with AAV-CMV-F.IX was successful, and hF.IX levels secreted by transduced human hepatoma cells were similar to data reported for primary myoblasts stably transduced with a retroviral vector4 and considerably higher than levels documented for an AAV vector with expression of hF.IX controlled by the Moloney murine leukemia virus (MLV) long terminal repeat.18 However, efficient in vitro transduction of immortalized cells with an AAV vector is not necessarily a useful indicator of the efficiency of transduction with AAV in vivo.32
After obtaining high titers of AAV-CMV-F.IX and showing hF.IX secretion in in vitro transduction experiments, we tested this vector for hF.IX expression in an optimal in vivo setting; ie, in the presence of helper adenovirus. Transient expression of therapeutic levels (4% to 10% of normal in human plasma) was evident during the first week postinjection in adult mice. The presence of adenoviral antigens in hepatocytes elicits an immune response that results in the destruction of transduced cells and therefore explains at least partly the transient nature of expression.8,9 No hF.IX was detected in parallel experiments, when adult CD-1 mice were injected with AAV-CMV-F.IX alone. Data from several studies indicate that the number of adult hepatocytes that express a transgene from an AAV vector may be increased after modification of the cellular milieu by the introduction of adenoviral gene products E1 and E4 orf6, topoisomerase inhibitors, or by the application of γ-irradiation to the liver.14 18
In contrast to the situation in adult animals, substantial expression of F.IX after transduction with AAV-CMV-F.IX was evident in neonatal hepatocytes even in the absence of adenovirus. Human F.IX was present at therapeutically useful levels in mouse plasma during the first week after injection of newborn animals. Transgene expression was detectable in 3% of hepatocytes at this time point. Fisher et al14showed that despite efficient delivery of the single-stranded AAV genome to adult hepatocytes, only a negligible percentage of hepatocytes (<0.01%) expressed the transgene at early time points. Snyder et al19 report a similar result, and additionally demonstrate that the number of expressing hepatocytes increases to 2% to 5% over a period of 3 to 5 weeks. It appears that neonatal hepatocytes provide a setting in which expression from an rAAV is achieved very rapidly, possibly because of a rapid conversion of single-stranded to double-stranded AAV. While neonatal hepatocytes are still proliferating, only 0.005% to 0.05% of adult hepatocytes are in S phase.33 Russell et al,30 using in vitro experiments with cultured fibroblasts, reported that recruitment of single-stranded AAV vector for transgene expression was approximately 200-fold higher for cells that were in S phase than for cells that were not.
Immunofluorescence studies on the livers of animals injected with AAV-CMV-F.IX as newborns show that the decrease of plasma levels of hF.IX was due in large part to the transient nature of transgene expression in hepatocytes. In addition, at least some animals developed circulating antibodies to human F.IX at later time points (Fig 3). In our experience, 10% to 30% of CD-1 mice develop antibodies to human F.IX after intravenous injection with viral vectors (this study and unpublished data, February, 1997). However, in all animals examined we found stable gene transfer and expression of F.IX in approximately 0.25% of hepatocytes for at least 10 months (Table1). PCR analysis indicates that the 12-fold decrease in the proportion of hepatocytes expressing hF.IX did not correlate with a loss of the hF.IX gene in mouse liver. These data are consistent with a specific shutdown of the CMV promoter. Recent data from transgenic animals and adenovirus vectors have shown that transgene expression driven by the CMV enhancer/promoter is suppressed in vivo in the liver and lung.34-36 Similar results had been reported in the context of retroviral vectors.37 38
Doses as low as 1 × 1010 particles of AAV-CMV-F.IX are sufficient to obtain plasma levels of hF.IX above 50 ng/mL in mice after intramuscular injection.22 In agreement with data discussed above for experiments with CD-1 mice, no hF.IX was detected in plasma of C57BL/6 mice injected into the portal vein with AAV-CMV-F.IX. In contrast to experiments with constructs using the CMV promoter, portal vein injection of AAV-EF1α-F.IX resulted in therapeutic plasma levels of hF.IX. The EF1α promoter has been shown to direct persistent high levels of transgene expression in liver in the context of an adenovirus vector.33 Expression of hF.IX clearly originated from hepatocytes when AAV-EF1α-F.IX was injected into adult mice, as shown by immunofluorescence staining (Fig 6B and C). Therefore, adult hepatocytes are capable of converting the single-stranded AAV vector genome to a transcriptionally active form, but expression is critically dependent on the choice of the promoter driving transgene expression. We conclude that adult hepatocytes can be efficiently transduced with AAV vectors without the need to modify the cellular milieu by introduction of adenovirus or by γ-irradiation.
Therapeutic levels of expression (4% to 6% of normal in human plasma) were reached within 5 weeks and were stable for at least 6 months after injection of 6.3 × 1010 particles of AAV-EF1α-F.IX. The highest dose of this vector that was injected (2.7 × 1011 particles) resulted in plasma levels that were on average 1,700 ng/mL (35% of human plasma levels). Assuming that vector titers documented in earlier studies are comparable with titers given in this study, the amount of hF.IX produced in mice per particle of AAV vector injected was twofold to fourfold higher for liver-derived expression from AAV-EF1α-F.IX (this study) than reported for muscle-derived expression from AAV-CMV-F.IX,22 and twofold to fourfold lower than reported for an AAV-MFG-F.IX construct.19 However, a comparison of efficiencies of gene transfer and expression with a particular vector in mice might not reflect efficiencies in other species.
The time course of expression as outlined in Fig 5A is similar to those reported for expression of hF.IX and erythropoietin in the systemic circulation of mice after intramuscular injection of recombinant AAV,22,26 and therefore appears to represent a general pattern for the expression of a secreted protein after in vivo transduction of nondividing cells with AAV. After an initial lag phase, plasma levels increase gradually over several weeks until a stable plateau is reached. The gradual increase likely results from the slow rate of AAV second-strand synthesis.10 22 Persistent expression can be monitored conveniently in C57BL/6 because of the absence of antibody production against human F.IX. Stable expression suggests the absence of cellular immune responses to hepatocytes transduced with AAV vector.
Transduction of cells in S phase with recombinant AAV in vitro has been attributed to integration of the vector into chromosomal DNA.30 The ITR sequences are the only cis elements required for this process.39 The persistence of the vector genome as well as the presence of clusters of hepatocytes expessing hF.IX at day 240 postinjection may be the result of integrative events during transduction of neonatal hepatocytes. Preliminary results indicate a high molecular weight form of vector DNA after transduction of adult mouse liver similar to data previously published for muscle (H.N., unpublished results, November, 1997).10 22 However, additional experiments will be necessary to determine whether the vector genome persists in an episomal or integrated form.
This work shows that AAV can be used to direct expression of therapeutic levels of F.IX in liver. Ongoing experiments are aimed at the optimization of the human F.IX expression cassette, of the injection dose, and of the route of vector administration , as well as the testing of liver-specific promoters.
ACKNOWLEDGMENT
The authors thank Drs R.C. Eisensmith and S.L.C. Woo for providing the EF1α promoter, and for sharing results before publication. The assistance of the Morphology Core of the Institute for Human Gene Therapy at the University of Pennsylvania is gratefully acknowledged. Cell line 84-31 was kindly provided by the Vector Core of the Institute for Human Gene Therapy.
Supported by National Institutes of Health Grants No. R01 Hl53668 and P50 HL54500 to K.A.H. H.N. was supported by a grant from the Ryoichi Naito Foundation for Medical Research.
Address reprint requests to Katherine A. High, MD, Abramson Research Center, Room 310A, The Children's Hospital of Philadelphia, 34th St and Civic Center Blvd, Philadelphia, PA 19104.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal