Abstract
Human umbilical cord blood (UCB) hematopoietic stem cells (HSC) receive increased attention as a possible target for gene-transfer in gene therapy trials. Diseases affecting the lymphoid lineage, as adenosine deaminase (ADA) deficiency and acquired immunodeficiency syndrome (AIDS) could be cured by gene therapy. However, the T-cell progenitor potential of these HSC after gene-transfer is largely unknown and was up to now not testable in vitro. We show here that highly purified CD34++ Lineage marker-negative (CD34++Lin−) UCB cells generate T, natural killer (NK), and dendritic cells in a severe combined immunodeficient mouse fetal thymus organ culture (FTOC). CD34++Lin− and CD34++CD38−Lin− UCB cells express the retroviral encoded marker gene Green Fluorescent Protein (GFP) after in vitro transduction with MFG-GFP retroviral supernatant. Transduced cells were still capable of generating T, NK, and dendritic cells in the FTOC, all expressing high levels of GFP under control of the Moloney murine leukemia virus (MoMuLV) long terminal repeat promotor. We thus present an in vitro assay for thymic T-cell development out of transduced UCB HSC, using GFP as a marker gene.
IN HUMANS AND other mammals, mature cells of the erythroid, myelomonocytic, and lymphoid lineages are the progeny of hematopoietic progenitor cells (HPC), the lineage-committed descendants of hematopoietic stem cells (HSC).1 This concept was clearly supported by use of retroviral marking.2 The self-renewing HSC are the ultimate target of gene therapy for long-lasting gene-transfer to the hematopoietic system.3-12 During human ontogeny, HSC are first found to be associated with embryonic aortic endothelium and later home to fetal liver and eventually bone marrow.13 At least during prenatal and early postnatal life, HSC or HPC seed the thymus to generate T, natural killer (NK), and dendritic cells.1,14At birth, a substantial number of HSC/HPC are present in umbilical cord blood (UCB).5,10,15 Several reports7,8 have shown that UCB HSC can be used for allologous or autologous transplantation to reconstitute the hematopoietic system after bone marrow ablation in children. The potential use of UCB HSC in adults is currently being studied.16 Kohn et al8 have shown that, in the absence of cytoablative therapy, retrovirally transduced autologous UCB HSC/HPC expressing adenosine deaminase (ADA) can engraft ADA-deficient newborns. Although this study showed the presence of ADA in selected granulocyte-macrophage colony-forming unit (GM-CFU) colonies 1 year after transplantation, it failed to show activity or presence of ADA in ex vivo expanded peripheral T lymphocytes. However, the investigators communicated that transduced circulating T cells were observed in these patients upon reduction of the PEG-ADA substitution therapy dosage.17 Because lymphocytes are the lineage affected by the ADA deficiency, gene therapy may fail if transduced UCB HSC can not generate T cells. This shows the need to assay T progenitor potential of ex vivo transduced HSC. In vivo assays using SCID-hu,3,12,18NOD/SCID,19 and beige/nude/XID (bnx) mice20have been used to demonstrate T-cell development out of transduced human UCB HSC/HPC. In contrast to assays for myelopoiesis and B-lymphocyte development, a simple in vitro culture assay for thymic T-cell development from HSC after gene-transfer was still lacking.
We previously described a SCID mouse fetal thymus organ culture (FTOC) that supports differentiation of human CD34++ Lineage marker-negative (Lin−) fetal liver cells into mature thymocytes.21,22 In this report, we show that this organ culture supports T-cell, NK cell, and dendritic cell development from highly purified cord blood CD34++Lin−cells. We also show that purified UCB CD34++Lin− and CD34++CD38−Lin− cells express the retroviral encoded marker gene Green Fluorescent Protein23 (GFP) after in vitro transduction. Finally, we show that transduced UCB CD34++ cells can still generate mature thymocytes in vitro expressing high levels of GFP. The Moloney murine leukemia virus (MoMuLV) long terminal repeat (LTR) promotor was shown to be sufficiently active in transduced UCB CD34++cells and thymocytes. In this report, an in vitro assay for thymic T-cell development out of transduced UCB HSC/HPC using GFP as a marker gene is presented as a versatile alternative to the laborious in vivo murine models. Without the need for human fetal tissue and in vivo testing, this assay will allow us to define transduction protocols for human cord blood HSC that combine optimal gene-transfer and preservation of T-lymphoid progenitor potential.
MATERIALS AND METHODS
Monoclonal antibodies (MoAbs).
Mouse antihuman MoAbs used were CD1a (OKT6, fluoresceı̈n isothiocyanate [FITC]-labeled, from Ortho Diagnostic Systems [Ortho], Raritan, NJ; or T6-RD1, phycoerythrin [PE]-labeled, from Coulter, Hialeah, FL), CD2 (Leu-5b FITC, from Becton Dickinson Immunocytometry Systems [BDIS], Mountain View, CA), CD3 (Leu-4 FITC or PE, from BDIS; or S4.1 tricolor [TC] from Caltag Laboratories [Caltag], San Francisco, CA), CD4 (Leu-3a FITC, or PE from BDIS or OKT4 FITC from American Tissue Type Collection [ATCC], Rockville, MD; or S3.5 TC from Caltag), CD5 (Leu-1 FITC from BDIS), CD7 (Leu-9 FITC from BDIS), CD8α (OKT8 FITC, from ATCC; or Leu2a FITC or PE from BDIS), CD10 (anti-CALLA FITC from BDIS); CD16 (3G8 FITC from PharMingen, San Diego, CA), CD19 (Leu-12 from ATCC), CD33 (WM-54 FITC from Dako, Glostrup, Denmark), CD34 (HPCA-2 PE from BDIS), CD38 (OKT10 FITC from Ortho or OKT10 biotin [BIO] from ATCC), CD44 (3F12 FITC, kind gift of Dr W. De Smet, Innogenetics, Ghent, Belgium), CD45 (anti–HLe-1 FITC from BDIS), CD45RA (MD4.3 BIO, kind gift of Dr F. Koning, University Hospital, Leiden, The Netherlands), CD56 (NCAM 16-2 PE from BDIS), anti–c-kit (CD117, 95C3 BIO from Caltag), anti–glycophorin-A (10F7MN, kind gift of Dr L. Lanier, DNAX, Palo Alto, CA), anti–T-cell receptor (TCR)-γδ (anti–TCR-γ/δ-1 PE from BDIS), and anti–HLA-DR (L243 BIO from ATCC)
Rat antimouse MoAb CD45-Cychrome (30F11.1 from PharMingen) was used to gate out mouse cells in flow cytometry and anti-FcγRII/III MoAb (Clone 2.4.G224; kind gift of Dr J. Unkeless, Mount Sinai School of Medicine, New York, NY) was used to block murine Fc receptors.
Isotypic control antibodies were IgG1 (FITC and PE from BDIS or BIO from Caltag) and IgG2a (FITC and PE from BDIS or BIO from Caltag). Biotinylated antibodies were shown by streptavidin-TC (SA-TC; Caltag). MoAbs from ATCC were FITC or BIO conjugated in our laboratory using standard methods.
SCID mice.
C.B.-17 scid/scid (SCID) mice, originally purchased from Iffa Credo (L'arbresle, France), were bred in our specific pathogen-free breeding facility. After overnight mating, the appearance of vaginal plugs was noted as day 0 of pregnancy. Fourteen- to 15-day pregnant mice were killed by cervical dislocation, and fetal thymic lobes were dissected out.
Animals were treated according to the guidelines of the Laboratory Animal Ethical Commission of the University Hospital of Ghent.
Purification of CD34++ cord blood cells.
UCB was obtained from full-term, normal newborns and used following the guidelines of the Medical Ethical Commission of the University Hospital of Ghent. Within 6 hours after collection, the mononuclear cell fraction (median, 1.4 × 106 CD45+cells/mL EDTA cord blood) was isolated over a Lymphoprep density-gradient (Nyegaard, Oslo, Norway), resuspended in 9 vol fetal calf serum (FCS; Life Sciences, Paisley, UK) and 1 vol dimethyl sulfoxide (Serva, Heidelberg, Germany), and cryopreserved in liquid N2 until use. After thawing, cells were kept at 4°C at all time until the initiation of culture. Washed cells were resuspended in phosphate-buffered saline/2% (vol/vol) FCS and stained with glycophorin-A, CD19, and CD7 FITC. Labeled cells were depleted using sheep antimouse Ig-coated Dynabeads (Dynal AS, Oslo, Norway; 4 beads per cell) in a magnetic particle concentrator (Dynal AS). Unlabeled cells were recovered and stained with CD1 FITC, CD3 FITC, CD4 FITC, CD8 FITC, CD34 PE, and, in some experiments, CD38 BIO. Cells that were CD34 PE++ and FITC− (called CD34++Lin−) were sorted on a FACS Vantage (BDIS) cell sorter equipped with an argon-ion laser (488 nm). On the average, 15 CD34++Lin− cells were obtained per 104 mononuclear cells. In some experiments, cells sorted were CD34 PE++, FITC−, and CD38 BIO/SA-TC− or CD38 BIO/SA-TC+(called CD34++CD38−Lin−or CD34++CD38+Lin−, respectively). Sorted populations are collectively called CD34++ cells. Sorted cells were checked for purity, which was always at least 99.5%.
Cell culture.
All cultures were performed at 37°C in a humidified atmosphere containing 7.5% (vol/vol) CO2 in air. The cells were cultured in Iscove's modified Dulbecco's medium (IMDM), supplemented with penicillin (100 IU/mL), streptomycin (100 μg/mL), and 10% heat-inactivated FCS (complete IMDM; all products from Life Sciences). FTOC was performed in complete IMDM containing 10% heat-inactivated human AB serum (BioWhittaker, Walkersville, MD) instead of FCS.
MFG-GFP retrovirus.
The marker gene Green Fluorescent Protein23 (EGFP; Clontech, Palo Alto, CA) was Nco I-Not I cloned in the pBluescript vector (Stratagene, La Jolla, CA). An NcoI-BamHI fragment containing GFP was inserted between the unique corresponding restriction sites of the MFG retroviral vector backbone.25 All enzymes used and DH5α an Escherichia coli host bacteria were bought from Life Sciences. Propagated plasmids were purified with resin columns (Qiagen, Hilden, Germany). The Phoenix-A, a 293 cell line (human fetal kidney)-based amphotropic packaging cell line that was kindly provided by Dr P. Achacoso and Dr G.P. Nolan (Stanford University School of Medicine, Stanford, CA), was transfected with the MFG-GFP plasmid using calcium-phosphate precipitation (Life Sciences). GFP-expressing cells (usually ∼70% of the transfected cells, 2 days posttransfection) were selected by electronic cell sorting using a FACS Vantage cell sorter (BDIS). After 3 rounds of sorting over 7 weeks of time, a stable transfected cell line was established. Cell culture supernatant was harvested from confluent cultures in 150 cm2 tissue culture flasks (Falcon, Becton Dickinson Labware, Franklin Lakes, NJ) 24 hours after refreshment of the medium. Pooled supernatants were spun (10 minutes for 350g at 21°C) and aliquots were stored at −70°C until use. The batch used in this report was shown to be free of replication competent retrovirus and contained after thawing 5 × 106 transforming units/mL (titrated on Jurkat T cells [ATCC]; data not shown).
Gene transduction into CD34++ cord blood cells.
Sorted CD34++ cord blood cells were resuspended in complete IMDM supplemented with 100 ng/mL recombinant human c-kit ligand (stem cell factor [SCF]; R&D Systems Europe, Abingdon, UK) and 500 U/mL (50 U/mL in the case of CD34++CD38−Lin− and CD34++CD38+Lin− cells) recombinant human IL-3 (Innogenetics, Antwerp, Belgium) and seeded in 96-well round bottom tissue culture plates (Falcon) at 1.5 to 2 × 104 cells in 150 μL medium per well. After 24 hours of culture, half of the medium volume was replaced with viral supernatant, supplemented with cytokines (to keep final cytokine concentration unchanged) and with the liposome complex DOTAP (Boehringer Mannheim, Mannheim, Germany) to a final concentration of 20 μg/mL. After resuspension of the cells, the 96-well plates were spun26(1.5 hours for 950g at 32°C) and put back in culture for 48 hours. After this period, a fraction of the cells was used to assay transduction efficiencies, while the remainder was used in FTOC. In certain experiments, cells were sorted for GFP expression before transfer to FTOC.
FTOC.
In Terasaki plates, hanging drops were prepared by adding per well 25 μL complete IMDM containing 1 × 104 freshly sorted CD34++ UCB cells or the progeny of 1 to 1.5 × 104 CD34++ UCB cells transduced as described above. In some experiments, 104 transduced CD34++Lin− UCB cells, sorted after transduction based on GFP expression, were used. To each of these wells, 1 fetal thymic lobe was added and the plates were inverted to form hanging drops and incubated during 48 or 72 hours. After this incubation, at day 0 of FTOC, the lobes were removed, washed in complete medium, put on the surface of a nuclepore filter (Nuclepore, Costar, Cambridge, MA), resting on a Gelfoam sponge (Upjohn, Kalamazoo, MI) soaked in complete medium in a 6-well tissue culture plate (Falcon), and cultured for 11 to 38 days. At the end of the culture, lobes were mechanically disrupted with a small tissue grinder to obtain a single cell suspension. Thymocytes were stained with Trypan Blue and counted with a hematocytometer. The cells, of which at least 80% were viable, were used for flow cytometry.
Flow cytometry.
Before labeling, cells were suspended in phosphate-buffered saline containing 1% (wt/vol) bovine serum albumin and 0.1% (wt/vol) NaN3. In all cases in which human cells were stained in the presence of mouse cells, the mixture of cells was preincubated for 15 minutes with saturating amounts of anti-FcγRII/III MoAb (Clone 2.4.G2)24 to avoid nonspecific binding of MoAbs by the murine cells. Subsequently, the cells were stained with a panel of MoAbs, as indicated. If present, murine and dead cells were gated out by mouse CD45 Cychrome and propidium iodide, respectively. Negative controls included isotype MoAbs conjugated with the corresponding fluorochrome. The cells were analyzed on a FACScan flow cytometer (FACS; BDIS) with an argon-ion laser tuned at 488 nm. Forward light scattering, orthogonal scattering, and three fluorescence signals were determined and stored in list mode data files. Data acquisition and analysis was performed with Lysis 2.0 software (BDIS).
RESULTS
Human CD34++Lin− cord blood cells differentiate into mature T, NK, and dendritic cells in a mouse fetal thymus organ culture.
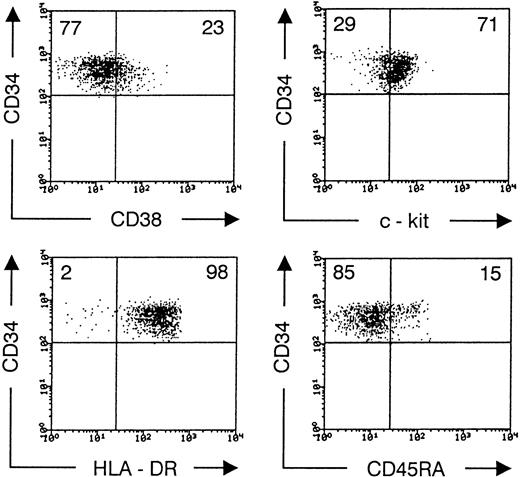
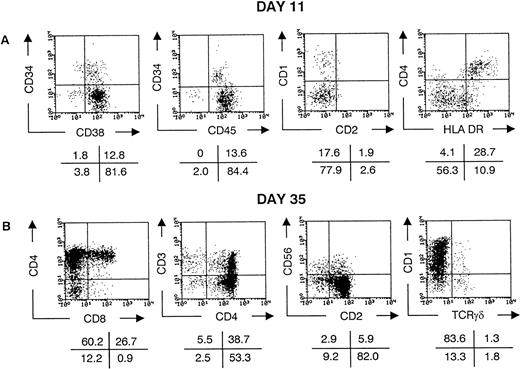
On the average, 1.6% of the CD45+ cells in the UCB mononuclear cell fraction were CD34+. About 10% of these CD34+ cells were recovered after sorting for high expression of CD34 and absence of lineage markers. Immunophenotyping of highly purified CD34++CD1−CD3−CD4−CD7−CD8−CD19−gpA−cells showed no expression of other lineage surface markers CD2, CD5, CD16, or CD33 (data not shown). CD38 expression was low on most cells and undetectable on some. In contrast, the cells were almost homogeneous HLA-DR+ and c-kitlow(Fig 1). This CD34++Lin− phenotype includes the most primitive HSC found in cord blood5,15,19 but does not contain cells with rearranged TCR loci27 and therefore no pro-T cells.1 A minor fraction of the purified CD34++Lin− cells expressed CD45RA (Fig1). After transfer to fetal thymic lobes of SCID mice, most cells downregulated CD34 and upregulated CD38, so that after 11 days, 80% of the human cells were CD34−CD38+(Fig 2A). About 20% of the human cells expressed the marker CD1, most of them in coexpression (not shown) with CD4 but not with CD2 (Fig 2A). The cells with highest expression of CD4 coexpressed high levels of HLA-DR. Cells with this phenotype in FTOC cultures were shown to have a dendritic morphology21 and are high efficient stimulators of allogenic mature lymphocytes (manuscript in preparation). Together with the absence of both the myeloid marker CD14 and the T-cell marker CD3, this indicates that these CD4++HLA-DR++ cells represent thymic dendritic cells. At this time of FTOC (days 11 to 14), the dendritic cells are a major component of the thymocyte population (Fig 2A). Their absolute number does not increase later in culture, so that their contribution to the number of thymocytes recovered decreases to less than 5% at days 35 to 38 of FTOC (data not shown). As we showed using this model with fetal liver CD34++Lin−cells,21 with increasing duration of culture, CD4+CD8+ double-positive cells start to appear, the TCR-associated antigen CD3 is gradually upregulated and some CD4 or CD8 single-positive cells expressing high levels of CD3 are generated (Fig 2B). After 35 days of culture, on the average 4 × 104 human cells were recovered per lobe. Most of the cells expressing CD3 were TCRαβ positive, but some were TCRγδ positive (Fig 2B). After this time of culture, some of the TCRαβhi, CD4, or CD8 single-positive cells have downregulated CD1 (data not shown) and thus represent a mature thymocyte phenotype in human T-cell development.28 As shown in Fig 2B, the TCRγδ+ cells were also either CD1+ or CD1−. We recently showed that the latter population represents the most mature human TCRγδ T cells.29 Some of the human cells were CD3−CD56+ NK cells. This population, which becomes predominant if FTOC is performed in the presence of interleukin-15 (IL-15; manuscript in preparation), has an NK-like morphology upon isolation from FTOC (not shown).
Phenotype of sorted CD34++Lin− UCB cells. Flow cytometric analysis of sorted CD34 PE++CD1−CD3−CD4−CD7−CD8−CD19−gpA−cells, stained after sorting with biotinylated antibodies recognizing CD38, c-kit, HLA-DR, and CD45RA, shown in a second step by SA-TC. Dot plots shows CD34 PE versus TC staining, gated on live cells. Values in the quadrants indicate the percentage of cells present in the corresponding quadrant. Quadrants were set to include 99.5% of sorted cells stained, with isotypic control antibody in the upper left quadrant. The data shown are representative of four independent experiments.
Phenotype of sorted CD34++Lin− UCB cells. Flow cytometric analysis of sorted CD34 PE++CD1−CD3−CD4−CD7−CD8−CD19−gpA−cells, stained after sorting with biotinylated antibodies recognizing CD38, c-kit, HLA-DR, and CD45RA, shown in a second step by SA-TC. Dot plots shows CD34 PE versus TC staining, gated on live cells. Values in the quadrants indicate the percentage of cells present in the corresponding quadrant. Quadrants were set to include 99.5% of sorted cells stained, with isotypic control antibody in the upper left quadrant. The data shown are representative of four independent experiments.
CD34++Lin− UCB cells generate T, NK, and dendritic cells in the FTOC. Flow cytometric analysis of thymocytes recovered from FTOC initiated with freshly sorted CD34++Lin− UCB cells. Cells were stained with CD1 PE, CD2 FITC, CD3 PE, CD4 PE, CD8α FITC, CD34 PE, CD38 FITC, CD45 FITC, CD56 PE, HLA-DR FITC, and anti-TCRγδ FITC. Dot plots show staining of live, human cells recovered after 11 (A) and 35 (B) days of culture. The values in the crosses indicate the percentage of cells present in the corresponding quadrant. Quadrants were set to include 99% of the cells stained, with isotypic control antibody in the lower left quadrant, except for the CD4 PE versus HLA-DR FITC dot plot in (A), in which quadrants are set to contain the CD4++HLA-DR++ dendritic cells in the upper right quadrant. The data shown are representative of eight independent experiments.
CD34++Lin− UCB cells generate T, NK, and dendritic cells in the FTOC. Flow cytometric analysis of thymocytes recovered from FTOC initiated with freshly sorted CD34++Lin− UCB cells. Cells were stained with CD1 PE, CD2 FITC, CD3 PE, CD4 PE, CD8α FITC, CD34 PE, CD38 FITC, CD45 FITC, CD56 PE, HLA-DR FITC, and anti-TCRγδ FITC. Dot plots show staining of live, human cells recovered after 11 (A) and 35 (B) days of culture. The values in the crosses indicate the percentage of cells present in the corresponding quadrant. Quadrants were set to include 99% of the cells stained, with isotypic control antibody in the lower left quadrant, except for the CD4 PE versus HLA-DR FITC dot plot in (A), in which quadrants are set to contain the CD4++HLA-DR++ dendritic cells in the upper right quadrant. The data shown are representative of eight independent experiments.
Collectively, these data indicate that purified UCB CD34++Lin− cells give rise to mature TCRαβ+ and TCRγδ+ T, NK, and thymic dendritic cells after introduction in a fetal SCID thymus in vitro. In this way, we have at our disposal an in vitro model that allows the study of precursor potential of purified UCB HSC/HPC for the lineages cited above. This is of particular interest for HSC, residing in the CD34++CD38− fraction,15 19genetically modified for fundamental research or gene therapy purposes. Therefore, to follow the fate of such cells in the FTOC, we next examined the ability of retrovirally transduced UCB CD34++Lin− cells to express a marker gene after retroviral transduction in vitro. These experiments were followed by a comparison between CD34++CD38−Lin− and CD34++CD38+Lin− cells in FTOC after transduction.
Human CD34++Lin− cord blood cells express the retroviral encoded GFP after transduction in vitro.
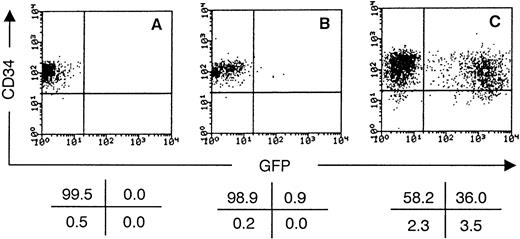
Sorted CD34++Lin− cord blood cells were cultured in medium supplemented or not with the cytokines IL-3 and SCF. After 1 day of culture, cell-free MFG-GFP retroviral supernatant was added. This retrovirus encodes a GFP variant23 under control of the MoMuLV LTR promotor. After another 2 days of culture, the cells were restained with CD34 PE and assayed for GFP expression. As shown in Fig 3, cells grown in complete medium without cytokine or virus were still uniformly CD34+ and not fluorescent above autofluorescence level in the FITC channel (FL1). If no cytokines, but only viral supernatant was added, all cells were still CD34+, but only a few cells expressed the marker gene 48 hours postinfection. This observation is in line with earlier reports that show that more than 90% of CD34++CD38−/dim cord blood cells are not in cell cycle upon isolation15 and need cytokine stimulation to allow retroviral transduction.10Cells grown in medium supplemented with IL-3 and SCF could be transduced at significant efficiencies (Fig 3). The marker gene expression level was sufficient to delineate a clear distinct population of transduced cells on FACS analysis. Cord blood donor to donor variations were observed in transduction percentages (average, 40%; range, 15% to 51%; number of experiments [n] = 8). After 3 days of culture, cytokine-stimulated cells always downregulated CD34 in at least a part of the population (Fig 3). On the average, 3 times (range, 2.3 to 4; n = 3) more cells were recovered in the cytokine-stimulated cultures compared with nonstimulated cultures after 3 days of culture. There was no obvious correlation between cell number expansion and transduction percentages. An additional round of transduction 2 days after initiation of the culture increased the average percentage of GFP-expressing cells to 45%. However, a decreased cell number expansion was observed compared with a single round of transduction, despite medium replacement with fresh, cytokine-supplemented complete IMDM, 4 hours postinfection after each round of transduction. Therefore, only one round of transduction was used in all subsequent experiments.
GFP is expressed by cytokine-stimulated CD34++Lin− UCB cells transduced with MFG-GFP retroviral supernatant. Flow cytometric analysis of the progeny of CD34++Lin− UCB cells after culture for 3 days, either without cytokines and retroviral supernatant (A), without cytokines but with retroviral supernatant (B), or with both cytokines (100 ng/mL SCF and 500 U/mL IL-3) and retroviral supernatant (C). Retroviral supernatant was added after 1 day of culture. Dot plots shows CD34 PE staining versus GFP expression gated on live cells. The values in the crosses indicate the percentage of cells present in the corresponding quadrant. Quadrants were set to include 99.5% of the cells from staining showed in (A) in the upper left quadrant. The data shown are representative of six independent experiments.
GFP is expressed by cytokine-stimulated CD34++Lin− UCB cells transduced with MFG-GFP retroviral supernatant. Flow cytometric analysis of the progeny of CD34++Lin− UCB cells after culture for 3 days, either without cytokines and retroviral supernatant (A), without cytokines but with retroviral supernatant (B), or with both cytokines (100 ng/mL SCF and 500 U/mL IL-3) and retroviral supernatant (C). Retroviral supernatant was added after 1 day of culture. Dot plots shows CD34 PE staining versus GFP expression gated on live cells. The values in the crosses indicate the percentage of cells present in the corresponding quadrant. Quadrants were set to include 99.5% of the cells from staining showed in (A) in the upper left quadrant. The data shown are representative of six independent experiments.
These data show that CD34++Lin− UCB cells can be transduced at high frequency using cell-free, high-titer supernatant after 1 day of stimulation with IL-3 and SCF. Furthermore, the MoMuLV LTR promotor activity in these HSC/HPC was sufficient to generate an intense fluorescence of the GFP marker.
Transduced human CD34++Lin− cord blood cells generate mature T, NK, and dendritic cells expressing high levels of the marker gene in FTOC.
As the CD34++Lin− UCB cells expressed GFP after transduction, we wondered if the transduction protocol conserved T-cell precursor potential of these cells. To test this, precultured CD34++Lin− cord blood cells were transferred without prior selection for marker gene expression to SCID mouse fetal thymic lobes to initiate organ cultures. After 14 days of FTOC, less than 5,000 human thymocytes were recovered (n = 2). As shown in Fig 4, half of the human cells stained positive for HLA-DR. About one in three of these cells were CD4+ (Fig 4) but CD14− and CD3− (not shown) and thus represent the dendritic cells. In the experiment shown, half of the dendritic cells expressed the GFP marker. After 30 to 38 days of culture, on the average 5 × 104 human thymocytes (range, 1.5 to 8 × 104; n = 8) were recovered per lobe. Flow cytometry showed generation of T cells expressing low, intermediate, and high levels of CD3 (Fig 5), and some cells expressing high levels of CD3 were negative for CD1. Of this most mature subset, half of the cells expressed a γδ-TCR (data not shown). As shown by other groups, this indicates that UCB CD34++Lin− cells retain the ability to generate T cells after several days of cytokine-supplemented suspension culture5,12,18,19 or cryopreservation.5Likewise, FTOC started with CD34++Lin−cord blood cells, precultured in complete medium without cytokines, were successful and phenotypically comparable (data not shown).
Transduced CD34++Lin− UCB cells generate dendritic cells expressing high levels of GFP in FTOC. Flow cytometric analysis of thymocytes recovered from FTOC initiated with transduced CD34++Lin− UCB cells that were not selected for GFP expression (in this experiment, 30% GFP+ cells at initiation of FTOC) and used to initiate FTOC. After 14 days of culture, thymocytes were recovered from FTOC and prestained with antimouse CD45 Cychrome (see the Materials and Methods). Subsequently, aliquots were stained with IgG1 PE and IgG2a BIO/SA-TC (not shown) or CD4 PE and HLA-DR BIO/SA-TC and analyzed by flow cytometry. The left dot plot shows the forward scatter (FSC) versus TC + Cychrome staining of all cells recovered. R1 is set to include less than 1% of the human cells in isotypic control antibody staining and contains the HLA-DR+ human cells. Above R1, the cluster of Cychrome+ murine cells is seen, which are at 14 days of FTOC still more numerous than human cells. The right dot plot shows CD4 staining versus GFP expression gated on HLA-DR+ human cells (R1). The values in the crosses indicate the percentage of cells present in the corresponding quadrant. Quadrants were set to include 99% of cells stained, with isotypic control antibody in lower quadrants, gated on all human cells. The data shown are representative of two independent experiments.
Transduced CD34++Lin− UCB cells generate dendritic cells expressing high levels of GFP in FTOC. Flow cytometric analysis of thymocytes recovered from FTOC initiated with transduced CD34++Lin− UCB cells that were not selected for GFP expression (in this experiment, 30% GFP+ cells at initiation of FTOC) and used to initiate FTOC. After 14 days of culture, thymocytes were recovered from FTOC and prestained with antimouse CD45 Cychrome (see the Materials and Methods). Subsequently, aliquots were stained with IgG1 PE and IgG2a BIO/SA-TC (not shown) or CD4 PE and HLA-DR BIO/SA-TC and analyzed by flow cytometry. The left dot plot shows the forward scatter (FSC) versus TC + Cychrome staining of all cells recovered. R1 is set to include less than 1% of the human cells in isotypic control antibody staining and contains the HLA-DR+ human cells. Above R1, the cluster of Cychrome+ murine cells is seen, which are at 14 days of FTOC still more numerous than human cells. The right dot plot shows CD4 staining versus GFP expression gated on HLA-DR+ human cells (R1). The values in the crosses indicate the percentage of cells present in the corresponding quadrant. Quadrants were set to include 99% of cells stained, with isotypic control antibody in lower quadrants, gated on all human cells. The data shown are representative of two independent experiments.
Transduced CD34++Lin− UCB cells generate T cells expressing high levels of GFP in FTOC. Flow cytometric analysis of thymocytes recovered from FTOC initiated with transduced CD34++Lin− UCB cells that were not selected for GFP expression (in this experiment, 40% GFP+ cells at initiation of FTOC) before initiation of FTOC. After 35 days of culture, thymocytes recovered from FTOC were stained with IgG1 PE (not shown), IgG2a PE, CD1 PE, CD3 PE, CD4 PE, CD8α PE and analyzed by flow cytometry. Dot plots show forward scatter (FSC) and staining versus GFP expression of live, human cells recovered. The values in the crosses indicate the percentage of cells present in the corresponding quadrant. Quadrants were set to include 99.5% of the cells stained, with isotypic control antibody in the lower quadrants. The data shown are representative of six independent experiments.
Transduced CD34++Lin− UCB cells generate T cells expressing high levels of GFP in FTOC. Flow cytometric analysis of thymocytes recovered from FTOC initiated with transduced CD34++Lin− UCB cells that were not selected for GFP expression (in this experiment, 40% GFP+ cells at initiation of FTOC) before initiation of FTOC. After 35 days of culture, thymocytes recovered from FTOC were stained with IgG1 PE (not shown), IgG2a PE, CD1 PE, CD3 PE, CD4 PE, CD8α PE and analyzed by flow cytometry. Dot plots show forward scatter (FSC) and staining versus GFP expression of live, human cells recovered. The values in the crosses indicate the percentage of cells present in the corresponding quadrant. Quadrants were set to include 99.5% of the cells stained, with isotypic control antibody in the lower quadrants. The data shown are representative of six independent experiments.
In FTOC initiated with transduced CD34++Lin− cord blood cells, part of the human cells expressed high levels of the GFP marker gene at days 30 to 35 of FTOC (Fig 5). The ratio of percentage of cells expressing the marker gene in the thymocyte progeny cells over that in the CD34++Lin− cells that initiated the FTOC varied from experiment to experiment (average, 0.5; range, 0.1 to 1.2; n = 8). Analysis of coexpression of GFP with CD1, CD3, CD4, and CD8 showed that the expression of the marker gene did not hamper T-cell development of transduced cells (Fig 5). Most cultures also contained NK cells (Fig 6E) and γδ-T cells (data not shown) expressing the marker gene. The level of GFP expression in the thymocytes was very high, up to more than 1,000 times the autofluorescence level. Murine cells recovered from FTOC (mouse CD45-Cychrome+) were always FL1−, indicating the absence of replication competent virus in the FTOC. We concluded that transduced CD34++Lin− cord blood cells could generate mature T, NK, and dendritic cells expressing high levels of the marker gene in FTOC.
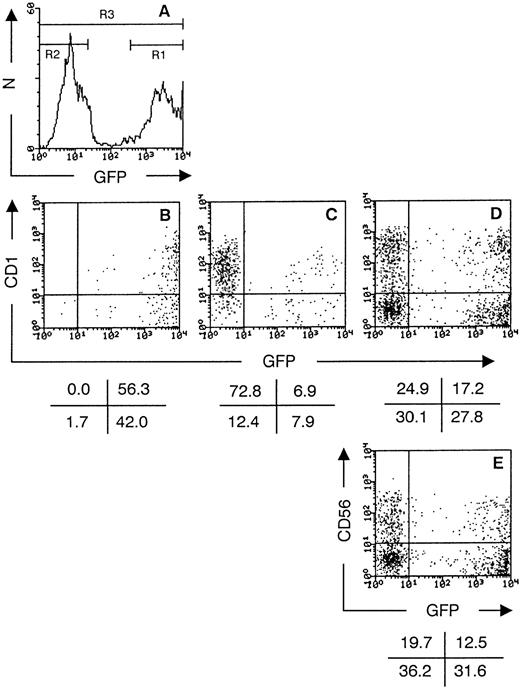
GFP+ transduced CD34++Lin− UCB cells do not downregulate GFP expression during thymic development in FTOC. Histogram (A) shows the GFP expression of transduced CD34++Lin− UCB 48 hours postinfection (in this experiment, 49% GFP+ cells; N = number of cells). The cells were purified in a GFP+ fraction (sort gate R1) and a GFP− fraction (sort gate R2) by cell sorting. The unseparated population (indicated by R3) served as a control. Dot plots show flow cytometric analysis of thymocytes recovered after 21 days of FTOC and stained with CD1 PE for FTOC initiated with the GFP+ fraction (B), the GFP− fraction (C), and the unseparated population (D). Flow cytometric analysis of thymocytes stained with CD56 PE, gated on CD3 TC− cells, after 21 days of FTOC initiated with the unseparated population (E) is also shown. Dot plots show staining versus GFP expression of live, human cells recovered. The values in the crosses indicate the percentage of cells present in the corresponding quadrant. Quadrants were set to include 99.5% of the cells stained, with isotypic control antibody in lower quadrants. The data shown are representative of two experiments.
GFP+ transduced CD34++Lin− UCB cells do not downregulate GFP expression during thymic development in FTOC. Histogram (A) shows the GFP expression of transduced CD34++Lin− UCB 48 hours postinfection (in this experiment, 49% GFP+ cells; N = number of cells). The cells were purified in a GFP+ fraction (sort gate R1) and a GFP− fraction (sort gate R2) by cell sorting. The unseparated population (indicated by R3) served as a control. Dot plots show flow cytometric analysis of thymocytes recovered after 21 days of FTOC and stained with CD1 PE for FTOC initiated with the GFP+ fraction (B), the GFP− fraction (C), and the unseparated population (D). Flow cytometric analysis of thymocytes stained with CD56 PE, gated on CD3 TC− cells, after 21 days of FTOC initiated with the unseparated population (E) is also shown. Dot plots show staining versus GFP expression of live, human cells recovered. The values in the crosses indicate the percentage of cells present in the corresponding quadrant. Quadrants were set to include 99.5% of the cells stained, with isotypic control antibody in lower quadrants. The data shown are representative of two experiments.
Transduced human CD34++Lin− cord blood cells, sorted for GFP expression, generate only thymocytes expressing high levels of the marker gene in FTOC.
As described above, unselected transduced CD34++Lin− UCB cells generate a mixed thymocyte population expressing or not expressing GFP after FTOC (Figs4 and 5). On the average, the original transduction percentages of CD34++Lin− cells was two times that observed in their thymocyte progeny. This phenomenon could be due to either downregulation of GFP expression early after introduction in the thymic lobe or to a difference in progenitor potential between transduced and nontransduced cells. To dissect these phenomena, we performed experiments with transduced CD34++Lin− cells that were sorted for presence or absence of GFP expression. As shown in Fig 6A, sort gates were set to purify transduced CD34++Lin−cells expressing (R1) or not expressing (R2) GFP. Also, the total population (indicated by R3) was used in comparison. An equal number of sorted cells (>99% pure) was cultured in FTOC. After 21 days of culture, the thymocyte progeny of transduced CD34++Lin− cells expressing GFP was homogeneously GFP+. No single human CD1+GFP− cell (Fig 6B) or CD4+GFP− cell (data not shown) was detected. The level of GFP expression in the thymocytes was very high (GFP++), up to more than 1,000 times the autofluorescence level. Interestingly, in the thymocyte progeny of transduced CD34++Lin− cells not expressing GFP, a minor fraction expressed moderate levels of GFP (GFP+; Fig6C). FTOC initiated with GFP− transduced CD34++Lin− cells yielded as many thymocytes as FTOC initiated with GFP+ cells, at least after 21 days of culture. This is not clear from the dot plots, because many GFP++ cells are clustered at the last FL1 channel and project on the frame of the plot (Fig 6D).
As observed earlier, cultures initiated with transduced CD34++Lin− that were not selected for GFP expression generated a mixed progeny of GFP−, GFP+, and GFP++ cells (Fig 5D). In this FTOC, most cells do not yet express CD3 after 21 days of culture. The CD3−CD56+ population is then still prominent, with both a GFP+ and GFP−subset (Fig 6E).
Both CD38− and CD38+CD34++Lin− cord blood cells generate mature T cells expressing high levels of the marker gene in FTOC.
From our experiments we concluded that CD34++Lin− UCB cells expressed GFP after transduction and generated mature T cells in FTOC expressing the marker gene. As shown in Fig 1, these CD34++Lin−UCB cells are heterogeneous in CD38 expression. Because UCB HSC reside in the CD34+CD38− cell fraction,15 19 we analyzed whether this subset of the CD34++Lin− UCB cells was transduced and could generate T cells in the FTOC. As shown in Fig 7, transduction was about half as efficient in CD34++CD38−Lin− as compared with CD34++CD38+Lin−UCB cells. Also, on the average, 3 times more (range, 1.5 to 6; n = 5) cells were recovered from CD38+ compared with CD38− CD34++Lin− UCB cells after the 3 days of culture in the transduction protocol, ie, posttransduction only as many cells as the input number were recovered per well from CD34++CD38−Lin− UCB cells.
Both CD38+ and CD38−CD34++Lin− UCB cells generate T cells expressing high levels of GFP in FTOC. Before FTOC indicates flow cytometric analysis of the progeny of CD38+ and CD38− CD34++Lin− UCB cells after culture for 3 days in medium with SCF (100 ng/mL) and IL-3 (50 U/mL) supplemented with retroviral supernatant after 1 day of culture. Dot plots shows CD34 PE staining versus GFP expression gated on live cells. The values in the crosses indicate the percentage of cells present in the corresponding quadrant. Quadrants were set arbitrarily. FTOC day 30 indicates flow cytometric analysis of thymocytes recovered from FTOC initiated with transduced CD38+ and CD38− CD34++Lin− UCB cells shown before FTOC that were not selected for GFP expression before initiation of FTOC. After 30 days of culture, thymocytes recovered from FTOC were stained with IgG1 PE (not shown), CD1 PE, CD4 TC, and CD3 PE and analyzed by flow cytometry. Dot plots show staining versus GFP expression of live, human cells recovered. The values in the crosses indicate the percentage of cells present in the corresponding quadrant. Quadrants were set to include 99.5% of the cells stained, with isotypic control antibody in the lower quadrants. The data shown are representative of three independent experiments.
Both CD38+ and CD38−CD34++Lin− UCB cells generate T cells expressing high levels of GFP in FTOC. Before FTOC indicates flow cytometric analysis of the progeny of CD38+ and CD38− CD34++Lin− UCB cells after culture for 3 days in medium with SCF (100 ng/mL) and IL-3 (50 U/mL) supplemented with retroviral supernatant after 1 day of culture. Dot plots shows CD34 PE staining versus GFP expression gated on live cells. The values in the crosses indicate the percentage of cells present in the corresponding quadrant. Quadrants were set arbitrarily. FTOC day 30 indicates flow cytometric analysis of thymocytes recovered from FTOC initiated with transduced CD38+ and CD38− CD34++Lin− UCB cells shown before FTOC that were not selected for GFP expression before initiation of FTOC. After 30 days of culture, thymocytes recovered from FTOC were stained with IgG1 PE (not shown), CD1 PE, CD4 TC, and CD3 PE and analyzed by flow cytometry. Dot plots show staining versus GFP expression of live, human cells recovered. The values in the crosses indicate the percentage of cells present in the corresponding quadrant. Quadrants were set to include 99.5% of the cells stained, with isotypic control antibody in the lower quadrants. The data shown are representative of three independent experiments.
As described above for total CD34++Lin−UCB cells, the progeny of precultured, transduced CD38− and CD38+ subsets of CD34++Lin− UCB cells was transferred without prior selection for marker gene expression to FTOC. After 30 days of FTOC, gene-marked thymocytes were recovered from cultures initiated with both subsets (Fig 7). For the markers tested, no phenotypical differences were observed between the thymocyte progenies from transduced CD38− versus CD38+CD34++Lin− UCB. However, relatively more GFP+ cells in cultures initiated with CD34++CD38−Lin− cells stained negative for the surface markers tested, as compared with FTOC initiated with CD34++CD38+Lin− UCB cells.
We conclude from these data that both the CD38− and CD38+ subsets of CD34++Lin−UCB cells can be transduced and can generate T cells in FTOC after transduction.
DISCUSSION
In this report, we show that highly purified CD34++Lin− UCB cells can generate dendritic, NK, and mature T cells in an FTOC. This ability is maintained after transduction of IL-3/SCF–stimulated CD34++Lin− UCB cells with retrovirus encoding GFP, the thymocyte progeny expressing high levels of the marker gene. Both the CD38− and CD38+subsets of CD34++Lin− UCB cells can be transduced and can generate T cells in FTOC posttransduction. We thus present an in vitro assay for thymic T-cell development from transduced HSC/HPC.
Phenotypical analysis of the purified UCB CD34++CD1−CD3−CD4−CD7−CD8−CD19−gpA−cells shows no expression of other lineage markers such as CD2, CD5, CD7, CD16, and CD33. In contrast, the cells are homogeneously positive for CD44 (not shown) and HLA-DR (Fig 1). Their immature phenotype is underscored by the expression of c-kit30 and the low or absent expression of CD38.15 30
Also, the absence of transduction without cytokine stimulation10 sustains the notion that these cells are immature. Part of the cells stained positive for CD45RA. A low expression of CD10 (on the average 5% of CD34++Lin− UCB cells; our unpublished observations) was detected only on some of these CD34+CD45RA+Lin− cells. A previous study14 showed that CD34++CD38+CD45RA+CD10+Lin−cells from adult bone marrow are nonprimitive progenitors for lymphoid and dendritic cells. Further experiments will teach us what the contribution of this and other subsets within the CD34++Lin− UCB cells is to the colonization of the thymic lobes in FTOC. However, our data (Fig 7) indicate that CD34++CD38−Lin− UCB can also generate T cells in the FTOC, indicating that more primitive cells than the aforementioned population can contribute to the thymocyte progeny in FTOC. The development into T and dendritic cells described in this report (Fig 2) is very similar to that as we described for CD34++Lin− fetal liver cells.21,22 The development of T cells in this system allows a kinetic analysis and study of T-cell development pathways.22 The T cells develop from a CD3−CD4−CD8−stage, via CD3−CD4+CD8− and CD3−/dim CD4+CD8+ cells into CD3++CD4+ or CD3++CD8+mature T cells, as reported for human T-cell development observed in SCID-hu mice and human thymus organ culture (reviewed by Shortman and Wu1). Several reports31,32 show the generation of CD3++CD4+ or CD3++CD8+ cells from CD34+ bone marrow cells in vitro by culture on thymic stroma in the absence of the three-dimensional thymic microenvironment. However, it is unclear whether these models reflect T-cell development as observed in the thymus. In the experiments of Freedman et al,31 no clear CD4+CD8+ double-positive intermediate was observed. Interestingly, a recent report shows the existence of an extra thymic pathway of CD4+ T-cell development from peripheral blood progenitors that also do not develop by a CD4+CD8+ double-positive intermediate.33 Such an intermediate is present in the data reported by Rosenzweig et al,32 who use cultures of human HSC/HPC on primate thymic stroma. However, this model is less readily available and less studied than FTOC.
Staal et al34 reported the development of CD34+thymocytes, transduced by coculture with Ψ-CRIP packaging cells producing MFG-LacZ retrovirus, into more mature thymocytes using FTOC. Using FACS detection of β-galactosidase expression, they show staining of part of the CD4+CD8− and CD4+CD8+ thymocytes after 3 weeks of culture. In our hands, transduction with retrovirus generated with the MFG-LacZ vector in Ψ-CRIP packaging cell line suffered from major drawbacks (our unpublished results). Similar to the findings of Staal et al,34 we obtained a uniform peak shift in FITC-fluorescence of CD34++ cells after coculture with Ψ-CRIP/MFG-LacZ cells. However, in the majority of cells, this was due to enzyme rather than gene-transfer of and did not mirror stable transduction. Mixing of cells showed that, in our hands, enzyme was transferred from nontransduced to transduced cells, hampering or eliminating discrimination between both populations. This problem was not observed by using β-galactosidase engineered to contain nuclear localization sequences (Dr C. Bagnis, Institut Paoli Calmettes, Marseille, France; personal communication, June 1997), possibly by preventing enzyme leakage. Overestimation of transduction due to protein rather than gene-transfer has also been reported with retrovirus-encoding cell surface markers.35 With the retroviral supernatant that was used in the present report, Jurkat cells were transduced (data not shown). In these experiments, the first GFP-expressing cells were detected only after more than 8 hours posttransduction, and GFP fluorescence intensity was only maximal after 48 hours. These observations argue against detectable passive protein transfer and suggest active protein synthesis. By using mixing experiments with homogeneous populations of GFP+ and GFP− cells, transfer of GFP to nontransduced cells was not detected. Finally, cloning of transduced Jurkat cells showed stable expression after gene-transfer. In our experiments with CD34++Lin− UCB cells, very few GFP+ cells were detected, unless the cells were stimulated with cytokines (Fig 3). This suggests that UCB CD34++ cells have to be put into cell cycle, before gene-transfer can occur, as is believed to be necessary for nonlentiviral retroviral vectors.5,10 We therefore conclude that FACS GFP detection is a reliable marker of productive gene-transfer in our experiments. As with cell surface markers,12,18,36 GFP expression allows the identification and separation of effectively transduced cells soon after infection. In this way, competition between nontransduced and transduced cells in subsequent assays can be excluded. By sorting transduced CD34++Lin− cells for GFP expression, we showed that the MoMuLV LTR is not at all downregulated in the development of T cells from transduced UCB HSC/HPC (Fig 6). This observation is in line with in vivo observations of other groups.11,12 18 Interestingly, GFP−transduced CD34++Lin− cells generated some GFP+ thymocytes. Although not detected, we never can exclude contamination during sorting. However, because unselected transduced CD34++Lin− generate both GFP+ (FL1 ≤4,000) and GFP++ (FL1 >4,000) thymocytes (Fig 5D) and GFP+ transduced CD34++Lin− cells generate mainly GFP++ thymocytes (Fig 5B), we favor the idea that at least part of the GFP+ thymocytes are the progeny of effectively transduced CD34++Lin− cells not expressing GFP at the moment of analysis. This would indicate an upregulation rather than downregulation of the MoMuLV LTR during T-cell development. The increase of average GFP fluorescence (250 stronger than autofluorescence in transduced UCB CD34++ cells to more than 1,000 times stronger than autofluorescence in thymocytes) sustains this conclusion. Also, protein accumulation is suggested by the fact that GFP fluorescence intensity in transduced UCB CD34++ cells is higher 48 hours compared with 24 hours posttransduction (data not shown). Because GFP is an intracellular, spontaneously fluorescent protein, no antibody staining is needed to detect its expression. This easy detection has the advantage that no background fluorescence is present in nontransduced cells. However, in contrast to cell surface markers, transduced cells can only be purified by cell sorting and cannot be phenotyped with FITC-labeled antibodies.
In some of our experiments, we used the bulk of CD34++Lin− cells exposed to virus, containing both cells expressing or not the transgene, to start FTOC (Figs 4 and 5). We observed the development of both transduced and nontransduced dendritic, NK, and T cells. The markers CD56, CD1, and CD4 appeared with the same kinetics in GFP+ and GFP− cells. However, CD3 and CD8 expression was less in the GFP+ compared with the GFP−populations after 35 days of FTOC. Moreover, in one experiment with transduced CD34++Lin− cells sorted for GFP expression before FTOC, we recovered fewer thymocytes after 14 days of FTOC in cultures initiated with GFP+ cells, compared with cultures initiated with GFP− cells (data not shown). This difference was not evident after 21 and 30 days. Further experiments will teach us if these observations might indicate a slower development of mature GFP+ compared with that of GFP− T cells. A toxic effect of GFP expression in the transduced CD34++ cells or thymocytes cannot be excluded. However, although reduced proliferation was observed in yeast cells expressing S65T GFP,37 no reports show a toxic effect of S65T GFP or of EGFP expressed in mammalian cells. Still, both GFP+ and GFP− mature T cells were present after 35 days of FTOC. This indicates that neither expression of retrovirally encoded GFP nor exposure to the retroviral supernatant blocks the development of CD34++Lin−cells in the FTOC.
Several diseases that affect the hematopoietic system, such as ADA deficiency,8,10 Gaucher disease,4 chronic granulomatous disease,9 and acquired immunodeficiency syndrome,12 are considered curable by gene therapy if the transduced gene is expressed in mature T cells. However, T-cell progenitor potential of transduced HSC is largely unknown in most gene therapy protocols. In clinical trials reported so far,6,8the in vivo fraction of cells transduced in myeloid and lymphoid lineages is low compared with that in in vitro assays, such as methyl cellulose cultures, started with an aliquot of the same transduced HSC. Also, in in vivo models such as SCID-hu mice intrathymically injected with transduced cord blood, the level of gene marking in the thymocytes is considerably lower than that of the input HSC/HPC (80-fold11 to 5-fold18 reduction). A similar (∼40-fold) reduction20 in gene marking levels was observed in human T cells found in the bone marrow of bnx mice repopulated with transduced CD34+ cells from normal human bone marrow or mobilized peripheral blood. The reduced marking of generated T cells in vivo compared with that of the input HSC/HPC population is probably due to the fact that true HSC, responsible for long-term repopulation of the acceptor, are less frequently or not transduced. In our experiments with the bulk of CD34++Lin− cells exposed to virus, containing both cells expressing or not expressing the transgene, an average of twofold reduction in gene marking of the thymocyte progeny was observed. We showed that this phenomenon was not due to downregulation of GFP expression, because the thymocyte progeny of GFP+ transduced CD34++Lin−cells was homogeneously GFP+ (Fig 6). We therefore currently consider the possibility that transduced CD34++Lin− cells have, on the population level, a reduced T-cell progenitor potential compared with their nontransduced counterparts. This might be an indirect consequence of a more limited survival. More experiments are needed to evaluate the impact of cytokines, cell cycle induction and differentiation kinetics on the observed differences in progenitor potential. Also, we are setting up inverse PCR experiments20 to assay (oligo)clonality of the transduced T, NK, and dendritic cells generated in the FTOC.
The CD34++Lin− cord blood cells used in some of our experiments were CD38− and thus encompass both the most primitive HSC.15 These cells could be transduced, albeit at lower efficiency compared with CD34++CD38+Lin− cells (Fig7). Our preliminary data show that gene-transfer to CD34++CD38−Lin− UCB cells is higher if the virus is added only after 2 days instead of 1 day of cytokine stimulation, whereas this difference was not clear for CD34++CD38+Lin− UCB cells (data not shown). This probably reflects differences in cell cycle entry between the two subsets. After transduction, both the CD38+ and the CD38− subset generate T cells expressing high levels of the marker gene in the FTOC (Fig 7). We observed that relatively more GFP+ cells in cultures initiated with CD34++CD38−Lin− cells stained negative for the surface markers tested, as compared with FTOC initiated with CD34++CD38+Lin− UCB cells. Future experiments will inquire if this phenomenon indicates non–T-cell development in the FTOC of transduced CD34++CD38−Lin− UCB cells. Our observations consistently show (7 independent experiments with different donors) T-cell development from freshly sorted CD34++CD38−Lin− UCB cells in FTOC, in contrast to the data of Blom et al.27These investigators report that CD34++CD38− UCB cells from some but not all donors fail to generate CD3+CD4+ cells in FTOC. However, because these investigators isolate CD34 FITC++CD38 PE− cells for FTOC using 2-deoxyguanosine–treated RAG-1−/− fetal thymic lobes, we cannot directly compare our results. Differences in fluorochrome of the antibody used can affect the expression level from which a surface marker is considered to be expressed or not. To resolve this issue, the two staining and isolation protocols should be compared in parallel.
Besides failure of transduction of HSC, one can infer that a transduction protocol affects T-lymphoid progenitor capacity of transduced cells that become nonlymphoid HPC rather than stay HSC. We have indeed observed that certain cytokine mixes, while resulting in comparable transduction efficiencies as those reported here, completely abrogate T-cell development in vitro of transduced HSC/HPC (manuscript in preparation).
In conclusion, we show in this report that retrovirally transduced human CD34++Lin− and CD34++CD38−Lin− UCB cells can generate a thymocyte progeny expressing the transgene in vitro. GFP, under control of the MoMuLV LTR promotor, was shown to be a suitable marker gene. The FTOC model we use allows an array of cytokine combinations and concentrations to be compared in parallel in their effect on transduction and T-cell development of transduced cells. The in vitro results could prove valuable in establishing a transduction protocol for in vivo studies and gene therapy. As in the SCID-hu model, developmental kinetics can be studied. This allows us to study the effect of genes of interest, such as (anti-)human immunodeficiency virus genes, on the development of transduced HSC/HPC.
ACKNOWLEDGMENT
The authors thank Christian De Boever for artwork, Achiel Moerman and Veronique Debacker for animal care, and the Department of Obstetrics (Prof M. Dhont) of the University Hospital of Ghent for the supply of human tissue. We also gratefully acknowledge the support of Dr H. Spits and collaborators (The Netherlands Cancer Institute, Amsterdam, The Netherlands) in introducing us in retroviral vector construction. We are greatly indebted to Dr R.C. Mulligan (Howard Hughes Medical Institute, Boston, MA) for making the MFG vector backbone and Ψ-CRIP packaging cell line available to us and to Dr G.P. Nolan (Stanford University School of Medicine, Stanford, CA) for the generous gift of the Phoenix-A packaging cell line. Finally, we thank our colleagues Drs B. Vandekerckhove and D. Vanhecke for critical reading of the manuscript.
Supported by grants from the Gezamelijk Overlegde Actie (GOA) and the Fund for Scientific Research-Flanders (Belgium). B.V. is a research assistant of the Fund for Scientific Research-Flanders (Belgium).
Address reprint requests to Bruno Verhasselt, MD, Department of Clinical Chemistry, Microbiology and Immunology, University of Ghent, University Hospital of Ghent, 4 Blok A, De Pintelaan 185, B-9000 Ghent, Belgium.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal