Abstract
Leukocyte rolling is the earliest observable event in their recruitment from the circulation to inflamed tissue. This rolling is mediated largely by interaction between the selectin family of adhesion molecules and their glycosylated ligands. Although the nature of these ligands and their interaction with the selectins is not fully understood, it is accepted that expression of fucosylated sialylated glycans such as sialyl Lewisx (sLex) is required for function. Despite findings that sLex inhibits binding of leukocytes to E-selectin in vitro, and has beneficial effects in inflammatory disease models, inhibition of E-selectin–dependent leukocyte rolling in vivo has not been described. Functional overlap between the selectins has been noted and reduction of rolling by E-selectin antibodies only occurs if P-selectin is absent or blocked. We demonstrate that leukocyte rolling velocity in tumor necrosis factor alpha (TNFα)-stimulated mouse cremaster is increased following treatment with either sLex or the sLex-mimetic CGP69669A and that rolling is dramatically reduced if CGP69669A is applied in the presence of anti–P-selectin antibody. These effects are characteristic of E-selectin antagonism. In contrast, surgically stimulated (L- or P-selectin–dependent) rolling is unaffected by either sLex or CGP69669A. Our data demonstrate that CGP69669A is an effective and selective antagonist of E-selectin in vivo.
USING INTRAVITAL MICROSCOPY, leukocytes can be observed to travel from the microcirculation to inflamed tissue according to a regulated chain of events that include rolling, firm adhesion, diapedesis, and migration through the interstitium.1-4 The earliest link in this chain (rolling) is mediated by interaction of the selectin family of adhesion molecules with their carbohydrate-bearing physiologic ligands.4-7 The three members of the selectin family (E-selectin expressed on endothelium, P-selectin on platelets and endothelium, and L-selectin on leukocytes) share a common modular structure characterized by an N-terminal lectin domain linked to an epidermal growth factor–like domain, a variable number of consensus repeats attached to a transmembrane segment, and a short cytoplasmic tail. While there is evidence demonstrating contributions of the epidermal growth factor–like8 and consensus repeat9 domains to optimal selectin function, this has been questioned,10 and it is binding of the lectin domain to physiologic carbohydrate-bearing ligands that is crucial for function.10-15 Thus, although physiologic selectin ligands are emerging as a diverse group of glycosylated proteins that recognize one or more of the selectins,2,5 it has been shown that the minimal carbohydrate epitope recognized by all three selectins is the tetrasaccharide sialyl Lewisx(sLex)11-15 to which E-selectin appears to bind with higher affinity. In line with this, treatment with glycosidases that remove important monosaccharides from sLex,16-18 or the use of sLex-blocking antibodies,19 limits the adhesion of treated cells to selectin-bearing substrates.
A contribution of carbohydrates to selectin function in vivo has also been demonstrated: large fucose-bearing polysaccharides (eg, fucoidin) can inhibit leukocyte rolling in vivo,20-22 treatment of human neutrophils with sialidase or with monoclonal antibodies specifically recognizing sLex prevent them from rolling in rat mesenteric venules,23,24 and, more recently, it was shown that mice genetically lacking the fucosyltransferase VII (Fuc-TVII), required for correct assembly of sLex,25 have numerous phenotypic similarities with mice that lack P- and E-selectins,26,27 an observation consistent with a requirement for Fuc-TVII in the correct assembly of physiologic selectin ligands. The importance of correct glycosylation of selectin ligands in humans is evident in leukocyte adhesion deficiency type II (LAD II),28 in which a deficiency of fucose metabolism prevents correct assembly of sLex and thus of selectin ligands. Deficient sLex expression correlates with the inability of neutrophils from LAD II patients to roll on inflamed endothelium,29 and results in poor resistance to infection in patients.28
Inhibition of selectin/selectin ligand interaction is an attractive approach to antiinflammatory drug design pursued by numerous groups.30-40 Beneficial effects of soluble sLexand various derivatives thereof have been demonstrated in models of inflammatory disease, including lipopolysaccharide (LPS)-induced leukocyte adhesion in rat mesenteric venules,41 acute lung injury in rats,42 and cardiac ischemia-reperfusion injury in cats43 and dogs.44
A direct effect of soluble sLex against P-selectin–dependent leukocyte rolling in vivo has been described,45,46 indicating that the described antiinflammatory effects of sLex are due to reduced leukocyte rolling. Despite the fact that sLex binds better to E-selectin than to L- or P-selectins,30 effects of inhibiting sLex on E-selectin–dependent rolling in vivo have not been described. One reason for this is that models allowing study of E-selectin–dependent rolling in vivo have, until recently, been unavailable. Because of functional overlap between the selectins, combined block of P-selectin with either E-, or L-selectin is required to completely inhibit thioglycollate-induced neutrophil recruitment to the peritoneal cavity.47 In tumor necrosis factor alpha (TNFα)-stimulated mouse cremaster muscle, blocking E-selectin alone has been shown to increase leukocyte rolling velocity,48 while blocking either L- or E-selectin reduces leukocyte rolling fraction if administered to mice that lack P-selectin.49 50 Equipped with this knowledge, selective antibodies, and/or P-selectin knockout mice, effects of different treatments on E-selectin–dependent rolling can now be studied using intravital microscopy.
Since rolling in the mouse cremaster is so well characterized with respect to its selectin dependence, we have used this system to investigate the inhibitory effects of sLex and a novel sLex mimetic, CGP69669A, on E-, L-, and P-selectin–dependent rolling in vivo. We demonstrate that sLex treatment increases leukocyte rolling velocities in TNFα-stimulated tissue (characteristic of E-selectin blockade). We also show that CGP69669A treatment increases rolling velocity if applied alone, and completely blocks E-selectin–dependent rolling in mouse cremaster if applied in combination with anti–P-selectin antibody. In apparent contrast to conclusions drawn from earlier work,45 46 we find no effect of sLex or CGP69669A on either P- or L-selectin–dependent rolling. Our data establish that soluble sLex is a weak inhibitor of E-selectin in vivo, while CGP69669A selectively and effectively abolishes E-selectin–dependent rolling in vivo.
MATERIALS AND METHODS
Antibodies and cytokines.
Murine recombinant TNFα (rmTNFα), purchased from R&D systems (Abingdon, Oxon, UK), was dissolved at 0.1 mg/mL in phosphate-buffered saline containing 0.1% bovine serum albumin (BSA), sterile-filtered, and stored in 500 ng aliquots at −20°C. The rat-antimouse-P-selectin antibody RB40.34 (rat IgG1) and the rat-antimouse-L-selectin antibody Mel-14 (Rat IgG2a), which have been shown previously to block rolling in mouse cremaster dependent on P- and L-selectin, respectively,49were purchased from Pharmingen Deutschland GmbH (Hamburg, Germany). Whole rat IgG (used as a control for RB40.34 and Mel-14) was purchased from Sigma (Buchs, Switzerland). Antibodies were stored at 1 mg/mL in 10-μg aliquots at −20°C. Before injection into mice, cytokine and antibody aliquot volumes were expanded to 150 μL by addition of sterile saline containing 0.1% BSA.
Test compounds.
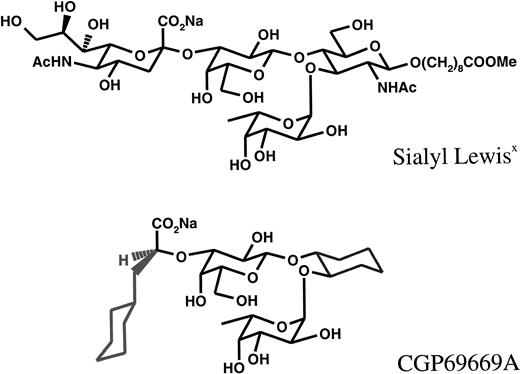
The structures of sLex(Siaα(2-3)galβ(1-4)[FUCα1-3]GlcNAcβ-), which was attached to a Lemieux spacer, and CGP69669A ((2S)-3-Cyclohexyl-2O-@1-O-[(1R,2R)2-O-(α-l-fucopyranosyl) -cyclohexyl]-β-d-galactopyrano-3-yl@-2-methyl-propanoate) are compared in Fig 1. In CGP69669A GlcNac, which in sLex acts as a spacer upon which Gal and Fuc are fixed, has been replaced by cyclohexandiol, and S-cyclohexylactic acid has been substituted for sialic acid. These two modifications were found to increase in vitro–binding activity to E-selectin by a factor of 10 (data not shown). 3-Sialyl N-acetyllactosamine was used as a negative control for sLex and CGP69669A. Compounds, which were stored as lyophilized solids, were dissolved in sterile saline and filtered (0.2 μm) before injection into mice.
Animals.
Male C57BL/6 mice weighing between 25 and 35 g were used in these experiments. All procedures performed were approved by the Kantonales Veterinäramt, Basel-Stadt, Switzerland. Mice were anesthetized with an intraperitoneal injection of 100 mg/kg ketamine hydrochloride (Ketalar; Parke-Davis, Baar, Switzerland) after premedication with 30 mg/kg sodium pentobarbital (Serva, Heidelberg, Germany) and 0.1 mg/kg atropine sulfate (Fluka, Buchs, Switzerland). Some mice received an intrascrotal injection of 500 ng rmTNFα in 150 μL saline approximately 2 hours before intravital microscopic observation. Where such injections were performed, mice were first briefly anesthetized with inhaled isofluorane. Before preparation of the cremaster muscle for intravital microscopy, the following cannulations were performed: trachea to facilitate breathing; left jugular vein to allow intravenous injection of compounds, antibodies, and supplementary anesthesia (pentobarbital 5 mg/mL, intravenously, 0.2 mL/h); and left carotid artery to allow blood sampling. Body temperature was automatically maintained at 37°C using a feedback temperature regulator (Model TCI-88; Systag AG, Thalwill, Switzerland) linked to a small heating pad.
Intravital microscopy.
The cremaster muscle was prepared for intravital microscopy as described.49 Briefly, the testis, the epididymis, and the cremaster muscle that encloses them, were gently extracted through a small incision in the scrotum. The tip of the cremaster muscle was pinned such that the testis and cremaster were positioned across a 10-mm Ø cover glass that comprised part of a specialized microscopy stage. After careful removal of fat and connective tissue, an incision was made through the cremaster beginning at the pinned tip and taking a route toward the point of exit from the scrotum that, where possible, avoided severing blood vessels in the muscle. The cremaster was then spread over the cover glass and pinned in place. During this procedure, which was typically complete within 10 minutes, and during intravital microscopic observation, the cremaster was superfused with thermocontrolled (36°C) bicarbonate-buffered solution (131.9 mmol/L NaCl, 18 mmol/L NaHCO3, 4.7 mmol/L KCl, 2.0 mmol/L CaCl2, and 2 mmol/L MgSO4) through which a gas mixture of 5% CO2 in N2 was bubbled.
Microscopic observations were made using a light microscope (Axioskop; Carl Zeiss AG, Zurich, Switzerland) equipped with a saltwater immersion objective (40×/0.75W). Venules with diameters between 15 and 50 μm were selected for observation and recorded via a CCD camera system (Model BC-6; AVT-Horn, Aalen, Switzerland) onto sVHS video cassettes using a sVHS video cassette recorder with a built-in time base corrector (Model AG 4700; Panasonic, Osaka, Japan). A time/date function was added to the recorded images by passing the signal from the camera through a video timer (Model C-VTG-33; FORA, Natik, MA), which was accurate to 1/100th of a second. Venules were typically recorded in 1-minute segments, except where treatments (antibody or oligosaccharides) were applied, in which case recording began 1 minute before treatment, was continued during treatment injection, and terminated 5 minutes after injection. Centerline red blood cell velocity was determined by the “two-slit” photometric technique using a dual photodiode linked via preamplifiers (Model 79D + Model 7P122 Amplifier; Grass Instrument, Quincy, MA) and an analog-digital converting board (Labmaster DMA; Scientific Solutions, Solon, OH) to a Pentium PC (IBM350-P133; Also-Comsyt AG, Basel, Switzerland) running a digital on-line cross correlation program.51 Mean blood flow velocity (Vb) was estimated from centerline red blood cell velocity by dividing by an empirical factor of 1.6 as described.52 Newtonian wall shear rate was calculated as 8Vb/vessel diameter. Blood samples (10 μL) were drawn from the carotid artery at regular (∼45 minutes) intervals throughout the experiment and at time points immediately before and after compound or antibody treatments. Blood samples were analyzed for total leukocyte concentration using an automatic cell counter (Model F-300; Sysmex, Kobe, Japan) calibrated for mouse leukocytes.
The bulk of analyses were performed on data extracted off-line from the video recordings of venules. Rolling flux was determined by counting the number of cells rolling past a plane perpendicular to the vessel axis. Where comparison of fluxes before and after treatment was made, values were obtained from recordings taken 1 minute before injection and 1 to 2 minutes after injection. Vessel diameter was measured using the public domain NIH-Image program (available on the internet at http:/rsb.info.nih.gov/nih-image), ported to the PC platform by Scion Corp (Frederick, MD), on a commercially available setup (P75 PC; Gateway, Des Moines, IA; LG3 Video capture card; Scion). Vessel diameter together with blood flow velocity and whole-blood leukocyte concentration was used to estimate total leukocyte flux (assuming cylindrical vessel geometry and uniform distribution of leukocytes in the microcirculation). Flux data are presented as rolling flux %, which is defined as the rolling leukocyte flux expressed as a percentage of total leukocyte flux.
In each vessel studied, leukocyte rolling velocities were determined immediately before and after different treatments for five randomly selected leukocytes by measuring the distance traveled (measured using NIH-image as earlier) in a given time (indicated by the video timer). For cells rolling at low velocities (<30 μm/s), distance traveled over a 2-second period was measured, whereas for faster moving cells (>30 μm/s), distance traveled in 0.1 to 0.5 seconds was typically measured.
Flow cytometry.
For analysis of the effect of intravenously injected sLexand CGP69669A on surface expression of L-selectin on leukocytes, 10-μL samples of mouse blood were collected via the carotid artery either before, or 2 minutes subsequent to injection of these compounds into mice at 100 mg/kg. These samples were mixed with 400 μL Dulbecco's modified Eagle's medium (DMEM) plus 10% fetal calf serum (FCS) containing 50 U/mL heparin sulfate and 2 μg fluorescein isothiocyanate (FITC) or phycoerythrin (PE)-conjugated antibodies (Mel-14, mouse granulocyte marker GR-1, antimouse CD3, Pharmingen isotype-matched control rat IgG; Sigma) in 7 mL Styropor FACS tubes (Becton Dickinson, Mountain View, CA). Samples were incubated in the dark for 30 minutes at 4°C. Positive control shedding of L-selectin was induced by incubating a 200-μL blood sample with 1 μm fMLP (Sigma) ex vivo for 1 hour at 37°C. Before our measurement of surface L-selectin expression by FACS (Becton Dickinson; FAC-Star Plus), red blood cells were lysed by addition of a 20-fold volume excess of sterile-filtered, ice-cold, lysis buffer (NH4Cl 155 mmol/L, KHCO3 10 mmol/L, EDTA 140 μm, pH 7.3), washed twice in phosphate-buffered saline and finally resuspended in Hank's balanced salt solution containing 0.1% wt/vol sodium azide (Sigma) and 1% wt/vol FCS (GIBCO, Basel, Switzerland). A total of 10,000 gated events were collected and relative fluorescence intensity versus cell number histograms constructed after gating on either lymphocyte or granulocyte regions determined by forward- and side-scatter profiles. The positions of granulocyte and lymphocyte regions were independently confirmed using samples stained with the pan-granulocyte marker GR-1 (FITC-labeled) or anti-CD3 (PE-labeled) monoclonal antibodies.
Statistical analysis.
Leukocyte rolling flux % values before and after treatment were compared using Student's t test with the Bonferroni correction for multiple comparison where appropriate. Leukocyte rolling velocity distributions were compared using the Kolmagorov-Smirnov test for goodness of fit.
RESULTS
Hemodynamic factors.
Since hemodynamic factors such as vessel diameter and blood flow velocity (both of which influence wall shear rate), together with systemic leukocyte concentration variation, can potentially alter the flux of rolling leukocytes, these factors were measured and are summarized in Table 1. Any effect of venular diameter variation on rolling was avoided in the present studies by making all comparisons in the same vessels. Blood flow velocity, estimated from centerline red blood cell velocity, was not significantly altered by antibody/compound treatments.
Influence of Injected Treatments on Wall Shear Rates in Observed Venules and Systemic Leukocyte Concentration
| Experiment . | Treatment . | Wall Shear Rate . | Systemic (white blood cell) Cells/μL . |
|---|---|---|---|
| 30-minute surgical stimulation (P-selectin–dependent) | Control | 912 ± 98 | 7,000 ± 860 |
| sLex | 891 ± 87 | 6,400 ± 340 | |
| Control | 776 ± 112 | 3,800 ± 380 | |
| CGP69669A | 763 ± 91 | 4,600 ± 900 | |
| 60-minute surgical stimulation (L-, P-selectin–dependent) | Control | 749 ± 114 | 5,600 ± 1,027 |
| sLex | 856 ± 154 | 5,900 ± 850 | |
| Control | 550 ± 112 | 7,767 ± 1,332 | |
| CGP69669A | 578 ± 118 | 7,900 ± 1,906 | |
| TNFα-induced rolling (E-, L-, P-selectin–dependent) | Control | 577 ± 133 | 2,000 ± 115 |
| RB40.34 | 430 ± 53 | 1,900 ± 289 | |
| RB40.34 + sLex | 646 ± 45 | 2,000 ± 346 | |
| Control | 918 ± 332 | 1,400 ± 180 | |
| RB40.34 | 708 ± 210 | 1,500 ± 160 | |
| RB40.34 + CGP69669A | 909 ± 353 | 2,200 ± 340* |
| Experiment . | Treatment . | Wall Shear Rate . | Systemic (white blood cell) Cells/μL . |
|---|---|---|---|
| 30-minute surgical stimulation (P-selectin–dependent) | Control | 912 ± 98 | 7,000 ± 860 |
| sLex | 891 ± 87 | 6,400 ± 340 | |
| Control | 776 ± 112 | 3,800 ± 380 | |
| CGP69669A | 763 ± 91 | 4,600 ± 900 | |
| 60-minute surgical stimulation (L-, P-selectin–dependent) | Control | 749 ± 114 | 5,600 ± 1,027 |
| sLex | 856 ± 154 | 5,900 ± 850 | |
| Control | 550 ± 112 | 7,767 ± 1,332 | |
| CGP69669A | 578 ± 118 | 7,900 ± 1,906 | |
| TNFα-induced rolling (E-, L-, P-selectin–dependent) | Control | 577 ± 133 | 2,000 ± 115 |
| RB40.34 | 430 ± 53 | 1,900 ± 289 | |
| RB40.34 + sLex | 646 ± 45 | 2,000 ± 346 | |
| Control | 918 ± 332 | 1,400 ± 180 | |
| RB40.34 | 708 ± 210 | 1,500 ± 160 | |
| RB40.34 + CGP69669A | 909 ± 353 | 2,200 ± 340* |
Values are presented as the mean ± SEM of 3 or 4 mice per group.
Significantly different from control, P < .05.
Total systemic leukocyte concentrations in untreated mice were similar to previously reported values49,50 and, in most groups, were unaltered by antibody and carbohydrate treatments given. Interestingly, total leukocyte concentrations in TNFα-stimulated mice were somewhat lower than those in mice stimulated merely by the surgery required for intravital microscopy. This lowering of systemic leukocyte count in response to TNFα, which has been observed in some48 but not all previous experiments,49,50using this system, most likely reflects the fact that large numbers of cells were involved in leukocyte endothelial interaction in the cremaster and other tissues exposed to the effects of TNFα. Treatment of TNFα-stimulated mice with RB40.34 plus CGP69669A caused a slight but significant increase in systemic leukocyte concentration. This effect, which was not seen after combined treatment with RB40.34 and sLex, possibly reflects redistribution of rolling leukocytes to the systemic circulation. Blocking P-selectin function has been previously demonstrated to increase systemic leukocyte counts, presumably by interfering with basal rolling in the skin and other tissues.47 53
TNFα-induced rolling (effect on rolling flux %).
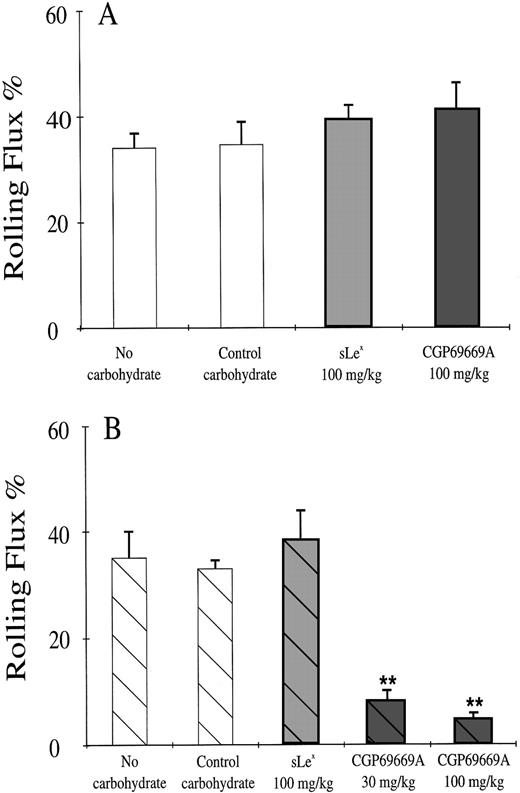
Treatment of the cremaster muscle with TNFα (500 ng intrascrotal) stimulates leukocyte rolling in venules that are dependent on E-, L-, and P-selectins.49,54 Because of some functional redundancy between the selectins, a reduction in the percentage of cells rolling through TNFα-stimulated venules is apparent only when a lack of P-selectin (ie, in knockout or anti–P-selectin antibody-treated mice) is combined with lack of E- or L-selectin.26,27,49 50 We wanted to assess the potential of CGP69669A as an E-selectin antagonist in vivo compared with its naturally occurring counterpart, sLex. We therefore gave these agents to TNFα-stimulated mice in combination with either control IgG or the blocking anti–P-selectin antibody RB40.34. In the present study, 2 hours after stimulation with TNFα, approximately 40% of leukocytes passing through observed venules were engaged in rolling interaction with the vessel wall (Fig 2A). This rolling fraction was not altered by sLex or by CGP69669A given alone (Fig2A). The anti–P-selectin antibody RB40.34 given alone or in combination with sLex was also without effect on rolling flux fraction (Fig 2B). In contrast, combined treatment with RB40.34 and CGP69669A at either 30 or 100 mg/kg intravenously reduced rolling flux fraction to less than 6% (Fig 2B). Although detailed kinetic analyses were not performed as part of this study, reduced rolling appeared to be maintained for approximately 20 minutes after application of RB40.34 plus CGP69669A. The control carbohydrate 3-sialyl N-acetyllactosamine, alone or in combination with RB40.34, did not alter leukocyte rolling flux %.
Leukocyte rolling in TNFα-stimulated mouse cremaster muscle. Shown are mean rolling flux % values (±SEM) in at least 8 venules from 3 or 4 TNFα-stimulated mouse cremaster muscles per group following treatments with (A) control IgG combined with control carbohydrate (100 mg/kg) sLex (100 mg/kg) or CGP69669A (100 mg/kg), and (B) RB40.34 combined with control carbohydrate (100 mg/kg) sLex (100 mg/kg) or CGP69669A (30 & 100 mg/kg). Only a combination of RB40.34 and CGP69669A reduces rolling flux % in this system. **Significantly different from control (P < .01).
Leukocyte rolling in TNFα-stimulated mouse cremaster muscle. Shown are mean rolling flux % values (±SEM) in at least 8 venules from 3 or 4 TNFα-stimulated mouse cremaster muscles per group following treatments with (A) control IgG combined with control carbohydrate (100 mg/kg) sLex (100 mg/kg) or CGP69669A (100 mg/kg), and (B) RB40.34 combined with control carbohydrate (100 mg/kg) sLex (100 mg/kg) or CGP69669A (30 & 100 mg/kg). Only a combination of RB40.34 and CGP69669A reduces rolling flux % in this system. **Significantly different from control (P < .01).
TNFα-induced rolling (effect on rolling velocity).
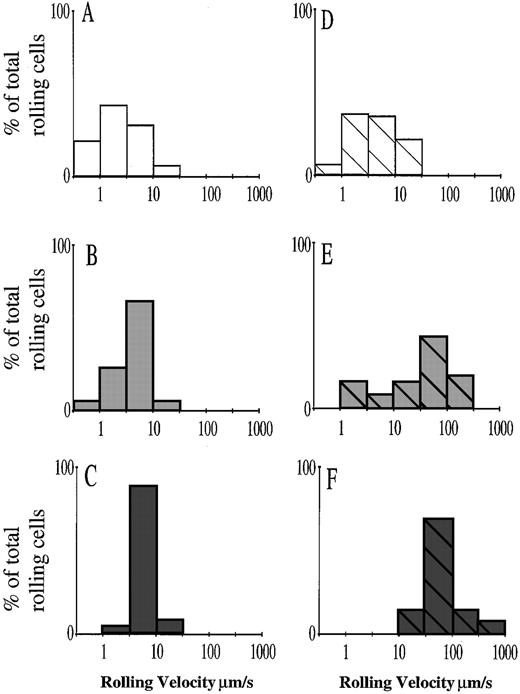
The effect of CGP69669A combined with RB40.34 described earlier is consistent with the function of this agent as either an E- and/or L-selectin antagonist. We noticed that the cells continuing to roll after combined RB40.34/CGP69669A treatment did so at a higher velocity than before treatment, suggesting that although some cells were able to form adhesive interactions with the vessel wall, these interactions were significantly less efficient. Although combined treatment with RB40.34/sLex did not affect the proportion of rolling cells, it was apparent that this treatment also increased rolling velocity and was thus interfering with the adhesion of these cells. We therefore performed further analysis of the experiments presented in Fig 2 by measuring leukocyte rolling velocities before and after treatments. Figure 3 illustrates the distribution of rolling cell velocities for leukocytes in TNFα-stimulated mouse cremaster. In the present study, the distribution of rolling cell velocities after TNFα stimulation (Fig3A) was similar to that described in an earlier study.48Following treatment with the anti–P-selectin antibody RB40.34, slightly fewer cells tended to roll below 3 μm/s (Fig 3D), although the overall distribution of rolling velocities was not significantly different from that measured in control IgG-treated mice. This is also consistent with previous findings.48 Treatment with either sLex or CGP69669A (in combination with control IgG) increased rolling cell velocities (Fig 3B and C). When sLexor CGP69669A was combined with RB40.34, rolling velocities were increased still further, with combined sLex and RB40.34 increasing rolling velocity approximately 30-fold and combined CGP69669A and RB40.34 treatment increasing rolling velocities approximately 100-fold compared with untreated controls or with mice treated with RB40.34 alone. CGP69669A given either separately or in combination with RB40.34 had a greater effect on leukocyte rolling velocity than the respective treatments with sLex, suggesting improved anti–E-selectin activity. Treatment with control carbohydrate 3-sialyl N-acetyllactosamine, alone or in combination with RB40.34, did not affect leukocyte rolling velocity (data not shown).
Effects of sLex and CGP69669A on leukocyte rolling velocity in TNFα-stimulated mouse cremaster muscle. Velocity distributions are shown for leukocytes rolling in TNFα-stimulated cremaster muscle of control IgG-treated mice (A), and for leukocytes rolling in TNFα-stimulated cremaster of mice treated with (B) sLex (100 mg/kg), (C) CGP69669A (100 mg/kg), (D) RB40.34 (10 μg), (E) RB40.34 (10 μg) + sLex (100 mg/kg), and (F) RB40.34 (10 μg) + CGP69669A (100 mg/kg). Visibly interacting leukocytes were assigned to groups rolling at <1 μm/s, 1 to 3 μm/s, 3 to 10 μm/s, 10 to 30 μm/s, 30 to 100 μm/s, 100 to 300 μm/s, and 300 to 1,000 μm/s. A log scale was selected to allow direct comparison of rolling over a wide range of velocities. Each histogram represents at least 40 individual cell velocities measured in 8 to 10 cremasteric venules from 3 or 4 mice, except for the RB40.34 + CGP69669A–treated group, which represents fewer cells (15 cell velocities in 9 venules from 4 mice), since rolling flux % was reduced by treatment.
Effects of sLex and CGP69669A on leukocyte rolling velocity in TNFα-stimulated mouse cremaster muscle. Velocity distributions are shown for leukocytes rolling in TNFα-stimulated cremaster muscle of control IgG-treated mice (A), and for leukocytes rolling in TNFα-stimulated cremaster of mice treated with (B) sLex (100 mg/kg), (C) CGP69669A (100 mg/kg), (D) RB40.34 (10 μg), (E) RB40.34 (10 μg) + sLex (100 mg/kg), and (F) RB40.34 (10 μg) + CGP69669A (100 mg/kg). Visibly interacting leukocytes were assigned to groups rolling at <1 μm/s, 1 to 3 μm/s, 3 to 10 μm/s, 10 to 30 μm/s, 30 to 100 μm/s, 100 to 300 μm/s, and 300 to 1,000 μm/s. A log scale was selected to allow direct comparison of rolling over a wide range of velocities. Each histogram represents at least 40 individual cell velocities measured in 8 to 10 cremasteric venules from 3 or 4 mice, except for the RB40.34 + CGP69669A–treated group, which represents fewer cells (15 cell velocities in 9 venules from 4 mice), since rolling flux % was reduced by treatment.
P-selectin–dependent leukocyte rolling.
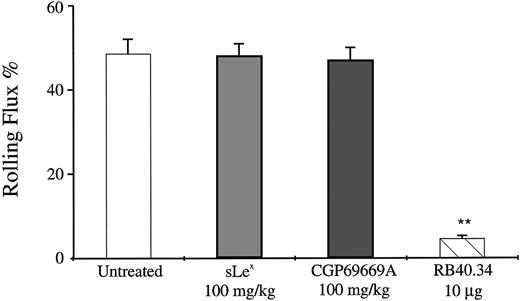
The results described for CGP69669A and sLex so far are consistent with function of these compounds as E-selectin antagonists. To investigate antagonism of P-selectin by these agents, CGP69669A and sLex were compared with the anti–P-selectin antibody RB40.34 in a system in which rolling is largely P-selectin–dependent. Exteriorization of the cremaster muscle and the subsequent manipulation required for intravital microscopic observation causes leukocyte rolling, which is mostly dependent on P-selectin at time points up to 30 minutes after surgery.49 We have used this system to determine what, if any, effect sLex and CGP69669A have on P-selectin–dependent rolling in the mouse cremaster. In the present study, surgical preparation of the cremaster resulted in marked leukocyte rolling, which was stable during the period of observation (Fig 4). Injection of the anti-P–selectin monoclonal antibody, RB40.34, at 30 minutes after tissue exteriorization, resulted in almost total block of rolling, with less than 5% of cells interacting with the vessel wall following treatment (Fig 4). In contrast to the striking inhibition of rolling given by RB40.34, injection of either sLex or CGP69669A at 100 mg/kg had no significant effect on P-selectin–dependent rolling (Fig 4). Treatment with either sLex or CGP69669A at 100 mg/kg had no significant effect on leukocyte rolling velocities in this system (data not shown).
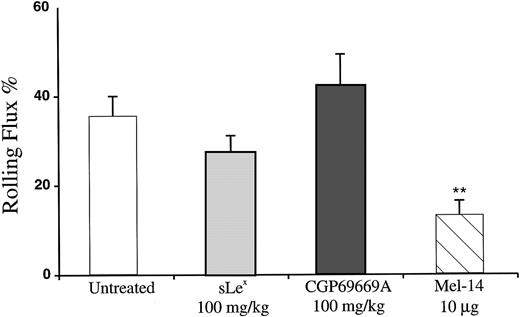
Effects of sLex and CGP69669A on surgically stimulated, P-selectin–dependent leukocyte rolling in vivo. Mouse cremaster muscle was prepared for intravital microscopy and superfused with bicarbonate buffer for 30 minutes to produce P-selectin–dependent rolling. Shown are the effects on leukocyte rolling flux of treatment with sLex (100 mg/kg), CGP69669A (100 mg/kg), and anti–P-selectin antibody RB40.34 (10 μg). Data are presented as the mean ±SEM of values from at least 9 venules from 3 or 4 mice per treatment group. **Significantly different from control (P< .01).
Effects of sLex and CGP69669A on surgically stimulated, P-selectin–dependent leukocyte rolling in vivo. Mouse cremaster muscle was prepared for intravital microscopy and superfused with bicarbonate buffer for 30 minutes to produce P-selectin–dependent rolling. Shown are the effects on leukocyte rolling flux of treatment with sLex (100 mg/kg), CGP69669A (100 mg/kg), and anti–P-selectin antibody RB40.34 (10 μg). Data are presented as the mean ±SEM of values from at least 9 venules from 3 or 4 mice per treatment group. **Significantly different from control (P< .01).
L-selectin–dependent leukocyte rolling.
It has been demonstrated that, following the surgical stimulation necessary to prepare the mouse cremaster muscle for microscopic observation, rolling is initially P-selectin–dependent, while over time, an increasing requirement for L-selectin becomes apparent.49 To test the effects of sLex and CGP69669A against L-selectin, these agents were compared with the anti–L-selectin antibody Mel-14 in venules observed 1 hour after surgical exteriorization of the cremaster. Approximately 35% of leukocytes passing through venules were engaged in rolling interaction when observed 1 hour after surgery (Fig 5).Treatment with Mel-14 reduced this value to approximately 12%. Although Mel-14 treatment also resulted in marked leukopenia (mean ± SEM systemic leukocyte concentration immediately before Mel-14, 2,250 ± 1,125; systemic leukocyte concentration immediately after Mel-14, 790 ± 400), this did not account for the reduction in rolling flux %, since systemic leukocyte concentration is accounted for in the calculation of this value (ie, rolling flux % = rolling cells/total cells). The effect of Mel-14 on leukocyte rolling flux % measured 60 minutes after surgical stimulation of tissue therefore demonstrates a significant contribution of L-selectin to leukocyte rolling at this time as described.49 Under identical conditions, treatment with either sLex or CGP69669A did not reduce leukocyte rolling.
Effects of sLex and CGP69669A on surgically stimulated L-selectin–dependent leukocyte rolling in vivo. Mouse cremaster muscle was prepared for intravital microscopy and superfused with bicarbonate buffer for 60 minutes to produce rolling that was partially L-selectin–dependent. Shown are the effects of treatment with sLex (100 mg/kg), CGP69669A (100 mg/kg), and anti–L-selectin antibody Mel 14 (10 μg). Data are presented as the mean ±SEM of values from at least 9 venules from 4 mice per treatment group. **Significantly different from control (P < .01).
Effects of sLex and CGP69669A on surgically stimulated L-selectin–dependent leukocyte rolling in vivo. Mouse cremaster muscle was prepared for intravital microscopy and superfused with bicarbonate buffer for 60 minutes to produce rolling that was partially L-selectin–dependent. Shown are the effects of treatment with sLex (100 mg/kg), CGP69669A (100 mg/kg), and anti–L-selectin antibody Mel 14 (10 μg). Data are presented as the mean ±SEM of values from at least 9 venules from 4 mice per treatment group. **Significantly different from control (P < .01).
Flow cytometry.
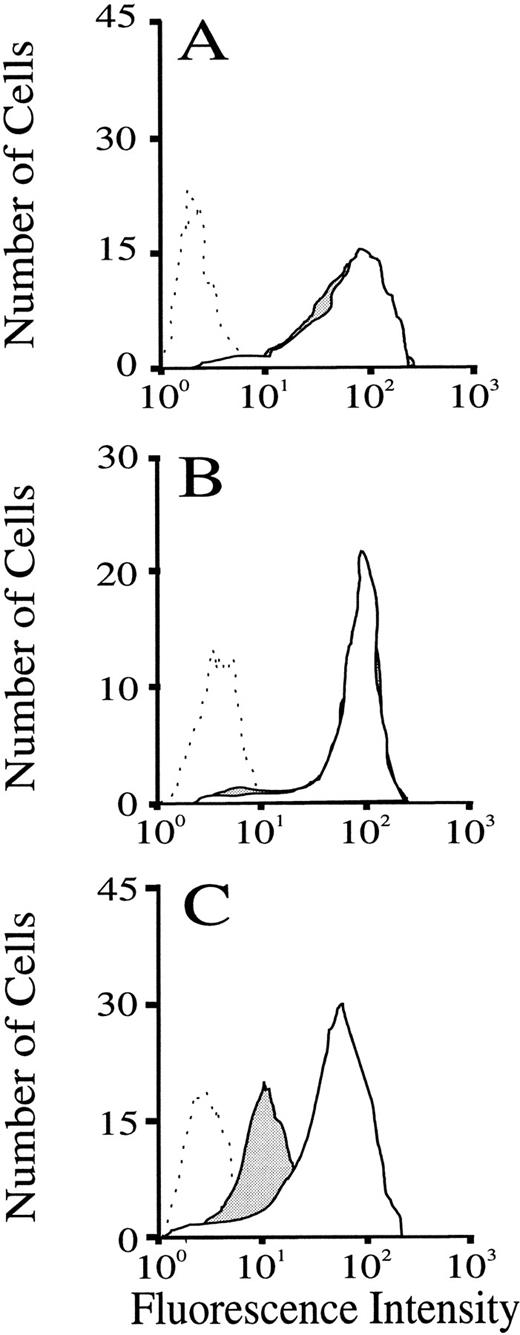
To determine whether the treatments given were activating leukocytes, and particularly polymorphonuclear leukocytes, which form the bulk of the rolling population under the conditions studied,48,55we performed FACS analysis on blood samples drawn from the carotid artery immediately before and 2 minutes after injection of compounds. Samples were stained for leukocyte L-selectin expression as described in the Methods. The results depicted in Fig6 show fluorescence intensity distributions gated for granulocytes (on the basis of forward- and side-scatter properties, together with negative staining for the mouse lymphocyte-selective marker Thy1.2). All cells stained with FITC-conjugated Mel-14. Cells collected and stained with FITC–Mel-14 following either sLex (Fig 6A, solid fill) or CGP69669A (Fig 6B, solid fill) had fluorescence intensity distributions that were indistinguishable from cells collected before compound injections. To validate that the method used was sensitive to changes in L-selectin expression, blood samples were treated in vitro with fMLP, which causes granulocytes to shed L-selectin.56 The effects of fMLP on granulocyte expression of L-selectin are shown in Fig 6C. Treatment of whole-mouse blood with fMLP caused a leftward shift of the fluorescence intensity distribution for Mel-14 staining of granulocytes, indicating loss of L-selectin from these cells.
Flow-cytometric analysis of the effect of sLex and CGP69669A on granulocyte expression of sLex. Blood samples drawn from mice immediately before and 2 minutes after injection of either sLex (100 mg/kg) or CGP69669A (100 mg/kg) were labeled and gated as described in the Methods. Lack of effect of sLex and CGP69669A injections on granulocyte expression of L-selectin is shown in A and B, respectively (Dashed line, negative control staining; solid line + no fill, cells drawn before sLex or CGP69669A; solid lines + solid fill, cells drawn 2 minutes after injections of sLex or CGP69669A). Positive shedding of L-selectin by granulocyte activation with fMLP (10 μm) is shown in C (dashed line, staining with negative control antibody; solid line + no fill, positive Mel-14 staining; solid line + solid fill, Mel-14 staining following fMLP treatment).
Flow-cytometric analysis of the effect of sLex and CGP69669A on granulocyte expression of sLex. Blood samples drawn from mice immediately before and 2 minutes after injection of either sLex (100 mg/kg) or CGP69669A (100 mg/kg) were labeled and gated as described in the Methods. Lack of effect of sLex and CGP69669A injections on granulocyte expression of L-selectin is shown in A and B, respectively (Dashed line, negative control staining; solid line + no fill, cells drawn before sLex or CGP69669A; solid lines + solid fill, cells drawn 2 minutes after injections of sLex or CGP69669A). Positive shedding of L-selectin by granulocyte activation with fMLP (10 μm) is shown in C (dashed line, staining with negative control antibody; solid line + no fill, positive Mel-14 staining; solid line + solid fill, Mel-14 staining following fMLP treatment).
DISCUSSION
We have shown that soluble sLex selectively disrupts E-selectin–dependent rolling in vivo, causing increased rolling velocity when applied alone or in combination with anti–P-selectin antibody. Furthermore, we describe a mimetic of sLex, CGP69669A, which has improved activity against E-selectin, causing a striking reduction in the number and a marked increase in the velocity of rolling leukocytes in TNFα-stimulated cremaster muscle when combined with anti–P-selectin antibody. These activities are consistent with function of sLex and CGP69669A as E-selectin antagonists.48 Although previous studies46 have detailed that sLex treatment can prevent an increase in leukocyte rolling flux and lowering of leukocyte rolling velocity induced by pharmacologic agents,45 46 our data represent the first description of a direct effect of sLex or sLex mimetics on E-selectin–dependent leukocyte rolling in vivo.
Previously described effects of sLex or other oligosaccharides in inflammatory models41-44 or against leukocyte rolling in vivo have been attributed to effects on P-selectin45,46 or “unidentified adhesion molecules”46 based on comparative effects of P-selectin antibodies in the models studied. In contrast, under the experimental conditions used in the present study, we find no effect of sLex or CGP69669A against P-selectin–dependent rolling in the mouse. The model we have used (intravital microscopy, 30 minutes after surgical stimulation) to test effects of injected agents against P-selectin–dependent leukocyte rolling is certainly sensitive to P-selectin inhibition as previously documented49 and demonstrated by the positive effect of anti–P-selectin antibody described here. Obvious differences between our study and those describing inhibitory effects of sLex against P-selectin lie in the nature of the sLex used, as well as in the animal species studied and specific methods employed. Thus, the term sLex is applied to a family of molecules (rather than to a specific molecular structure) that share the common tetrasaccharide shown in Fig 1, but which can vary in the reducing end modification (eg, galactose or aglycon). The studies describing effects of sLex on P-selectin–dependent leukocyte rolling45,46 were performed in rats, which might differ from mice with regard to which selectin pathways are affected by sLex and/or analogs thereof. The methods used in the earlier studies also differ from those described here with respect to how rolling was stimulated and to the timing of compound administration. Particularly, in these studies, sLexpretreatment was found to prevent an increase in leukocyte rolling flux induced by pharmacologic agents (histamine45 or leukotriene C446). The approach used in the present study holds certain advantage over these earlier studies in that sLex and CGP69669A were directly observed to inhibit existing E-selectin–dependent rolling in an exquisitely characterized system, while, in an equally well-defined system, no effect was seen against P-selectin–dependent rolling. The lack of effect of these agents against P-selectin was also directly compared with a striking effect of the well-characterized anti–P-selectin antibody RB40.34.47,49 50
The reduction of leukocyte rolling flux in TNFα-stimulated mouse cremaster muscle venules by CGP69669A combined with RB40.34 was quantitatively similar to that given by antibodies against either E-50 or L-selectin49 in combination with P-selectin blockade. This suggests that CGP69669A, but not sLex, is an efficient inhibitor of either E- or L-selectin function at the concentrations used in the present study. The finding that treatment with sLex or CGP69669A increased leukocyte rolling velocity in TNFα-stimulated mouse cremaster when given in combination with anti–P-selectin or control antibodies is consistent with antagonism of E- rather than L-selectin, as only antibodies against E-selectin have been observed to increase rolling velocity in this system.48,55 To rule out the additional possibility of L-selectin antagonism by sLex and CGP69669A, these compounds were tested against rolling 1 hour after surgical trauma. A clear contribution of L-selectin to rolling induced under these conditions has been demonstrated previously49 and is confirmed here by the effect of Mel-14 antibody. Injection of either CGP69669A or sLex exactly 60 minutes after surgical trauma produced no significant reduction in leukocyte rolling flux, while injection of the anti–L-selectin antibody reduced rolling flux by greater than 60%. This provides convincing evidence that CGP69669A and sLex are not exerting their effects on TNFα-induced rolling via inhibition of L-selectin.
It has been known for some time that neutrophil activation results in shedding of L-selectin from the cell surface.56 More recent studies have demonstrated that functional expression of a P-selectin ligand can also be altered by chemotactic stimulation.57 To address the possibility that sLex and CGP69669A were producing effects against rolling indirectly, by activating cells, we measured the effect of intravenous injections of sLex and CGP69669A on mouse leukocyte activation state, using expression of L-selectin as a sensitive marker of activation relevant to the present study. When samples drawn immediately before and 2 minutes after injection of sLex and CGP69669A were compared, no difference was detected with respect to granulocyte expression of L-selectin. At 2 minutes sLex and CGP69669A had clear effects on leukocyte rolling in venules of TNFα-stimulated cremaster. This strongly suggests that the effects of these agents on leukocyte rolling in vivo resulted from specific inhibition of E-selectin, rather than nonspecific granulocyte activation.
Here, we characterize CGP69669A as a selective and efficient E-selectin antagonist that can reduce the number and increase the velocity of rolling leukocytes in vivo. Classical models of acute inflammation such as thioglycollate-induced peritonitis and delayed type hypersensitivity predict that antiinflammatory approaches aimed at the selectins should address both P- and E-selectins, requiring CGP69669A to be combined with anti–P-selectin treatment. However, with the field of selectin biology unfolding so rapidly, it is possible that an absolute requirement for E-selectin in certain diseases might be found, in which case CGP69669A might provide clinical benefit.
ACKNOWLEDGMENT
The expert assistance of M. Wesp in performing FACS measurements and analysis is gratefully recognized.
Address reprint requests to Keith E. Norman, PhD, Novartis AG, S386.638, Basle 4002, Switzerland.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal