Abstract
Blood coagulation factor X plays a pivotal role in the clotting cascade. When administered intravenously to mice, the majority of activated factor X (factor Xa) binds to α2-macroglobulin (α2M) and is rapidly cleared from the circulation into liver. We show here that the low-density lipoprotein receptor-related protein (LRP) is responsible for factor Xa catabolism in vivo. Mice overexpressing a 39-kD receptor-associated protein that binds to LRP and inhibits its ligand binding activity displayed dramatically prolonged plasma clearance of 125I-factor Xa. Preadministration of α2M-proteinase complexes (α2M*) also diminished the plasma clearance of125I-factor Xa in a dose-dependent fashion. The clearance of preformed complexes of 125I-factor Xa and α2M was similar to that of 125I-factor Xa alone and was also inhibited by mice overexpressing a 39-kD receptor-associated protein. These results thus suggest that, in vivo, factor Xa is metabolized via LRP after complex formation with α2M.
FACTOR X, A PLASMA glycoprotein involved in the blood coagulation cascade, can be converted to its active form (factor Xa) by both the intrinsic pathway (via factors IXa and VIIIa) and extrinsic pathway (via factor VIIa together with tissue factor).1 Activated factor X generated from either pathway participates in the prothrombinase complex in which factors Xa and Va activate prothrombin to thrombin in the presence of calcium and phospholipid. Thus, control of factor Xa levels by plasma protease inhibitors may be pivotal in the regulation of the coagulation process. Although little is known regarding factor Xa catabolism, a number of plasma serine protease inhibitors are thought to inhibit factor Xa activity. These include α1-protease inhibitor (α1PI),2 α2-macroglobulin (α2M),3 antithrombin III (AT-III),4 and tissue factor pathway inhibitor (TFPI).5 In vitro, factor Xa is mainly inactivated by AT-III and α1-PI, as observed in a purified system and in plasma.6-8 Although α2M accounts for only 10% to 15% of the factor Xa inactivation in vitro, Fuchs and Pizzo7 showed that, by 2 minutes after injection of125I-factor Xa (125I-Xa) into mice, 90% of the radioactivity was bound to α2M. This finding suggests that α2M is a major inhibitor of factor Xa in vivo. After intravenous administration, 125I-Xa is cleared rapidly into the liver, with a plasma half-life of approximately 2 minutes in rabbits.7 However, the biology underlying this clearance process has not been elucidated.
The low-density lipoprotein receptor-related protein (LRP), initially identified by its structural homology with the low-density lipoprotein (LDL) receptor,9 is a large multifunctional endocytosis receptor10 having several structurally and functionally distinct ligands. Ligands of LRP include α2M-protease complexes (α2M*),11 tissue-type plasminogen activator (t-PA),12,13 and TFPI.14A unique ligand, the 39-kD protein, which copurifies with LRP, inhibits all known ligand interactions with LRP.15 16
In vivo, the role of LRP in the clearance of the circulating ligands such as t-PA and TFPI has been shown after overexpression of the 39-kD protein in mice using an adenoviral mediated gene transfer technique.17-19 Because several protease-inhibitor (eg, α2M) complexes are endocytosed and degraded via LRP, we examined the role of LRP in the catabolism of factor Xa in vivo. Our results show that LRP mediates the plasma clearance of factor Xa after complex formation with protease inhibitors and thus suggest strategies for regulation of its catabolism.
MATERIALS AND METHODS
Reagents.
N-Succinimidyl 3-(4-hydroxy-5-[125I] iodophenyl) propionate (Bolten and Hunter reagent) was purchased from Amersham (Arlington Heights, IL). Human α2M was purified and activated (α2M*) as described previously.20 Balb/c mice were obtained from Jackson Laboratories (Bar Harbor, ME). AT-III was purchased from Kabi Pharmacia Diagnostics (Piscataway, NJ). Factor VII- and X-deficient plasma and phospholipid (rabbit brain cephalin) were obtained from Sigma (St Louis, MO). Rabbit brain thromboplastin was from Ortho (Raritan, NJ). The chromogenic substrate Spectrozyme FXa (methoxycarbonyl-D-hexahydrotyrosyl-L-alanyl-L-arginine-p-nitroanilide-diacetate) was obtained from American Diagnostica Inc (Greenwich, CT). Factor X-deficient plasma was from George King Bio-Medicals Inc (Overland Park, KS).
Coagulation factors.
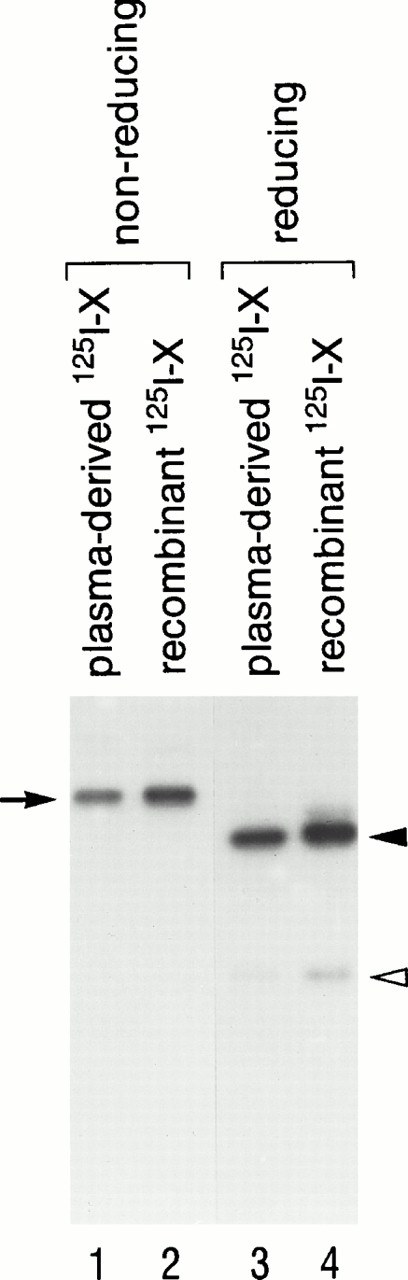
Recombinant factor X was prepared and purified as described by Miletich et al,21 with minor modifications.22 The cDNA encoding human factor X was obtained by polymerase chain reaction amplification from first-strand cDNA template from a human hepatocyte library, ligated into the mammalian expression vector ZMB3, and stably transfected into human kidney 293 cells. Factor X was isolated from the culture media by absorption to barium citrate, followed by resuspension in 32% saturated (NH4)2SO4 in the presence of 5 mmol/L diisopropyl fluorophosphate and incubation for hour at 4°C. The precipitate was collected by centrifugation and the resulting supernatant was dialyzed into 10 mmol/L HEPES, pH 7.0, 100 mmol/L NaCl, and 1 mmol/L benzamidine. The dialyzed supernatant was concentrated and immunoaffinity purified using antibody 3698.1A8.10, which is a calcium-dependent monoclonal antibody directed against the Gla domain of factor X. Factor X was further purified using a Pharmacia Mono Q FPLC column. Plasma (human)-derived factor X was isolated by the same procedure. In some experiments, factor X was activated using factor X coagulant protein (XCP) from Russell's viper venom in a factor X:XCP ratio (wt/wt) of 100:1 in 10 mmol/L HEPES, pH 7.4, 100 mmol/L NaCl, 5 mmol/L CaCl2. XCP was purified from crude viper venom as described previously.23 Because native factor X is composed of two polypeptide chains joined by a disulfide bond, the gel pattern in the presence of β-mercaptoethanol is particularly useful to confirm successful processing from the 1-chain form to the 2-chain native form. On nonreducing sodium dodecyl sulfate (SDS)-gels (Fig 1, lanes 1 and 2), the apparent molecular weight of both plasma-derived and recombinant factor Xs was approximately 68 kD (noted by arrow). On the other hand, under reducing conditions (lanes 3 and 4), the apparent molecular weight of both factor Xs was 49 kD for the heavy chain (solid arrowhead) and 23 kD for the light chain (open arrowhead). Plasma-derived (lanes 1 and 3) and recombinant (lanes 2 and 4) factor X migrated identically on both reducing and nonreducing conditions, indicating that they have the same apparent molecular size. Furthermore, no difference in functional activity between plasma-derived and recombinant factor X was detected.24
SDS-PAGE of plasma-derived 125I-X and recombinant 125I-X in 15% polyacrylamide gels. Plasma-derived 125I-X (lanes 1 and 3) and recombinant125I-X (lanes 2 and 4) were subjected to 15% SDS-PAGE and autoradiography, under nonreducing conditions (lanes 1 and 2) and reducing conditions (lanes 3 and 4) (68-kD single chain; 47-kD heavy chain; 23-kD light chain).
SDS-PAGE of plasma-derived 125I-X and recombinant 125I-X in 15% polyacrylamide gels. Plasma-derived 125I-X (lanes 1 and 3) and recombinant125I-X (lanes 2 and 4) were subjected to 15% SDS-PAGE and autoradiography, under nonreducing conditions (lanes 1 and 2) and reducing conditions (lanes 3 and 4) (68-kD single chain; 47-kD heavy chain; 23-kD light chain).
Mutagenesis of factor X.
The S379A factor X mutant was constructed as follows. The mutation was engineered into the cDNA of factor X using a Transformer Site-Directed Mutagenesis Kit from Clontech (Palo Alto, CA). The codon encoding the active site serine at position 379 was mutated to alanine: (GAC)(AGC)(GGG)′(GAC) (GCT)(GGG). The DNA sequence of the constructs was verified by the dideoxy chain termination technique. Mutated constructs were shuttled into the ZMB3 mammalian expression vector, transfected into the 293 cells for expression, and purified as described above.
Protein iodination.
Factor X was iodinated using the Bolton and Hunter reagent according to the manufacturer's instructions. Specific radioactivities were 5 to 10 μCi/μg of protein (0.13 to 0.26 mol 125I/mol protein). Functional activity of recombinant 125I-labeled and unlabeled factor X was evaluated using the modified prothrombin time (PT) as follows. Various dilutions of factor X samples were mixed with factor X-deficient plasma and incubated at 37°C for 5 minutes in a fibrometer. Clotting was initiated by the addition of thromboplastin and 15 mmol/L CaCl2. No difference was seen in the coagulant activity between labeled and unlabeled protein. Enzymatic activity of recombinant 125I-labeled and unlabeled factor X was also determined by measurement of factor Xa activity against the chromogenic peptide substrate, Spectrozyme FXa. No difference was seen in the enzymatic activity between labeled and unlabeled protein. These results show that iodination using Bolten and Hunter reagent does not affect functional and enzymatic activity of factor X and Xa.
Adenovirus purification.
In vivo viral delivery.
In vivo viral delivery was performed via intravenous administration as described previously.18 Various viral particle doses were examined. Optimal expression was achieved after the administration of 4 × 1011 particles (4 × 109plaque-forming units) of either Ad-39-kD or Ad-β-Gal. All experiments were performed on day 5 after viral delivery, because optimal expression of the 39-kD protein was at day 4 or 5 after virus administration.
In vivo plasma clearance in mice.
Twelve- to 16-week-old BALB/c mice (weighing 20 to 25 g) were anesthetized with sodium pentobarbital (1 mg/ 20 g body) during the course of the experiment. The indicated radiolabeled protein (∼10 pmol of factor 125I-X, 125I-Xa, or125I-S379A Xa) in sterile saline (total volume, 100 μL) was injected into a tail vein over 30 seconds. At the indicated times, 40 to 50 μL of blood was collected by periorbital bleeding. The amount of radiolabeled protein in the plasma samples was determined as described previously.18 After 20 minutes, the animals were killed, and the liver, spleen, kidneys, and lungs were removed and weighed. 125I-radioactivity was determined in a gamma counter from Packard Instrument Co (Meriden, CT). Studies were performed in 2 to 7 animals for each condition described. Recombinant factor Xa was used in all experiments in Figs 2-5.
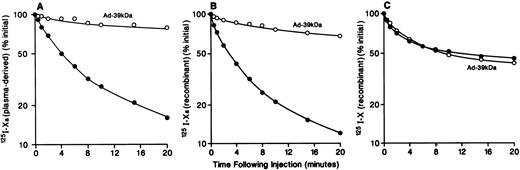
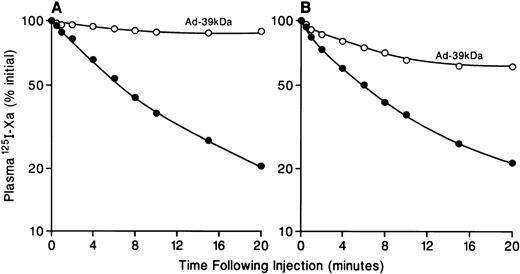
Effect of Ad-39-kD on the plasma clearance of125I-Xa and 125I-X. As described in the Materials and Methods, mice were injected with 10 pmol of plasma-derived 125I-Xa (A) or recombinant125I-Xa (B) or 125I-X (C) with (○) or without (•) preinjection of 4 × 1011 particles of Ad-39-kD 5 days previously. Blood samples were collected at the indicated times and trichloroacetic acid-insoluble radioactivity was determined.
Effect of Ad-39-kD on the plasma clearance of125I-Xa and 125I-X. As described in the Materials and Methods, mice were injected with 10 pmol of plasma-derived 125I-Xa (A) or recombinant125I-Xa (B) or 125I-X (C) with (○) or without (•) preinjection of 4 × 1011 particles of Ad-39-kD 5 days previously. Blood samples were collected at the indicated times and trichloroacetic acid-insoluble radioactivity was determined.
Reaction of factor Xa with purified plasma proteinase inhibitors.
Complexes of 125I-Xa with human α2M and AT-III were prepared by reacting 125I-Xa with increasing concentrations of each inhibitor. The optimal AT-III:factor Xa binding ratio was determined by quantitation of the residual factor Xa activity against the chromogenic peptide substrate, Spectrozyme FXa. Factor Xa inhibition by α2M could not be determined by this method, because the complex still retains partial activity toward peptide substrates. Therefore, the optimal α2M:factor Xa ratio was determined by the clotting method of Fujikawa et al,25using factor VII- and X-deficient plasma in the presence of CaCl2 and phospholipid. Inhibition reactions were performed at room temperature for 30 minutes in the buffer containing 10 mmol/L HEPES, 100 mmol/L NaCl, 5 mmol/L CaCl2, 1 mg/mL PEG 8000, 1 mg/mL bovine serum albumin, pH 7.0. The initial factor Xa concentration was kept constant at 1.0 μmol/L, and the concentration of the inhibitors varied to obtain the desired molar ratio. After incubation, aliquots were removed and tested for residual factor Xa activity. Residual factor Xa activity decreased linearly with increasing inhibitor concentration. An AT-III:factor Xa ratio of 1:1.8 yielded complete inhibition of factor Xa. Complete inhibition of factor Xa by α2M was obtained at a 1:1 molar ratio.
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography.
SDS-PAGE was performed according to Laemmli.26 Dried gels were exposed to Kodak XAR film (Eastman Kodak, Rochester, NY) at −80°C for autoradiography.
RESULTS
125I-factor Xa and 125I-factor X clearance in normal mice and Ad-39-kD–injected mice.
The plasma clearance of plasma-derived 125I-Xa after intravenous administration of 10 pmol of 125I-Xa in normal mice and Ad-39-kD–injected mice is shown in Fig 2A. The initial plasma half-life of125I-Xa was approximately 3 minutes, with 10% of the administered dose remaining in the circulation at 20 minutes. Figure 2B shows the plasma clearance of 125I-recombinant Xa. The plasma clearance curves for 125I-plasma–derived Xa and125I-recombinant Xa are identical. The plasma clearance of125I-X was substantially slower than that of125I-Xa, with a half-life of approximately 10 minutes (Fig2C). The plasma clearance curve for 125I-Xa seen in Fig 2A was similar to that reported earlier by Fuchs and Pizzo.7Approximately 60% of the injected 125I-X and125I-Xa was associated with the liver at 20 minutes (Table 1 and data not shown, see below). These data show rapid hepatic clearance of 125I-Xa and are consistent with a receptor-mediated endocytosis mechanism. Among those candidate receptors is LRP, which mediates the endocytosis of several protease-protease inhibitor complexes such as α2M-complexes, uPA:PAI-1, t-PA:PAI-1, and protease nexin:uPA. To examine the role of LRP in 125I-Xa and125I-X clearance in vivo, we took advantage of an adenoviral delivery vector to express an LRP-antagonist, the 39-kD protein, in mouse liver. Overexpression of the 39-kD protein in liver results in plasma accumulation of the 39-kD protein. Previously, we showed that mice receiving 4 × 1011 particles of Ad-39-kD expressed sufficient 39-kD protein in plasma to completely inhibit LRP, and we also showed that viral infection induced no gross or microscopic morphologic changes in the liver.18 19 This dose of Ad-39-kD was administered intravenously to mice via tail vein. Five days after administration, plasma clearance studies of recombinant125I-Xa and 125I-X were performed. As seen in Fig 2, administration of Ad-39-kD prolonged the plasma clearance of125I-Xa significantly (Fig 2A and B), whereas administration of Ad-39-kD did not alter the plasma clearance of125I-X (Fig 2C). To confirm that the viral infection did not induce adverse effects on the clearance of 125I-Xa, studies were performed in mice after administration of Ad-β-Gal. The plasma clearance of 125I-Xa in Ad-β-Gal–injected mice was essentially the same as that of the noninfected mice (data not shown). These results indicate that the rapid hepatic clearance of125I-Xa, but not of 125I-X, is mediated by LRP.
Organ Distribution of 125I-Xa and125I-S379A Xa
| . | 125I-Xa . | 125I-S379A Xa . |
|---|---|---|
| Liver | 56 | 32 |
| Spleen | 3.0 | 3.2 |
| Kidney | 3.0 | 2.4 |
| Blood | 11 | 25 |
| Lung | 0.5 | 0.9 |
| . | 125I-Xa . | 125I-S379A Xa . |
|---|---|---|
| Liver | 56 | 32 |
| Spleen | 3.0 | 3.2 |
| Kidney | 3.0 | 2.4 |
| Blood | 11 | 25 |
| Lung | 0.5 | 0.9 |
Values are the percentage of the injected dose. Mice were injected with approximately 10 pmol of 125I-Xa and125I-S379A Xa as described in the Materials and Methods. After 20 minutes, the animals were killed, organs and plasma were obtained, and their content of 125I-radioactivity was determined by gamma counting.
In vivo association of 125I-Xa among proteinase inhibitors.
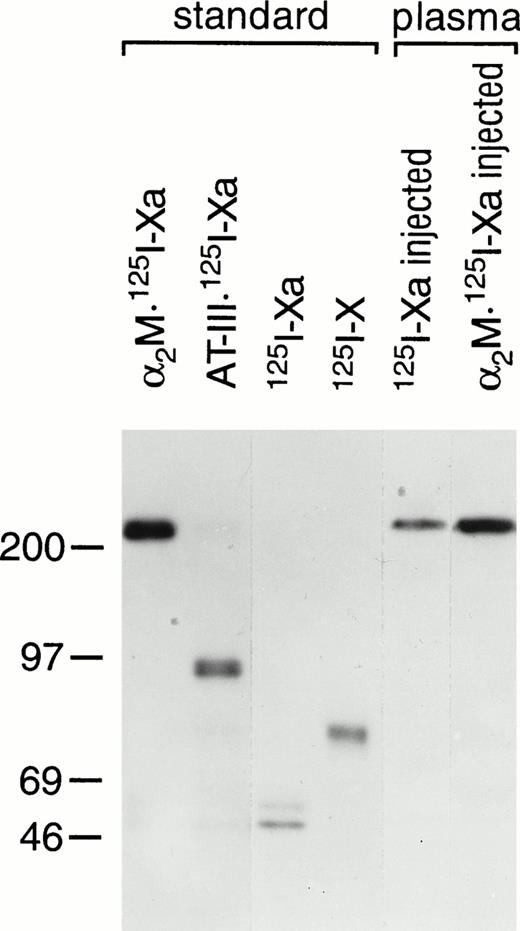
Previous studies have shown that, 2 minutes after the injection of125I-Xa into mice, 90% of that bound within the plasma was associated with α2M.7 To confirm and extend this observation, plasma samples obtained 30 seconds after the injection of 125I-Xa or the injection of the preformed complex of α2M and 125I-Xa (α2M:125I-Xa) were subjected to SDS-PAGE (Fig 3). Standards containing α2M:125I-Xa, AT-III:125I-Xa,125I-Xa, and 125I-X were also analyzed. In both plasma samples, complexes of α2M with 125I-Xa were observed at the identical size as the α2M:125I-Xa standard. These results show that the vast majority of exogenously administered 125I-Xa is complexed to α2M.
Plasma species of 125I-Xa and α2M:125I-Xa after injection to mice. As described in the text, plasma samples (3 μL) obtained 30 seconds after the injection of 125I-Xa or α2M:125I-Xa were subjected to 7.5% SDS-PAGE and autoradiography. Standards containing α2M:125I-Xa, AT-III:125I-Xa,125I-Xa, and 125I-X were also analyzed as indicated. Molecular weight markers in kilodaltons are indicated on the left.
Plasma species of 125I-Xa and α2M:125I-Xa after injection to mice. As described in the text, plasma samples (3 μL) obtained 30 seconds after the injection of 125I-Xa or α2M:125I-Xa were subjected to 7.5% SDS-PAGE and autoradiography. Standards containing α2M:125I-Xa, AT-III:125I-Xa,125I-Xa, and 125I-X were also analyzed as indicated. Molecular weight markers in kilodaltons are indicated on the left.
Effect of preadministration of α2M* or α2M on the clearance of 125I-Xa in mice.
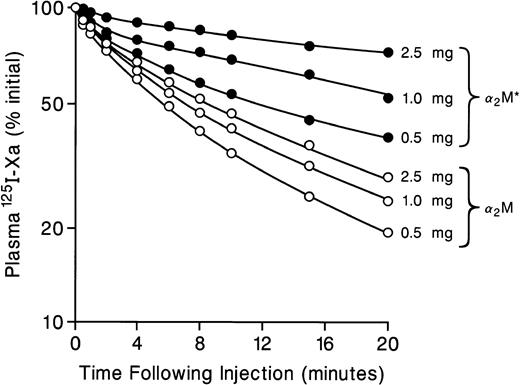
Because in vivo the vast majority of factor Xa is bound to α2M (Fuchs and Pizzo7 and Fig 3) and because α2M:protease complexes and activated α2M (α2M*) but not free or uncomplexed α2M are metabolized via LRP,11 we next examined whether preadministration of α2M* (which competes for the α2M* binding site on LRP) can influence on the plasma clearance of 125I-Xa. As seen in Fig 4, preinjection of various amount of α2M* (0.5, 1, and 2.5 mg) altered the plasma half-life of 125I-Xa from approximately 3 minutes to >>20 minutes in a dose-dependent manner. On the other hand, preinjection of various amount of α2M (which does not bind to LRP) essentially did not change the plasma half-life of 125I-Xa. These results further support the hypothesis that factor Xa complexed to α2M is cleared via LRP.
Effect of preadministration of α2M* and α2M on the plasma clearance of 125I-Xa. Various doses (0.5, 1.0, and 2.5 mg) of α2M*(•) or α2M (○) were preadministered 1 minute before injection of 125I-Xa. Plasma 125I-Xa radioactivities were determined at the indicated times, as described in Fig 2.
Effect of preadministration of α2M* and α2M on the plasma clearance of 125I-Xa. Various doses (0.5, 1.0, and 2.5 mg) of α2M*(•) or α2M (○) were preadministered 1 minute before injection of 125I-Xa. Plasma 125I-Xa radioactivities were determined at the indicated times, as described in Fig 2.
Clearance of α2M:125I-Xa and AT-III:125I-Xa.
To further characterize the proteinase inhibitor-dependent phase of125I-Xa clearance, complexes of 125I-Xa with α2M (α2M:125I-Xa) were prepared and examined in clearance studies (Fig 5A). The clearance of α2M:125I-Xa in normal mice was rapid, with a plasma half-life of approximately 5 minutes. On the other hand, the clearance of α2M:125I-Xa in Ad-39-kD–injected mice was extremely slow. The characteristics of these clearance curves are essentially the same as those of125I-Xa in normal mice and Ad-39-kD–injected mice, as described above (Fig 2A and B). Next, the role of proteinase inhibitor, AT-III, was examined. Complexes of 125I-Xa with AT-III (AT-III:125I-Xa) were prepared and evaluated in clearance studies (Fig 5B). Plasma clearance of AT-III:125I-Xa was rapid, with a half-life of approximately 5 minutes. Clearance was also prolonged in Ad-39-kD mice but not to the extent observed with α2M:125I-Xa. Fuchs et al27 have previously reported that AT-III:protease complexes are rapidly cleared, with a half-life of approximately 6 minutes, and that this rapid clearance is independent of α2M*. Our data suggest that α2M:125I-Xa is metabolized solely via LRP and, in addition, that LRP plays an important role in the metabolism of AT-III:125I-Xa. This latter conclusion is consistent with recent observations of Kounnas et al28 in studies of AT-III-thrombin and LRP. However, our observation that, after the injection of AT-III:125I-Xa, the clearance of approximately 75% of the 125I-Xa was prolonged, suggests that another clearance receptor may well be involved. Future studies will address this issue further.
Effect of Ad-39-kD on the plasma clearance of α2M:125I-Xa or AT-III:125I-Xa. Mice were injected with α2M:125I-Xa (A) or AT-III:125I-X (B) with (○) or without (•) preinjection of Ad-39-kD 5 days previously. Clearance studies were performed as described in Fig 2.
Effect of Ad-39-kD on the plasma clearance of α2M:125I-Xa or AT-III:125I-Xa. Mice were injected with α2M:125I-Xa (A) or AT-III:125I-X (B) with (○) or without (•) preinjection of Ad-39-kD 5 days previously. Clearance studies were performed as described in Fig 2.
Clearance of a factor Xa mutant that cannot bind proteinase inhibitors.
Previously, Fuchs and Pizzo7 showed that the clearance of diisopropyl fluorophosphoryl (DFP)-inactivated factor Xa, which cannot bind to the protease inhibitors, was more rapid than factor Xa alone, suggesting that DFP-inactivated factor Xa has a binding site(s) that is distinct from LRP. To further characterize the role of the proteinase inhibitors in factor Xa clearance, we prepared mutant factor Xa in which the active-site serine of factor Xa has been substituted by alanine. The resultant mutant (S379A Xa) cannot bind to the proteinase inhibitors (α2M, AT-III, etc). Plasma samples obtained 30 seconds after intravenous administration of 125I-Xa and125I-S379A Xa were subjected to SDS-PAGE and autoradiography. The injected 125I-S379A Xa migrated at the same molecular size as the uninjected standard, indicating that S379A Xa does not form SDS-stable complexes with proteinase inhibitors in vivo (data not shown). Clearance studies were then performed using this mutant. The clearance of 125I-S379A Xa was extremely rapid, with a plasma half-life of approximately 1 minute, which was essentially the same as that of DFP-inactivated factor Xa (data not shown). Ad-39-kD did not affect the clearance of 125I-S379A Xa (data not shown). These data suggest that a pathway(s) distinct from LRP is involved in the clearance of 125I-S379A Xa.
Organ distribution of 125I-Xa and 125I-S379A Xa in normal mice.
The organ distributions of radioactivity at 20 minutes are summarized in Table 1. 125I-Xa is found primarily in liver (56%). However, recovery of 125I-S379A Xa in liver (32%) was significantly less than that of 125I-Xa. Lung distribution was also examined. The distribution of radioactivity was low in the lung for both 125I-Xa (0.5%) and 125I-S379A Xa (0.9%).
DISCUSSION
The present observations show that (1) inhibition of LRP in vivo by gene transfer of a 39-kD protein prolongs the plasma half-life of125I-Xa; (2) the plasma half-life of 125I-X is slow and unaltered by the 39-kD protein; (3) the vast majority of exogenously administered 125I-Xa is bound to α2M; (4) preadministration of α2M*, but not α2M, prolongs the plasma half-life of125I-Xa; and (5) the clearance of preformed α2M:125I-Xa complexes is the same as that of125I-Xa. Taken together, these results indicate that, in vivo, LRP mediates the hepatic clearance of 125I-Xa after complex formation with α2M.
Factor Xa plays a pivotal role in the clotting cascade, because it can be activated by both the intrinsic and the extrinsic pathways. After intravenous administration, 125I-Xa was rapidly cleared from the circulation into liver, whereas clearance of125I-X was much slower. The observation that the expression of the 39-kD protein altered the clearance of 125I-Xa indicates that the clearance of 125I-Xa, but not125I-X, is mediated by LRP. The mechanism(s) responsible for the clearance of 125I-X is currently unknown. Previously, using adenoviral gene transfer of the 39-kD protein, we showed that LRP mediates the clearance of both t-PA and TFPI in vivo.18 19 TFPI is cleared as the free species, whereas t-PA is cleared as both the free species as well as that complexed to PAI-1.
LRP did not mediate the plasma clearance of 125I-S379A Xa, an active-site serine factor Xa mutant that cannot bind serine-site proteinase inhibitors, including α2M, AT-III, α1PI, etc. This finding suggests that LRP does not modulate the active coagulation process occurring in the periphery such as the microvascular environment, but once circulating coagulation factors are inactivated by protease inhibitors, these may be eliminated from the circulation via LRP.
Regulation of the blood coagulation cascade is a complex, multifactorial process. Several proteinase inhibitors are active participants in this process via inhibition of the activated coagulation factors. In vitro, factor Xa is primarily inactivated by AT-III and α1PI, as has been observed in both purified systems as well as whole plasma.6-8 Although α2M only accounts for 10% to 15% of factor Xa inactivation in vitro, Fuchs and Pizzo7 showed that, 2 minutes after injection of 125I-Xa into mice, 90% of the radioactivity was associated with α2M. This finding suggested that α2M is a major inhibitor of factor Xa in vivo. Our present observations are consistent with these findings.
The vascular endothelium also plays an important role in the coagulation process. Activation of prothrombin to thrombin by factor Xa and factor Va is believed to occur not only on platelets but also on vascular endothelial cells. Lollar and Owen29 reported that thrombin binds to active site-independent high-affinity binding sites on the endothelial cell surface. This interaction catalyzes the inactivation of thrombin by AT-III. Thereafter, thrombin–AT-III complexes are selectively removed by the liver. Because both thrombin and factor Xa are inactivated by AT-III on the vascular endothelial cell surface, factor Xa inactivation by α2M may also occur on or in proximity to the vascular endothelial cell surface.
Numerous cellular binding sites/receptors have been implicated in the recognition of factor Xa (reviewed in Altieri30). These include those on the surface of platelets,31 endothelial cells,32 alveolar macrophages,33 leukocytes, and hepatoma cells. Specific recognition (inhibitory) molecular candidates include cell-associated AT-III, TFPI,34 protease nexin-1,34 and effector cell protease-1 (EPR-1).35 Each of these binding sites has been implicated in intravascular activation or inhibition of coagulation via interaction with factor Xa. For example, platelets and vascular endothelial cells provide cell surface sites for the generation of prothrombin as described above. Recently, in in vitro studies in the absence of other protease inhibitors, we have identified a new factor Xa cellular binding/uptake pathway that requires TFPI and is independent of LRP.36 However, in vivo, LRP appears to play a distinctly different role, because it functions as the major pathway responsible for elimination of inactivated Xa from the circulation.
ACKNOWLEDGMENT
The authors thank J.S. Traush-Azar for preparing the virus. We also thank Dave Wilson for critical reading of the manuscript.
Supported in part by National Institutes of Health Grants No. HL14147 (to J.P.M.) and HL 53280 and HL 52040 (to A.L.S.).
Address reprint requests to Masaaki Narita, MD, PhD, Department of Pediatrics, Box 8116, Washington University School of Medicine, One Children's Place, St Louis, MO 63110.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal