Abstract
We performed a retrospective analysis of 35 patients with primary diffuse large B-cell lymphoma of the mediastinum treated with high-dose cyclophosphamide, carmustine, and etoposide (CBV) plus autologous hematopoietic cell transplantation to determine outcome and prognostic features for progression-free survival (PFS). Thirty-five patients with primary diffuse large B-cell lymphoma of the mediastinum in first response (complete remission [CR] or partial remission [PR]) with poor prognostic features, with primarily refractory disease, or with relapsed disease following conventional chemotherapy, were treated with CBV and autologous hematopoietic cell transplantation. PFS and overall survival were assessed by the Kaplan-Meier method. Patient characteristics before transplantation were examined by univariate analysis using the log-rank test and by Cox's proportional hazards regression analysis to determine predictors of PFS. Estimated 5-year PFS varied significantly with patient disease status at transplantation. Patients transplanted in first response had an estimated 5-year PFS rate of 83%, compared with 58% and 27% for primarily refractory and relapsed patients, respectively (P = .02). The strongest predictor of PFS was chemotherapy responsiveness immediately before transplantation. Patients with chemotherapy-responsive disease had a significantly greater PFS rate than patients with chemotherapy-nonresponsive disease (risk ratio, 3.60; 95% confidence interval [CI], 1.14 to 11.4). No other factors were found to be significant on univariate or multivariate analysis. Patients with primary diffuse large B-cell lymphoma of the mediastinum can achieve prolonged PFS following high-dose chemotherapy and autologous hematopoietic cell transplantation. Outcomes are strongly correlated with disease status (first response v refractoryv relapsed) at transplantation and chemotherapy responsiveness immediately before transplantation.
PRIMARY DIFFUSE LARGE B-cell lymphoma of the mediastinum (mediastinal large-cell lymphoma) is a distinct clinicopathologic entity recognized in the revised European-American classification of lymphoid neoplasms (REAL classification).1-13 Patients present with an aggressive, locally invasive anterior mediastinal mass, believed to originate from thymic B cells,14 and frequently develop symptoms of airway compromise or superior vena cava (SVC) syndrome. The tumors are usually bulky (>10 cm in diameter), and confined to the thorax at presentation.13 Bone marrow involvement is rare.7,9,11-13 Histology shows large cells with variable nuclear features, B-cell immunophenotype, and frequent compartmentalizing sclerosis.15,16 There is a female predominance and a median age of diagnosis in the fourth decade.7,9,13 Mediastinal large-cell lymphoma may represent 3% to 7% of all diffuse large-cell lymphomas.7,9 11
Response to treatment and clinical outcome have varied from one series to another, and may be explained by the small number of patients in most series, variable patient characteristics, inconsistent histologic inclusion criteria, and the heterogeneity of therapy. Early studies suggesting that mediastinal large-cell lymphomas were unusually aggressive, with a poorer prognosis than other large-cell lymphomas,2-4 have been contradicted by more recent reports. Complete remission (CR) rates of 53% to 80% have been reported following initial therapy,7,12 with a 50% to 65% overall survival rate at 5 years.7,12,13 Management can be complicated by the presence of residual masses of uncertain significance. Radiation therapy has been administered following chemotherapy, with unclear benefit. Relapses are frequently extranodal, including involvement of the lung, liver, gastrointestinal tract, kidneys, adrenals, ovaries, and CNS.7,9,11 Outcome following additional chemotherapy (salvage therapy) has not been systematically studied. Patients whose disease has relapsed, or who have primarily refractory disease, are often resistant to salvage therapy, with a poor survival rate.7,11 13
High-dose chemotherapy with autologous bone marrow transplantation (ABMT) has been successfully used in aggressive non-Hodgkin's lymphoma (NHL) as treatment intensification for patients with poor prognostic features and as salvage treatment for patients with refractory or relapsed disease following conventional chemotherapy.17-19The role of ABMT in patients with mediastinal large-cell lymphoma is not well defined. The outcome following ABMT and an analysis of prognostic features in this subset of patients is the focus of this report.
MATERIALS AND METHODS
Thirty-five patients who underwent ABMT between January 1987 and June 1995 at two Harvard tertiary care hospitals (Brigham and Women's Hospital and Beth Israel Hospital) for treatment of mediastinal large-cell lymphoma were included in this study. Patients were treated in accordance with protocols approved by the human research protection committees at each hospital. This group represents all known patients who underwent ABMT with this diagnosis at these institutions. All patients had histologically confirmed diffuse large-cell NHL, with B-cell antigen expression and varying degrees of sclerosis, and a predominant mediastinal mass at the time of initial presentation.
Prior therapy and response.
Patients received various chemotherapeutic regimens as initial treatment, as determined by their primary physicians. One patient received mantle irradiation alone as initial therapy. All other patients received a doxorubicin-containing regimen; 13 patients received regimens that contained six or more drugs. One patient received intrathecal methotrexate for CNS prophylaxis. Eight patients received consolidating radiation therapy, including five patients who achieved an initial CR to chemotherapy (two of whom were transplanted in first response and three of whom were transplanted following relapse) and three patients with refractory disease. It should be noted that the provision of radiation therapy, following either initial chemotherapy, salvage chemotherapy, or ABMT, was determined at the discretion of physicians involved and was not based on uniform criteria.
Patients who had complete resolution of their disease by all available imaging techniques (plain radiographic imaging, with or without computed tomographic [CT] scans, and with or without gallium scans) for at least 3 months' duration were considered to be in CR. Patients who had a greater than 50% reduction in measurable disease for at least 3 months were considered to be in partial remission (PR). Based on physician preferences, a subset of patients assumed to be at high risk of relapse was selected a priori to proceed to ABMT in first response (CR or PR). Response to initial chemotherapy in these patients was based on restaging evaluation performed before ABMT. Patients who did not achieve a CR or PR, or who developed progressive disease within 3 months of completion of initial therapy, were considered to have primarily refractory disease. Patients who developed disease progression off chemotherapy, following an initial CR or PR, were considered to have relapsed disease. For the purpose of analysis, patients were classified into three disease status groups based on their response to initial chemotherapy and timing of ABMT: first response (CR or PR), primarily refractory, and relapsed.
Gallium scans were not performed routinely on all patients, and therefore gallium avidity was not included in the definition of response. However, if a gallium scan was performed, it must be negative for a patient to be considered in CR. The predictive value of gallium scanning, before and after ABMT, was examined for the cohort of patients who underwent studies. Gallium scan results were obtained from official radiologists' interpretation. All false-positive scans (scans interpreted as positive in patients who remain progression-free) were reviewed with a radiologist to exclude the possibility of physiologic perihilar uptake. The majority of scans (86%) were performed with single-photon emission computed tomography (SPECT) imaging.
Ten patients with poor prognostic features went straight to ABMT following initial therapy, all of whom had achieved a CR or substantial PR. The remaining 25 patients, including two patients with a PR and all refractory and relapsed patients, received further chemotherapy before ABMT. Nine patients received more than two salvage regimens. Twenty-three patients were treated with 36 courses of conventional-dose cytoreductive salvage chemotherapy, such as ifosfamide, carboplatin, and etoposide (ICE),20 n = 12; etoposide, methylprednisolone, cytarabine, and cisplatin (ESHAP),21 n = 6; prednisone, doxorubicin, cyclophosphamide, etoposide, cytarabine, bleomycin, vincristine, methotrexate, and leucovorin (ProMACE-CytaBOM),22 n = 3; dexamethasone, cytarabine, and cisplatin (DHAP),23 n = 3; etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone (EPOCH),24 n = 2; or a modified regimen, n = 10. Two patients were treated on a salvage protocol with high-dose ICE chemotherapy and peripheral blood hematopoietic cell support.25 Four patients (including one patient transplanted in first PR, two relapsed patients, and one refractory patient) received consolidating radiation therapy following salvage therapy and before ABMT.
Patient characteristics.
Patient characteristics are listed in Table1. The median age at ABMT was 29 years (range, 17 to 50). Twenty-one of 35 patients (60%) were female.
Patient Characteristics
| Total no. | 35 |
| Age (yr) | |
| Median | 29 |
| Range | 17-50 |
| Follow-up (mo) | |
| Median | 47 |
| Range | 19-84 |
| Sex | |
| Male | 14 |
| Female | 21 |
| Status at ABMT | |
| First CR | 4 |
| First PR | 8 |
| Primarily refractory | 12 |
| Relapsed | 11 |
| Response to salvage chemotherapy | |
| CR | 2 |
| PR | 7 |
| Stable or progressive disease | 16 |
| No. of chemotherapy regimens pre-ABMT | |
| 1 | 10 |
| 2 | 16 |
| ≥3 | 9 |
| Chemotherapy responsiveness at ABMT | |
| Responsive | 20 |
| Nonresponsive | 15 |
| Prior marrow involvement | 2 |
| Source of progenitor cells | |
| Peripheral blood hematopoietic cells | 2 |
| Marrow alone | 22 |
| Both | 11 |
| Radiation therapy | |
| Initial therapy | 8 |
| Salvage therapy | 4 |
| ABMT consolidation | 14 |
| Total no. | 35 |
| Age (yr) | |
| Median | 29 |
| Range | 17-50 |
| Follow-up (mo) | |
| Median | 47 |
| Range | 19-84 |
| Sex | |
| Male | 14 |
| Female | 21 |
| Status at ABMT | |
| First CR | 4 |
| First PR | 8 |
| Primarily refractory | 12 |
| Relapsed | 11 |
| Response to salvage chemotherapy | |
| CR | 2 |
| PR | 7 |
| Stable or progressive disease | 16 |
| No. of chemotherapy regimens pre-ABMT | |
| 1 | 10 |
| 2 | 16 |
| ≥3 | 9 |
| Chemotherapy responsiveness at ABMT | |
| Responsive | 20 |
| Nonresponsive | 15 |
| Prior marrow involvement | 2 |
| Source of progenitor cells | |
| Peripheral blood hematopoietic cells | 2 |
| Marrow alone | 22 |
| Both | 11 |
| Radiation therapy | |
| Initial therapy | 8 |
| Salvage therapy | 4 |
| ABMT consolidation | 14 |
Twelve patients were transplanted in first response. Four patients were transplanted in first CR. These included two patients with stage IV disease at presentation, both of whom had large mediastinal masses, one with bone marrow involvement and the other with multiple pulmonary nodules. The remaining two patients transplanted in first CR had locally extensive stage IIE disease and bilateral pleural effusions: one with pericardial involvement and the other with extension into the chest wall.
Eight patients were transplanted in first PR. These included five patients with stage IV disease at presentation, four of whom had diffuse pulmonary involvement and one with characteristic liver and renal involvement. The remaining three patients transplanted in first PR had stage II disease, one of whom had a large mass at presentation (>20 cm), and another who remained persistently gallium-positive before ABMT. Two patients in PR received additional cytoreductive therapy before ABMT, which resulted in a slight reduction in mass size in one and a second PR with conversion to gallium negativity in the other. Both of these patients were considered to have chemotherapy-responsive disease at ABMT. One of seven PR patients evaluated was gallium-positive immediately before ABMT. Following ABMT, six of eight patients in first PR had a further reduction in measurable disease, including two further PRs, two CRs, and one with resolution of the previously positive gallium scan. The two remaining PR patients had stable gallium-negative disease, and may have been in first CR with residual nonmalignant radiographic abnormalities at ABMT.
Twelve patients had primarily refractory disease and 11 patients had relapsed disease, and received salvage chemotherapy before ABMT. Of 12 patients with primarily refractory disease, eight remained refractory after salvage treatment, while two achieved a CR and two achieved a PR. Of 11 patients with relapsed disease, seven were refractory to salvage therapy, while four achieved a PR.
Patients who achieved a CR or PR to chemotherapy immediately before ABMT were considered to have chemotherapy-responsive disease. All others were considered to have chemotherapy-nonresponsive disease. At ABMT, 15 patients (43%) had chemotherapy-nonresponsive disease.
Two patients had prior bone marrow involvement and received peripheral blood hematopoietic cells alone at ABMT, 22 patients received marrow alone, and 11 received both marrow and peripheral blood hematopoietic cells. Fourteen patients received consolidating radiation therapy following ABMT, including five patients transplanted in first PR, four relapsed patients, and five refractory patients.
Therapy.
Details of the transplant procedure have been previously described,26 27 and consisted of cyclophosphamide twice daily concomitant with twice-daily etoposide and daily carmustine (BCNU) on days −7 to −3. Total doses of cyclophosphamide ranged from 6,000 to 7,200 mg/m2 (the majority received 6,000 mg/m2), etoposide 1,200 to 2,000 mg/m2 (the majority received 1,600 mg/m2), and BCNU 450 mg/m2. Marrow and peripheral blood hematopoietic cells were collected, frozen, and thawed using standard techniques. Peripheral blood hematopoietic cells were mobilized with granulocyte colony-stimulating factor, usually during the recovery phase from chemotherapy. All patients received unpurged, unmanipulated stem cells, with the exception of one patient who received B1-purged marrow and one patient who received marrow primed with interleukin-3.
ABMT response criteria and follow-up.
Patients were restaged within 3 months of engraftment with plain radiographs and CT scans, with or without gallium scans. Patients with no measurable disease at ABMT were considered nonassessable for response. CR and PR were considered as previously defined. New sites of disease following ABMT or a greater than 25% increase in previously noted disease constituted progressive disease. All other patients were considered to have stable disease. Patients were monitored for potential relapse using a variety of tests, including routine blood tests, serial radiographs, CT scans, and gallium scans, at variable time intervals (∼ every 3 months in the first year, followed by every 6 to 12 months thereafter).
Statistical analysis.
Data were analyzed using the SAS statistical package (SAS Institute, Cary, NC). Progression-free survival (PFS) and overall survival estimates were obtained by the Kaplan-Meier method.28 Time to disease progression was calculated as the time from stem-cell reinfusion to the time of documented disease progression, relapse, or date last known alive. Overall survival times were calculated as the time from stem-cell reinfusion until death or date last known alive. Univariate comparisons of groups defined by patient characteristics determined before reinfusion for predicting PFS, and the value of pre- and post-ABMT gallium scan results for predicting relapse rates were evaluated using the log-rank test.29 Prognostic variables were considered simultaneously in a proportional hazards regression analysis30 and through the use of a backward selection model.
RESULTS
Toxicity.
Among 35 autotransplants, there were no early treatment-related deaths. One patient died of myelodysplastic syndrome without evidence of disease progression at 67 months following ABMT, which likely represents a late complication. Another patient has undergone successful resection of an early-stage lung cancer.
Response.
The median follow-up time is 47 months (range, 19 to 84). Six patients were transplanted with unmeasurable disease and were not assessable for response. Following ABMT, four additional patients had CRs, eight had PRs, seven had stable disease, and nine had disease progression. One additional patient was not assessable secondary to bronchial collapse and lung consolidation, making accurate radiographic restaging impossible. Ten of 15 patients with PR or stable disease underwent gallium scanning after ABMT, and four were persistently positive. Three of these four patients with positive gallium scans received consolidating radiation therapy post-ABMT, and all four had gradual resolution of gallium positivity and remain without disease progression at last follow-up evaluation.
PFS and overall survival.
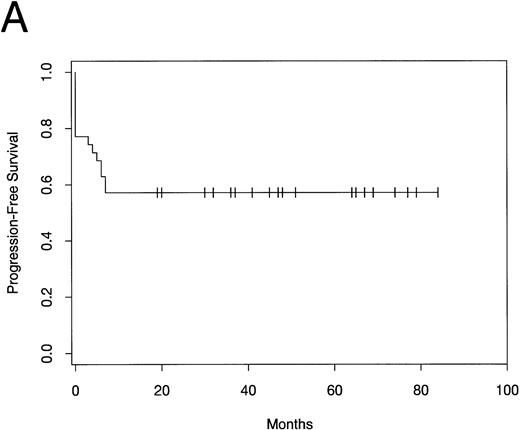
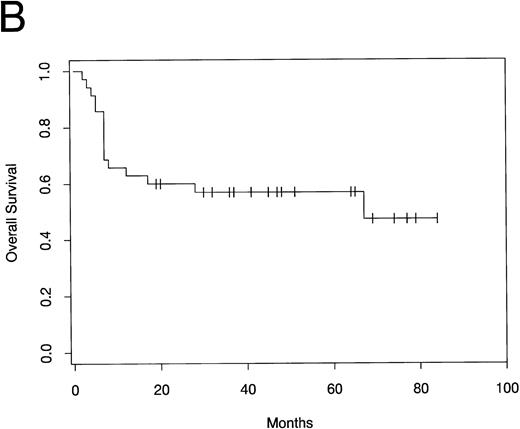
PFS and overall survival are shown in Fig1. Fifteen patients have developed progressive disease following ABMT, while 20 patients (57%) remain free of disease progression. All relapses occurred within 7 months of ABMT. The median PFS has not yet been reached. The median overall survival time is 67 months. The estimated 5-year PFS and overall survival rate by Kaplan Meier analysis is 57% (95% confidence interval [CI], 40% to 73%).
(A) PFS and (B) overall survival in patients with mediastinal large-cell lymphoma following ABMT.
(A) PFS and (B) overall survival in patients with mediastinal large-cell lymphoma following ABMT.
The following factors were evaluated by univariate analysis as prognostic variables for PFS: age, sex, age-adjusted International Index31 at diagnosis (low/low-intermediate vhigh-intermediate/high), mediastinal mass size at diagnosis (<10 cmv ≥10 cm), presence of pleural effusion at diagnosis, B-symptoms at diagnosis, SVC syndrome at diagnosis, disease stage at ABMT (localized v advanced), presence of extranodal disease at ABMT, radiation therapy (at any time during treatment), chemotherapy responsiveness immediately before ABMT, and disease status at ABMT (first response v primarily refractory vrelapsed). Of these factors, only chemotherapy responsiveness and disease status at ABMT were found to be statistically significant (Table 2 and Figs 2 and3).Although multiple testing was performed, the only two variables found to be significant were both highly significant, and were anticipated a priori.
Univariate Analysis (N = 35)
| Factor . | No. of Patients . | P Value . |
|---|---|---|
| Age (yr) | 35 | .097 |
| Sex | ||
| Male | 14 | .408 |
| Female | 21 | |
| Age-adjusted international index | ||
| High | 21 | .403 |
| Low | 14 | |
| Mediastinal mass size (cm) | ||
| <10 | 12 | .668 |
| ≥10 | 14 | |
| Pleural effusion at presentation | ||
| Yes | 12 | .930 |
| No | 17 | |
| B-symptoms | ||
| Yes | 16 | .480 |
| No | 18 | |
| SVC syndrome | ||
| Yes | 13 | .264 |
| No | 22 | |
| Stage at ABMT | ||
| Localized | 26 | .079 |
| Advanced | 9 | |
| Radiation therapy | ||
| Yes | 24 | .646 |
| No | 11 | |
| Extranodal sites at ABMT | ||
| Yes | 8 | .205 |
| No | 27 | |
| Chemotherapy responsiveness at ABMT | ||
| Responsive | 20 | .007 |
| Nonresponsive | 15 | |
| Disease status at ABMT | ||
| First response (CR or PR) | 12 | |
| Primarily refractory | 12 | .023 |
| Relapsed | 11 |
| Factor . | No. of Patients . | P Value . |
|---|---|---|
| Age (yr) | 35 | .097 |
| Sex | ||
| Male | 14 | .408 |
| Female | 21 | |
| Age-adjusted international index | ||
| High | 21 | .403 |
| Low | 14 | |
| Mediastinal mass size (cm) | ||
| <10 | 12 | .668 |
| ≥10 | 14 | |
| Pleural effusion at presentation | ||
| Yes | 12 | .930 |
| No | 17 | |
| B-symptoms | ||
| Yes | 16 | .480 |
| No | 18 | |
| SVC syndrome | ||
| Yes | 13 | .264 |
| No | 22 | |
| Stage at ABMT | ||
| Localized | 26 | .079 |
| Advanced | 9 | |
| Radiation therapy | ||
| Yes | 24 | .646 |
| No | 11 | |
| Extranodal sites at ABMT | ||
| Yes | 8 | .205 |
| No | 27 | |
| Chemotherapy responsiveness at ABMT | ||
| Responsive | 20 | .007 |
| Nonresponsive | 15 | |
| Disease status at ABMT | ||
| First response (CR or PR) | 12 | |
| Primarily refractory | 12 | .023 |
| Relapsed | 11 |
Patients with missing data for a given factor were excluded.
PFS in patients with mediastinal large-cell lymphoma following ABMT by disease status at transplantation (first response, primarily refractory, or relapsed).
PFS in patients with mediastinal large-cell lymphoma following ABMT by disease status at transplantation (first response, primarily refractory, or relapsed).
PFS in patients with mediastinal large-cell lymphoma following ABMT by chemotherapy responsiveness immediately before transplantation.
PFS in patients with mediastinal large-cell lymphoma following ABMT by chemotherapy responsiveness immediately before transplantation.
Ten of 12 patients in first response, seven of 12 patients with primarily refractory disease, and three of 11 patients with relapsed disease remain free of disease progression. The 5-year estimated PFS for patients transplanted in first response is 83% (95% CI, 62% to 100%), compared with 58% (95% CI, 30% to 86%) and 27% (95% CI, 1% to 53%) for primarily refractory and relapsed patients, respectively (P = .02). Fifteen of 20 patients with chemotherapy-responsive disease at ABMT remain free of disease progression, compared with five of 15 patients with chemotherapy-nonresponsive disease. The estimated 5-year PFS for chemotherapy-responsive patients is 75% (95% CI, 56% to 94%), compared with 33% (95% CI, 9% to 57%) for chemotherapy-nonresponsive patients (P = .007).
A multivariate analysis was performed using a Cox proportional hazards regression model assessing PFS. The two variables found to be significant on univariate analysis were included in the model (chemotherapy responsiveness and disease status at ABMT), as well as variables that almost reached significance or were believed to be potential confounders (age, International Index at diagnosis, and disease stage at ABMT). Due to a strong correlation between chemotherapy responsiveness and disease status at ABMT, these two factors canceled each other out on multivariate analysis. A backward selection process resulted in disease status being dropped from the model, suggesting that chemotherapy responsiveness was the strongest of the two significant predictors. Controlling for age, International Index at presentation, and disease stage at ABMT, patients with chemotherapy-responsive disease have a significantly greater PFS than patients with chemotherapy-nonresponsive disease (risk ratio, 3.60; 95% CI, 1.14 to 11.4). No other predictors were found to be significant on multivariate analysis.
Gallium scans and residual disease.
Twenty patients with previously positive gallium scans had gallium scans performed immediately before ABMT (including six PR, 10 primarily refractory, and four relapsed patients). Four of nine gallium-positive patients have developed progressive disease, compared with one of 11 gallium-negative patients. The 5-year estimated relapse rate for patients with positive scans before ABMT is 44%, compared with 9% for patients with negative scans (P = .06). Although this does not meet statistical significance, these results suggest that gallium scans performed immediately before ABMT may offer some predictive value. Twenty-three patients underwent gallium scanning on initial restaging following ABMT (including six PR, 12 primarily refractory, and five relapsed patients). Three of seven patients with positive gallium scans have developed progressive disease, compared with five of 16 with negative gallium scans. The 5-year estimated relapse rate is 43% and 31% for patients with positive and negative scans, respectively (P = .55). The predictive value of gallium scans performed for initial restaging following ABMT is questionable.
Thirteen of 19 responding or stable-disease patients (68%) had residual mediastinal abnormalities following ABMT. Only four of these 13 patients (31%) with residual abnormalities have developed progressive disease, suggesting that a large proportion of residual abnormalities following ABMT are nonmalignant.
DISCUSSION
Clinical characteristics and response following conventional chemotherapy for mediastinal large-cell lymphoma have been well documented.2-13 There have been no systematic studies assessing outcome following salvage chemotherapy. Patients who have progressive disease or relapse following conventional chemotherapy appear to be highly refractory to salvage chemotherapy, with poor survival.7,11,13 Patients with mediastinal large-cell lymphoma have been observed to have low rates of bone marrow involvement, which may make them amenable for treatment strategies that include ABMT.7,9 11-13
We performed a retrospective analysis of 35 patients with mediastinal large-cell lymphoma following ABMT. Overall, the patient characteristics appear representative of previous cohorts described undergoing conventional therapy, including female predominance, young age, frequent bulky tumors, and low incidence of marrow involvement. The treatment was well tolerated and there were no early treatment-related deaths. Of note, one patient died of myelodysplastic syndrome and another has undergone successful resection of an early-stage lung cancer.
Twelve patients with poor prognostic features underwent ABMT in first response, with an estimated 5-year PFS rate of 83%. Haioun et al18 found a 5-year PFS rate of 59% in high-risk NHL patients treated with ABMT in first CR, while Martelli et al19 found a PFS rate at 55 months of 73% for patients treated with ABMT in first PR for aggressive NHL. Our results also compare favorably to the 71% 3-year PFS reported for initial responders following conventional chemotherapy for mediastinal large-cell lymphoma, which included both low- and high-risk patients.13 Thus, patients with mediastinal large-cell lymphoma appear to do remarkably well following ABMT in first response. It is important to recognize that some patients in clinical PR may actually represent patients in CR with residual fibrotic changes, which can continue to resolve over time. In our study, six of eight patients in PR had evidence of further response following ABMT.
Eleven patients with relapsed disease underwent ABMT with an estimated 5-year PFS of 27%. Four of 11 patients had chemotherapy-responsive disease (responsive relapse), while seven of 11 had chemotherapy-nonresponsive disease (resistant relapse), but outcomes were similar between the two groups. This result is similar to the 3-year PFS rate of 30% reported by Philip et al17following ABMT in relapsed patients with aggressive NHL.
Twelve patients were transplanted with primarily refractory disease with an estimated 5-year PFS of 58%. This result is strikingly better than the 3-year PFS of 0% reported by Philip et al17following ABMT in refractory patients with aggressive NHL. It is also remarkably better than the 0% 3-year PFS reported by Lazzarino et al13 for patients with refractory mediastinal large-cell lymphoma treated with a variety of salvage regimens, only three of whom underwent ABMT. This high survival rate may in part be explained by several factors. While all refractory patients in the studies by Philip et al17 and Lazzarino et al13 remained nonresponsive to further chemotherapy, four of 12 patients in our study were chemotherapy-responsive to a subsequent regimen. Three of these four patients received CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) as first-line therapy, which has been suggested to be an inferior regimen in mediastinal large-cell lymphoma.9 It is also possible that some patients with bulky disease and large residual nonmalignant masses were incorrectly classified as refractory. However, eight of 11 refractory patients had positive gallium scans following initial chemotherapy, while the remainder had enlarging masses on CT scan or biopsy-proven active disease. Overall, it would appear that treatment intensification with ABMT for patients with primarily refractory mediastinal large-cell lymphoma results in a high response rate, and may reflect a more favorable biology in this subset.
The strongest predictor of PFS in our study was the presence of chemotherapy-responsive disease immediately before ABMT. Patients with chemotherapy-responsive disease had a significantly greater estimated 5-year PFS compared with patients who were chemotherapy-nonresponsive (75% v 33%, P = .007). The prognostic importance of chemotherapy responsiveness at ABMT has previously been reported for aggressive NHL.17 No other variables analyzed were found to be significant on multivariate analysis. Bulky mediastinal masses (>10 cm)7,13 and the presence of a pleural effusion at presentation12 have been associated with poorer outcome following initial chemotherapy in mediastinal large-cell lymphoma. This association was not found following ABMT. However, due to the small sample size, there is limited power to detect significant prognostic factors.
In this study, there was no significant difference in outcome between patients who received radiation therapy before or after ABMT, and those who did not. However, the patients who received radiation therapy were heterogeneous in character and were treated at varying time points. The role of radiation therapy in the treatment of mediastinal large-cell lymphoma cannot be directly assessed from this study and is yet to be defined.
Due to the difficulty in interpreting residual radiographic abnormalities, we examined retrospectively the correlation between pre- and post-ABMT gallium scan results to long-term outcome following ABMT. Gallium scan imaging has been reported to be a strong predictor of residual tumor viability following therapy for diffuse large-cell lymphoma.32 In our study, patients who were gallium-negative pre-ABMT had a lower likelihood of relapse than patients who were gallium-positive (5-year estimated relapse rate of 9% v 44%, P = .06). Gallium scans performed for restaging following ABMT were a less reliable indicator of outcome. The 5-year estimated relapse rate for patients with positive gallium scans post-ABMT is 43%, compared with 31% for patients with negative scans (P = .55). This is in contrast to results reported by Vose et al,33 who found that SPECT gallium scans performed on day +100 following ABMT for NHL had a high predictive value, especially for patients with residual mediastinal masses. False-positive results may reflect residual inflammation following high-dose chemotherapy. The majority of restaging scans were performed between 2 and 3 months following engraftment. Thus, the earlier timing may in part be responsible for these discrepant results. Nonetheless, our study suggests that early posttransplant gallium scans should be interpreted with caution.
Despite the small patient number and retrospective format of this study, we believe that our results help to clarify clinical response and outcome following ABMT in patients with mediastinal large-cell lymphoma. A preliminary analysis of 29 patients by Popat et al34 provides the only available data on ABMT in this subgroup. With a median follow-up duration of 958 days, they reported a 51% PFS rate in induction-failure patients, and a 75% and 33% PFS rate for patients with sensitive and refractory relapse, respectively.
We conclude that outcome following ABMT in patients with mediastinal large-cell lymphoma appears to be as good, and perhaps better than in patients with other aggressive NHLs. Early intensification of poor-prognosis patients in first response results in a high incidence of durable remissions. In addition, primarily refractory patients can achieve long-term survival following ABMT, and should strongly be considered for early intensification. The role of ABMT as part of primary therapy for patients with mediastinal large-cell lymphoma should be studied further in a prospective fashion.
ACKNOWLEDGMENT
We gratefully acknowledge E. John Orav for providing biostatistical assistance.
Supported by National Institutes of Health Grants no. CA38493 and CA39542.
Address reprint requests to Catherine Wheeler, MD, Hematology-Oncology Division, Beth Israel Hospital, 330 Brookline Ave, Boston, MA, 02214.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal