Abstract
During granulocyte differentiation in the bone marrow (BM), neutrophilic leukocyte precursors synthesize large amounts of lysosomal enzymes. These enzymes are sequestered into azurophilic storage granules until used days later for digestion of phagocytized microorganisms after leukocyte emigration to inflamed tissues. This azurophil granule population has previously been defined as a primary lysosome, ie, a membrane-bound organelle containing acid hydrolases that have not entered into a digestive event. In this study, azurophil granules were purified and shown to contain large amounts of mannose 6-phosphate-containing glycoproteins (Man 6-P GP) but little lysosome-associated membrane proteins (LAMP). In addition, the fine structural localization of Man 6-P GP and LAMP was investigated at various stages of maturation in human BM and blood. Man 6-P GP were present within the azurophilic granules at all stages of maturation and in typical multivesicular bodies (MVB) as well as in multilaminar compartments (MLC), identified by their content of concentric arrays of internal membranes. LAMP was absent in all identified granule populations, but was consistently found in the membranes of vesicles, MVB, and MLC. The latter compartment has not been previously described in this cell type. In conclusion, the azurophilic granules, which contain an abundance of lysosomal enzymes and Man 6-P GP, lack the LAMP glycoproteins. By current criteria, they therefore cannot be classified as lysosomes, but rather may have the functional characteristics of a regulated secretory granule. Rather, the true lysosomes of the resting neutrophil are probably the MVB and MLC. Finally, the typical “dense bodies” or mature lysosomes described in other cells are not present in resting neutrophils.
ENZYME CYTOCHEMISTRY and subcellular fractionation have shown that lysosomal enzymes are synthesized early in the maturation of neutrophilic leukocytes in bone marrow (BM).1-5 These enzymes are stored within azurophilic (or primary) granules for 10 to 14 days before being used during phagocytosis. The azurophilic granules were considered by de Duve6 to be classic examples of primary lysosomes since they are membrane-bound organelles that contain acid hydrolases that have not yet entered into a digestive event. These granules, which are formed at the promyelocytic stage (see Table1),7-9 are the major source of acid hydrolases and many other proteins such as myeloperoxidase (MPO), granulocyte elastases, and nonglycosylated defensins. However, little is known of their membranes. Only CD6310,11 and CD6812 have been demonstrated in the membrane of azurophilic granules. Two other granule populations, which are nonlysosomal and peroxidase-negative, form later in maturation: specific or secondary granules,13 which contain lactoferrin and many other substances, and the gelatinase or tertiary granules, which form mainly at the band cell stage.14 Also, highly mobilizable secretory vesicles that contain endocytosed plasma proteins such as albumin have recently been characterized.15 16
Granules of the Human Neutrophil
| . | Characteristic Protein(s) . | Time of Formation . |
|---|---|---|
| Azurophilic/primary granules | Myeloperoxidase elastase, defensins | Promyelocyte |
| Specific/secondary granules | Lactoferrin, NGAL, B12-binding protein | Myelocyte, metamyelocyte |
| Gelatinase/tertiary granule | Gelatinase | Band cell |
| . | Characteristic Protein(s) . | Time of Formation . |
|---|---|---|
| Azurophilic/primary granules | Myeloperoxidase elastase, defensins | Promyelocyte |
| Specific/secondary granules | Lactoferrin, NGAL, B12-binding protein | Myelocyte, metamyelocyte |
| Gelatinase/tertiary granule | Gelatinase | Band cell |
Historically, two major types of storage granules were definitively identified by the presence or absence of MPO. The MPO-positive azurophil (primary) granules are formed only during the promyelocyte stage and are reduced in number by mitosis. It has recently been shown that the peroxidase negative granules constitute a continuum from early appearing granules (myelocyte stage) that contain lactoferrin but no gelatinase to granules that contain both lactoferrin and gelatinase (metamyelocyte stage) to granules that contain gelatinase but no lactoferrin (band cell stage). The aforementioned granules that contain lactoferrin are referred to as specific (secondary) granules while the granules that contain gelatinase but not lactoferrin are referred to as gelatinase (tertiary) granules. Thus, the mature circulating neutrophil contains the three major granule types. When appropriately stimulated, these cells move from blood to tissues and, within seconds, the granules may release their contents in endocytic vacuoles or, by fusion with the plasma membrane, to the exterior of the cell.
Since de Duve's original discovery and description of lysosomes, considerable progress has been made in defining the components of lysosomes in many cell types.17 In most other previously investigated cell types, the synthesis and post-translational processing of the lysosomal enzymes occur over the relatively short time of a few hours.18 During their transport from the endoplasmic reticulum to the Golgi complex, the newly synthesized lysosomal hydrolases are modified to contain mannose 6-phosphate (Man 6-P), which binds to Man 6-P receptors in the Golgi complex effecting the targeting of the hydrolases to an acidic endosome where the complexes dissociate.17,19 The receptors recycle back to the Golgi complex or to the plasma membrane, while the lysosomal enzymes reach the mature lysosome where they are rapidly dephosphorylated. Thus, at steady state, the lysosome contains the bulk of the dephosphorylated hydrolases while the phosphorylated forms are in endosomes.20 Another defining feature of lysosomes is the presence of unique transmembrane glycoproteins, identified as closely related lysosome-associated membrane proteins (LAMP) LAMP-1 and LAMP-221,22 reviewed by Kornfeld and Mellman.17 LAMPs are among the most densely glycosylated glycoproteins known, with carbohydrate comprising 55% to 65% of the total mass.23,24 While the function of these proteins is unknown, it has been speculated that one of their roles is to protect the lysosomal membrane from degradation by lysosomal acid hydrolyses, since their luminal domains are very resistant to proteolysis.25 However, it is unlikely that this simple explanation defines the complete role of the glycoproteins, because, for one thing, it does not explain the different levels of tissue expression of LAMP 1 and 2. Moreover, Cuervo and Dice26have recently reported that LAMP-2 is a receptor for the selective uptake of proteins into lysosomes for subsequent degradation.
As a result of these advances, lysosomes are now defined as vesicular compartments with (1) a high concentration of LAMPs, (2) a full complement of mature dephosphorylated lysosomal enzymes, (3) the absence of cation-independent Man 6-P receptor, and (4) an acid pH.17 This definition has largely been observed from studies of rapidly dividing tissue culture cell lines and leaves open a number of questions concerning lysosomal biosynthesis, including the pathway of lysosomal synthesis in neutrophilic leukocytes, an important professional phagocyte. We decided to reexamine the concept in this important cell type by asking two questions: (1) Where are the LAMPs and Man 6-P GP located in developing and mature neutrophils? (2) Does the azurophilic granule fit the more modern definition of a lysosome?
We have addressed these questions by application of fractionation methods and immunolabeling using thawed cryosections to study the distribution of the Man 6-P marker and distribution of LAMP-1 and LAMP-2 in mature human blood neutrophils. In addition, human BM neutrophils at different stages of maturation were examined by light and electron microscopy. These studies show the absence of LAMP-1 and LAMP-2 in membranes of azurophil granules. The findings suggest that, despite the high content of lysosomal acid hydrolases, the azurophil granule has the characteristics of a regulated secretory granule rather than being a true lysosome. LAMP proteins were identified, for the first time in neutrophils, in a multilaminar compartment (MLC) identified by its content of concentric arrays of internal membranes, and in typical multivesicular bodies (MVB). These multilaminar and multivesicular bodies are possibly the true precursors to the “housekeeping” lysosomes of this cell type, during its relatively short life span of 2 to 3 weeks.
MATERIALS AND METHODS
Antibodies and Marker Proteins
Monoclonal antibodies (MoAbs) H5G11 and H4A3 (antihuman LAMP-1), H4B4 (anti-LAMP-2) (all IgG1,K) were derived from mice immunized with human adherent peripheral blood (PB) cells.21,22 Polyclonal rabbit antibodies against human gelatinase and against human albumin were raised as described by Kjeldsen et al27 and Borregaard et al,16 respectively. Lactoferrin antibodies were obtained from Accurate Chemical Co (Westbury, NY).
Azurophil granules present in subcellular fractions were identified by myeloperoxidase and their contents were measured by enzyme linked immunosorbent assay (ELISA).28 Specific and gelatinase granules were identified by lactoferrin and gelatinase, respectively, and measured by ELISA.27,29 Secretory vesicles were identified by albumin and measured by ELISA.16 Plasma membranes were identified by HLA class I and measured by ELISA.30
Subcellular Fractionation
Subcellular fractionation was performed as previously described.16 31 Briefly, isolated neutrophils were disrupted by nitrogen cavitation in 13 mL relaxation buffer (100 mmol/L KCl, 3 mmol/L NaCl, 1 mmol/L ATPNa2, 3.5 mmol/L MgCl2, 10 mmol/L Piperazine N,N′-bis2 [ethane-sulfonic acid] [Pipes] pH 7.2 containing 0.5 mmol/L phenylmethylsulfonyl fluoride (PMSF). Nuclei and intact cells were sedimented by centrifugation at 400g for 15 minutes (P1) and 10 mL of the postnuclear supernatant (S1) was applied on top of a 28 mL two-layer Percoll density gradient (1.05/1.12 g/mL) containing 0.5 mmol/L PMSF and centrifuged at 35,000g for 30 minutes. This resulted in the generation of separate fractions that could be visually identified in the gradient: the bottom band (α-band), containing azurophilic granules; the intermediate band (β-band), containing specific and gelatinase granules; the top band (γ-band), containing plasma membranes and secretory vesicles. The gradient was aspirated from the bottom and dispersed into fractions of 1.4 mL each through a fraction collector.
Detection of Man 6-P Containing Glycoproteins (Man 6-P GP) in Subcellular Fractions
Samples of 100 μL from each fraction were diluted with 150 μL saline. This mixture was added to 250 μL sodium dodecyl sulfate (SDS)-reducing sample buffer. After boiling for 5 minutes, the proteins of 125-μL samples were resolved on a 5% to 20% acrylamide SDS gradient gel and transferred to 0.2-μm nitrocellulose filters.32 The filters were probed with radioiodinated sCI-MPR as described by Valenzano et al.33 Briefly, membranes were treated at 4°C with blocking buffer (phosphate-buffered saline [PBS] containing 1 mg/mL bovine serum albumin [BSA] and 0.2% Tween-20) for two hours to quench nonspecific binding sites, incubated with 3 nmol/L 125I-labeled sCI-MPR (∼1 Ci/μmol) in blocking buffer for 16 hours, and then rinsed 10 times for 30 seconds in PBS containing 0.2% Tween-20. The binding of sCI-MPR was detected and quantified with a phosphorimager (Molecular Dynamics, Sunnyvale, CA). The probe detects the phosphorylated glycoproteins which retain the Man 6-P recognition marker and are subsequently called Man 6-P GP.
Detection of LAMP in Subcellular Fractions
LAMP were detected as previously described by Mane et al.22Briefly, SDS-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described by Laemmli and proteins were transferred to 0.2-μm nitrocellulose filters (Bio-Rad Laboratories, Richmond, CA) essentially as described by Towbin et al.34 Mouse-MoAbs H4A3 and H4B4 directed against LAMP-1 and LAMP-2, respectively, were then applied at a dilution of 1:3,000 (from stock of 1 mg/mL) in PBS containing 0.1% Tween-20 and incubated overnight. The nitrocellulose filters were then washed in PBS, 0.1%Tween-20, and developed by the Amersham Chemiluminescence Method as described by the manufacturer. The films were scanned using the CREAM system, version 4.0 (Kem-En-Tec, Copenhagen, Denmark).
Immunoprecipitation
Immunoprecipitation was performed after lysis of 3 × 107 cells/mL in lysis buffer (10 mmol/L HEPES, 100 mmol/L KCl, 25 mmol/L NOG [N-octylglucoside]; 0.2% cetyl-trimethyl ammonium bromide (CTAB), 1 mmol/L PMSF, 200 KIU Aprotinin, 100 μg/mL leupeptin, 1 mmol/L EGTA). Samples of 200 μL were incubated with anti-MPO coupled to Sepharose particles. The precipitates were washed five times and resuspended to 200 μL of SDS sample buffer. Each sample was divided in two, each of which was further run on SDS-PAGE, one of which was further processed for transfer to nitrocellulose and quantitation of Man 6-P content.
Preparation of Human Leukocytes for Morphologic Examination
Normal human leukocytes from PB, anticoagulated in heparin and freed of erythrocytes by sedimentation in Dextran, were washed in Hanks' balanced salt solution. The cells were fixed in 4% paraformaldehyde-lysine, using the buffers of McLean and Nakane35 or in 2% paraformaldehyde, 0.05% glutaraldehyde, in 0.1 mol/L phosphate buffer (pH 7.4), for 30 minutes, at 4°C. The cells were then washed thoroughly in the same buffer containing 3% (wt/vol) sucrose.
Immunolabeling Using Thawed Cryosections
The cell pellet was embedded in a 20% Polyvinyl pyrrolidone (PVP-10; Sigma, St Louis, MO)/2.1 mol/L sucrose solution, frozen and stored in liquid nitrogen. Sections were cut on a Reichert-Jung Ultracut FC-4 (Buffalo, NY). The techniques used for preparing ultrathin cryosection and the immunocytochemistry have been previously described.36 The primary antibody was used at the following dilutions: all LAMP MoAbs were used as undiluted ascites fluid; antigelatinase, 1/100; antialbumin, 1/50; antilactoferrin, 1/1,000 in .5 mol/L NaCl/PBS. After several washes, the immunogold probes were used at a dilution of 1/50 in .1% BSA/PBS, pH 8.2. The gold probes were goat antimouse IgG and IgM-gold, 5 nm or 10 nm (GAM-5 or GAM-10), and goat antirabbit IgG-gold, 5 nm or 10 nm (GAR-5 or GAR-10) with optical density (O.D.) at 520 nm of 2.5 (Amersham, Arlington Heights, IL). A nonimmune purified rabbit IgG was used as a control for polyclonal antibodies, and normal mouse serum or the control hybridoma, for MoAbs. Double-labeling experiments to localize H4B4 and albumin were performed by combining the monoclonal H4B4 and polyclonal albumin in the primary antibody incubation, washing, and subsequently combining GAM and GAR for the immunogold incubation. After several washes, the sections were then stained with a neutral pH uranyl acetate oxalate and embedded in methyl cellulose/uranyl acetate as described previously.37 38
Detection of Man 6-P GP on Ultrathin Cryosections of Human BM and Blood by Electron Microscopy
Leukocytes from five normal donors were separated from blood by Dextran sedimentation, or from BM from two normal donors and enriched for different maturation stages, as described previously.14 The ultrathin cryosections were incubated for 2 hours at 4°C, in 1% BSA, 0.2% Tween-20 and PBS: and then incubated overnight with the biotinylated sC1-MRP (1 mg/100 mL) in PBS plus 1% BSA and 5 mmol/L β-glycerolphosphate. The phosphorylated enzymes were detected the next day by streptavidin-gold, 5 or 10 with O.D. at 520 nm of approximately 5.0 (Sigma) diluted to 1:20. The sections were then further processed as described above. For double-label, the antibody was applied first, after washing; the biotinylated sC1-MPR was then applied and allowed to incubate overnight. For controls, the sections were preincubated with 10 mmol/L of Man 6-P for 1 hour before applying the biotinylated sC1-MPR.The grids were then further processed as described above.
Detection of Man 6-P GP on Blood or BM Smears by Light Microscopy
Samples of human blood or BM were spread on a cover slip, fixed in acetone: methanol: 37% formaldehyde (19:19:2) at 4°C for 90 seconds, then washed in PBS and further processed as previously described by Sleat et al.39 Briefly, the slides were treated for 2 hours at 4°C in PBS, 1% BSA, 2% Tween 20, and then incubated overnight with the biotinylated sCI-MPR (1 mg/100 mL) in PBS, 1% BSA, and 5 mmol/L β-glycerophosphate. For controls, slides were incubated with 10 mmol/L of Man 6-P 1 hour before the biotinylated sCI-MPR was applied. The phosphorylated enzymes were detected the next day by avidin-linked alkaline phosphatase and the substrate Vector Red (Vectastain ABC kit; Vector, Burlingame, CA). Levamisole was also present to inhibit neutrophil endogenous alkaline phosphatase (Vectastain ABC kit; Vector).
RESULTS
Subcellular Localization of Man 6-P GP and LAMP in Mature Neutrophils
Subfractionation Studies
The subcellular localization of Man 6-P GP and LAMP was determined in the different neutrophilic fractions separated by density on a Percoll gradient. The fractions were characterized by use of the following markers (Table 2): myeloperoxidase for azurophilic granules, lactoferrin for specific/secondary granules, gelatinase for gelatinase/tertiary granules, albumin for the secretory vesicles, and HLA class I for the plasma membrane.
Specific Markers for Neutrophil Compartments
| Granules . | Content Markers . | Membrane Markers . |
|---|---|---|
| Azurophilic granules | Lysosomal enzymes, myeloperoxidase, M6P-glycoproteins | CD63, CD68 |
| Specific granules | Lactoferrin | CD11b, cytochrome b |
| Gelatinase granules | Gelatinase | CD11b, cytochrome b |
| Other Compartments | ||
| Secretory vesicles | Albumin | Alkaline phosphatase, CD35, CD11b, cytochrome b |
| Multivesicular bodies & multilaminar compartments | M6P-glycoproteins | LAMP-2/LAMP-1 |
| Plasma membrane | HLA-1 |
| Granules . | Content Markers . | Membrane Markers . |
|---|---|---|
| Azurophilic granules | Lysosomal enzymes, myeloperoxidase, M6P-glycoproteins | CD63, CD68 |
| Specific granules | Lactoferrin | CD11b, cytochrome b |
| Gelatinase granules | Gelatinase | CD11b, cytochrome b |
| Other Compartments | ||
| Secretory vesicles | Albumin | Alkaline phosphatase, CD35, CD11b, cytochrome b |
| Multivesicular bodies & multilaminar compartments | M6P-glycoproteins | LAMP-2/LAMP-1 |
| Plasma membrane | HLA-1 |
References are found in the text, except for CD35.65
On blots of subcellular fractions, Man 6-P–containing proteins were quantitatively analyzed by use of the sCI-MPR probe. This probe is a biotinylated or radioiodinated derivative of a soluble form of the cation-independent Man 6-P receptor isolated from fetal bovine serum.33,39 The probe binds Man 6-P containing proteins with nanomolar affinity, and is used in much the same manner as an antibody is to detect a specific substrate. The biotinylated receptor binds its ligand; subsequently the biotin moiety binds with either avidin-conjugated alkaline phosphatase for detection at the light microscopy level or streptavidin-gold for detection at the fine structural level. This assay of Valenzano et al33 on fractions also allows for the detection of phosphorylated lysosomal enzymes and other glycoproteins.
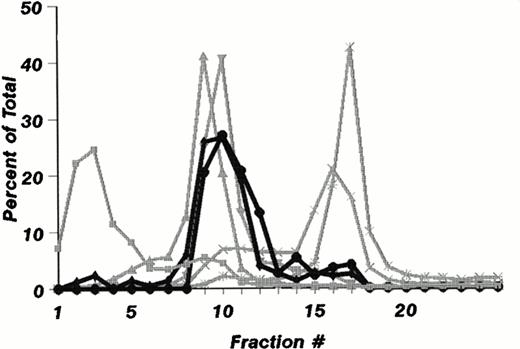
The subcellular distribution of LAMP-1 and 2 (Fig 1A) and Man 6-P GP (Fig 1B) in fractions from mature neutrophils are shown in Fig 1. It is striking that these do not colocalize to the same fractions. While Man 6-P GP essentially colocalizes with myeloperoxidase in the most dense fraction (Fig 1B), LAMP-1 and 2 are found in less dense fractions that also contain the markers lactoferrin and gelatinase (Fig 1A), and in small amounts in fractions 15 and 16.
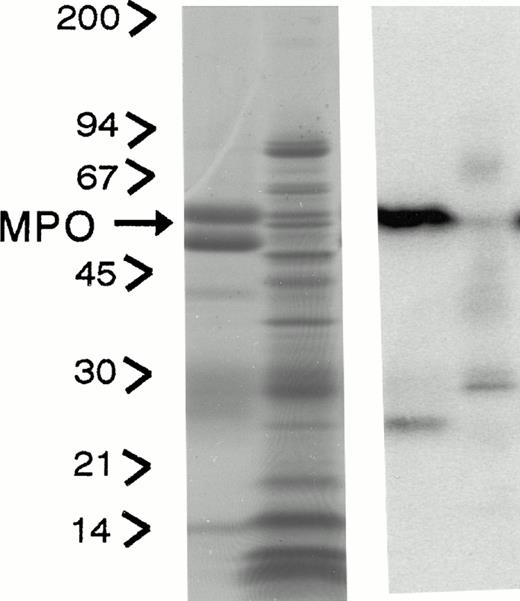
Subcellular distribution of LAMP 1 and 2 and Man 6-P GP in neutrophils. Neutrophils from PB were isolated, disrupted by nitrogen cavitation, and fractionated on a two-layer Percoll Density gradient. (A) LAMP 1 and 2 in subcellular fractions. Other fractions of 1.4 mL were collected and assayed for markers of azurophil granules (MPO, ▪), specific granules (lactoferrin, ▴), gelatinase granules (gelatinase, ▾), secretory vesicles (albumin, X), and plasma membranes (HLA, class I, *). Samples from each fraction were centrifuged to remove Percoll and subjected to SDS-PAGE. The separated proteins were blotted to nitrocellulose membranes and probed with antibody against LAMP 1 (•) and LAMP 2 (⧫). The immunoblots were developed by chemiluminescence and quantitated by scanning (bold lines). (B) Man 6-P GP in subcellular fractions. Fractions of 1.4 mL were collected and assayed for markers of azurophil granules (MPO, ▪), specific granules (lactoferrin, •), gelatinase granules (gelatinase, ⧫), secretory vesicles (albumin, X ), and plasma membranes (HLA, class I, *). Samples from each fraction were centrifuged to remove Percoll and subjected to SDS-PAGE. The separated proteins were blotted to nitrocellulose membranes and probed with the sCI-MPR (★) and quantified by phosphorimager (bold line). (C) Man 6-P GP in relation to myeloperoxidase. Isolated neutrophils were disrupted by nitrogen cavitation and the postnuclear supernatant was immunoprecipitated with anti-MPO coupled to sepharose particles, divided into two, and subjected to SDS-PAGE and Coomassie blue staining for protein (left panel) or to SDS-PAGE followed by transfer to nitrocellulose and probed with sCI-MPR (right lane). From left to right: Lane 1, protein profile in immunoprecipitate; lane 2, protein profile in supernatant after immunoprecipitation; lane 3, Man 6-P GP in immunoprecipitate; lane 4, Man 6-P GP in supernatant after immunoprecipitation. (D) Profile of Man 6-P GP in fraction #3 and #10.
Subcellular distribution of LAMP 1 and 2 and Man 6-P GP in neutrophils. Neutrophils from PB were isolated, disrupted by nitrogen cavitation, and fractionated on a two-layer Percoll Density gradient. (A) LAMP 1 and 2 in subcellular fractions. Other fractions of 1.4 mL were collected and assayed for markers of azurophil granules (MPO, ▪), specific granules (lactoferrin, ▴), gelatinase granules (gelatinase, ▾), secretory vesicles (albumin, X), and plasma membranes (HLA, class I, *). Samples from each fraction were centrifuged to remove Percoll and subjected to SDS-PAGE. The separated proteins were blotted to nitrocellulose membranes and probed with antibody against LAMP 1 (•) and LAMP 2 (⧫). The immunoblots were developed by chemiluminescence and quantitated by scanning (bold lines). (B) Man 6-P GP in subcellular fractions. Fractions of 1.4 mL were collected and assayed for markers of azurophil granules (MPO, ▪), specific granules (lactoferrin, •), gelatinase granules (gelatinase, ⧫), secretory vesicles (albumin, X ), and plasma membranes (HLA, class I, *). Samples from each fraction were centrifuged to remove Percoll and subjected to SDS-PAGE. The separated proteins were blotted to nitrocellulose membranes and probed with the sCI-MPR (★) and quantified by phosphorimager (bold line). (C) Man 6-P GP in relation to myeloperoxidase. Isolated neutrophils were disrupted by nitrogen cavitation and the postnuclear supernatant was immunoprecipitated with anti-MPO coupled to sepharose particles, divided into two, and subjected to SDS-PAGE and Coomassie blue staining for protein (left panel) or to SDS-PAGE followed by transfer to nitrocellulose and probed with sCI-MPR (right lane). From left to right: Lane 1, protein profile in immunoprecipitate; lane 2, protein profile in supernatant after immunoprecipitation; lane 3, Man 6-P GP in immunoprecipitate; lane 4, Man 6-P GP in supernatant after immunoprecipitation. (D) Profile of Man 6-P GP in fraction #3 and #10.
To test whether or not myeloperoxidase was the major Man 6-P–containing protein, neutrophils were solubilized and precipitated with polyclonal antibodies against MPO. This precipitation was effective as judged by reduction of myeloperoxidase from 93 μg/mL in the solubilized cells to 0.31 μg/mL in the supernatant after immunoprecipitation. The precipitation was specific for MPO since the MPO bands at 62 kD and 12 kD, and the IgG at 50 kD are the only major protein bands seen in the SDS-PAGE profiles (Fig 1C). By comparing the content of Man 6-P in supernatant and pellet as revealed by binding of the radioiodinated fragment of the CI-mannose 6-P receptor to the blotted proteins we found that the majority of the receptor binding is associated with myeloperoxidase. Other, smaller bands probably represent lysosomal enzymes. The Man 6-P GP profiles differ in the different fractions of Fig 1B. This can be observed in Fig 1D, which gives the Man 6-P GP profile of fraction #3 and #10, the latter showing a much more diverse profile than #3, which is dominated by MPO.
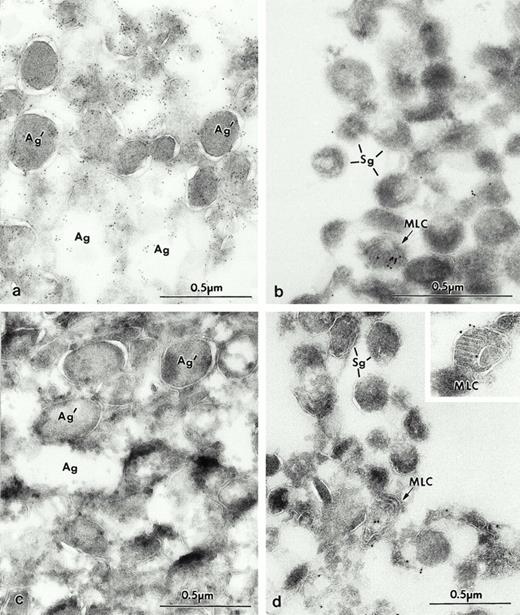
We next performed immunocytochemistry for Man 6-P GP and LAMP on frozen thin sections of individual fractions characterized in Fig 1. The azurophilic granules from fractions 2 and 3 (Fig 2a) were shown to be strongly labeled for Man 6-P GP. However, the small amount of Man 6-P GP present in fractions 9 and 10 was not in specific granules but was seen in vacuoles containing concentric arrays of internal membranes, called MLC (Fig 2b), in MVB, and in rare small granules (not shown). Fractions 15 and 16 contained no label for Man 6-P GP (not illustrated). When the fractions were examined for LAMP, no label was found in the azurophil granule fraction (Fig 2c). The most interesting finding was in fraction 10, the one highest in LAMP; the marker was not found in the numerous specific granules located there, but was detected in MLC (Fig 2d and inset) and in rare MVB (not illustrated).
Electron micrographs of frozen thin sections from fractions containing azurophil granules, section of fractions # 2 (a and c), and specific granules # 9 (b and d). (a) Frozen thin section of fraction #2, which is labeled for Man 6-P GP with biotinylated probe, and detected with streptavidin gold-10. Most of the organelles in fraction #2 appear to be azurophilic granules labeled for Man 6-P GP (Ag). Some of the granules have extracted contents (Ag), while others retain their content (Ag′). This variation in content preservation has been previously observed in intact cells. (b) Fraction #10 contained typical specific granules (Sg) negative for Man 6-P GP. Indeed, compared to (a), only a few gold particles were present, and they were in MLC (arrow) and rare granular structures. Fractions 2 (c) and 10 (d) were labeled for LAMP-2 with 10 nm gold. Fraction 2 consisted mostly of azurophilic granules (Ag and Ag′); these granules were devoid of immunoreactivity (c). Most of the organelles in fraction 10 appear to be specific granules (Sg), which are not labeled, whereas the gold labeling is present in MLC (arrows). Tissue preparation for figures of electron micrographs, except 8: Fractions or cells were fixed in 2% paraformaldehyde, 0.05% glutaraldehyde for 1 hour at 4°C embedded in sucrose, frozen and processed for ultrathin-cryosectioning. For Fig 8b and c: leukocytes were fixed in 4% paraformaldehyde-lysine, using the buffers of McLean and Nakane.35
Electron micrographs of frozen thin sections from fractions containing azurophil granules, section of fractions # 2 (a and c), and specific granules # 9 (b and d). (a) Frozen thin section of fraction #2, which is labeled for Man 6-P GP with biotinylated probe, and detected with streptavidin gold-10. Most of the organelles in fraction #2 appear to be azurophilic granules labeled for Man 6-P GP (Ag). Some of the granules have extracted contents (Ag), while others retain their content (Ag′). This variation in content preservation has been previously observed in intact cells. (b) Fraction #10 contained typical specific granules (Sg) negative for Man 6-P GP. Indeed, compared to (a), only a few gold particles were present, and they were in MLC (arrow) and rare granular structures. Fractions 2 (c) and 10 (d) were labeled for LAMP-2 with 10 nm gold. Fraction 2 consisted mostly of azurophilic granules (Ag and Ag′); these granules were devoid of immunoreactivity (c). Most of the organelles in fraction 10 appear to be specific granules (Sg), which are not labeled, whereas the gold labeling is present in MLC (arrows). Tissue preparation for figures of electron micrographs, except 8: Fractions or cells were fixed in 2% paraformaldehyde, 0.05% glutaraldehyde for 1 hour at 4°C embedded in sucrose, frozen and processed for ultrathin-cryosectioning. For Fig 8b and c: leukocytes were fixed in 4% paraformaldehyde-lysine, using the buffers of McLean and Nakane.35
Fractions 15 and 16 contained numerous small vesicles, some of which were undoubtedly vesiculated plasma membranes as well as occasional large vacuoles. No Man 6-P GP was found in these fractions, consistent with the quantitative data presented in Fig 1B. Occasionally, label for LAMP-2 could be seen as isolated gold particles on the membranes of small vesicles. On the other hand, small vesicles heavily labeled for albumin could be found, as one would have predicted (not illustrated).
Localization of the Man 6-P GP and LAMP in Developing BM Neutrophils
Light Microscopy
Man 6-P GP in BM smears was labeled and visualized by biotinylated sCI-MPR in combination with streptavidin-alkaline phosphatase. A bright red reaction product was detected in cells at all stages of neutrophil maturation (from promyelocytes to mature neutrophils) as well as on eosinophils. This labeling was especially strong in promyelocytes and myelocytes. Other cells contained lesser amounts of reaction product, such as in megakaryocytes, or none at all, eg, in nucleated erythroblastic or mature red blood cells. The specificity of the probe was shown in a control experiment by a marked decrease in labeling when the sCI-MPR was preincubated with Man 6-P. Staining was also absent when the probe was omitted. The MoAb against LAMP-2 was applied to the same BM smears, and was detected in the neutrophilic cells at all stages of maturation.
Electron Microscopy
Man 6-P GP.
At the ultrastructural level, in immature promyelocytes and myelocytes (Fig 3a) Man 6-P GP was present in the Golgi complex (Fig 3b) and, to a greater extent, in the newly formed azurophilic granules presumably because they are concentrated in that organelle. The labeling was especially strong within the matrix of the azurophilic granules (Fig 3c and d), as predicted by the data previously presented in the fractions. In areas near azurophilic granules with high labeling, we frequently found “spillage” of the antigen in the nearby cytoplasm (Fig 3c and d). This artifact has been previously observed with the low molecular weight defensins40 and proteinase 3.41 A possible explanation is that the membranous compartments are cut open by the cryostat method, and some of the granule contents become soluble and may partially relocate during the subsequent incubation procedures. Man 6-P GP was also detected in structures containing concentric arrays of internal membranes, the so-called MLC, which had not been described in neutrophils, but was observed in a related cell line, HL60.22 Other organelles, with the exception of rare multivesicular bodies, were not labeled. Intense labeling for Man 6-P GP within the matrix of azurophilic granules of intact mature neutrophils was also confirmed (Fig 4a and b). No staining was observed when control preparations were preincubated with 10 mmol/L of Man 6-P, or when the probe or streptavidin-gold label were omitted (not shown).
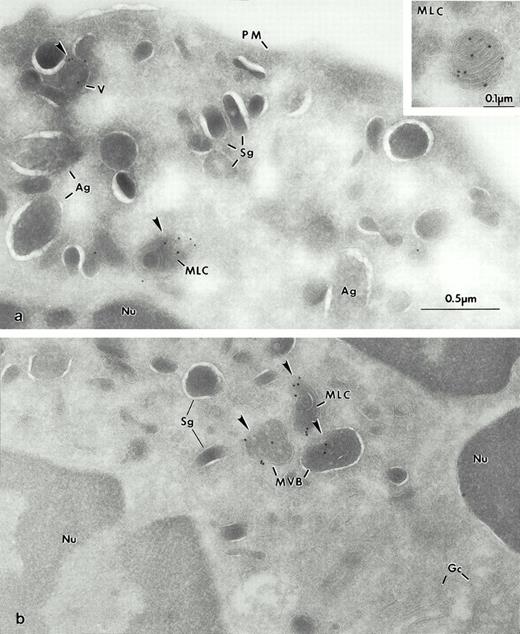
Electron micrographs of frozen thin sections from human BM labeled by a biotinylated sCI-MPR probe. The Man 6-P GP are visualized with streptavidin 10 nm. (a) Early immature granulocytic cell of the BM. (b) The Golgi complex (GC) of an immature cell is labeled for Man 6-P GP. (c and d) In this immature neutrophil from BM (portion of the promyelocyte or an early myelocyte from [a]), the highest levels of Man 6-P GP are found within the newly formed azurophilic granules (Ag), some of which have retained their density (Ag′) as in (c), while others appear extracted (d) but retain immunolabel. At intermediate stages of maturation (myelocytes), an MLC is first observed (Inset, [d]).
Electron micrographs of frozen thin sections from human BM labeled by a biotinylated sCI-MPR probe. The Man 6-P GP are visualized with streptavidin 10 nm. (a) Early immature granulocytic cell of the BM. (b) The Golgi complex (GC) of an immature cell is labeled for Man 6-P GP. (c and d) In this immature neutrophil from BM (portion of the promyelocyte or an early myelocyte from [a]), the highest levels of Man 6-P GP are found within the newly formed azurophilic granules (Ag), some of which have retained their density (Ag′) as in (c), while others appear extracted (d) but retain immunolabel. At intermediate stages of maturation (myelocytes), an MLC is first observed (Inset, [d]).
Electron micrograph of frozen thin section of mature blood neutrophils (a), Man 6-P GP is detected with the sCI-MPR–biotinylated probe and visualized with streptavidin gold (5 nm). (b) High levels of Man 6-P GP are found within the Ag, and in vesicular structures (V) probably an MVB, but not in Sg.
Electron micrograph of frozen thin section of mature blood neutrophils (a), Man 6-P GP is detected with the sCI-MPR–biotinylated probe and visualized with streptavidin gold (5 nm). (b) High levels of Man 6-P GP are found within the Ag, and in vesicular structures (V) probably an MVB, but not in Sg.
LAMP.
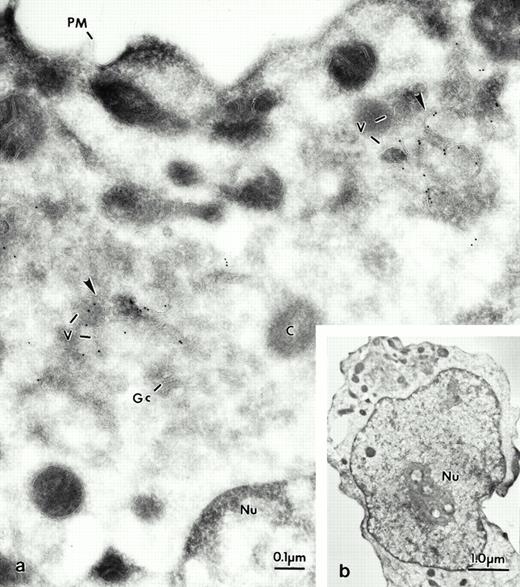
At the early stages of maturation, in promyelocytes (Fig 5a and b), LAMP labeling was found in vesicles near the Golgi complex (Fig 5a). Later (at the myelocyte stage), LAMP was found mainly in vesicles of various sizes, and the multilaminar compartments (Fig 6a, inset), as well as in typical multivesicular bodies (Fig 6b). In contrast, as could be predicted from the fractionation data, LAMP labeling was absent from azurophilic granules (Figs 6 and7), but was found in vesicles near the Golgi cisternae and in larger vesicles (Fig 7a and b) as well as in the multilaminar compartment and multivesicular bodies (Fig 7c and d). LAMP was not seen in the azurophilic granules stained in Fig 7d for peroxidase. No label was seen in other organelles, plasma membrane, Golgi cisternae, or mitochondria. Negative controls were seen when the antibody was omitted or a normal mouse serum or X63 were used. In general, H4B4, the antibody for LAMP-2, was more suitable for immunolabeling than the other LAMP-1 antibodies. In the different maturational stages of neutrophils, at no time was LAMP seen in azurophilic granules or in the two other granule populations.
Electron micrograph of a frozen thin section of an immature neutrophil labeled for LAMP-2, using the MoAb H4B4. At lower magnification the cell can be identified as a promyelocyte (see b). The Golgi region of this cell can be seen at higher magnification in a. Most of the gold grains are present in vesicular structures (arrows). The vesicles (v) are concentrated near the Golgi complex (Gc) and the centriole (C). Note the absence of label on the plasma membrane (PM). Nucleus (Nu).
Electron micrograph of a frozen thin section of an immature neutrophil labeled for LAMP-2, using the MoAb H4B4. At lower magnification the cell can be identified as a promyelocyte (see b). The Golgi region of this cell can be seen at higher magnification in a. Most of the gold grains are present in vesicular structures (arrows). The vesicles (v) are concentrated near the Golgi complex (Gc) and the centriole (C). Note the absence of label on the plasma membrane (PM). Nucleus (Nu).
Electron micrograph of a frozen thin section of myelocytes immunolabeled for LAMP-2. In (a), large vesicles (V) and the MLC are labeled with GAM-10 (arrowheads). Note the absence of labeling in large Ag as well as in the Sg and PM. In inset, higher magnification of another multilaminar compartment positive for LAMP-2. (b) Two organelles contain label (arrowheads). The typical MVB are labeled for LAMP-2 as is the MLC. The membranes of the small granules (Sg) are not labeled nor is the Gc. Nucleus (Nu).
Electron micrograph of a frozen thin section of myelocytes immunolabeled for LAMP-2. In (a), large vesicles (V) and the MLC are labeled with GAM-10 (arrowheads). Note the absence of labeling in large Ag as well as in the Sg and PM. In inset, higher magnification of another multilaminar compartment positive for LAMP-2. (b) Two organelles contain label (arrowheads). The typical MVB are labeled for LAMP-2 as is the MLC. The membranes of the small granules (Sg) are not labeled nor is the Gc. Nucleus (Nu).
Electron micrograph of frozen thin section of blood neutrophils labeled with LAMP-2. (a) LAMP-2 (arrows) is found in small vesicles around the Gc. (b) In larger vesicular structures (V). (c) LAMP-2 is present in this MLC, and in (d) the MVB. The Ag are stained for myeloperoxidase and appear dense. Nucleus (Nu).
Electron micrograph of frozen thin section of blood neutrophils labeled with LAMP-2. (a) LAMP-2 (arrows) is found in small vesicles around the Gc. (b) In larger vesicular structures (V). (c) LAMP-2 is present in this MLC, and in (d) the MVB. The Ag are stained for myeloperoxidase and appear dense. Nucleus (Nu).
As mentioned above, the LAMP-positive organelles are vesicular in nature and their numbers vary with the stage of maturation as follows: (1) in promyelocytes and early myelocytes, small vesicles (50 to 150 nm) 16%; larger vesicles (150 to 500 nm) 83%; and rare MLC or typical MVB. (2) In late myelocytes and metamyelocytes, small vesicles (17%), larger vesicles (73%) and the appearance of MLC (9%), and typical MVB (1%). (3) In mature neutrophils, small vesicles (16%), larger vesicles (76%), MLC (3%), and typical MVB (4%). A total of 520 positive compartments were counted.
Double labeling.
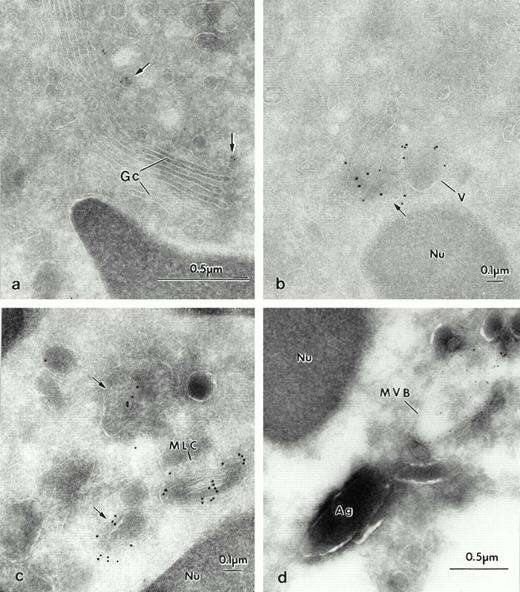
The differential localization of the specific probes was further investigated by double-labeling experiments (Fig 8). Double labeling with M6-P GP probe and LAMP-2 showed colocalization in the multilaminar compartment (Fig 8a, inset). Double labeling with a rabbit polyclonal antibody against lactoferrin clearly showed that there was no colocalization of lactoferrin (goat antirabbit-gold 5 nm) with the Man 6-P GP (streptavidin gold-10nm) (Fig 8a). The reverse labeling of lactoferrin with goat antirabbit-gold (10 nm) and Man 6-P GP with streptavidin gold (5 nm) confirmed this differential localization (not shown). Man 6-P GP did not colocalize with albumin (not shown). Double labeling with antibodies against lactoferrin and LAMP-2 (Fig 8b) and gelatinase and LAMP (Fig 8c) confirmed that the LAMP glycoproteins were in different compartments from the granules. Furthermore, albumin was found in vesicular structures other than those labeled for LAMP (Fig8d). We conclude that in mature intact cells, as predicted by the fractionation data, the major repository of Man 6-P GP is the azurophil granule, that of LAMP is the multilaminar compartment, and that both are found in the multilaminar compartment.
Portions of human neutrophils with double labeling to identify various organelles. (a) Double labeling with sCI-MPR–biotinylated and Lactoferrin 1/1,000. The specific granules are characterized by the presence of lactoferrin (lf), with the small gold-5 nm, (arrowhead), which does not colocalize with the Man 6-P GP, as visualized with streptavidin 10 nm (*) inside the azurophilic granules. Inset: the multilaminar compartment is double labeled for the Man 6-P GP, as observed with streptavidin 10 nm (*), and LAMP-2, gold-5 nm, (arrow). (b) Double labeling with LAMP-2 (arrows). Portions of human neutrophils, which demonstrate that LAMP-2 is localized in membranes of vesicular structures, and do not colocalize with lf, the marker for specific granules. The large gold (GAM-10) labels the location of LAMP-2 in vesicles (arrows), whereas the small gold (GAR-5), (arrowheads) detects lf. (c) LAMP-2 (GAM-5), (arrows) and gelatinase (gel), (GAR-10), (arrowheads) do not colocalize either. (d) Albumin (alb) (GAR-5), (arrowheads) is found in different vesicular structures from LAMP-2 (GAM-10), (arrows).
Portions of human neutrophils with double labeling to identify various organelles. (a) Double labeling with sCI-MPR–biotinylated and Lactoferrin 1/1,000. The specific granules are characterized by the presence of lactoferrin (lf), with the small gold-5 nm, (arrowhead), which does not colocalize with the Man 6-P GP, as visualized with streptavidin 10 nm (*) inside the azurophilic granules. Inset: the multilaminar compartment is double labeled for the Man 6-P GP, as observed with streptavidin 10 nm (*), and LAMP-2, gold-5 nm, (arrow). (b) Double labeling with LAMP-2 (arrows). Portions of human neutrophils, which demonstrate that LAMP-2 is localized in membranes of vesicular structures, and do not colocalize with lf, the marker for specific granules. The large gold (GAM-10) labels the location of LAMP-2 in vesicles (arrows), whereas the small gold (GAR-5), (arrowheads) detects lf. (c) LAMP-2 (GAM-5), (arrows) and gelatinase (gel), (GAR-10), (arrowheads) do not colocalize either. (d) Albumin (alb) (GAR-5), (arrowheads) is found in different vesicular structures from LAMP-2 (GAM-10), (arrows).
DISCUSSION
The goal of these studies was to acquire additional information on the composition of lysosomes of human neutrophil leukocytes. The significant new findings of this article are as follows: (1) The azurophil granules contain phosphorylated glycoproteins, which retain the Man 6-P recognition marker for several days. (2) Azurophilic granules, despite their high content of lysosomal enzymes, do not contain LAMPs. (3) A multilaminar structure (MLC) that had not been previously observed in neutrophilic leukocytes contains both Man 6-P GP and LAMP and appears early in the maturation of neutrophils, but its function is unknown.
Man 6-P GP
Detailed studies by other investigators42conducted mainly in fibroblasts, have shown the Man 6-P recognition marker as necessary for the sorting of newly synthesized acid hydrolases. Man 6-P–containing acid hydrolases bind to the Man 6-P receptors and are targeted from the secretory pathway to the lysosomal pathway. The Man 6-P is hydrolyzed to yield the dephosphorylated hydrolases found in mature lysosomes. In most cultured cells, Man 6-P recognition marker is a transient modification that is rapidly removed from lysosomal enzymes when they reach a compartment where Man 6-P is cleaved. For instance, kinetic data from mouse lymphoma cells showed that the Man 6-P recognition marker remained on lysosomal enzymes within the cells for only 1.4 hours.43 However, this is not universally the case, as in mouse L(Rec-) cells that lack the sCI-MPR, where the Man 6-P has a half-life of 19 hours.44 Supporting these in vitro results, a preliminary survey of mouse tissues using the sCI-MPR probe at the light level indicates that there are low steady state levels of proteins that retain the Man 6-P recognition marker in most cell types, but some cell types (eg, neurons and Sertoli cells) also contain relatively high levels.45
It has long been recognized that MPO is formed early in neutrophil maturation, during the promyelocyte stage, and that synthesis ceases when the cell enters the myelocyte stage of maturation. Since MPO is one of the major glycoproteins with Man 6-P, this is consistent with our observation that Man 6-P GP is present within the azurophilic granules of neutrophils from the early stages of maturation and is maintained even in the mature circulating cell. This means that proteins with the Man 6-P recognition markers are stored in the azurophilic granules for at least 14 days. This is much longer than has been noted in other cell types to date. The fact that the Man 6-P GP content is very high in azurophilic granules indicates that the conditions that allow for the removal of Man 6-P may not exist in these granules.
The sorting mechanism of lysosomal enzymes was initially demonstrated by the finding that fibroblasts from patients with I-cell disease (mucolipidosis) were unable to sort acid hydrolases because the cells were deficient in a phosphotransferase, an enzyme needed to add the Man 6-P recognition marker.42 However, this mechanism is not exclusively used by all cell types. For example, hepatocytes and leukocytes from patients with this deficiency successfully target enzymes to the lysosome.42 There is accumulating evidence for alternative lysosomal enzyme targeting pathways.46,47The sorting pathway of lysosomal enzymes in neutrophils is controversial. Nauseef et al,48 have shown that MPO in HL60 cells, a promyelocytic cell line, contains the Man 6-P marker, but there is no evidence that the Man 6-P receptor is involved in the MPO targeting to the azurophilic granules. Moreover, the defensins, other azurophilic granule proteins, lack the Man 6-P marker, hence, they cannot use this mechanism for sorting to the azurophilic granules.40,49,50 It is likely that the content of different granules reflects the profile of proteins synthesized and transported to the trans-Golgi network at the time of formation of the individual granules during different stages of maturation. This is supported by the finding that the heterogeneity of granules reflects the known order of biosynthesis of granule proteins,5,13and by the finding that the granule protein neutrophil gelatinase-associated lipocalin, which is found exclusively in specific granules of normal neutrophils51 will localize to azurophilic granules when expressed in promyelocytic HL60 cells.52
LAMP and Lysosomes
Combining immunolabeling with cell fractionation studies of mature blood neutrophils, we demonstrate that the typical membrane markers of lysosomes (LAMP-1 and LAMP-2) are confined to the MLC as well as to small vesicles and typical multivesicular bodies of human neutrophils, and are not present in the three major granule types. These data are in agreement with those of Dahlgren et al53 who also recently studied the distribution of LAMP in subcellular fractions of human neutrophils and concluded that LAMP-1 and LAMP-2 are present “in the specific granule-enriched fraction and in the light membrane fraction, but not in the azurophil granules.” Our direct analysis of the specific granule fraction itself clarifies the situation and shows that LAMPs are not present in specific granules. The only known azurophil granule membrane proteins are CD6310 and CD68 (see Table1). CD63, also referred to as granulophysin,11ME491,54 or LIMP-1C, has been identifed by Fukuda24 to be another lysosomal membrane marker, LAMP-324 because it shares a cytoplasmic Gly-Tyr motif essential for lysosomal trafficking during receptor-mediated endocytosis with LAMP-1 and -2. It differs, however, in that it is predicted to traverse the plasma membrane four times (the tetraspan family), unlike LAMP-1 or 2 which are typical type 1 transmembrane proteins. Vischer and Wagner55 recently showed the presence of CD63 in the membranes of Weibel-Palade bodies, which are the regulated secretory granules in endothelial cells. In both endothelial cells and neutrophils, granules containing CD63 can be relocated to the plasma membrane by activation,11 whereas in neutrophils, LAMP-1 and LAMP-2 are not translocated after activation (unpublished data). CD63 seems to be expressed mainly in hematopoietic and endothelial cells. Additionally, Saito et al12 have shown that CD68 is present on the membranes of azurophil granules in neutrophils. Less is known about CD68; it is a 110-kD transmembrane glycoprotein recently cloned by Holness and Simmons.56 It seems that CD68 is a member of a growing family of hematopoietic mucin-like molecules, including CD43, CD34, P-selectin glycoprotein ligand-1, and Gly CAM-1. CD68 can also be found in liver and kidney.
Although the phagocytic pathway of neutrophils has been clearly defined, it is less well-appreciated that neutrophils are also active in pinocytosis of soluble proteins and recycling of plasma membrane receptors. Multivesicular bodies have already been described as LAMP-positive in HL60.22 Multivesicular bodies are not easily seen in resting neutrophils that have not been exposed to chemoattractants, but in activated cells they are important and serve as sites of internalization of markers of fluid phase pinocytosis.57 Berger et al58 showed that multivesicular bodies from resting neutrophils express few LAMP vesicles, but they subsequently fuse with LAMP-positive structures, rendering them positive as well, and they then mature as prelysosomal compartments.58 In related studies, the uptake of eosinophil peroxidase into vesicular structures in neutrophils was observed by Zabucchi et al.59 Of more relevance, Borregaard et al16 showed that neutrophils contain endocytosed plasma proteins, particularly albumin, although this occurs mainly at the myelocyte stage of maturation.
It is likely that the special structures, the multilaminar compartment and the multivesicular bodies, are the prelysosomal structures of the neutrophils, and may serve in the early or late endosomal compartment. Mane et al22 showed a marked accumulation of LAMP on the membrane of phagocytic vacuoles containing opsonized erythrocytes in U937 cells, apparently delivered to these sites in multivesicular vacuoles. We also observed this in human neutrophils that have phagocytized bacteria (unpublished observation, January 1997). This means that MVB and MLC must fuse with the newly formed endosome because the vacuole containing degraded bacteria contains LAMPs. Finally, it should be mentioned that we did not observe “dense bodies” that have been referred to as mature lysosomes in most other cells.6 17
Azurophil Granules Correspond to Regulated Secretory Granules
The absence of LAMP-1 and LAMP-2 in azurophilic granule membranes suggests that, despite its high content of lysosomal enzymes, the neutrophil azurophilic granule has the characteristics of a regulated secretory granule membrane rather than being a true lysosome. This may also be true in other cell types such as the acrosomes of sperm cells,2 but this awaits further investigation. The lack of LAMP in azurophil granules suggests that these granules are not part of a dynamic endosomal lysosomal compartment, but behave more as regulated storage granules that are mobilized to the phagosome during ingestion of microorganisms.
The Multilaminar Compartment of the Neutrophils
This compartment has not been previously recognized in this cell type in Epon embedded material. However, this multilaminar compartment was previously observed in ultracryosections of neutrophils60and in the promyelocytic cell line HL60.22 Our data show that this compartment is present in BM neutrophils from intermediate to late stages of maturation, and in circulating neutrophils, but is most easily sampled in myelocytes. The compartment that contains the lysosomal membrane glycoproteins LAMP-1 and LAMP-2 and with Man 6-P recognition markers is morphologically similar to the prelysosomal compartment first observed in normal rat kidney cells by ultracryosectioning and believed to be part of the endocytic pathway. This compartment was found to be positive for the Man 6-P receptor.20,61 62
This compartment was also similar to the multilaminar compartment termed MII C63 characterized by the colocalization of the major histocompatibility class II (MHC II) protein, lysosomal hydrolases, and the lysosomal membrane glycoproteins, but lacking the CI-MPR. Gosselin et al64 found that stimulated neutrophils express MHC II molecules; however, using immunocytochemical methods, we were unable to detect sufficient labeling to draw any definite conclusions.
As mentioned previously, O'Brien et al45 using the same sCI-MPR probe at the light microscopic level on mouse tissues found that Man 6-P GP is abundant in vesicular structures of mouse testis and brain but was absent from most of the other tissues tested. Furthermore, the distribution of LAMP and Man 6-P GP was distinct. Also, malignant cells in a subset of human breast carcinomas exhibited high levels of Man 6-P GPs.39 This suggests that a special class of granules contains proteins which retain the Man 6-P recognition marker and may serve unique functions. Additional studies will be necessary to fully appreciate the meaning of this distribution in various cell types.
ACKNOWLEDGMENT
We thank Ivy Hsieh and Yvonne Jacques for the excellent technical assistance and David Geller for his help editing, and Silvia Molina for preparation of the manuscript.
Supported by Grants No. HLB-31610 and DK-10486 from the National Institutes of Health (Bethesda, MD); The Danish Medical Research Council; and The Danish Cancer Society, Copenhagen, Denmark.
Address reprint requests to D.F. Bainton, MD, University of California, San Francisco School of Medicine, San Francisco, CA 94143-0400.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.



![Fig. 3. Electron micrographs of frozen thin sections from human BM labeled by a biotinylated sCI-MPR probe. The Man 6-P GP are visualized with streptavidin 10 nm. (a) Early immature granulocytic cell of the BM. (b) The Golgi complex (GC) of an immature cell is labeled for Man 6-P GP. (c and d) In this immature neutrophil from BM (portion of the promyelocyte or an early myelocyte from [a]), the highest levels of Man 6-P GP are found within the newly formed azurophilic granules (Ag), some of which have retained their density (Ag′) as in (c), while others appear extracted (d) but retain immunolabel. At intermediate stages of maturation (myelocytes), an MLC is first observed (Inset, [d]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/3/10.1182_blood.v91.3.1044/3/m_blod4033603.jpeg?Expires=1765956291&Signature=gsmlbpFtoWuCTRgXzg-ZFstWThTTeI5mujv48thJnPkDsK2k10juevTGeB6HHTyZzHDzF5NQsR43jB01RGOv-cCV3bQYPqEhWg4ZzPlm~B4L1OxYuNuODgzuuI1G~icPJYI8vzaJs06ZRO2LCqyLXxD4du~tkCU9Rte68WfI4RB8N86aozUZiWfBcVqM~sdbuiaOEc7aF-O53KU8Xz31SB8fr21Q76pAa5ODQ2boEjFflTZoYlbt0LNLYIs9trbTuYDAaeQQYatL8PcGlWaZqn0CtszdYqy~fngvceszrOH8coyxU24TbTPjaYl3zftCS3tGy-~H8kiGKd4A6QyUSw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal