Abstract
Iron overload in Africa was previously regarded as purely due to excessive iron in traditional beer, but we recently found evidence that transferrin saturation and unsaturated iron binding capacity may be influenced by an interaction between dietary iron content and a gene distinct from any HLA-linked locus. To determine if serum ferritin follows a genetic pattern and to confirm our previous observations, we studied an additional 351 Zimbabweans and South Africans from 45 families ranging in size from two to 54 members. Iron status was characterized with repeated morning measurements of serum ferritin, transferrin saturation, and unsaturated iron binding capacity after supplementation with vitamin C. For each measure of iron status, segregation analysis was consistent with an interaction between a postulated iron-loading gene and dietary iron content (P < .01). In the most likely model, transferrin saturation is 75% and serum ferritin is 985 μg/L in a 40-year-old male heterozygote with an estimated beer consumption of 10,000 L, whereas the saturation is 36% and serum ferritin is 233 μg/L in an unaffected individual with identical age, sex, and beer consumption. This segregation analysis provides further evidence for a genetic influence on iron overload in Africans.
IRON OVERLOAD of a severity to cause damage to the liver is common among sub-Saharan Africans, with a prevalence in some communities that is over 10 times that of the homozygous state for HLA-linked hemochromatosis in populations derived from Europe.1 Although increased dietary iron from traditional home-brewed beer has been regarded as the sole cause of iron overload in Africa,2 we recently conducted a study of 36 Zimbabwean and Zambian pedigrees that suggested a gene, distinct from any gene linked to the HLA region on chromosome 6, may be implicated in the pathogenesis of this condition.3 In that study, likelihood analysis was used to test for an interaction between a gene (the hypothesized iron-loading locus) and an environmental factor (increased dietary iron) that would determine the levels of transferrin saturation and unsaturated iron binding capacity, two indirect measures of iron status.
Our original study3 had some potential limitations in the measurement of iron status: (1) Serum ferritin was not used in the genetic analysis. Because serum ferritin reflects body iron stores,4 this measure would be expected to display a genetic pattern in African iron overload as it does in HLA-linked hemochromatosis.5 (2) Single determinations of transferrin saturation and unsaturated iron binding capacity were made on blood samples obtained from nonfasting subjects at various times of the day rather than repeated determinations on specimens obtained in the morning from fasting subjects. Because serum iron has a diurnal variation,6,7 the transferrin saturation and unsaturated iron binding capacity may vary markedly according to the time of day. Furthermore, ingestion of an iron-rich food or beverage within 6 hours of venesection could conceivably increase transferrin saturation and decrease unsaturated iron binding capacity8,9 (3) The vitamin C status of the subjects was unknown, and they did not receive vitamin C supplementation before venesection. Serum iron and transferrin saturation may be inappropriately decreased and unsaturated iron binding capacity inappropriately increased in iron-loaded subjects with vitamin C deficiency.10 (4) The subjects were not asked to abstain from alcohol before venesection. Simultaneous ingestion (within 6 hours) of alcohol in the form of traditional beer might substantially increase the serum iron and transferrin saturation and decrease the unsaturated iron binding capacity because of the iron content of the beverage, as mentioned, and recent ingestion (within days) of substantial amounts of alcohol of any type might increase serum iron and transferrin saturation levels and decrease unsaturated iron binding capacity through bone marrow suppression and impaired folate metabolism.11 12
We now report the results of a study of 45 additional pedigrees that was designed to address the potential limitations of our original investigation. Iron status was measured in a detailed manner in these families, and serum ferritin was included in the segregation analysis.
SUBJECTS AND METHODS
Study sample.
Written informed consent was obtained from all study subjects. The sample consisted of 351 black Africans older than 12 years of age: 150 from five of Zimbabwe's 10 provinces and 201 from one of South Africa's nine provinces. The research subjects were predominantly rural dwellers of Shona, Swazi, and Shangaan ethnic origin, and none had European ancestry. We ascertained 21 pedigrees, ranging in size from six to 54 members and comprising a total of 271 subjects, through index subjects exhibiting iron overload (hepatocellular iron grade, 2 to 4)13 14 on diagnostic liver biopsy specimens. To maximize the sample size and to strengthen the estimates of the parameters of the genetic model and of the gene frequency, we included an additional 24 pedigrees, ranging in size from two to 13 members, that were not ascertained through index subjects with iron overload but comprised 70 subjects who were rural traditional beer drinkers. We visited the villages of the study participants and determined precise relationships within the pedigrees in interviews.
Estimation of traditional beer consumption.
Traditional beer was defined as a beverage that is brewed at home in nongalvanized iron drums and is known to have a high iron content.2 In 48 samples of this beverage collected from the subjects' households, the mean ± SD alcohol concentration was 3.2% ± 0.4% and the mean iron content was 46 ± 17 mg/L. Each subject was asked to estimate his or her consumption of traditional beer: the amount ingested on a typical day, the number of days in a typical month the beverage was consumed, the year the subject began drinking traditional beer, and if no longer drinking, the year he or she stopped. The estimate only provides a broad approximation of lifetime traditional beer consumption, because consumption was probably not uniform over time and information was obtained by recollection.
Collection of blood samples.
To confirm that elevated serum iron levels were not a sporadic event,15 venous blood samples were obtained on 3 separate days in 322 (92%) of the subjects. In the remaining 29 subjects, blood samples were collected on 1 or 2 days. The blood samples were obtained in the morning to avoid the effect of diurnal variation in serum iron.4,5 Samples were drawn from fasting individuals to avoid the transient increase in serum iron potentially seen after ingestion of an iron-rich meal.6 Because serum iron and serum ferritin may be inappropriately decreased in iron-loaded subjects with vitamin C deficiency,8 1.0 or 2.0 g vitamin C were given orally to each subject 24 hours before collection of the second and third blood samples. To minimize alcohol-related increases in serum iron and ferritin,9,10 16 the subjects were asked to abstain from drinking any alcoholic beverage for at least 24 hours before collection of the first blood sample and to continue to abstain throughout the study period.
Laboratory tests.
The serum iron level and total iron binding capacity were determined by methods modified from those recommended by the International Committee for Standardisation in Haematology.17,18 The serum ferritin level was measured using an enzyme immunoassay (Ramco, Houston, TX). Full blood cell counts (Coulter, Hialeah, FL), reticulocyte counts, and erythrocyte sedimentation rates (Westergren) were determined. Liver function tests were performed with a Cobas Bio autoanalyzer (Roche Diagnostic Systems, Montclair, NJ) using reagents from Roche Diagnostic Systems (Johannesburg, South Africa). Hepatitis B and hepatitis C markers were screened using enzyme immunoassay techniques (Abbott Laboratories, North Chicago, IL). Leukocyte ascorbic acid levels were determined by a method modified from Dennson and Bowers.19 20 Leukocyte ascorbic acid levels were determined on all 3 days in 298 of the subjects; in the remainder, samples were lost during transport or processing.
Indirect measures of iron status.
Transferrin saturation was calculated as the serum iron divided by the total iron binding capacity times 100, with a maximum value of 100%. Unsaturated iron binding capacity was calculated by subtracting the serum iron from the total iron binding capacity, with a minimum value of 0 μg/dL. Where possible, we averaged transferrin saturation, unsaturated iron binding capacity, and serum ferritin measurements obtained on 2 different days (days 2 and 3) following vitamin C supplementation. The ratio of serum ferritin to aspartate aminotransferase (AST) was calculated by dividing the serum ferritin on day 1 by the AST measured on day 1, with the minimum value for AST set at the upper limit of normal of our assays (30 to 35 IU/L) and a minimum value for the ratio set at 1.0.
Statistical analysis.
The effect of vitamin C supplementation on leukocyte ascorbic acid levels and on indirect serum measures of iron status was examined with repeated-measures analysis of variance. In subjects other than index cases, variables were compared according to whether traditional beer was consumed by the Student t-test, Mann-Whitney Utest, or Fisher's exact test. Measures of iron status were compared according to whether subjects were first-degree relatives of index cases using analysis of variance with adjustment for age, sex, and estimate of lifetime traditional beer consumption. Values for ferritin, the ratio of ferritin to AST, and estimated traditional beer consumption were logarithmically transformed for the analysis of variance procedures.
Segregation analysis of families.
We eliminated trait values for 16 individuals including seven probands because anemia (hemoglobin <13 g/dL for men and <12 g/dL for women)21,22 could not be excluded as the cause of elevations in transferrin saturation and the ratio of ferritin to AST. We followed the example of an assessment of iron nutritional status in the US population23 and regarded an increased serum ferritin as greater than 150 μg/L in women 20 to 44 years of age, greater than 200 μg/L in men aged 20 to 44 and women aged 45 to 65 years, greater than 300 μg/L in men aged 45 to 64 and women over 64 years, and greater than 400 μg/L in men aged over 64 years. The corresponding elevated ferritin to AST ratio would be the indicated serum ferritin divided by a minimum AST of 30 or 35. In a conservative application of the definition of iron overload recommended by Dr C.A. Finch24 and used in the US nutritional survey, we designated as affected the 21 probands and 25 other individuals with a high serum ferritin and a transferrin saturation greater than 80%. We designated as unaffected 50 individuals who had consumed at least 1,000 L homemade beer yet had a serum ferritin that was not elevated and a transferrin saturation less than 42%, or who had consumed at least 10,000 L beer and had a serum ferritin that was not elevated. A transferrin saturation of 42% is about 2 standard deviations above the mean for African-Americans in the second National Health and Nutrition Examination Survey.23 Adult/postmenopausal age was computed as the years exceeding 20 for men and the years exceeding 50 for women. Trait values for three individuals were eliminated because they had serum ferritin levels over 10,000 μg/L, extreme elevations that would more likely reflect hepatocellular necrosis or secretion by a tumor rather than body iron stores.25 Lifetime beer consumption, serum ferritin, and the ferritin to AST ratio were each natural logarithmically transformed because of positive skew. Transferrin saturation and unsaturated iron binding capacity were eliminated in 12 individuals because of a serum ferritin greater than 400 μg/L and a ferritin to AST ratio less than 11.4 μg/IU; with the combination of an elevated ferritin and a normal ratio, an elevated transferrin saturation may represent hepatocellular damage rather than iron stores.
We applied segregation analysis to transferrin saturation, unsaturated iron binding capacity, serum ferritin, and the ferritin to AST ratio. Each segregation analysis was a bivariate analysis of a quantitative trait measured on 332 individuals and affection status designated on 96 individuals. Likelihoods26 of the genetic model were computed using the Pedigree Analysis Package27 and the maxima obtained with Non-linear Programming: Systems Optimization Laboratory (NPSOL).28 Correction was made for the ascertainment of each of 21 pedigrees selected through a proband with increased hepatic iron by dividing the likelihood by the probability of affection for each proband. Tests of hypotheses compared the maximized likelihood of a general model with the maximized likelihood of a submodel formed by restricting parameters. Negative two multiplied by the natural logarithm of the ratio of the submodel likelihood to the general model likelihood approximates a chi-square distribution if certain assumptions hold. Briefly, the approximation requires a large number of independent and identically distributed observations. The degrees of freedom for the chi-squared test equal the difference in the number of parameters estimated in the general model and the submodel.
We assumed that a single major locus with two alleles determined both affection status and the quantitative trait. The prevalence of affection was fixed at 0.059. This prevalence is based on our previous community survey in rural Zimbabwe and is the average of the proportion of iron overload in men and in women.29 A recent survey of iron status in a South African population gives a similar estimated prevalence of iron overload of 0.05.30 Affection status was assumed to reflect an underlying quantitative liability scale; the difference between mean values for the two homozygotes was fixed at 10 within-genotype standard deviations. Each quantitative trait was transformed as part of the analysis using power transformation.31 Natural logarithmically transformed lifetime beer consumption and adult/postmenopausal age were each assumed to have a linear effect on the mean transformed quantitative trait level. Genotype-specific effects were assumed for beer consumption, and a single effect was assumed for age. The liability scale and each quantitative trait, after transformation and correction for lifetime beer consumption and age, were each assumed to be distributed as a mixture of normal densities with the mixture proportions equal to the genotype frequencies. Within major locus genotypes, the variation was attributed to polygenes and random environmental effects specific to the individual; we assumed a within-genotype correlation of .9 between the quantitative trait and affection status. Differences between the present analysis and our previuos study3 include the following: traditional beer consumption modeled as a continuous rather than a categorical variable, the designation of affection status in certain individuals, and fixing the prevalence of affection at 5.9%.
The parameters of the model included allele frequency (q), transmission probabilities (τ1, τ2, and τ3), mean level of the trait (μ1, μ2, and μ3), within-genotype standard deviation of the trait (ς), effect on the trait of beer consumption by genotype (b1, b2, and b3), effect on the trait of age (a), location and scale parameters of the transformation (L and S), polygenic heritability of the trait (ha2). Transmission probability τi represents the probability that a parent of genotype i transmits allele 1, where genotypes i = 1, 2, and 3 correspond to homozygotes lacking the iron-loading allele, heterozygotes, and homozygotes with the iron-loading allele, respectively. Polygenic heritability ht2 and ha2 represents the proportion of within-genotype variance attributed to polygenes. The likelihood of the model was approximated.32
Major locus inheritance was inferred by rejecting the hypotheses of no major locus and of environmental nontransmission, but not rejecting the hypothesis of Mendelian transmission. The hypothesis of no major locus (q = 0) was tested by comparing the likelihood maximized fixing q = 0, μ1 = μ2 = μ3, b1 = b2 = b3, d = 0, τ1 = 1, τ2 = ½, τ3 = 0 to the likelihood maximized with τ1 = 1, τ2 = ½, τ3 = 0. The hypothesis of environmental nontransmission (1-q = τ1 = τ2 = τ3) was tested by comparing the likelihood maximized fixing 1-q = τ1 = τ2 = τ3 to the likelihood maximized with no constraints. The hypothesis of Mendelian transmission (τ1 = 1, τ2 = ½, τ3 = 0) was tested by comparing the likelihood maximized fixing τ1 = 1, τ2 = ½, τ3 = 0 to the likelihood maximized with no constraints.
The mode of inheritance was inferred by rejecting either the hypothesis of recessive inheritance, the hypothesis of dominant inheritance, or both. The hypothesis of recessive inheritance (μ1 = μ2, b1 = b2, d = 0) was tested by comparing the likelihood maximized fixing μ1 = μ2, b1 = b2, d = 0, τ1 = 1, τ2 = ½, τ3 = 0 to the likelihood maximized with τ1 = 1, τ2 = ½, τ3 = 0. The hypothesis of dominant inheritance (μ2 = μ3, b2 = b3, d = 1) was tested by comparing the likelihood maximized fixing μ2 = μ3, b2 = b3, d = 1, τ1 = 1, τ2 = ½, τ3 = 0 to the likelihood maximized with τ1 = 1, τ2 = ½, τ3 = 0.
RESULTS
Administration of vitamin C led to significant increases in leukocyte ascorbic acid levels, but had no effect on indirect measures of iron status (Table 1). Table2 summarizes the clinical characteristics of 21 index subjects who were chosen because of a liver biopsy demonstrating grade 2+ to 4+ hepatocellular iron. These patients had markedly abnormal values for the indirect measures of iron status, and they had some evidence of hepatic dysfunction. Table3 presents the clinical characteristics of other study subjects according to whether there was any history of traditional beer consumption. The indirect measures of iron status were significantly different between drinkers and nondrinkers. Table4 presents iron measures according to whether the research subjects were first-degree relatives of the index cases with liver biopsy-proven iron overload. The mean values for serum ferritin and the ratio of ferritin to AST were significantly higher in first-degree relatives, and the transferrin saturation tended to be higher and unsaturated iron binding capacity lower. Similar to our previous study,3 the frequency distributions of transferrin saturation and unsaturated iron binding capacity in males were bimodal in the presence of increased dietary iron. In contrast to our previous study,3 these frequency distributions were not obviously bimodal for women in the presence of increased dietary iron.
Effect of Vitamin C Supplementation on Leukocyte Ascorbic Acid Levels and Indirect Serum Measurements of Iron Status
| Parameter . | No. of Subjects . | Day 1 . | Day 2 . | Day 3 . | F . | P . |
|---|---|---|---|---|---|---|
| Ascorbic acid (μg/108 leukocytes, mean ± SD) | 298 | 26.8 ± 14.8 | 30.8 ± 16.3 | 32.0 ± 16.9 | 43.0 | <.001 |
| Transferrin saturation (%, mean ± SD) | 322 | 45 ± 25 | 45 ± 26 | 45 ± 26 | 0.3 | .58 |
| Unsaturated iron binding capacity (μg/dL, mean ± SD) | 322 | 166 ± 92 | 168 ± 96 | 163 ± 93 | 0.9 | .34 |
| Serum ferritin (μg/L, geometric mean and SD range) | 322 | 211 (35-1,279) | 214 (37-1,245) | 212 (36-1,253) | 0.2 | .78 |
| Parameter . | No. of Subjects . | Day 1 . | Day 2 . | Day 3 . | F . | P . |
|---|---|---|---|---|---|---|
| Ascorbic acid (μg/108 leukocytes, mean ± SD) | 298 | 26.8 ± 14.8 | 30.8 ± 16.3 | 32.0 ± 16.9 | 43.0 | <.001 |
| Transferrin saturation (%, mean ± SD) | 322 | 45 ± 25 | 45 ± 26 | 45 ± 26 | 0.3 | .58 |
| Unsaturated iron binding capacity (μg/dL, mean ± SD) | 322 | 166 ± 92 | 168 ± 96 | 163 ± 93 | 0.9 | .34 |
| Serum ferritin (μg/L, geometric mean and SD range) | 322 | 211 (35-1,279) | 214 (37-1,245) | 212 (36-1,253) | 0.2 | .78 |
One to 2 g vitamin C was administered orally 24 hours before the day 2 blood sample and 24 hours before the day 3 sample were collected. Statistical analysis was performed with repeated-measures analysis of variance.
Clinical Characteristics of 21 Index Subjects With Iron Overload
| Characteristic . | Result . | Normal Range . |
|---|---|---|
| Age (yr, mean ± SD) | 64 ± 12 | |
| Sex (male:female) | 15:6 | |
| Estimated traditional beer consumption (L, median and range)* | 19,200 (6,048- 186,150) | |
| Hemoglobin (g/dL, mean ± SD) | 13.3 ± 1.5 | 12.0-18.0 |
| Erythrocyte sedimentation rate (mm/h, mean ± SD) | 52 ± 29 | 0-20 |
| Aspartate aminotransferase (IU/L, median and range) | 64 (14-105) | 10-30 |
| Alanine aminotransferase (IU/L, median and range) | 40 (7-172) | 6-37 |
| Gamma-glutamyl transpeptidase (IU/L, median and range) | 244 (31-1,836) | 5-35 |
| Transferrin saturation (%, mean ± SD) | 90 ± 18 | 20-50 |
| Unsaturated iron binding capacity (μg/dL, mean ± SD) | 22 ± 38 | 125-320 |
| Ferritin (μg/L, median and range) | 3,745 (1,170->10,000) | 20-400 |
| Ferritin to AST ratio (μg/IU, median and range) | 65.5 (7.8-127.3) | 0.7-11.4 |
| Characteristic . | Result . | Normal Range . |
|---|---|---|
| Age (yr, mean ± SD) | 64 ± 12 | |
| Sex (male:female) | 15:6 | |
| Estimated traditional beer consumption (L, median and range)* | 19,200 (6,048- 186,150) | |
| Hemoglobin (g/dL, mean ± SD) | 13.3 ± 1.5 | 12.0-18.0 |
| Erythrocyte sedimentation rate (mm/h, mean ± SD) | 52 ± 29 | 0-20 |
| Aspartate aminotransferase (IU/L, median and range) | 64 (14-105) | 10-30 |
| Alanine aminotransferase (IU/L, median and range) | 40 (7-172) | 6-37 |
| Gamma-glutamyl transpeptidase (IU/L, median and range) | 244 (31-1,836) | 5-35 |
| Transferrin saturation (%, mean ± SD) | 90 ± 18 | 20-50 |
| Unsaturated iron binding capacity (μg/dL, mean ± SD) | 22 ± 38 | 125-320 |
| Ferritin (μg/L, median and range) | 3,745 (1,170->10,000) | 20-400 |
| Ferritin to AST ratio (μg/IU, median and range) | 65.5 (7.8-127.3) | 0.7-11.4 |
Clinical Characteristics in Subjects Other Than Index Cases According to History of Traditional Beer Consumption
| Characteristic . | Traditional Beer Consumption . | P . | |
|---|---|---|---|
| No (n = 129) . | Yes (n = 201) . | ||
| Age (yr, mean ± SD) | 35 ± 15 | 54 ± 16 | <.0005 |
| Sex (male:female) | 36:93 | 111:90 | <.0005 |
| Estimated traditional beer consumption (L, median and range) | 0 | 9,600 (7-148,920) | — |
| Hemoglobin (g/dL, mean ± SD) | 13.4 ± 1.7 | 14.0 ± 1.4* | .003 |
| Erythrocyte sedimentation rate (mm/h, mean ± SD) | 37 ± 29† | 32 ± 29* | .1 |
| Aspartate aminotransferase (IU/L, median and range) | 24 (7-323)‡ | 28 (7-274)2-153 | <.0005 |
| Alanine aminotransferase (IU/L, mean ± SD) | 16 (4-169)2-155 | 21 (4-124)* | <.0005 |
| Gamma glutamyl transpeptidase (IU/L, mean ± SD) | 21 (4-168)‡ | 33 (3-1,086)¶ | <.0005 |
| Transferrin saturation (%, mean ± SD) | 28 ± 11 | 50 ± 24 | <.0005 |
| Unsaturated iron binding capacity (μg/dL, mean ± SD) | 240 ± 67 | 139 ± 77 | <.0005 |
| Ferritin (μg/L, median and range) | 49 (2-640) | 415 (5->10,000) | <.0005 |
| Ferritin to AST ratio (μg/IU, median and range) | 1.4‡ (0.02-9.8) | 11.6 (0.2-149.0) | <.0005 |
| Characteristic . | Traditional Beer Consumption . | P . | |
|---|---|---|---|
| No (n = 129) . | Yes (n = 201) . | ||
| Age (yr, mean ± SD) | 35 ± 15 | 54 ± 16 | <.0005 |
| Sex (male:female) | 36:93 | 111:90 | <.0005 |
| Estimated traditional beer consumption (L, median and range) | 0 | 9,600 (7-148,920) | — |
| Hemoglobin (g/dL, mean ± SD) | 13.4 ± 1.7 | 14.0 ± 1.4* | .003 |
| Erythrocyte sedimentation rate (mm/h, mean ± SD) | 37 ± 29† | 32 ± 29* | .1 |
| Aspartate aminotransferase (IU/L, median and range) | 24 (7-323)‡ | 28 (7-274)2-153 | <.0005 |
| Alanine aminotransferase (IU/L, mean ± SD) | 16 (4-169)2-155 | 21 (4-124)* | <.0005 |
| Gamma glutamyl transpeptidase (IU/L, mean ± SD) | 21 (4-168)‡ | 33 (3-1,086)¶ | <.0005 |
| Transferrin saturation (%, mean ± SD) | 28 ± 11 | 50 ± 24 | <.0005 |
| Unsaturated iron binding capacity (μg/dL, mean ± SD) | 240 ± 67 | 139 ± 77 | <.0005 |
| Ferritin (μg/L, median and range) | 49 (2-640) | 415 (5->10,000) | <.0005 |
| Ferritin to AST ratio (μg/IU, median and range) | 1.4‡ (0.02-9.8) | 11.6 (0.2-149.0) | <.0005 |
*n = 197.
n = 127.
n = 128.
n = 200.
n = 129.
¶n = 199.
Iron Measures in First-Degree Relatives of Index Subjects Compared With Other Subjects
| Measure . | First-Degree Relatives (n = 108) . | Other Research Subjects (n = 222) . | P . |
|---|---|---|---|
| Transferrin saturation (%, mean ± SE) | 44 ± 2 | 40 ± 1 | .067 |
| Unsaturated iron binding capacity (μg/dL, mean ± SE) | 170 ± 6 | 184 ± 4 | .066 |
| Ferritin (μg/L, geometric mean and SE range) | 220 (199-243) | 148 (137-158) | .001 |
| Ferritin to AST ratio (μg/IU, geometric mean and SE range) | 6.1 (5.5-6.8) | 4.03-150 (3.7-4.3) | .001 |
| Measure . | First-Degree Relatives (n = 108) . | Other Research Subjects (n = 222) . | P . |
|---|---|---|---|
| Transferrin saturation (%, mean ± SE) | 44 ± 2 | 40 ± 1 | .067 |
| Unsaturated iron binding capacity (μg/dL, mean ± SE) | 170 ± 6 | 184 ± 4 | .066 |
| Ferritin (μg/L, geometric mean and SE range) | 220 (199-243) | 148 (137-158) | .001 |
| Ferritin to AST ratio (μg/IU, geometric mean and SE range) | 6.1 (5.5-6.8) | 4.03-150 (3.7-4.3) | .001 |
The analysis is adjusted for age, sex, and estimated lifetime traditional beer consumption.
n = 221.
Table 5 shows that for each measure of iron status, segregation analysis was consistent with an interaction between a postulated iron-loading gene and dietary iron content (P < .01). We inferred major locus inheritance by rejecting the hypotheses of no major locus and of environmental nontransmission while failing to reject the hypothesis of Mendelian transmission for all four traits.
Chi-Square Statistics Testing the Major Locus Hypotheses for Each Trait
| Hypothesis . | Transferrin Saturation . | Unsaturated Iron Binding Capacity . | Serum Ferritin . | Ferritin to AST Ratio . |
|---|---|---|---|---|
| No major locus* | 40.194-153 | 40.734-153 | 72.624-153 | 31.714-153 |
| Mendelian inheritance4-151 | 5.57 | 4.79 | 1.72 | 0.16 |
| Environmental nontransmission4-151 | 12.90‡ | 15.93‡ | 69.674-153 | 12.04‡ |
| Hypothesis . | Transferrin Saturation . | Unsaturated Iron Binding Capacity . | Serum Ferritin . | Ferritin to AST Ratio . |
|---|---|---|---|---|
| No major locus* | 40.194-153 | 40.734-153 | 72.624-153 | 31.714-153 |
| Mendelian inheritance4-151 | 5.57 | 4.79 | 1.72 | 0.16 |
| Environmental nontransmission4-151 | 12.90‡ | 15.93‡ | 69.674-153 | 12.04‡ |
*6 df.
3 df.
P < .01.
P < .001.
Table 6 shows that we inferred dominant inheritance for transferrin saturation, unsaturated iron binding capacity, serum ferritin, and the ferritin to AST ratio by rejecting recessive but not dominant inheritance. All four traits showed nonsignificant evidence for different parameter values in homozygotes versus heterozygotes for the iron-loading allele. This finding could result either because homozygotes do not have more severe iron-loading than heterozygotes or because the sample includes too few homozygotes to allow us to detect a difference.
Chi-Square Statistics Testing Mode of Inheritance for Each Trait
| Hypothesis* . | Transferrin Saturation . | Unsaturated Iron Binding Capacity . | Serum Ferritin . | Ferritin to AST Ratio . |
|---|---|---|---|---|
| Recessive | 14.245-151 | 12.535-151 | 12.545-151 | 12.385-151 |
| Dominant | 1.62 | 4.76 | 1.52 | 0.02 |
| Hypothesis* . | Transferrin Saturation . | Unsaturated Iron Binding Capacity . | Serum Ferritin . | Ferritin to AST Ratio . |
|---|---|---|---|---|
| Recessive | 14.245-151 | 12.535-151 | 12.545-151 | 12.385-151 |
| Dominant | 1.62 | 4.76 | 1.52 | 0.02 |
*3 df.
P < .01.
Table 7 presents the estimated mean values for each measure by genotype, age, and lifetime beer consumption for men and women. Only normal homozygotes and heterozygotes are included because of the lack of support for homozygotes for the iron-loading allele, as shown in Table 6. Nondrinking heterozygotes, although having marginally higher iron status than homozygotes, do not qualify as iron-loaded. As little as 1,000 L traditional beer results in fourfold differences in serum ferritin and the ratio of ferritin to AST between the genotypes. Similarly, 1,000 L or more of traditional beer consumption leads to marked elevations in transferrin saturation and reductions in unsaturated iron binding capacity in heterozygotes compared with nonaffected homozygotes.
Estimated Trait Means by Age and by Lifetime Beer Consumption for Normal Homozygotes (genotype 1) and Heterozygotes (genotype 2) for Dominant Inheritance
| Age (yr) . | Beer (L) . | Saturation (%) . | UIBC (μg/dL) . | Serum Ferritin (μg/L) . | Ferritin to AST Ratio (μg/IU) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men . | Women . | 1 . | 2 . | 1 . | 2 . | 1 . | 2 . | 1 . | 2 . | |
| 20 | ≤50 | 0 | 25 | 29 | 254 | 230 | 44 | 46 | 1.4 | 1.4 |
| 20 | ≤50 | 1,000 | 31 | 59 | 205 | 100 | 109 | 347 | 2.5 | 10.7 |
| 20 | ≤50 | 10,000 | 33 | 71 | 190 | 66 | 145 | 641 | 3.2 | 21.8 |
| 20 | ≤50 | 100,000 | 35 | 83 | 175 | 36 | 192 | 1,158 | 4.3 | 35.4 |
| 40 | 70 | 0 | 27 | 31 | 232 | 209 | 74 | 77 | 1.9 | 1.9 |
| 40 | 70 | 1,000 | 33 | 63 | 185 | 85 | 177 | 542 | 3.9 | 18.0 |
| 40 | 70 | 10,000 | 36 | 75 | 170 | 53 | 233 | 985 | 5.4 | 30.7 |
| 40 | 70 | 100,000 | 38 | 87 | 156 | 25 | 306 | 1,754 | 8.6 | 46.5 |
| 60 | 90 | 0 | 30 | 34 | 211 | 189 | 122 | 126 | 2.7 | 2.7 |
| 60 | 90 | 1,000 | 36 | 66 | 166 | 71 | 282 | 836 | 7.1 | 26.4 |
| 60 | 90 | 10,000 | 39 | 78 | 152 | 41 | 369 | 1,497 | 11.2 | 41.1 |
| 60 | 90 | 100,000 | 41 | 91 | 139 | 15 | 480 | 2,634 | 15.7 | 59.1 |
| 80 | 0 | 33 | 37 | 190 | 170 | 197 | 204 | 4.1 | 4.1 | |
| 80 | 1,000 | 39 | 70 | 148 | 58 | 443 | 1,276 | 13.9 | 36.1 | |
| 80 | 10,000 | 42 | 82 | 135 | 29 | 575 | 2,255 | 18.6 | 53.1 | |
| 80 | 100,000 | 44 | 94 | 122 | 5 | 742 | 3,923 | 23.7 | 73.6 | |
| Age (yr) . | Beer (L) . | Saturation (%) . | UIBC (μg/dL) . | Serum Ferritin (μg/L) . | Ferritin to AST Ratio (μg/IU) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men . | Women . | 1 . | 2 . | 1 . | 2 . | 1 . | 2 . | 1 . | 2 . | |
| 20 | ≤50 | 0 | 25 | 29 | 254 | 230 | 44 | 46 | 1.4 | 1.4 |
| 20 | ≤50 | 1,000 | 31 | 59 | 205 | 100 | 109 | 347 | 2.5 | 10.7 |
| 20 | ≤50 | 10,000 | 33 | 71 | 190 | 66 | 145 | 641 | 3.2 | 21.8 |
| 20 | ≤50 | 100,000 | 35 | 83 | 175 | 36 | 192 | 1,158 | 4.3 | 35.4 |
| 40 | 70 | 0 | 27 | 31 | 232 | 209 | 74 | 77 | 1.9 | 1.9 |
| 40 | 70 | 1,000 | 33 | 63 | 185 | 85 | 177 | 542 | 3.9 | 18.0 |
| 40 | 70 | 10,000 | 36 | 75 | 170 | 53 | 233 | 985 | 5.4 | 30.7 |
| 40 | 70 | 100,000 | 38 | 87 | 156 | 25 | 306 | 1,754 | 8.6 | 46.5 |
| 60 | 90 | 0 | 30 | 34 | 211 | 189 | 122 | 126 | 2.7 | 2.7 |
| 60 | 90 | 1,000 | 36 | 66 | 166 | 71 | 282 | 836 | 7.1 | 26.4 |
| 60 | 90 | 10,000 | 39 | 78 | 152 | 41 | 369 | 1,497 | 11.2 | 41.1 |
| 60 | 90 | 100,000 | 41 | 91 | 139 | 15 | 480 | 2,634 | 15.7 | 59.1 |
| 80 | 0 | 33 | 37 | 190 | 170 | 197 | 204 | 4.1 | 4.1 | |
| 80 | 1,000 | 39 | 70 | 148 | 58 | 443 | 1,276 | 13.9 | 36.1 | |
| 80 | 10,000 | 42 | 82 | 135 | 29 | 575 | 2,255 | 18.6 | 53.1 | |
| 80 | 100,000 | 44 | 94 | 122 | 5 | 742 | 3,923 | 23.7 | 73.6 | |
Table 8 shows that the estimates of the iron-loading allele frequency (p) ranged from 0.029 to 0.050. When estimates of the frequencies of heterozygotes and homozygotes are calculated from allele frequencies by the Hardy-Weinberg equation, the summed frequency of heterozygotes (2pq or 2p[1-p]) and of homozygotes (p2) exceeds the assumed prevalence of 0.059 for transferrin saturation and unsaturated iron binding capacity. The segregation analysis permits different allele frequency estimates (p) from the assumed prevalence (2pq + p2) because affection status was assigned in only 28% of the subjects studied; in the remainder, the probability of affection was estimated. The small number of independent individuals in our pedigree samples does not allow good frequency estimation of the iron-overload allele.
Estimate (mean ± SE) of Allele Frequency for Each Trait
| . | Transferrin Saturation . | Unsaturated Iron Binding Capacity . | Serum Ferritin . | Ferritin to AST Ratio . |
|---|---|---|---|---|
| Estimate of allele frequency (p) from the present analysis, autosomal dominant model | 0.050 ± 0.017 | 0.034 ± 0.006 | 0.029 ± 0.003 | 0.030 ± 0.001 |
| Calculated frequency of heterozygotes (2pq or 2p[1-p]) | 0.095 | 0.066 | 0.056 | 0.058 |
| Calculated frequency of homozygotes (p2) | 0.003 | 0.001 | 0.001 | 0.001 |
| Summed frequency of heterozygotes and homozygotes | 0.097 | 0.067 | 0.057 | 0.059 |
| . | Transferrin Saturation . | Unsaturated Iron Binding Capacity . | Serum Ferritin . | Ferritin to AST Ratio . |
|---|---|---|---|---|
| Estimate of allele frequency (p) from the present analysis, autosomal dominant model | 0.050 ± 0.017 | 0.034 ± 0.006 | 0.029 ± 0.003 | 0.030 ± 0.001 |
| Calculated frequency of heterozygotes (2pq or 2p[1-p]) | 0.095 | 0.066 | 0.056 | 0.058 |
| Calculated frequency of homozygotes (p2) | 0.003 | 0.001 | 0.001 | 0.001 |
| Summed frequency of heterozygotes and homozygotes | 0.097 | 0.067 | 0.057 | 0.059 |
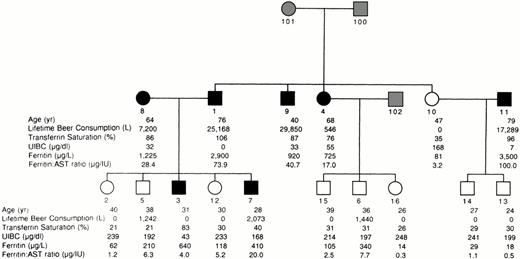
Pedigree Z-34 is shown in Fig 1. Purely environmental causation of high iron levels is refuted by relatively normal values for transferrin saturation and ferritin for individual no. 5 despite substantial beer consumption. Individual no. 3 is the only member of this pedigree to express iron overload in the absence of beer consumption. He may be a homozygote for the iron-loading allele that our previous analysis3 suggests leads to iron loading in the absence of beer consumption, but more individuals are needed to confirm this. The high frequency of the disorder is supported by the presence of two spouses of pedigree members with iron overload.
Pedigree Z-34. Filled symbols designate iron overload defined from liver biopsy (individual no. 1), high transferrin saturation and ferritin (individuals no. 8, 9, and 11), a probability >90% of heterozygosity in the analyses of transferrin saturation and unsaturated iron binding capacity (individual no. 4), or a probability >70% in the analyses of ferritin and the ferritin to AST ratio (individuals no. 3, 4, and 7). Open symbols designate studied individuals for whom iron overload criteria were not met. Shaded symbols designate unstudied individuals. Individual no. 5 was assigned unaffected status in the analysis because of low transferrin saturation and ferritin to AST ratio despite consuming >1,000 L beer. Individual no. 6 had a probability >90% of being a normal homozygote in the analyses of transferrin saturation and unsaturated iron binding capacity.
Pedigree Z-34. Filled symbols designate iron overload defined from liver biopsy (individual no. 1), high transferrin saturation and ferritin (individuals no. 8, 9, and 11), a probability >90% of heterozygosity in the analyses of transferrin saturation and unsaturated iron binding capacity (individual no. 4), or a probability >70% in the analyses of ferritin and the ferritin to AST ratio (individuals no. 3, 4, and 7). Open symbols designate studied individuals for whom iron overload criteria were not met. Shaded symbols designate unstudied individuals. Individual no. 5 was assigned unaffected status in the analysis because of low transferrin saturation and ferritin to AST ratio despite consuming >1,000 L beer. Individual no. 6 had a probability >90% of being a normal homozygote in the analyses of transferrin saturation and unsaturated iron binding capacity.
DISCUSSION
This segregation analysis of African pedigrees provides evidence that in some individuals exposed to increased dietary iron, a genetic defect allows an elevation in serum ferritin and in transferrin saturation and a decrease in unsaturated iron binding capacity. The present findings extend the results of our original genetic analysis of African pedigrees,3 and are especially important because the present study was specifically designed to address potential shortcomings of our initial investigation. In particular, the alterations in serum ferritin, transferrin saturation, and unsaturated iron binding capacity observed in these pedigrees do not appear to be explainable by purely environmental effects. Furthermore, these indirect measures of iron status do not appear to be unduly influenced by diurnal variations or by the presence of ascorbic acid deficiency or anemia. According to the most likely model, a 40-year-old male heterozygote for the postulated iron-loading locus with an estimated beer consumption of 10,000 L would have a transferrin saturation of 75% and a serum ferritin of 985 μg/L, as compared with a transferrin saturation of 36% and a serum ferritin of 233 μg/L in an unaffected individual of identical age, sex, and beer consumption (Table 7).
Because the chief source of iron in these subjects is a traditional beverage that contains alcohol,2 it was important to consider that changes in serum iron measures resulting from alcohol ingestion11,12 may have affected our results. This potential confounding factor was addressed in two ways: (1) the subjects were asked to refrain from alcohol ingestion for a minimum period of 24 hours before the first venesection and to continue to abstain throughout the study period, and (2) the ratio of ferritin to AST was calculated. This index has been shown to reflect hepatic iron stores in the setting of acute alcohol consumption, shortly after stopping consumption, and after prolonged abstention from alcohol.33 34
In our previous study,3 we inferred recessive inheritance of the iron-loading gene in the absence of increased dietary iron and dominant inheritance in the presence of increased dietary iron. The present set of pedigrees may not include any homozygotes for the iron-loading allele, leading to the inference of dominant inheritance in this analysis. The sample sizes in both studies were too small to make confident statements regarding the iron status of homozygotes for the hypothesized iron-loading locus. In addition, the performance of the analysis required that assumptions be made about the relationships among age, beer consumption, and iron measures. The conclusions are dependent on the assumptions.
Iron overload in Africans was first reported by A.S. Strachan, who conducted an autopsy series of blacks from across southern and central Africa and who died in Johannesburg, South Africa, in the 1920s.35 Professor Strachan found a high prevalence of iron overload: 19% of the subjects had “very marked to marked” iron deposition in the liver and in the spleen. Iron overload in southern African blacks was thought initially to be the result of some metabolic defect induced by chronic malnutrition,36 but the dietary intake of iron was subsequently shown to be very high, with most of the iron derived from the iron drums and cans used for brewing traditional alcoholic beverages.2 The histologic findings were distinctive, with the bulk of the iron being deposited in hepatocytes and in macrophages in most subjects.37 Several direct and indirect sequelae were documented. Direct sequelae included micronodular cirrhosis38-41 and diabetes mellitus,41 and indirect sequelae, ascorbic acid deficiency and osteoporosis.42-44 More recently, associations of iron overload in Africans with death from hepatocellular carcinoma and tuberculosis have been described.45 Although the condition is underrecognized or even unknown by many health care providers today, iron overload still has a high prevalence in many areas of Africa.1,29,46 47
The finding of a possible genetic pattern to iron overload in African can now be put in perspective with other inherited iron overload conditions. HLA-linked hemochromatosis is the predominant inherited iron-loading condition in populations derived from Europe.15,48 Thalassemia major and intermedia syndromes are inherited anemias common in historically malarious geographic areas that are characterized by high degrees of ineffective erythropoiesis, increased intestinal iron absorption, and development of iron overload even in the absence of blood transfusions.49-51 An apparently autosomal dominant and non–HLA-linked form of iron loading has been described in a large Melanesian family.52Congenital atransferrinemia is a sporadic autosomal recessive condition that leads to severe parenchymal iron overload.53 A single nucleic acid substitution of the ceruloplasmin gene leads to aceruloplasminemia and systemic iron overload in Japanese families in an autosomal recessive pattern.54 The finding of different genetically determined mechanisms for iron overload in different populations may suggest there is some type of advantage to these traits.
Recent studies have emphasized that primary iron overload occurs among African-Americans, but the genetics of this condition have not been examined.55 56 High dietary iron does not appear to be a contributing factor. Further studies will be required to determine whether primary iron overload in African-Americans is related in any way to the condition in Africa.
Supported by grants from the Office of Minority Health to the Cell Biology and Metabolism Branch, National Institute of Child Health and Human Development (NICHD); the JF Kapnek Charitable Trust; the Research Board of the University of Zimbabwe; the South African Medical Research Council; and the University of the Witwatersrand; and by NICHD Contract No. 1-HD 3-3196.
Address reprint requests to Victor R. Gordeuk, MD, Department of Medicine, The George Washington University Medical Center, 2150 Pennsylvania Ave NW, Washington, DC 20037.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal