Abstract
The in vivo roles of granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte (G)-CSF were studied in factor-deficient gene-targeted knockout mice infected with the facultative intracellular bacterium Listeria monocytogenes.Previous results showed that G-CSF−/− mice had an underlying selective deficiency in granulopoiesis, but GM-CSF−/− mice had little disturbance in resting hematopoiesis. Nevertheless, in this study it is revealed that 3 days after intraperitoneal infection with 2 × 105Listeria, GM-CSF−/− mice harbored 50-fold more organisms in their spleen and liver than similarly infected wild-type mice. This was accompanied by a severe depletion of bone marrow hematopoietic cells and a deficient inflammatory response in their peritoneal cavity. Thus, GM-CSF is essential for emergency, but not resting, hematopoiesis. In contrast, G-CSF−/− mice were markedly susceptible to low doses (2 × 104) ofListeria intraperitoneally. After infection, the acute (1 day) granulocyte infiltration to the peritoneal cavity was normal compared with wild type, but the more prolonged monocyte response was deficient, reflecting a continued decrease in bone marrow cellularity and hematopoiesis over 3 days, which was not observed in infected wild-type mice. It is thus apparent that G-CSF deficiency affects monocytopoiesis as well as granulopoiesis during infection.
THE COLONY-STIMULATING factors (CSFs) are an important group of cytokines discovered for their ability to support the culture of hematopoietic cells in vitro. In vitro studies indicated that CSFs support the survival, proliferation, differentiation, and end cell function of myeloid cells.1 For example, granulocyte-macrophage colony-stimulating factor (GM-CSF) supports the in vitro growth of neutrophilic granulocytes and of macrophages from their common progenitor cells and enhances the functions of granulocytes,2 macrophages,2 and dendritic cells,3 whereas G-CSF controls the survival, proliferation, differentiation, and function of mature neutrophilic granulocytes and their precursors.4 However, it has been difficult to confirm an in vivo physiological role for the various CSFs in hematopoiesis. One definitive approach to studying the in vivo role of cytokines is the development of gene-targeted knockout mice.5 In particular, the generation of mice with targeted disruption of the G-CSF6 or GM-CSF7,8 genes is aiding the study of the in vivo roles of these two CSFs.
We have previously reported that the G-CSF–deficient mice suffer a chronic neutropenia associated with a deficiency in granulopoietic precursor cells.6 The G-CSF−/− mice were highly susceptible to intravenous infection with the facultative intracellular bacterium Listeria monocytogenes. In contrast, although suffering a characteristic pulmonary disease,8 the GM-CSF–deficient mice show little disturbance in resting hematopoiesis, with normal numbers of colony forming cells when their bone marrow was cultured in any of a variety of hematopoietic growth factors, and normal numbers of granulocytes and monocytes in the blood.7 This surprisingly indicated that despite its potency in vitro, at least for baseline hematopoiesis, GM-CSF was apparently redundant in vivo.
Against this background, we undertook to study the response of the GM-CSF−/− mice to the facultative intracellular bacterium L monocytogenes. This organism provided the original definition of cell-mediated immunity (CMI) and has since been used extensively to study the control of CMI.9 In the murine infection, L monocytogenes survives within macrophages and liver parenchymal cells. The early inflammatory response is critically important in natural resistance, and the major gene governing resistance to this infection in mice determines the time of onset of that response,10,11 apparently via its effects on the number of hematopoietic cells in bone marrow and spleen.12 Macrophage activation to increased bactericidal activity by interferon-γ is a key event in acquired cellular resistance to this and other intracellular bacteria.13 Until recently, it was assumed that granulocytes played little role in resistance to intracellular bacteria, but depletion studies using monoclonal antibody (MoAb) to the Gr1 marker have shown that granulocytes are essential in both the primary and secondary infection with Listeria.14,15The granulocytes are believed to lyse infected parenchymal cells, releasing the bacteria and exposing them to killing by activated macrophages/monocytes.16 It is the importance of both granulocytes and macrophages which makes this infection a suitable choice to test the role of GM-CSF and G-CSF in emergency hematopoiesis in knockout mice. Despite the apparent redundancy of GM-CSF in resting hematopoiesis, the study of intraperitoneal Listeria infection in GM-CSF–deficient mice showed that there was a deficiency in the emergency hematopoietic response to Listeria infection, although such deficiency was not as striking as that seen in G-CSF–deficient mice.
MATERIALS AND METHODS
Mice and bacteria.
GM-CSF−/− and G-CSF−/− mice were produced by targeted disruption of the respective genes in 129 occipitolaeva anterior (OLA) embryonal stem cells which, after selection, were injected into C57BL/6 blastocysts.6 7 Both 129/OLA and C57BL/6 mice are genetically resistant to Listeria infection and show quantitatively similar responses to infection. The CSF−/− mice and wild-type control mice used in these experiments were comparably outbred and maintained in the Department of Microbiology, University of Melbourne. The mice were housed in isolation and fed sterile pellets and water to maintain their infection-free status.Listeria monocytogenes were maintained by weekly subculture on horse blood agar (HBA). Mice 6 to 8 weeks old were infected intraperitoneally with 2 × 104 or 2 × 105Listeria from a 24-hour HBA culture and the dose was checked retrospectively.
Quantitation of infection.
Mice were killed by CO2 narcosis. Spleen and liver were removed aseptically and individually homogenized in normal saline with an Ultra Turrax homogenizer (Janke and Kunkel KG, Breisgau, Germany). The numbers of Listeria in the organs were established by plating serial 10-fold dilutions of organ homogenates in saline on an HBA plate and incubating at 37°C for 24 hours.
Cell preparation.
Bone marrow cells were prepared by flushing the tibia with an enriched Dulbecco's modified Eagle's medium (GIBCO, Grand Island, NY) with 10% fetal calf serum (DMEM + 10% FCS) using a syringe with a 25-gauge needle. The cells were centrifuged at 800g for 7 minutes, resuspended in DMEM + 10% FCS, and counted. Peritoneal cells were prepared by washing peritoneal cavities with 5 mL DMEM + 10% FCS. Peritoneal cells were pelleted by centrifuge at 800gfor 7 minutes and resuspended in 10 mL DMEM + 10% FCS.
MoAbs.
Phenotypic analysis.
Bone marrow cells were washed once in phosphate-buffered saline (PBS, pH 7.2) with 5% FCS. Cells were then mixed with appropriately diluted MoAbs on ice for 30 minutes. After washing twice in PBS-5% FCS, cells were incubated with fluorescein isothiocyanate-labeled sheep anti-rat Ig (mouse absorbed) (Silenus, Hawthorn, Australia) on ice for 30 minutes. After two washes in PBS, cells were analyzed by FACSort (Becton Dickinson, San Jose, CA). Cell populations were analyzed and percentages calculated using the Becton Dickinson Immunocytometry system “Cell Quest” application. Because the F4/80 marker is downregulated on macrophage activation,18 it was not a satisfactory marker of inflammatory macrophages/monocytes in the peritoneal cavity. Therefore, these cells were assessed microscopically. Peritoneal cells were spun through an FCS cushion in a cytocentrifuge onto microscopic slides and stained with Diff-Quick (Lab-Aids, Narrabeen, New South Wales, Australia). For differential counts, 500 cells per slide were scored.
Assay for colony-forming cells (CFCs).
CFC assays were performed as described.19 Triplicate cultures containing 5 × 104 bone marrow cells in 1 mL semi-solid agar were placed in 35-mm Petri dishes (Becton Dickinson, Oxnard, CA). To this was added 0.1 mL of a 1 in 4 dilution of serum prepared from BALB/c mice which had been injected intraperitoneally with 5 mg lipopolysaccharide w (Escherichia coli 011:B4; Difco Laboratories, Detroit, MI) 6 hours earlier. This lipopolysaccharide (LPS) serum was used to support CFC growth because LPS serum reflects the cocktail of CSFs that are encountered during infection.20 Cultures were incubated at 37°C for 6 days and colonies containing more than 50 cells were counted.
Statistics.
The statistical significance of the experimental data was determined by Student's t-test.
RESULTS
Listeria infection in GM-CSF−/− mice.
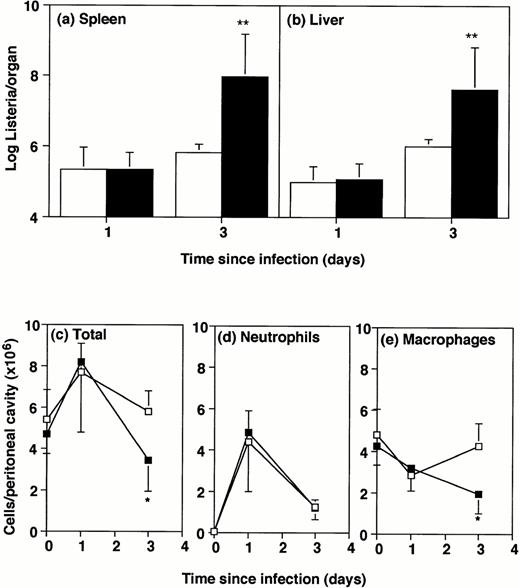
Sex- and age-matched GM-CSF−/− and wild-type mice were infected intraperitoneally with 2 × 105Listeriaorganisms. The intraperitoneal route of infection was chosen because it allows ready observation of the phagocytic cells drawn to that site. The rate of infiltration of cells to the peritoneal cavity correlates strictly with natural genetic resistance in different strains of mice.10 Mice were killed 1 or 3 days after infection for bacterial counts in liver and spleen (Fig1A and B). At day 1 postinfection, bacterial numbers in the GM-CSF−/− mice were similar to the numbers in wild-type mice, but by day 3 the GM-CSF−/− mice showed more than 50-fold exacerbation compared with wild-type mice (P < .01). The experiments were terminated at this time because this dose was uniformly lethal for GM-CSF−/− mice after 4 or more days, and lethal also for a proportion of wild-type controls.
Bacterial numbers and inflammatory responses in GM-CSF−/− mice. GM-CSF knockout mice (▪) or wild-type mice (□) were infected intraperitoneally with 2 × 105Listeria for 1 or 3 days. Bacterial load and peritoneal cells were quantitated. Data represent mean and standard deviation of groups of five mice. *P < .05 and **P < .01 compared with wild-type mice. Results were from one of three similar experiments. (a) Bacterial counts in spleen. (b) Bacterial counts in liver. (c) Total cells recovered per peritoneal cavity. (d) Neutrophils per peritoneal cavity. (e) Macrophages and monocytes per peritoneal cavity.
Bacterial numbers and inflammatory responses in GM-CSF−/− mice. GM-CSF knockout mice (▪) or wild-type mice (□) were infected intraperitoneally with 2 × 105Listeria for 1 or 3 days. Bacterial load and peritoneal cells were quantitated. Data represent mean and standard deviation of groups of five mice. *P < .05 and **P < .01 compared with wild-type mice. Results were from one of three similar experiments. (a) Bacterial counts in spleen. (b) Bacterial counts in liver. (c) Total cells recovered per peritoneal cavity. (d) Neutrophils per peritoneal cavity. (e) Macrophages and monocytes per peritoneal cavity.
Numbers of peritoneal cells in uninfected mice did not differ significantly between GM-CSF−/− and wild-type mice (Fig 1C). After intraperitoneal infection with 2 × 105Listeria,the number of cells in the peritoneal cavity of GM-CSF−/− mice was only half that in wild-type mice by 3 days after infection. There was no significant difference in numbers of neutrophils and macrophages between wild-type and GM-CSF−/− mice, either uninfected or 1-day infected (Fig 1D and E). However, by 3 days after infection the numbers of macrophages were significantly lower in GM-CSF−/− mice compared with wild-type mice (Fig 1E). The percentage of neutrophils and macrophages was not significantly different in the peritoneal cavity of uninfected or 1-day infected wild-type and GM-CSF−/− mice (Table1). By day 3 there was a significantly higher percentage of typical macrophages in the wild-type mice. It was of interest that approximately half of the peritoneal cells (53% ± 5%) from GM-CSF−/− mice contained visibleListeria organisms 3 days after infection. Only one quarter (27% ± 5%) of the cells from wild-type mice contained visible bacteria at this time and there were considerably fewer bacteria per cell.
Percentage of Cells in the Peritoneal Cavity and Bone Marrow of GM-CSF−/− Mice and Wild-Type Mice Before and After Intraperitoneal Infection
| Infection . | Mice . | Peritoneal Cells . | Bone Marrow Cells . | ||
|---|---|---|---|---|---|
| Neutrophilic Granulocytes . | Macrophages/ Monocytes . | GR1+ . | F4/80+ . | ||
| None | GM-CSF−/− | 1 ± 1 | 81 ± 2 | 58 ± 3 | 12 ± 2 |
| Wild type | 1 ± 1 | 81 ± 3 | 52 ± 5 | 8 ± 1 | |
| 1 day | GM-CSF−/− | 57 ± 11 | 41 ± 13 | 60 ± 7-150 | 7 ± 2 |
| Wild type | 56 ± 8 | 38 ± 10 | 48 ± 6 | 8 ± 2 | |
| 3 days | GM-CSF−/− | 34 ± 6 | 56 ± 6-151 | 62 ± 10 | 10 ± 2 |
| Wild type | 22 ± 8 | 73 ± 9 | 68 ± 2 | 9 ± 2 | |
| Infection . | Mice . | Peritoneal Cells . | Bone Marrow Cells . | ||
|---|---|---|---|---|---|
| Neutrophilic Granulocytes . | Macrophages/ Monocytes . | GR1+ . | F4/80+ . | ||
| None | GM-CSF−/− | 1 ± 1 | 81 ± 2 | 58 ± 3 | 12 ± 2 |
| Wild type | 1 ± 1 | 81 ± 3 | 52 ± 5 | 8 ± 1 | |
| 1 day | GM-CSF−/− | 57 ± 11 | 41 ± 13 | 60 ± 7-150 | 7 ± 2 |
| Wild type | 56 ± 8 | 38 ± 10 | 48 ± 6 | 8 ± 2 | |
| 3 days | GM-CSF−/− | 34 ± 6 | 56 ± 6-151 | 62 ± 10 | 10 ± 2 |
| Wild type | 22 ± 8 | 73 ± 9 | 68 ± 2 | 9 ± 2 | |
Mice were infected with 2 × 105Listeriaintraperitoneally and groups of five mice were killed at day 1 or 3 postinfection, or without infection. Peritoneal cells and bone marrow cells were prepared from individual animals. Peritoneal cells were assessed microscopically, while bone marrow cells were analyzed by FACS. The results are typical of four separate experiments.
P < .05 by Student's t-test.
P < .01 by Student's t-test.
Hematopoietic response of GM-CSF−/− mice to infection.
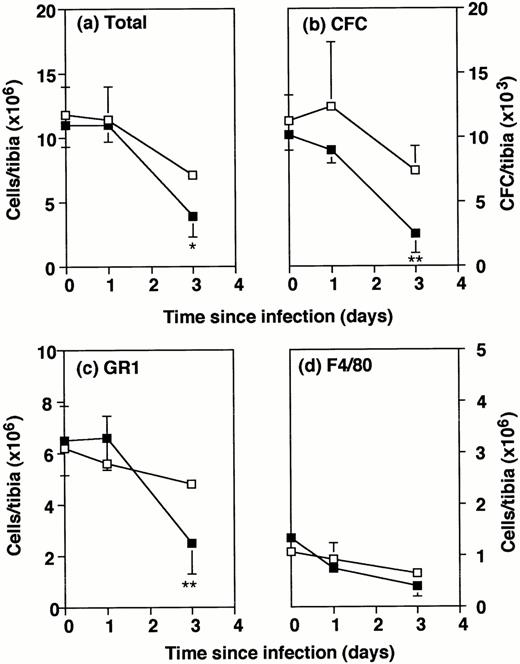
The explanation for this impairment in defense against infection was sought by analysis of the bone marrow progenitors. Consistent with the known data,7 the total numbers of bone marrow cells and the numbers of CFCs recovered from the tibia of uninfected GM-CSF−/− mice were similar to those from wild-type mice (Fig 2A and B). After intraperitoneal infection with 2 × 105Listeria organisms, bone marrow cellularity showed a steady depletion in both GM-CSF−/− and wild-type mice. By day 3, numbers in GM-CSF−/− mice were significantly lower than in wild-type mice (Fig 2A). The numbers of CFCs recovered from the tibia also showed a decline which was significantly greater in the GM-CSF−/− mice than wild-type mice (Fig 2B).
Number of bone marrow cells in wild-type and GM-CSF−/− mice following Listeria infection. GM-CSF−/− mice (▪) and wild-type mice (□) were either uninfected or infected intraperitoneally with 2 × 105Listeria for 1 or 3 days. Bone marrow cells were flushed from the tibia of individual mice. Data represent the mean and SD of individual tibia of five mice in each group. *P < .05 and **P < .01 compared with wild-type mice which were infected with the same dose ofListeria. Results were from one of three similar experiments. (a) Total cells recovered per tibia. (b) Colony forming cells per tibia. (c) Gr1+ cells per tibia. (d) F4/80+cells per tibia.
Number of bone marrow cells in wild-type and GM-CSF−/− mice following Listeria infection. GM-CSF−/− mice (▪) and wild-type mice (□) were either uninfected or infected intraperitoneally with 2 × 105Listeria for 1 or 3 days. Bone marrow cells were flushed from the tibia of individual mice. Data represent the mean and SD of individual tibia of five mice in each group. *P < .05 and **P < .01 compared with wild-type mice which were infected with the same dose ofListeria. Results were from one of three similar experiments. (a) Total cells recovered per tibia. (b) Colony forming cells per tibia. (c) Gr1+ cells per tibia. (d) F4/80+cells per tibia.
Phenotypic analysis of bone marrow cells by FACS showed no deficit in Gr1+ or F4/80+ cells in uninfected GM-CSF−/− mice compared with wild-type (Fig 2C and D). By day 3, total numbers of Gr1+ cells in GM-CSF−/− mice were only half those in wild-type mice, whereas no differences in the total numbers of F4/80+ cells were noted. The percentages of Gr1+ and F4/80+ cells in the bone marrow were not consistently different between the two types of mice (Table 1). The slight excess of granulocytes in GM-CSF−/− mice was not observed in other experiments.
It should be noted that all the above results involved challenge with a relatively high dose of Listeria (2 × 105). When mice were injected intraperitoneally with 2 × 104Listeria organisms, a dose which resulted in marked exacerbation in GM-CSF−/− mice (below), in four out of seven experiments there was no exacerbation of infection in the GM-CSF−/− mice. In those experiments in which no exacerbation was observed, similar numbers of bone marrow cells, CFCs, and peritoneal exudate cells were found in the two mouse strains 3 days postinfection. On the other hand, in the three experiments where exacerbation was observed there were deficiencies in the bone marrow cells and peritoneal exudate cells similar to those described above for higher doses ofListeria (results not shown).
Listeria infection in GM-CSF−/− mice.
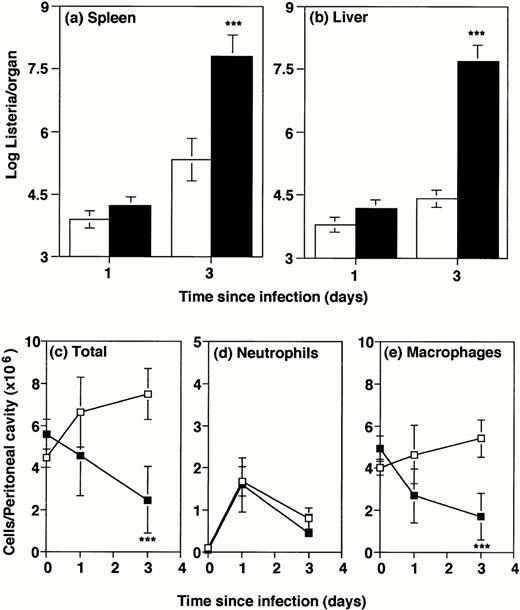
In contrast to GM-CSF−/− mice, G-CSF−/− mice have reduced numbers of granulocytes and hematopoietic cells under resting conditions.6 Our previous study showed that susceptibility of G-CSF−/− to intravenously injected Listeria was associated with a deficient blood inflammatory response to infection.6 To allow comparison with intraperitoneally infected GM-CSF−/− mice (above), GM-CSF−/− and wild-type controls were infected intraperitoneally in the present study with 2 × 104Listeria organisms. The low dose (compared with 2 × 105 for most of the GM-CSF−/− experiments) was necessitated by the marked susceptibility of the G-CSF−/− mice to infection.6 Groups of mice were killed 1 or 3 days later. Consistent with the previous study of intravenous infection, at 1 day after infection, bacterial counts in liver and spleen of G-CSF−/− mice were similar to counts in wild-type mice, indicating no impairment of the initial ability of resident macrophages to capture the organisms. However, by 3 days postinfection, spleens and livers of G-CSF−/− mice harbored at least 100 times more Listeriathan wild-type mice (Fig 3A and B).
Bacterial numbers and inflammatory responses in G-CSF−/− mice. G-CSF knockout mice (▪) or wild-type mice (□) were infected intraperitoneally with 2 × 104Listeria for 1 or 3 days. Bacterial load and peritoneal cells were quantitated. Data represent mean and standard deviation of groups of five mice. ***P < .001 compared with wild-type mice. Results were from one of three similar experiments. (a) Bacterial counts in spleen. (b) Bacterial counts in liver. (c) Total cells recovered per peritoneal cavity. (d) Neutrophils per peritoneal cavity. (e) Macrophages and monocytes per peritoneal cavity.
Bacterial numbers and inflammatory responses in G-CSF−/− mice. G-CSF knockout mice (▪) or wild-type mice (□) were infected intraperitoneally with 2 × 104Listeria for 1 or 3 days. Bacterial load and peritoneal cells were quantitated. Data represent mean and standard deviation of groups of five mice. ***P < .001 compared with wild-type mice. Results were from one of three similar experiments. (a) Bacterial counts in spleen. (b) Bacterial counts in liver. (c) Total cells recovered per peritoneal cavity. (d) Neutrophils per peritoneal cavity. (e) Macrophages and monocytes per peritoneal cavity.
To test the local inflammatory response to Listeria infection in G-CSF−/− mice, the total cellularity and cell subpopulations in the peritoneal cavity were monitored after intraperitoneal infection. Before infection, the numbers of cells in the peritoneal cavity were similar in G-CSF−/− mice and wild-type, and there were very few granulocytes in either strain (Fig 3C through E). One day postinfection, cell numbers were variable and differences not significant, but by day 3 the G-CSF−/− mice showed an approximately twofold deficit in macrophages (Fig 3C and E). This reflected the fact that total cells in the peritoneal cavity of wild-type mice 3 days postinfection were almost two times the normal number, whereas in G-CSF−/− mice they had actually decreased to half of the normal number. This was a reproducible finding, despite some mouse-to-mouse variation. Percentages of granulocytes and macrophages/monocytes in the peritoneal cavity (Table 2) showed no selective deficiency in the granulocyte inflammatory response, accentuating the monocyte/macrophage deficiency. Furthermore, histological examination of lesions in the infected liver and spleen revealed no selective deficiency in the cell subsets (data not shown).
Percentage of Cells in the Peritoneal Cavity and Bone Marrow of G-CSF−/− Mice and Wild-Type Mice Before and After Intraperitoneal Infection
| Infection . | Mice . | Peritoneal Cells . | Bone Marrow Cells . | ||
|---|---|---|---|---|---|
| Neutrophilic Granulocytes . | Macrophages/ Monocytes . | GR1+ . | F4/80+ . | ||
| None | GM-CSF−/− | 1 ± 1 | 88 ± 1 | 12 ± 3* | 11 ± 3 |
| Wild type | 2 ± 1 | 89 ± 1 | 48 ± 3 | 18 ± 7 | |
| 1 day | G-CSF−/− | 35 ± 8 | 57 ± 10 | 16 ± 4* | 8 ± 4 |
| Wild type | 27 ± 7 | 70 ± 4 | 46 ± 8 | 23 ± 17 | |
| 3 days | G-CSF−/− | 15 ± 10 | 70 ± 5 | 30 ± 7* | 11 ± 3 |
| Wild type | 10 ± 4 | 76 ± 8 | 54 ± 4 | 19 ± 9 | |
| Infection . | Mice . | Peritoneal Cells . | Bone Marrow Cells . | ||
|---|---|---|---|---|---|
| Neutrophilic Granulocytes . | Macrophages/ Monocytes . | GR1+ . | F4/80+ . | ||
| None | GM-CSF−/− | 1 ± 1 | 88 ± 1 | 12 ± 3* | 11 ± 3 |
| Wild type | 2 ± 1 | 89 ± 1 | 48 ± 3 | 18 ± 7 | |
| 1 day | G-CSF−/− | 35 ± 8 | 57 ± 10 | 16 ± 4* | 8 ± 4 |
| Wild type | 27 ± 7 | 70 ± 4 | 46 ± 8 | 23 ± 17 | |
| 3 days | G-CSF−/− | 15 ± 10 | 70 ± 5 | 30 ± 7* | 11 ± 3 |
| Wild type | 10 ± 4 | 76 ± 8 | 54 ± 4 | 19 ± 9 | |
Mice were infected with 2 × 104Listeriaintraperitoneally and groups of five mice were killed at day 1 or 3 postinfection, or without infection. Peritoneal cells and bone marrow cells were prepared from individual animals. Peritoneal cells were assessed microscopically, while bone marrow cells were analyzed by FACS.
P < .001 comparing G-CSF−/− and wild-type mice. Other differences between G-CSF−/− and wild-type mice were not significant. The results are typical of four separate experiments.
Hematopoietic response of GM-CSF−/− mice to infection.
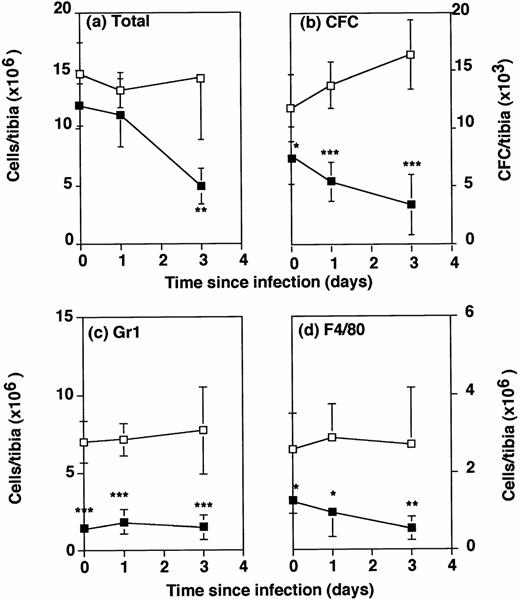
The total number of bone marrow cells recovered from the tibia cavity of G-CSF−/− mice and wild-type mice was not significantly different in uninfected mice (Fig 4A). Twenty-four hours after intraperitoneal infection with 2 × 104Listeria, there was a drop in numbers of bone marrow cells recovered from the tibia, which was not significantly or consistently different in G-CSF−/− and wild-type mice. By 3 days after infection, cell numbers in the tibia of wild-type mice were steady, but had decreased still further in G-CSF−/− mice, to less than half of the numbers in uninfected G-CSF−/−, or in 3-day infected wild-type mice.
Number of bone marrow cells in wild-type and G-CSF−/− mice following Listeria infection. G-CSF−/− mice (▪) and wild-type mice (□) were either uninfected or infected intraperitoneally with 2 × 104Listeria for 1 or 3 days. Bone marrow cells were flushed from the tibia of individual mice. Data represent the mean and standard deviation of individual tibia of five mice in each group. *P < .05, **P < .01, and ***P < .001 compared with wild-type mice which were infected with the same dose ofListeria. Results were from one of three similar experiments. (a) Total cells recovered per tibia. (b) Colony forming cells per tibia. (c) Gr1+ cells per tibia. (d) F4/80+cells per tibia.
Number of bone marrow cells in wild-type and G-CSF−/− mice following Listeria infection. G-CSF−/− mice (▪) and wild-type mice (□) were either uninfected or infected intraperitoneally with 2 × 104Listeria for 1 or 3 days. Bone marrow cells were flushed from the tibia of individual mice. Data represent the mean and standard deviation of individual tibia of five mice in each group. *P < .05, **P < .01, and ***P < .001 compared with wild-type mice which were infected with the same dose ofListeria. Results were from one of three similar experiments. (a) Total cells recovered per tibia. (b) Colony forming cells per tibia. (c) Gr1+ cells per tibia. (d) F4/80+cells per tibia.
The potential to produce granulocytes and monocytes/macrophages in the bone marrow of Listeria infected mice was tested by measuring CFCs. Even before infection there was a significant deficiency in CFCs in uninfected G-CSF−/− mice compared with wild-type controls (Fig4B). One day postinfection, the numbers in wild-type mice had increased over the numbers in uninfected mice, but in G-CSF−/− mice they had declined. By 3 days after infection, numbers of CFCs in wild-type mice were about 1½ times normal. However, the numbers of CFCs in G-CSF−/− mice had declined to half the numbers in uninfected mice and were only the numbers in wild-type mice.
Phenotypic analysis of bone marrow cells by FACS showed a striking deficit in the absolute numbers (Fig 4C) and the percentage (Table 2) of granulocytes in G-CSF−/− mice compared with wild-type mice. In uninfected mice, almost 50% of bone marrow nucleated cells in wild-type mice were Gr1+, whereas in G-CSF−/− mice only 12% of bone marrow cells were Gr1+. Although the percentage of Gr1+ cells in bone marrow increased somewhat during infection of the G-CSF−/− mice, absolute numbers remained low. The initial deficit in the percentage of F4/80+monocytic cells (Table 2) in the bone marrow of G-CSF−/− mice was less striking (11% compared with 18% in wild-type mice, and not significantly different). However, the difference in absolute numbers increased with time because of the decline in total cellularity of the bone marrow (Fig 4D).
DISCUSSION
The initial reports of normal baseline hematopoiesis in GM-CSF–deficient mice7 8 raised the possibility that, despite its potency in support of hematopoiesis in vitro, GM-CSF is not mandatory for balanced granulocyte and monocyte/macrophage development in vivo. Our data now show that, with a sufficient infective challenge, an essential hematopoietic role for GM-CSF can be shown in vivo, and hence GM-CSF is not wholly redundant with respect to hematopoiesis. Although infection with typical experimental challenge doses ofListeria did not reproducibly reveal a hematopoietic impairment in GM-CSF–deficient mice, when higher doses were used, GM-CSF−/− mice failed to control the infection. This failure of host defenses was accompanied by deficiencies in both the granulocyte lineage (in the marrow) and the monocyte/macrophage lineage (in the peritoneal cavity), consistent with the in vitro and in vivo effects of GM-CSF on both these lineages. The fact that the susceptibility of GM-CSF-deficient mice to Listeria was only manifest at high infecting doses indicates that emergency host defenses are intact, but it is an inability to sustain host defences or a lack of reserves of responsiveness that is missing.
Previously in experiments using the intravenous route of infection, we had shown that G-CSF–deficient mice were susceptible toListeria infection.6 In view of the baseline hematopoietic defect in G-CSF−/− mice, this suggested that a G-CSF–primed bone marrow was necessary for a normal defense against infection. The deficits observed in the intraperitoneally infected GM-CSF–deficient mice prompted a reappraisal of the response of G-CSF−/− mice to Listeria infection, using this route of infection. While confirming the previous observations,6several new insights emerged. Despite the image of G-CSF as a factor primarily supporting the development and function of neutrophilic granulocytes, the failure of hematopoiesis in Listeria-infected G-CSF–deficient mice clearly involves not only the granulocytic lineage but also the monocyte/macrophage lineage. Impaired monocyte/macrophage lineage production is evident in the blood,6 in the local peritoneal inflammatory response, and in the bone marrow itself (present studies).
Because of the different basal states of total- and granulocyte-hematopoiesis in these two CSF-deficient mouse lines, it is difficult to make direct comparisons. The marked baseline granulopoietic deficit in G-CSF–deficient mice precludes isolated evaluation of the nature of an emergency granulopoietic response, whereas in GM-CSF–deficient mice with normal baseline hematopoiesis, a high dose of Listeria unmasks an inability to sustain an emergency response without GM-CSF. On the other hand, monocytopoiesis shows little difference in the two strains before infection, and the impairment of monocytopoiesis in the face of infection is of a similar magnitude in either G-CSF- or GM-CSF–deficient mice.
The importance of G-CSF in listeriosis is consistent with the elevated serum levels (>1,000 U/mL) observed during infection.20In contrast, serum GM-CSF levels are low (<50 U/mL), although evidence is accumulating that endogenous mechanisms do not generally use GM-CSF as a freely circulating cytokine (G.J.L., unpublished, 1996). Indeed, more detailed analysis of events after infection throws light on the mechanisms of action of these two cytokines during infection. In both GM-CSF−/− and G-CSF−/− mice, the initial localization of bacteria in the spleen and liver was the same as in wild-type mice, indicating there was no deficiency in the ability of resident macrophages to capture the invading microorganisms. Defects become apparent later.
As might be expected in mice suffering no disturbance of resting hematopoiesis, the numbers of cells reaching the peritoneal cavity of GM-CSF mice were not deficient at 1 day postinfection, and only became significant by day 3, when numbers were only half those in wild-type mice. By this time the neutrophil response had waned, and the deficiency was reflected particularly in macrophage numbers. It is difficult to say whether this final failure of the inflammatory system in the GM-CSF−/− mice allowed the increase in bacterial numbers, or if the failure in hematopoiesis and inflammation is secondary to the high bacterial load reached by 3 days. The fact that GM-CSF−/− mice given a lower dose of Listeria often showed no exacerbation of infection and no deficiency in their 3-day hematopoietic or inflammatory responses favors the latter interpretation. What, then, exacerbates infection? Although a deficient inflammatory response can certainly exacerbate infection,11 the peritoneal cells of GM-CSF−/− mice showed functional deficiencies (Y.Z. and C.C., manuscript in preparation), in particular a reduced capacity to produce nitric oxide. Nitric oxide is of primary importance in macrophage killing of intracellular bacteria21 and the deficiency would undoubtedly contribute to the exacerbation of infection. This result, suggesting a role for GM-CSF in macrophage activation, is the converse of the activation of peritoneal cell function observed in transgenic mice overexpressing GM-CSF.22
It is of interest that at the site of infection in the peritoneal cavity of G-CSF−/− mice there was no significant difference in numbers of granulocytes compared with wild-type mice. However, by 3 days postinfection, there was a marked deficit in total cellularity and in the number of monocytes/macrophages in the G-CSF−/− mice. Our earlier observation of the inflammatory response in the blood after intravenous infection also showed a deficit in the monocytic response.6 However, mature monocytes and macrophages do not carry receptors for G-CSF. This raises the possibility that, after infection, G-CSF contributes to the expansion of stem cell precursors common to all of these hematopoietic cells. G-CSF has been shown in vitro to act synergistically with interleukin-3 (IL-3), IL-1, and IL-6 in different systems to promote the proliferation or survival of primitive multipotent progenitor cells.23 In mice whose bone marrow was depleted with 5-fluorouracil, G-CSF and IL-1 act synergistically to stimulate multilineage hematologic recovery.24
It is not surprising that a halving of the numbers of CFCs in the bone marrow of G-CSF−/− mice could increase susceptibility to L monocytogenes infection. Naturally susceptible BALB/c mice have only half the numbers of bone marrow CFCs compared with resistant C57BL mice, and this forms the basis of the major gene governing resistance of mice to listeriosis.12 What is more surprising is the fact that the deficiency of neutrophilic granulocytes in the G-CSF−/− mice is not more profound. This indicates a redundancy in the action of CSFs or other cytokines controlling myelopoiesis. It is relevant to note that the CSFs are not the only cytokines which control myelopoiesis during infection. Depletion of IL-6 during listeriosis markedly exacerbates infection25and may act at least in part by controlling granulocyte production.26 Thus, it would be of interest to test the effect of further depletion of IL-6 in these mice.
These experiments with gene-targeted knockout mice indicate that although a particular factor may initially appear redundant for some expected functions, closer examination is likely to reveal that it does play a specialized role under some circumstances. In GM-CSF–deficient mice, the in vivo hematopoietic role of GM-CSF only becomes crucial under the stress of a high-infecting dose of bacteria.
ACKNOWLEDGMENT
We thank Dr Sunanda Basu for helpful discussions.
Supported by the National Health and Medical Research Council of Australia Project Grant No. 921126.
Address reprint requests to Christina Cheers, PhD, Department of Microbiology, University of Melbourne, Parkville, Victoria, 3055, Australia.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance wth 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal