HEMATOPOIESIS IS A life-long process responsible for replenishing both hematopoietic progenitor cells and mature blood cells from a pool of pluripotent, long-term reconstituting stem cells.1 The daily turnover in a normal adult of approximately 1012 blood cells is tightly regulated, involving, in part, a complex interaction between soluble and membrane-bound stimulatory and inhibitory cytokines and their corresponding receptors.2-4 The molecular cloning of these hematopoietic growth factors (HGFs) and their receptors has been instrumental in delineating the pathways that lead from a single hematopoietic stem cell to the various terminally differentiated cells in the hematopoietic system.
Although a number of cytokines have effects on progenitor and stem cells in vitro or in vivo, two cytokines discovered in the early 1990s, c-kit ligand and flt3 ligand, appear to have unique and nonredundant activities on primitive progenitor/stem cells.
Because of the broad range of hematopoietic activities mediated through interaction of c-kit ligand (KL) and flt3 ligand (FL) with their receptors, it is beyond the scope of this report to review the effects of these proteins outside of the hematopoietic system. Rather, we will focus on the discovery, structure, function, expression, and biological roles of these two ligand-receptor pairs. Special attention will be directed towards hematopoietic activities in which KL and FL show either distinct or synergistic effects. For a more detailed overview of other hematologic and immunologic effects of KL and FL, other reviews can be recommended.5-8 Two subjects have been deliberately left out of this report, because they are deserving of their own separate reviews (signal transduction pathways involving c-kit and flt3 and activities of KL and FL outside of the hematopoietic system).
DISCOVERY OF THE DOMINANT WHITE SPOTTING (W) LOCUS AND ITS RELATIONSHIP TO THE c-kit TYROSINE KINASE RECEPTOR
The W (dominant White spotting) locus in mice was first described in the early 1900s.9,10 Mice afflicted with mutations at the W locus were originally identified, as the name implies, by the presence of a white spot on the bellies of pigmented mice. Detailed examination of these mice showed that the mutation was pleiotropic. The mice suffer from defects in germ cell development (manifested as reproductive difficulties) and in hematopoiesis (characterized by a macrocytic anemia). Over the years, at least 20 allelic variants of the W locus have been described; most have a similar, although not identical, phenotype.9,10 The W locus is on chromosome 5 and is one of the most mutable loci in mice.9 10
A central question that remained was what kind of protein the Wlocus encoded, and how did it affect so many different tissues. A breakthrough came in 1988 when it was shown that the W locus encoded a tyrosine kinase receptor known as c-kit.11,12 The c-kit protein has the same general structure as four other tyrosine kinase receptors: c-fms, the receptor for macrophage colony-stimulating factor (M-CSF)13-15; flt316-19; and both of the receptors for platelet-derived growth factor (PDGF; designated as A and B).20-23 Each of these receptors is approximately 1,000 amino acids in length, has five Ig-like domains in the extracellular region, and contains a split catalytic domain in the cytoplasmic region that phosphorylates tyrosine residues in specific target proteins after activation of the receptor by ligand. The exact defect in the c-kit receptor has been identified at the molecular level for a number of alleles of theW locus24-28 (see section on genetic alterations in c-kit and KL genes).
THE STEEL (Sl) LOCUS AND ITS RELATIONSHIP TOW
Many years after the discovery of the W locus, a mutation in mice that had a phenotype virtually identical to W mice was identified.29 Despite the similarities in phenotype, this new mutation, designated Steel (Sl), was localized to mouse chromosome 10, so it was clearly not allelic with the W locus on chromosome 5.10,30 Because mutations on two different chromosomes had the same complex phenotype that affects pigmentation, germ cells, and hematopoiesis, researchers hypothesized that there would be some relationship between the proteins encoded at these two loci. Elizabeth Russell, who did much of the pioneering research on both of these mutations, suggested (years before the discovery that theW locus encoded c-kit and that c-kit was a receptor) that the W and Sl loci might encode a receptor and its cognate ligand.10
CLONING OF THE STEEL FACTOR (THE c-kit LIGAND, KL)
With the recognition that the W locus encoded c-kit,11,12 the search for the c-kit ligand began in earnest. A number of approaches were undertaken to identify the protein encoded at the Sl locus, including chromosome walking31 and expression cloning. However, the successful approach turned out to be the purification of the Steel factor protein.
The cloning of a cDNA encoding the Steel factor was reported simultaneously by three different groups, each of which discovered a different source of the factor.32-34 All three groups used a similar approach; they first purified the protein from medium conditioned by a cell line, obtained N-terminal amino acid sequence, and then made degenerate oligonucleotide primers based on the protein sequence to isolate cDNA clones by polymerase chain reaction (PCR). The three groups named this protein mast cell growth factor, stem cell factor, and c-kit ligand (see below). In this review, we will use the name c-kit ligand (KL) for the protein that binds to the c-kit receptor and is encoded at the Sl locus on mouse chromosome 10 (see below).32,35 36
DISCOVERY OF THE Flt3 TYROSINE KINASE RECEPTOR
In contrast to the discovery of c-kit, analysis of mouse mutations did not play a role in the discovery of the flt3 receptor. This receptor was isolated independently by two groups using distinct cloning strategies.18,19,41 One group used low stringency hybridization with a DNA probe from the M-CSF receptor (c-fms) to isolate a portion of a related DNA sequence that was named flt3 (fms-like tyrosine kinase 3).41 The partial clone was then used to isolate a full-length receptor clone.18
A second group used degenerate oligonucleotides (based on conserved regions within the kinase domain of tyrosine kinase receptors) in a PCR-based strategy to isolate a novel receptor fragment from highly purified murine fetal liver stem cells.19 This fragment was used to isolate a full-length receptor clone given the name flk-2 (fetal liver kinase 2). The flt3/flk-2 receptor has also been referred to as Stk-1 (stem cell kinase-1),17 but this name is not widely used, perhaps because it has been previously designated to denote a gene regulating stem cell kinetics42 as well as a different receptor tyrosine kinase of the met/sea/ron family.43
Comparison of the murine flt3 and flk-2 receptor sequences showed that these sequences differ by only two amino acids in their extracellular domains.44 In contrast, a large number of amino acid differences were seen in a region near their C-terminal ends. The murine flt3 receptor sequence has been independently confirmed by several groups,44-46 and the human receptor sequence is directly homologous to the murine flt3, but not the murine flk-2 sequence.16,17 No independent confirmation of the sequence of flk-2 has been reported. Differences between flt3 and flk-2 sequences are not a result of tissue-specific expression of distinct isoforms.46 The differences in the murine flt3 and flk-2 sequences have never been fully explained, and the validity of the sequence reported as flk-2 is still unclear.47 As a result of this, we refer to the receptor as flt3 and to its ligand as flt3 ligand (FL).
CLONING OF THE LIGAND (FL) FOR THE Flt3 RECEPTOR
A soluble form of the flt3 receptor was the key reagent used by two groups to clone FL. Lyman et al48 screened a variety of cell lines to look for one that expressed a ligand on the cell surface that was capable of binding the soluble receptor. A murine T-cell line was identified that specifically bound the soluble flt3 receptor. The ligand was then cloned from a cDNA expression library made from mRNA isolated from these cells.
An alternative approach employed by Hannum et al49 used an affinity column made with the mouse flt3 receptor extracellular domain to purify FL from medium conditioned by a murine thymic stromal cell line. N-terminal sequencing of the purified protein generated a short amino acid sequence, which was then used to design degenerate oligonucleotide primers to amplify a portion of the FL gene by PCR. Isolation of this FL gene fragment led to the cloning of a full-length murine cDNA.
SPECIES SPECIFICITY OF KL AND FL
No restriction in species specificity has been observed with regard to FL binding or biological activity. Both the mouse and human ligand proteins are fully active on cells bearing either the mouse or human receptors.51 The human FL protein has been found to stimulate mouse, cat (Janis Abkowitz, University of Washington, Seattle, WA, unpublished data), rabbit, nonhuman primate, and human cells. This lack of species specificity of FL is in marked contrast to KL, where the mouse protein is active on human cells but the human protein has limited activity on murine cells.33 Analysis of chimeric mouse/human KL proteins has helped define regions of the protein that regulate its species-specific action.52
STRUCTURE OF THE c-kit AND Flt3 RECEPTORS
The murine and human c-kit receptors are each 976 amino acids in length, have nine potential sites for N-linked glycosylation in their extracellular domains,53,54 and are glycosylated at one or more of these sites.54,55 Immunoprecipitation shows two proteins of approximately 140 kD and 155 kD54; the predicted size of the protein backbone alone is approximately 108 kD. Pulse-chase analysis has shown that the larger 155-kD protein arises from the smaller protein,56 presumably due to glycosylational processing of the protein from one containing high mannose carbohydrates to one containing complex carbohydrates. Furthermore, cell surface iodination of c-kit-expressing cells radiolabels only the larger protein.54 The size of the c-kit protein varies between tissues,55 although whether this is due to differential glycosylation or expression of different isoforms is unclear (see below).
The murine (1,000 amino acids) and human (993 amino acids) flt3 receptors have 9 and 10 potential sites for N-linked glycosylation, respectively, in their extracellular domains16-19 and are also glycosylated at one or more of these sites.44Immunoprecipitation shows two proteins of 130-143 kD and 155-160 kD44,57,58; the predicted size of the protein backbone alone is approximately 110 kD. As with c-kit, pulse-chase analysis has shown that the larger protein arises from the smaller protein44; again, this most likely results from glycosylational processing. Consistent with this interpretation is the finding that only the 158-kD species is found on the cell surface.44 There do not appear to be any O-linked sugars on the protein.59
BINDING OF KL AND FL TO THEIR RECEPTORS
A number of studies have measured the binding affinity of KL to the c-kit receptor60-64 and that of FL to the flt3 receptor.65 Both high (kd, 16 to 310 pmol/L) and low (kd, 11 to 65 nmol/L) affinity binding of KL to its receptor have been reported.60,61,63 Some primary cells and cell lines have only high- affinity sites, whereas others have both.61,63 Neither the number of receptors per cell nor the finding of one or two classes of receptors can be correlated with the ability of cells to proliferate in response to KL.60
The binding affinity of human FL for the flt3 receptor on human myeloid leukemia cells has been estimated to be 200 to 500 pmol/L,65 and only high-affinity binding is seen. The high binding affinity of FL for the flt3 receptor is therefore in the same range of affinities as the binding of KL to c-kit.
The c-kit and flt3 receptors each have five Ig-like domains in their extracellular regions. Mutagenesis studies on c-kit have shown that the first three domains are both necessary and sufficient for binding of ligand66 and that the fourth Ig-like domain is required for dimerization of the receptor,66 although this has recently been called into question.67 Several models have been proposed for binding of KL to c-kit,66-71 but it is beyond our scope to review these studies. Whatever the mechanism responsible for the formation of the complex, the ultimate result is that a dimeric form of the ligand is associated with a dimeric form of the receptor, which results in signal transduction. Although similar studies have not been performed with FL and flt3 receptors, a similar process most likely occurs with this ligand-receptor pair.
ISOFORMS OF THE c-kit AND Flt3 RECEPTORS
Analysis of independently derived cDNA clones has shown that there are two isoforms of both the murine and human c-kit-encoded protein.72 These c-kit receptor isoforms differ by four amino acids (glycine-asparagine-asparagine-lysine, abbreviated GNNK) that are either present or absent just upstream of the transmembrane domain. The different isoforms result from alternative splicing of c-kit mRNAs at a cryptic splice donor site located at the 3′ end of exon 9.73 Although it is not clear if physiologic differences occur because of ligand signaling via one c-kit isoform versus another, ligand-independent constitutive phosphorylation of the receptor occurs only in the isoform missing these four amino acids.72
Crosier et al74 examined expression of the two c-kitisoforms in both leukemic cell lines and in primary acute myeloid leukemias; both isoforms appeared to be expressed in all of the cells examined, with the ratio of GNNK− to GNNK+isoforms ranging from 10:1 to 15:1. A second study confirmed the expression of both isoforms in a series of acute myeloid leukemias.75
In addition to the isoforms discussed above, other variants have been seen in the c-kit receptor. Alternative splicing of mRNAs has been shown to insert an extra serine residue in the cytoplasmic domain at position 715; a survey of human cell lines and acute myeloid leukemia samples shows that both of these isoforms are normally expressed.74
Finally, soluble c-kit receptors are produced by some hematopoietic cell lines in culture,64 and a soluble version of c-kit has been found in human serum at high levels (324 ± 105 ng/mL).76 How this soluble c-kitreceptor is generated is unknown, although it does appear capable of binding KL.60 64 In each of the cases described above, the physiologic significance, if any, of the receptor variant is unknown.
Fewer isoforms of the flt3 receptor have been reported than have been seen with c-kit. One isoform of the murine flt3 receptor is missing the fifth of the five Ig-like regions in the extracellular domain as a result of the skipping of two exons during transcription.77 This alternative isoform is present at lower levels than the wild-type receptor, although it is able to bind ligand and is phosphorylated as a result of this binding. Thus, the fifth Ig domain of flt3 is not required for either ligand binding or receptor phosphorylation. Similarly, the c-kit receptor requires only the first three Ig-like domains for ligand binding.66 The physiologic significance of this flt3 receptor isoform is presently unknown, and a soluble version has not yet been identified in human serum.
STRUCTURES OF THE KL AND FL PROTEINS
The KL and FL proteins are structurally similar to each other (as described below)48-50 and to M-CSF.78 The primary translation product of the KL gene is a type 1 transmembrane protein, ie, the N-terminus of the protein is located outside of the cell. This protein is biologically active on the cell surface.79 The murine and human KL proteins are each 273 amino acids in length, with a 25 amino acid leader, a 185 amino acid extracellular domain, a 27 amino acid transmembrane domain, and a 36 amino acid cytoplasmic tail.
The murine32,79 KL protein has four potential sites for N-linked sugar addition; the human protein has five. KL made by Buffalo rat liver cells is N-glycosylated in a heterogeneous fashion and probably contains O-linked sugars. Analysis of human KL produced by Chinese hamster ovary (CHO) cells shows that it is glycosylated in a somewhat different manner than the rat protein and that it also contains O-linked sugars.80
Circular dichroism spectra of KL shows that it has considerable secondary structure, including both α helical and β sheets.80 There are four cysteine residues that are conserved between KL, FL, and M-CSF. In the case of KL, these form two intramolecular disulfide bonds that establish the three-dimensional structure of the protein.81 Although KL forms homodimers in solution, they are not covalently linked.80 KL is thus different from M-CSF, which contains three intramolecular disulfide bonds and an unpaired cysteine residue that forms an intermolecular disulfide bond.82 Preliminary data suggest that FL also contains three intramolecular disulfide bonds and exists as a noncovalently linked homodimer (Rick Remmele, Immunex, Seattle, WA; unpublished observation).
Mutagenesis studies of mouse and human KL have identified a core region that is required for biological activity; this region constitutes the major portion of the extracellular domain and encompasses all four of the cysteine residues conserved between KL, FL, and M-CSF.83,84 Neither the cytoplasmic, transmembrane, spacer, nor tether regions of KL (Fig 1) is required for biological activity. Similar studies on FL have yielded essentially identical results.85
Sequence alignment of human FL and KL proteins. The figure illustrates that both colony-stimulating factors are type I transmembrane proteins with short cytoplasmic domains; both are likely to be four helix bundle proteins (based on x-ray crystallography data in the case of M-CSF82). The approximate positions of the four helices are shown. The vertical red lines show the locations of introns (to the nearest amino acid) within the genes33,93,95,104 and illustrate their common genomic structure and ancestral origin. Conserved cysteine residues are shaded in color to reflect the formation of proposed intramolecular disulfide bonds (3 in the case of FL and 2 in the case of KL). Possible sites for N-linked glycosylation are boxed. The alignment is based on the one originally proposed by Bazan78 for KL and M-CSF.
Sequence alignment of human FL and KL proteins. The figure illustrates that both colony-stimulating factors are type I transmembrane proteins with short cytoplasmic domains; both are likely to be four helix bundle proteins (based on x-ray crystallography data in the case of M-CSF82). The approximate positions of the four helices are shown. The vertical red lines show the locations of introns (to the nearest amino acid) within the genes33,93,95,104 and illustrate their common genomic structure and ancestral origin. Conserved cysteine residues are shaded in color to reflect the formation of proposed intramolecular disulfide bonds (3 in the case of FL and 2 in the case of KL). Possible sites for N-linked glycosylation are boxed. The alignment is based on the one originally proposed by Bazan78 for KL and M-CSF.
The primary translation product of the FL gene is also a type 1 transmembrane protein. The mouse and human proteins contain 231 and 235 amino acids, respectively. The first 27 (mouse) or 26 (human) amino acids constitute a signal peptide that is absent from the mature protein, followed by a 161 (mouse) or 156 (human) amino acid extracellular domain, a 22 (mouse) or 23 (human) amino acid transmembrane domain, and a 21 (mouse) or 30 (human) amino acid cytoplasmic tail. The cytoplasmic domains of murine and human FL are only 52% identical and are much more divergent than the cytoplasmic domains of murine and human KL (92% identical). Why the cytoplasmic domains of mouse and human FL are so much more divergent in sequence than the cytoplasmic domains of mouse and human KL is unknown. The mouse and human FL proteins each contain two potential sites for N-linked glycosylation. The human FL protein contains N-linked sugars (Claudia Jochheim, Immunex; unpublished observation).
KL AND FL ISOFORMS
The mature mouse and human KL proteins (from which the amino acid signal sequence has been cleaved) undergo proteolytic cleavage to generate a soluble, biologically active, 164-165 amino acid protein.32,33,79,86 The primary site for proteolytic cleavage is encoded within exon six33; however, mutagenesis experiments have shown that there is a secondary proteolytic cleavage site just upstream of the transmembrane region within exon 7.87 This secondary site is used only if the primary site is missing, which can occur by splicing out the sixth exon.79,88 89
Splicing has been suggested to be a method of regulating the generation of soluble versus membrane-bound forms of the protein. Alternative splicing of the sixth exon of the KL gene has been reported in both mouse and human cells.40,79,88,90,91 The cell-bound form of KL appears to be required for normal development in mice since a mutation (Sld) that eliminates the membrane-bound form of the factor, but still makes a biologically active soluble form, results in developmental abnormalities.88,92 Huang et al90 showed that there is tissue-specific expression of the different isoforms. The physiologic significance of these altered isoform ratios is unknown but presumably reflects the capacity of each tissue to produce a form of KL that is capable of interacting with specific c-kit-expressing cells.
It is unclear what regulates the proteolytic cleavage of KL, and what, if any, the physiologic effects of this process are. The protease responsible for cleavage of KL has not been identified, and it is unknown if it is the same protease that generates soluble, biologically active forms of M-CSF and FL.48,49 93
Multiple isoforms of both mouse and human FL have been identified by analysis of multiple cDNA clones and PCR.48-50,94 The biological significance of these isoforms is presently unknown. The predominant isoform of human FL is the transmembrane protein that is biologically active on the cell surface.48-50 This isoform is also found in the mouse, although it is not the most abundant isoform in that species (see below). The transmembrane FL protein can be proteolytically cleaved to generate a soluble form of the protein that is also biologically active.48 Neither the protease responsible for this cleavage nor the exact site in the FL amino acid sequence where cleavage occurs has been identified.
The most abundant isoform of murine FL95 is an alternative, 220 amino acid form that is membrane bound, but is not a transmembrane protein.49,94 This form arises due to a failure to splice an intron from the mRNA. This leads to a change in the reading frame, which terminates in a stretch of hydrophobic amino acids that serve to anchor the protein in the membrane.50 This isoform is missing the spacer and tether regions that contain the proteolytic cleavage site seen in the transmembrane isoform. As a result, this membrane-associated isoform is resistant to proteolytic cleavage,94 although it is biologically active on the cell surface. This isoform has not been identified in any human FL cDNAs examined.
A third FL isoform identified in mouse94 and human95 tissues arises because of an alternatively spliced sixth exon. This exon introduces a stop codon near the end of the extracellular domain and thereby generates a soluble, biologically active protein that appears to be relatively rare compared with other isoforms.95 Another method of generating soluble FL in the human is to splice out the transmembrane domain,50 but the relative abundance of this isoform has not been quantitated.
There is a difference between KL and FL in regard to their alternatively spliced sixth exons. The amino acids in exon 6 of mouse and human KL are nearly identical, whereas those of mouse and human FL have virtually no homology.95 In the case of KL, the sixth exon is normally part of the transmembrane protein and contains the proteolytic cleavage site. In the case of FL, it is not a part of the transmembrane protein; introduction of the sixth exon results in the generation of a soluble protein due to a shift in the reading frame. Thus, evolution has made two different uses of the sixth exon of KL and FL, allowing the generation of a soluble protein by different mechanisms.
STRUCTURE OF THE GENOMIC LOCI ENCODING THE c-kit AND Flt3 RECEPTORS
The genomic loci encoding the c-kit, flt3, and c-fms receptors share overall conservation of exon size, number, sequence, and exon/intron boundary positions,96 and these genes have likely arisen from a common ancestral gene. The genomic loci encoding the mouse97 and human98-100 c-kit receptors show clear evidence of evolutionary conservation. The coding region of the c-kitreceptor encompasses 21 exons, and both the mouse and human loci span more than 70 kb of genomic sequence.
STRUCTURE OF KL AND FL GENOMIC LOCI
The genomic locus encoding KL has been cloned from the human,33 rat,33 and mouse.103 The human KL locus is more than 50 kb in length (Vann Parker, Amgen, Thousand Oaks, CA; personal communication) and consists of eight exons that contain the entire coding region of the protein. The intron:exon boundaries identified within the rat, human, and murine genes occur at identical positions. In the case of the mouse protein, a ninth exon is present and encodes the C-terminal end of the cytoplasmic domain.103
The genomic loci encompassing the coding regions of mouse and human FL are approximately 4.0 kb and 5.9 kb, respectively; the coding region comprises 8 exons.95 The human FL locus is thus significantly smaller than the human KL locus. The sizes of the individual FL exons are well conserved between species,95although the intron sizes are much more variable.
The genomic locus encoding M-CSF also contains eight exons.104 A comparison of exon sizes between FL, KL, and M-CSF shows that identically numbered exons are similar in size in all three proteins.95 If the sizes of the exons are taken as a measure of overall relatedness, then M-CSF and KL are more closely related to each other than they are to FL. For example, the sizes of exons 3 and 4 are identical between M-CSF and KL, but are not the same as the corresponding exons in FL. The location of the introns in the three genes are also fairly well conserved, indicating that these proteins are probably ancestrally related.
CHROMOSOMAL LOCATION OF c-kit AND Flt3 RECEPTORS
The murine c-kit locus is located in the D-E region of mouse chromosome 511,12 near two other tyrosine kinase receptors (PDGF A and flk-1/KDR). The murine flt3 receptor gene is also on chromosome 5, but at the G region.41 The flt3 receptor105 is located less than 350 kb from the murine flt tyrosine kinase receptor106 but is separated from the clustered c-kit, PDGF A, and flk-1/KDR receptors.
The human c-kit locus is on the centromeric region of chromosome 4, in the area of 4q31-34,534q11-21,54 and 4q11-12.107 The gene encoding the human flt3 receptor maps to chromosome 13q12,41 again near the flt receptor locus. The flt3 and flt genes are linked105 in a head to tail fashion and are separated by about 150 kb.101
CHROMOSOMAL LOCATION OF KL AND FL GENES
The KL gene is, as expected, encoded on mouse chromosome 10 and is deleted in some, but not all, Sl alleles.32,35,36The FL gene maps to the proximal portion of mouse chromosome 7.94
The gene encoding human KL has been mapped to chromosome 12q22-2440 and 12q14.3-qter108 in a region that is syntenic with mouse chromosome 10. The human FL gene maps to chromosome 19q13.3-13.4,94 109 which is syntenic with mouse chromosome 7. The chromosomal locations of KL, FL, M-CSF, and their receptors are summarized in Table 1.
Chromosomal Locations of the c-kit,c-fms, and Flt3 Receptors and Their Ligands
| . | Mouse . | Human . |
|---|---|---|
| Receptors | ||
| Flt3 | 5G | 13q12 |
| c-kit | 5D-E | 4q11-34 |
| c-fms | 18 | 5q32-33 |
| Ligands | ||
| FL | 7 | 19q13.3-13.4 |
| KL | 10 | 12q14.3-qter |
| M-CSF | 3 | 1p13-21 |
| . | Mouse . | Human . |
|---|---|---|
| Receptors | ||
| Flt3 | 5G | 13q12 |
| c-kit | 5D-E | 4q11-34 |
| c-fms | 18 | 5q32-33 |
| Ligands | ||
| FL | 7 | 19q13.3-13.4 |
| KL | 10 | 12q14.3-qter |
| M-CSF | 3 | 1p13-21 |
GENETIC ALTERATIONS IN c-kit AND KL GENES
The exact defect in the c-kit receptor has now been identified at the molecular level for a number of alleles of the Wlocus.24-28 Most of the alleles result from point mutations in the cytoplasmic domain of the receptor; these changes decrease the tyrosine-phosphorylating activity of the protein. However, in several cases, the mutations appear to be of a regulatory instead of a structural nature and result in reduced expression of the c-kitreceptor.
There is a rare, autosomal dominant genetic disease in humans known as piebald trait. Affected individuals have a white forelock and large, nonpigmented patches on the chest and/or other areas. All cases of piebald trait that have been molecularly analyzed result from missense or frameshift mutations in the c-kit tyrosine kinase receptor (Ezoe110 and references therein). Affected individuals are heterozygous for defects in the c-kit protein; the dominant nature of the trait reflects the dominant-negative effects of the mutant c-kit allele. The dominant-negative effects of these mutations are thought to result because receptor dimerization is required for proper biological function.
Because pigmentation defects in W and Sl mice are often indistinguishable, it would be reasonable to expect that at least some cases of piebald trait in humans would arise from mutations in the KL gene, ie, from a defect in the ligand instead of the receptor. However, no defects in the KL gene have been reported in piebald humans. Piebald trait thus represents the human homologue of the W mutation in mice.
Mutations at the Steel locus35 have occurred spontaneously or have been induced by chemical mutagenesis, x-ray irradiation, or transgene insertion.111 In addition to theSld mutation (see above), the molecular defect responsible for three other Sl mutations has been identified. In the Sl17H mutation,103 the cytoplasmic tail of KL is altered as a result of a splicing defect; in contrast, the Slcon and Slpanmutations are of a regulatory nature and result in altered, tissue-specific expression of mRNAs encoding KL.112
GENETIC ALTERATIONS IN Flt3 RECEPTOR AND FL GENES
In contrast to the well-described mutations in the c-kitreceptor and its ligand (see above), there are no reports of any genetic defects associated with either the flt3 receptor or its ligand.
As described above, FL maps to human chromosome 19q13.3. Trisomy 19 is strongly associated with myeloid malignancies.113 However, whether overexpression of FL plays a role in the increased incidence of leukemia in trisomy 19 remains to be determined.
EXPRESSION OF KL AND FL IN MOUSE AND HUMAN HEMATOPOIETIC TISSUES
The expression of the c-kit and flt3 receptors, and not their ligands, is the key to understanding the function of these growth factors. Numerous studies have shown that both KL and FL are widely expressed in different tissues, in contrast to their receptors, which are expressed on a more limited number of cells, especially in the case of flt3. KL is widely expressed during embryogenesis,114-116 suggesting that KL may affect the growth, survival, and/or differentiation of cells in addition to the three lineages (hematopoietic cells, germ cells, and melanocytes) shown to be affected in both W and Slmutant mice. Cells expressing KL are frequently contiguous with cells expressing c-kit, ie, ligand and receptor expression are complementary. KL is expressed on stromal cells,117,118fibroblast,26,79,119 endothelial cells,117visceral yolk sac,115 and other places.
FL, like KL, is widely expressed in both murine and human tissues.49,50,94 Highest levels of FL mRNA on human tissue Northern blots are in peripheral blood mononuclear cells, but the ligand is also expressed in almost every tissue that has been examined.48-50 Mouse developmental in situ hybridization studies have not yet been performed with FL, although it would be interesting to see how the distribution of FL would compare with flt3 receptor.120
EXPRESSION OF c-kit AND Flt3 RECEPTORS ON HEMATOPOIETIC CELL LINES
Expression of the c-kit receptor has been extensively surveyed on mouse and human hematopoietic cell lines (Table 2). It is seen on only a small percentage of myeloid and myeloblastic cell lines.121-124In contrast, the majority of erythroid and erythroleukemia cell lines express c-kit,121-123,125 as do virtually all megakaryocytic cell lines.121,123,125 Mast cell lines generally express c-kit.51,126-128 In contrast, expression of c-kit is generally not seen on lymphoid leukemia cell lines (including pre-B, B, and T cells),121,123,125 on B-cell or T-cell lymphoma cell lines,121,122,125 or on myeloma cell lines.121
Expression of c-kit and Flt3 Receptors on Murine and Human Cell Lines
| . | c-kit . | Flt3 . |
|---|---|---|
| Myeloid | Few positive | Mostly positive* |
| Monocytic | Few | About 50% |
| Erythroid | Most | Few |
| Megakaryocytic | Most | Few |
| Mast cell | All | None |
| Lymphoid | ||
| Pro-B | None | Most |
| Pre-B | None | Most* |
| B | None | Few |
| T | None | Few |
| Mature NK | ND | None |
| Lymphomas | None | About 25% |
| Myeloma | None | None |
| . | c-kit . | Flt3 . |
|---|---|---|
| Myeloid | Few positive | Mostly positive* |
| Monocytic | Few | About 50% |
| Erythroid | Most | Few |
| Megakaryocytic | Most | Few |
| Mast cell | All | None |
| Lymphoid | ||
| Pro-B | None | Most |
| Pre-B | None | Most* |
| B | None | Few |
| T | None | Few |
| Mature NK | ND | None |
| Lymphomas | None | About 25% |
| Myeloma | None | None |
Results tabulated from a large number of reports. For individual references, see the sections of this report detailing the expression patterns for each of these receptors.
Abbreviation: ND, not determined.
Different expression patterns have been reported on mouse versus human cells; see text for details.
Flt3 receptor expression on mouse and human cell lines is quite different from that of c-kit. No flt3 expression is seen on any of the mouse myeloid, macrophage, erythroid, megakaryocyte, or mast cell lines examined46,129 or most early mouse B-cell lines, but it has been reported on several mature B-cell lines.129This lack of expression is different from what is seen on most human pre-B-cell lines, which do express flt3 receptor.123,130 In addition, flt3 expression has been seen on only one mouse pro-T cell line, but not on any T-cell lines.46 129
A number of studies have been published that show expression of flt3 receptor on a limited range of human cell lines. The flt3 receptor is found on a high percentage of human myeloid and monocytic cell lines,123,129,130 in contrast to mouse cell lines.46,129 No flt3 expression is seen on myeloma cell lines,129,130 and only a few megakaryocytic cell lines are positive.123,129,130 All erythroid and erythroblastic cell lines are flt3 negative as well.129 130
EXPRESSION OF c-kit AND Flt3 RECEPTORS ON PRIMARY HUMAN LEUKEMIAS
Both the c-kit and flt3 receptors are frequently seen on acute myelogenous leukemia (AML) blasts. The c-kit protein is expressed on blast cells obtained from a high percentage of patients with AML from all French-American-British (FAB) subtypes.61,124,131-139 Receptor levels on AML blast cells are variable, but in general are similar to or less than c-kitlevels on normal stem and progenitor cells.140
Expression of the flt3 receptor in primary leukemias has also been investigated and recently reviewed.141 As with c-kit, the majority of adult AML samples from all FAB classes are positive for flt3 receptor expression.57 142-146
Among lymphoid leukemias, little or no expression of c-kit is observed on blast cells in acute lymphoblastic leukemia (ALL).133,143 c-kit is expressed on Reed-Sternberg cells in about half of Hodgkin's disease patients as well as on some anaplastic large-cell lymphoma samples.147
All B-lineage ALL samples examined are flt3 receptor positive,142-144 as are most hybrid (also known as mixed or biphenotypic) leukemia samples.144 The greatest variability reported in flt3 receptor expression is on T-lineage ALL, which have been reported to be all negative,142 have a small percentage that are positive,143 or have about half of the samples positive.144 In contrast, both T-cell and B-cell lymphomas are negative for flt3 receptor expression.144Tandem in-frame duplications in the juxtamembrane region of the human flt3 receptor have been reported to be associated with both leukocytosis148 and leukemic transformation.149
The c-kit receptor is expressed on a majority of samples from chronic myelogenous leukemia (CML) patients in blast crisis134,150 and at least some samples of chronic phase CML138 and CML in blast transition.151 In contrast, almost all chronic-phase or accelerated-phase CML samples are negative for flt3 receptor expression.143,144 However, about two thirds of the samples from CML patients in blast crisis are flt3 receptor positive.143 144
RESPONSIVENESS OF PRIMARY LEUKEMIA CELLS TO KL AND FL
AML.
Numerous studies have been performed on human leukemia samples to determine whether the cells proliferate in response to KL, FL, or other growth factors, although a lack of proliferation should not necessarily be considered negative expression. For example, a growth factor could drive differentiation or inhibit apoptosis; in fact, both KL152 and FL153 have been shown to have this latter effect. In the case of nonproliferative cells, the cells may be truly nonresponsive or may be producing endogenous ligand, and thus are refractory to exogenously added growth factor.
c-kit receptor expression is variable among AML FAB subtypes and does not predict responsiveness to KL.145 The majority of AML samples proliferate in response to KL.61,131,137,154,155 Many of these studies show that KL synergizes with other cytokines to enhance the proliferation of leukemic blast cells. Some AML cell lines express KL in addition to c-kit,140,156 suggesting that an autocrine loop may play a role in the transformation of these cells. However, the low level of KL expression on some AML cells has led one group to conclude that a c-kit and KL autocrine cycle is not common in AML.140
Whether flt3 receptor or its ligand play a causal role in the development of human leukemias has not been determined. A large percentage of AML cells from children142 and adults145,146 proliferate (as measured by both [3H]-thymidine incorporation or colony formation) in response to FL. Within age groups (children or adults), some FAB subtypes show a greater response compared with others.142 146 It is unclear whether there is a difference in the FL responsiveness of flt3 receptor-positive AML samples of different FAB subtypes from children and adults because not enough samples of each FAB subtype have been analyzed.
Primary AML samples that proliferate in response to FL also frequently proliferate in response to granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-3 (IL-3), and KL, and additive or synergistic responses are observed. Some AML cells are therefore similar to normal hematopoietic progenitor cells in that both show synergistic responses to FL in combination with other cytokines. Many of the AML samples that do not proliferate in response to FL do proliferate in response to other cytokines,142 indicating that the cells do not lack a general capacity to proliferate. In summary, flt3 receptor expression on AML samples is not predictive of FL responsiveness, just as c-kit expression is not predictive of KL responsiveness.
CML.
KL can weakly stimulate the proliferation of CML blast cells on its own and strongly stimulate them in the presence of IL-3 and/or GM-CSF.138 Culturing of bone marrow (BM) cells from CML patients in the presence of KL favors the growth of malignant progenitor cells.157 In contrast, preliminary results suggest that FL favors the outgrowth of benign progenitors from 5-FU-treated CD34+ CML BM cells at the expense of malignant cells158 and that FL generates a significantly greater percentage of normal progenitors (Philadelphia chromosome-negative cells) compared with KL.
ALL.
Because c-kit is not generally expressed on ALL cells,124,133,134,139 the capacity of these cells to proliferate in response to KL has not been examined. As mentioned above, all B-lineage ALL and some T-lineage ALL samples express flt3 receptor. However, only a small percentage of B-lineage ALL samples proliferate in response to FL.142
In one study, pediatric T-lineage ALL samples did not proliferate in response to FL, but none of these samples was positive for flt3 expression.142 In a separate study on a variety of ALLs, several flt3 receptor-positive samples proliferated in FL.159 However, the majority of samples failed to proliferate in FL, even though they were flt3 receptor positive.159 Flt3 receptor expression is therefore not predictive for proliferation of ALL cells to FL in vitro.
EXPRESSION AND FUNCTION OF c-kit AND Flt3 IN THE HEMATOPOIETIC HIERARCHY
Studies of cytokine receptor expression have proven valuable in pinpointing where specific ligand-receptor pairs have biological activities. Not only can such studies identify cell types in which a specific receptor might be important, they also allow functional characterization of distinct cell populations separated based on various levels of receptor expression. The expression of c-kitand flt3 in the hematopoietic system has been studied in detail, and in the following sections we review the findings of flt3 and c-kitexpression on various cell types (summarized in Fig 2), followed by the in vitro biological effects (summarized in Table 3) of FL and KL on the same cell types. It is important to emphasize that the extensive c-kitand flt3 expression studies to be described have inherent limitations. Most expression studies have been performed by flow cytometric evaluation of cell-surface c-kit and flt3 expression. Because flow cytometry has a rather high detection limit (∼500 molecules/cell), so- called c-kit− and flt3− populations might prove to express low levels of c-kit and flt3, respectively. On the other hand, reverse transcriptase-PCR (RT-PCR) detection of c-kitand flt3 mRNA has much greater sensitivity, but unless performed at the single-cell level does not provide a quantitative measurement of c-kit+ and flt3+ cells. Thus, a minor contaminating (nonrelevant) cell type might account for detected expression (particularly relevant for heterogenous primary cell populations).
c-kit and Flt3 expression in the hematopoietic hierarchy. The figure indicates expression of c-kit (red, upper symbol on side of each cell) and flt3 (green, lower symbol on side of each cell) on various classes of hematopoietic stem and progenitor cells as well as mature blood cells, as described in the text. Because most hematopoietic cell populations are heterogeneous and hard to purify, it is not possible to exclude c-kit and/or flt3 expression on a minority of cells in the different cell populations. Therefore, the figure illustrates the c-kit and flt3 receptor status on the majority of cells within a specific population, based on studies of receptor expression and/or functional studies. As discussed in the text, the proposed hierarchy of pluripotent stem cells is based solely on different levels of c-kit and flt3 expression and does not take into account other stem cell antigens/characteristics, which are likely to uncover additional heterogeneity. Symbols: (−) most/all cells appear to lack c-kit or flt3 expression; (+) most/all cells appear to express c-kit or flt3; (+/−) the cell type appears to consist of significant receptor-positive as well as receptor-negative populations; (?) sufficient expression or functional data not available; (high and low) cell populations have been separated based on high and low levels of c-kit expression. Abbreviations: BFU, burst-forming units; CFU, colony-forming units; E, erythroid; Mk, mega karyocyte; G, neutrophilic progenitor; M, monocyte/macrophage; DC, dendritic cell; Baso, basophil; RBC, red blood cell; NK, natural killer cell.
c-kit and Flt3 expression in the hematopoietic hierarchy. The figure indicates expression of c-kit (red, upper symbol on side of each cell) and flt3 (green, lower symbol on side of each cell) on various classes of hematopoietic stem and progenitor cells as well as mature blood cells, as described in the text. Because most hematopoietic cell populations are heterogeneous and hard to purify, it is not possible to exclude c-kit and/or flt3 expression on a minority of cells in the different cell populations. Therefore, the figure illustrates the c-kit and flt3 receptor status on the majority of cells within a specific population, based on studies of receptor expression and/or functional studies. As discussed in the text, the proposed hierarchy of pluripotent stem cells is based solely on different levels of c-kit and flt3 expression and does not take into account other stem cell antigens/characteristics, which are likely to uncover additional heterogeneity. Symbols: (−) most/all cells appear to lack c-kit or flt3 expression; (+) most/all cells appear to express c-kit or flt3; (+/−) the cell type appears to consist of significant receptor-positive as well as receptor-negative populations; (?) sufficient expression or functional data not available; (high and low) cell populations have been separated based on high and low levels of c-kit expression. Abbreviations: BFU, burst-forming units; CFU, colony-forming units; E, erythroid; Mk, mega karyocyte; G, neutrophilic progenitor; M, monocyte/macrophage; DC, dendritic cell; Baso, basophil; RBC, red blood cell; NK, natural killer cell.
In Vitro Effects of KL and FL in the Murine and Human Hematopoietic System
| Cell Type . | Response . | KL . | FL . |
|---|---|---|---|
| Primitive progenitors/candidate stem cells | Growth | Synergy | Synergy |
| Viability | + | + | |
| Adhesion | + | ND | |
| Erythroid progenitors | |||
| BFU-E | Growth | Synergy | − |
| Adhesion | + | − | |
| CFU-E | Growth | + | − |
| Myeloid (GM) progenitors | Growth | Synergy | Synergy |
| Viability | + | + | |
| Adhesion | + | ND | |
| Megakaryocytopoiesis | |||
| BFU-Mk/CFU-Mk | Growth | + | + |
| Mk maturation | + | − | |
| Mast cells | Growth | + | − |
| Maturation | + | − | |
| Adhesion | + | − | |
| Migration | + | − | |
| Activation | + | − | |
| B lymphopoiesis | |||
| Murine stem cells | Growth/ commitment | Weak | Strong |
| Murine pro-B cells | Growth | Synergy | Synergy |
| Human pro-B cells | Growth | − | Synergy |
| T lymphopoiesis | |||
| Murine pro-T cells | Growth | Synergy | Synergy |
| Human pro-T cells | Stromadependent growth | Synergy | Synergy |
| NK cells | |||
| NK cell progenitors | Growth | Synergy | Synergy |
| NK cells | Growth | Synergy | ND |
| Viability | + | ND | |
| Dendritic cells | |||
| DC progenitors | Growth | Synergy | Synergy |
| Cell Type . | Response . | KL . | FL . |
|---|---|---|---|
| Primitive progenitors/candidate stem cells | Growth | Synergy | Synergy |
| Viability | + | + | |
| Adhesion | + | ND | |
| Erythroid progenitors | |||
| BFU-E | Growth | Synergy | − |
| Adhesion | + | − | |
| CFU-E | Growth | + | − |
| Myeloid (GM) progenitors | Growth | Synergy | Synergy |
| Viability | + | + | |
| Adhesion | + | ND | |
| Megakaryocytopoiesis | |||
| BFU-Mk/CFU-Mk | Growth | + | + |
| Mk maturation | + | − | |
| Mast cells | Growth | + | − |
| Maturation | + | − | |
| Adhesion | + | − | |
| Migration | + | − | |
| Activation | + | − | |
| B lymphopoiesis | |||
| Murine stem cells | Growth/ commitment | Weak | Strong |
| Murine pro-B cells | Growth | Synergy | Synergy |
| Human pro-B cells | Growth | − | Synergy |
| T lymphopoiesis | |||
| Murine pro-T cells | Growth | Synergy | Synergy |
| Human pro-T cells | Stromadependent growth | Synergy | Synergy |
| NK cells | |||
| NK cell progenitors | Growth | Synergy | Synergy |
| NK cells | Growth | Synergy | ND |
| Viability | + | ND | |
| Dendritic cells | |||
| DC progenitors | Growth | Synergy | Synergy |
Cell types or responses in which neither KL nor FL are known to have an effect are not listed.
Abbreviations: −, no effect found on indicated response (in some cases not specifically investigated but cell type lacks receptor for indicated ligand); +, stimulatory effect of ligand alone on indicated response; synergy, effect predominantly through synergistic interaction with other cytokines; ND, not determined.
EXPRESSION OF c-kit AND Flt3 ON MATURE BLOOD CELLS
c-kit and flt3 expression in the hematopoietic system appear predominantly restricted to the progenitor/stem cell compartment (outlined in the following sections). However, some differentiated blood cells also express these receptors (Fig 2).
c-kit is expressed on primary mast cells as well as mast cell lines and primary neoplastic mast cells.160 In addition, c-kit is constitutively activated in a number of mast cell tumor lines (mastocytomas),127,161 but mast cells do not express flt3.128
There are other differentiated hematopoietic cells that express c-kit and/or flt3, although the functional significance is less clear. In mouse BM, very low levels of c-kit can be detected on promyelocytes and myelocytes, but not on neutrophils.162 Approximately 50% of murine BM eosinophils and monocytes express low levels of c-kit.162 Seven percent of lymphocytes in murine BM express high levels of c-kit.162 However, still other studies suggest that mature B and T cells do not express c-kit; therefore, this small fraction of c-kit+ cells might represent B- and T-cell precursors/progenitors.163-165
Similar studies have revealed that flt3 expression in murine BM is restricted to blast cells, monocytes, and a small fraction of lymphocytes.166 Nucleated murine erythroid cells lack both c-kit and flt3 expression.162,166 Early murine megakaryocytes (stage I and II) express c-kit, whereas the most mature (stage III) megakaryocytes appear to be c-kit−.167 Also, human megakaryocytes express c-kit,61,168 but not flt3.169 In addition, activated but not resting platelets express c-kit.170
Initial studies indicated that flt3 mRNA is expressed by murine B and T cells from thymus, spleen, and peripheral blood.18 However, several later studies of mature murine B and T cells suggest that these do not express flt3.166 171 Thus, the initial findings potentially were due to a small fraction of contaminating flt3+ cells, such as more primitive B- and T-cell progenitors.
Peripheral human blood cells contain less than 0.1% c-kit+ cells, suggesting that very few mature human blood cells express c-kit.172-174 c-kit is constitutively expressed on a small subset of resting human NK cells in peripheral blood that are characterized by high CD56 expression, whereas c-kit is not expressed on the larger fraction of more differentiated NK cells with low CD56 expression.175 These c-kit+ NK cells appear to be the only mature, resting lymphocytes that constitutively express c-kit.
No expression of flt3 mRNA has been reported on mature lympohematopoietic cells fractionated from human peripheral blood17 or B cells, T cells, monocytes, or granulocytes.144 However, in other studies, monocytes and granulocytes have been shown as weakly positive at the mRNA and cell-surface level.16 176
RESPONSE OF MAST CELLS TO KL, BUT NOT FL
The effects of KL on mast cell populations have been extensively reviewed6 and will be only briefly summarized here. KL regulates the migration, maturation, proliferation, and activation of mast cells in vivo.6 Injection of recombinant KL into rodents,86,177 primates,178 or humans179 results in an increase in mast cells at both the site of injection and at distant sites. Treatment of rats with KL generates both connective tissue mast cells and mucosal mast cells.177 Animals treated with KL generally do not appear to suffer from serious adverse events despite the large-scale expansion of mast cells in vivo.178 However, at least one study has shown that KL administration to mice leads to degranulation of mast cells in the lungs, which leads to acute respiratory distress.180 The effects of KL on mast cells may have a significant impact on the clinical potential of this molecule for humans.179,181 182
COMMITTED MYELOID PROGENITOR CELLS ARE c-kit+Flt3+ OR c-kit+Flt3−, WHEREAS EARLY ERYTHROID PROGENITOR CELLS APPEAR TO BE ONLY c-kit+Flt3−
Half of c-kit+ murine BM cells coexpress lineage-specific cell surface antigens such as GR-1 and MAC-1 (Lin+), characteristic of cells committed to the myeloid lineage, whereas the remaining half express higher levels of c-kit and are Lin−, suggesting that uncommitted progenitor cells might express higher levels of c-kit than those committed to the myeloid lineage.183 Indeed, murine in vitro clonogenic progenitor cells committed to the myeloid lineage and colony-forming units-spleen (CFU-S) progenitors are almost completely depleted in c-kit− BM cells, showing that most, if not all, clonogenic myeloid progenitor cells express c-kit.183-188
Most c-kit+ human BM and fetal liver cells express the progenitor-associated CD34 antigen,172-174 suggesting that overlapping (but not identical) populations each express these two progenitor cell antigens. c-kit+ human BM and fetal liver cells are highly enriched and contain all or most in vitro clonogenic progenitor cells with a myeloid (granulocyte/monocyte), megakaryocytic, and/or erythroid potential.172-174 189
CD34highCD64+ cells, which are virtually a pure population of human GM progenitor cells, express high levels of c-kit, whereas the more mature CD34lowCD64+ cells express lower levels of c-kit,190 suggesting downregulation of c-kit expression during GM differentiation. Similarly, erythroid progenitor cells (CD34highCD64−CD71high and CD34lowCD64−CD71high) also express high levels of c-kit.190 Although some studies have suggested that a subclass of mature erythroid progenitor cells (colony-forming units-erythroid [CFU-E]) might not be KL-responsive, c-kit expression has been demonstrated on human CFU-E and erythroblasts.174 The vast majority of human megakaryocyte progenitor cells (burst-forming unit-megakaryocyte [BFU-Mk] as well as colony-forming unit-megakaryocyte [CFU-Mk]) are also c-kit+.191
Whereas almost 90% of murine BM blast cells express c-kit,162 flt3 expression is restricted to 30% of murine BM blast cells.166 The majority of lineage-restricted murine myeloid and erythroid BM progenitor cells are Lin−Sca-1− and express c-kit.188 However, less than half of these Lin−Sca-1−c-kit+progenitors express flt3.166
More than 60% of flt3+ human BM cells coexpress CD33, a myeloid cell-surface antigen, suggesting that flt3 might be expressed on subsets of myeloid progenitor and/or mature cells.57 Most human CD34+ BM and cord blood cells express flt3, and most GM progenitors express flt3, whereas CD34+flt3+ cells are depleted in erythroid progenitors.176 The majority of CD34+c-kit+ BM and cord blood cells coexpress flt3, but a significant (10% to 25%) population is flt3−.
Flt3 appears to be shut off before erythroid differentiation and gradually downregulated during GM differentiation.192 In contrast, c-kit expression is gradually downregulated during both erythroid and GM differentiation.192 Thus, flt3 appears to be expressed on subpopulations of myeloid (GM) progenitor cells, but not on erythroid progenitor cells.
Myeloid-derived dendritic cell (DC) progenitors appear to express c-kit and flt3, because they respond to KL and FL in combination with other cytokines (see DC section for details). However, neither ligand has been shown to have effects on mature DC.193-196
ERYTHROID PROGENITOR CELLS: KEY ROLE OF KL AND ABSENCE OF FL RESPONSE
Besides the mast cell deficiency, the dominating hematopoietic defect resulting from severe mutations in the W or Sl loci is a macrocytic anemia.6,10 KL enhances the in vitro cloning frequency as well as the clonal size of murine79,197 and human33,172,174,198-200 erythroid progenitor cells. KL has its most potent growth promoting effects on early erythroid progenitor cells (BFU-E), whereas more mature progenitors (CFU-E) are less responsive to KL-stimulation.172-174,191 201
The effects of KL on the growth of BFU-E are predominantly synergistic and require costimulation with erythropoietin (EPO).79,172,174,197-200 However, KL can, in combination with IL-6 and soluble IL-6 receptor, promote EPO-independent growth of human BFU-E in vitro.202 Furthermore, c-kit might activate the EPO receptor by inducing its phosphorylation on tyrosine.203 KL also promotes the adhesion of human BFU-E to fibronectin.204
MEGAKARYOCYTE PROGENITOR CELLS: POTENT GROWTH-PROMOTING EFFECTS MEDIATED THROUGH c-kit BUT NOT Flt3
Although Sl/Sld mice have normal levels of platelets, their BM displays reduced numbers of mature megakaryocytes and megakaryocyte progenitor cells.209-211 Administration of KL to Sl/Sld mice not only reverses the macrocytic anemia, but results in enhanced platelet production.36 In vitro, KL enhances megakaryocyte progenitor cell cloning frequency and growth potential in combination with other cytokines, including GM-CSF, IL-3, IL-6, and IL-11.168,212-215 Whereas some studies have found little or no effect on megakaryocyte maturation and ploidy, others have suggested that KL can promote megakaryocyte maturation and ploidy,216and subsets of early megakaryocytes express c-kit.167
Thrombopoietin (TPO) is the primary regulator of megakaryocyte and platelet production,217 and KL appears to interact with TPO at two levels in the hematopoietic hierarchy. First, a synergistic interaction is observed on committed megakaryocyte progenitor cells, enhancing megakaryocyte production.217-221 In addition, KL and TPO interact synergistically on candidate murine and human stem cell populations to stimulate multilineage growth in vitro.222-226 Thus, the primary role of KL in platelet production might be through its interaction with TPO.
Unlike W/Wv andSl/Sld mice, flt3 knockout mice have not been reported to have any defects in megakaryocyte and platelet production,227 and FL alone or in combination with IL-3, KL, or TPO has no effect on in vitro growth of murine megakaryocyte progenitor cells.65 Similarly, FL has no effect on megakaryocyte ploidy by itself or in combination with TPO.65 In contrast, FL acts synergistically with TPO to enhance the growth of candidate murine stem cells.223
Some data suggest that FL might have effects on human megakaryocytopoiesis. Some megakaryocytic leukemic cell lines, as well as primary megakaryoblastic leukemic cells, express flt3, although less frequently than c-kit.65,123,130 In addition, studies of FL effects on primary BM cells have demonstrated effects on megakaryocyte formation.228 Unlike KL, FL has been reported to have no synergistic interaction with TPO on in vitro clonogenic growth of human megakaryocyte progenitor cells.169 Thus, the finding that FL and TPO synergistically promote prolonged megakaryocyte progenitor cell formation in long-term cultures of human CD34+ cord blood cells229 could result from a recruitment of primitive (uncommitted) progenitor cells that might subsequently become responsive to TPO alone.
EXPRESSION OF c-kit AND Flt3 ON LYMPHOID PROGENITORS AND PRECURSORS
About 25% of B220+ murine BM cells express c-kit, accounting for more than half of the total c-kit+cells.164 However, no BM cells (or fetal liver cells) expressing cytoplasmic μ coexpress c-kit, suggesting that c-kit expression is restricted to the earliest stages of B-cell progenitors, whereas the pre-B-cell and subsequent stages are c-kit−.163,164,230 231
Flt3 mRNA is expressed in early murine pre-pro and pro-B cells, whereas pre-B cells, as well as immature and mature B cells, are devoid of flt3 expression.171 A similar pattern of flt3 expression is seen at the cell surface of pro-B, pre-B, and mature B cells.166c-kit is also expressed at low levels on subsets of human pro-B cell progenitor cells (CD34+CD19+).173,189,190Twenty-five percent of BM CD34+CD19+ (pro-B cells) express flt3, as do subfractions of CD10+ and CD20+ B-cell precursors.176
c-kit is expressed at high levels on the most primitive subsets of murine fetal and adult thymocytes, including CD4−CD8−CD3−CD44+CD25+pro-T cells and more primitive CD4loCD8−CD3− thymocytes, the latter cells also having the potential to develop into B cells.165,232-235 When thymocytes develop into CD4−CD8−CD3−CD44−CD25+pre-T cells, they still express low levels of c-kit, which is lost in later stages of T-cell development.165
Like c-kit, flt3 expression is restricted to the most immature CD4−CD8− murine thymocytes, whereas more mature thymocytes expressing CD4 and/or CD8 are flt3−.19
Because human NK cell progenitor cells respond to KL or FL (see separate section), they most likely express c-kit and flt3. However, there is as yet no direct evidence for c-kit or flt3 expression on NK cell progenitor cells, and the few human NK cell lines examined lack flt3 expression.130 236
Multipotent lymphoid progenitor cells capable of producing DC express high levels of flt3.237 Because a DC-restricted lymphoid progenitor has not yet been identified, c-kit and flt3 expression on such a CFU-DC remains to be established.
EARLY B-CELL DEVELOPMENT: COEXPRESSION OF c-kit AND Flt3 AND APPARENT KEY ROLE OF Flt3/FL INTERACTION
Although no reduction in cells of the B-cell lineage has been reported in adult W mutant mice, embryonic mice deficient in c-kit or KL expression have reduced numbers of B-cell progenitor cells in fetal liver.238 Such a reduction could indicate a direct role of c-kit and its ligand in B lymphopoiesis or, alternatively, an indirect effect of a depleted pool of pluripotent stem cells and/or altered stromal cells in these mice.186
KL can synergize with IL-7 to promote stroma-independent growth of murine BM pro-B- and pre-B-cell progenitors unresponsive to IL-7 alone, whereas KL lacks proliferative activity on B220+cμ+ pre-B cells.33,118,239,240 One study found that KL in combination with IL-7 could promote development of pre-B cells and expression of μ-heavy chain118; other studies have not found KL plus IL-7 sufficient to allow differentiation of pro-B cells into pre-B cells in vitro, even though such pro-B cells coexpress c-kitand IL-7 receptors.231,239,240 Furthermore, a blocking antibody against c-kit inhibits the growth of murine pro-B cells cultured on stromal cells in the presence of IL-7, but has no effect on pre-B-cell differentiation supported by the same stroma cells.163,241,242 Similarly, KL in combination with IL-7 can replace the requirement for stroma to induce pro-B-cell proliferation, but not differentiation into pre-B cells.239In addition to its ability to promote growth of committed pro-B cells, KL in combination with IL-7 can stimulate stroma-independent B-cell progenitor cell development from candidate murine stem cells243-245 or from bipotent macrophage-B-cell progenitor cells.246
In vivo treatment of mice with a blocking antibody against c-kit results in an almost complete elimination of myeloid and primitive hematopoietic progenitor cells, leaving virtually no mature granulocytes and erythroblasts in the BM.164,183 However, the total number of BM cells are normal, of which the majority are B220+.164,183 A concomitant expansion in the number of pre-B-cell progenitor cells is observed,164,183suggesting that an interaction between c-kit and KL is not required for B-cell development in vivo. In support of this, W/Wstem cells are as efficient as wild-type stem cells at reconstituting BM B cells in RAG-2-deficient mice.247 Thus, unlike the critical role of c-kit/KL interaction in generation of the erythroid, myeloid, and T-cell lineages, c-kit-KL is not required for normal B-cell development in adult mice. The mechanism behind the intriguing observation that a c-kit antibody blocks the production of mature myeloid and erythroid progeny but enhances B-cell development remains unclear, although it appears to result from an indirect rather than a direct effect.
An important and distinct role of FL in early stages of B-cell development is supported by studies of flt3-deficient mice. These animals, unlike c-kit-deficient mice, have reduced numbers of pro-B cells in the BM, although the number of mature B cells is normal.227 These findings have also been confirmed in FL-deficient mice.248
FL promotes the in vitro growth of early B-cell progenitor cells in a pattern distinct from that of KL. Primitive (CD43+B220lowCD24−) B-cell progenitors in murine BM do not respond to either FL or IL-7 individually, but in combination the two cytokines induce a greater proliferative response than IL-7 plus KL.249 In contrast, more differentiated CD43+B220lowCD24+ B-cell progenitors fail to respond to FL, whereas KL enhances IL-7-induced proliferation, indicating that FL activity is restricted to an earlier stage of B-cell development than KL activity. Another important finding is the capacity of FL plus KL to promote the growth of CD43+B220lowCD24−B-cell progenitor cells in the absence of IL-7.249 This might help explain why IL-7 receptor-deficient mice have normal levels of these primitive B-cell progenitors, but dramatic reductions in more differentiated B-cell progenitors and mature B cells.250 It could also explain why mice with a combined deficiency in flt3 and c-kit have a more severe reduction in early B-cell progenitors than mice deficient in flt3 only.227
FL synergizes with IL-7 to enhance the production of B220+cells from B220+ as well as B220− murine BM cells.245 IL-7-independent B220+ cell development occurs in the presence of FL alone, but not KL alone, indicating a primary role of FL over KL in early murine B-cell development. Pro-B cells isolated from murine fetal liver also proliferate in response to either FL or KL in combination with IL-7, maintaining a population of early pro-B cells.251
Because the B-cell defect in flt3-deficient mice is restricted to a reduction in the most primitive B-cell progenitors, an essential role of flt3/FL might be to promote B-cell development from progenitor/stem cells not yet committed to the B-cell lineage. In support of this, FL and KL can each promote the growth of fetal liver and BM progenitor cells with a combined myeloid and lymphoid potential.251,252 FL and IL-7 synergize to enhance the growth of primitive murine Lin−Sca-1+ BM progenitors, resulting in production of almost exclusively pro-B cells, whereas KL plus IL-7 stimulate formation of 90% myeloid cells.252
Studies of the early stages of human B-cell growth have been hampered by the lack of optimized in vitro systems. Therefore, the potential roles of KL and FL in human B-cell development remain to be elucidated. A stimulatory effect of KL on committed human B-cell progenitors has been suggested,253 although stromal and IL-7-dependent early B lymphoid growth from BM or cord blood cells in vitro is neither stimulated by KL nor inhibited by a neutralizing anti-KL antibody.254-256 In contrast, FL in combination with IL-7 promotes stromal cell-independent growth of human fetal BM pro-B cells (CD34+CD19+), whereas KL has no effect.256
Although the precise roles of FL and KL in B lymphopoiesis remain to be determined, the available in vitro, in vivo, and knockout data suggest that flt3 and FL may be more critically involved in early B-cell development than c-kit and KL, perhaps identifying a physiologically important difference between KL and FL.
T-CELL PROGENITOR CELLS
In mice lacking functional c-kit expression, T-cell numbers in peripheral blood are normal,257 although a deficiency in fetal thymic development has been reported.258
One purified c-kit+ BM stem cell can reconstitute the thymus in more than 40% of sublethally irradiated mice, whereas c-kit− stem cells have little or no such ability.259 Although the BM population can produce myeloid/erythroid as well as T-cell progeny, thymus-derived c-kit+Lin−Thy-1lo cells appear to be lymphoid-restricted.260 Anti-c-kitantibodies completely block T-cell generation from BM, but not thymic cells, suggesting that T-cell generation from these primitive, lymphoid-committed stem cells in the thymus might not require signaling through c-kit.260
KL has little or no growth-promoting activity alone, but promotes IL-7-stimulated growth of primitive mouse CD4−CD8−CD3− thymocytes, but not CD4+CD8+ cells or single CD4+and CD8+ cells.234,261 Anti-c-kitantibodies dramatically inhibit in vitro fetal thymic T-cell production and differentiation from fetal liver progenitor cells.234Similarly, anti-c-kit antibodies reduce cell production and differentiation towards CD4+CD8+ cells in a reconstitution assay with fetal thymocytes into fetal thymus.232 This suggests that KL might be involved in promoting the growth and differentiation of immature thymocytes. IL-3 and IL-12 have been shown to synergize with KL to enhance the growth of primitive, but not more mature, thymocyte populations.235
T-cell numbers in peripheral blood are normal, but a reduction in early T-cell progenitors is seen postnatally in flt3-deficient mice, and flt3-deficient stem cells are impaired in their ability to reconstitute T cells in the thymus and peripheral blood.227
FL synergizes with IL-7 to stimulate the proliferation of unfractionated murine thymocytes, and a stimulatory effect can be seen in response to FL in the absence of IL-7.49 The most primitive CD4low thymic progenitor cells capable of generating multiple lymphoid lineages are growth stimulated by FL (in combination with IL-3, IL-6, and IL-7) more efficiently than with KL.262 In contrast, pro-T cells are more efficiently expanded with KL than FL, suggesting that FL might be more active than KL at an earlier stage of T-cell growth.262 In agreement with this, FL appears to preferentially promote self- renewal of CD4low cells in fetal thymic organ culture, whereas KL promotes early T-cell differentiation.262
Studies of cytokine effects on the regulation of human T-cell development have been difficult due to the lack of appropriate in vitro assays. However, KL enhances thymic stromal cell-supported production of human CD4+ and/or CD8+ cells from CD34+CD4−CD8− BM progenitor cells,263 whereas FL promotes IL-12-stimulated T-cell production from human CD34+ BM cells on thymic stromal layers.264
NK CELL PROGENITORS
c-kit is constitutively expressed on a small subset of resting human NK cells in peripheral blood characterized by high CD56 expression, but not on the larger fraction of more differentiated NK cells with low CD56 expression.175 These c-kitreceptors are functional because KL suppresses apoptosis, apparently through induction of bcl-2 expression, although it does not promote proliferation, differentiation, or cytotoxicity on its own.152,175 However, KL in combination with IL-2 promotes the growth, but not cytotoxicity, of this population of resting NK cells.175
KL enhances stroma-independent NK cell development from human BM progenitor cells stimulated by IL-2, IL-7, or IL-15 in vitro.265-267 An important regulatory role of flt3 and its ligand in NK cell development is supported by the finding that FL-deficient mice treated with poly IC or IL-15 are devoid of NK cell activity in the spleen.248 Furthermore, FL in combination with IL-15 promotes the expansion but not differentiation of CD3−CD56+ NK cells from human CD34+ progenitor cells.268
DC DEVELOPMENT: KEY ROLE OF FL
All DC express CD45 and arise from BM progenitor cells; evidence suggests that DC derive from myeloid and lymphoid progenitor cells.269,270 Myeloid-derived DC can be generated in vitro from progenitor cells isolated from BM, mobilized peripheral blood, or cord blood; GM-CSF appears to play a primary role in promoting their production.269,270 A number of cytokines, including tumor necrosis factor-α (TNF-α), IL-4, and KL, can enhance DC formation induced by GM-CSF.269,270 KL stimulates DC formation from human CD34+ BM and cord blood progenitor cells in combination with GM-CSF and TNF-α without affecting DC differentiation.193-195
FL increases the production of DC from CD34+ BM progenitor cells in combination with GM-CSF plus TNF plus IL-4.196This enhanced DC production is similar to that observed in response to KL, and when these two cytokines are combined, the effect is additive.196 As with KL, FL does not appear to affect the differentiation, but rather the production, of DC.196Production of DC from mobilized CD34+ peripheral blood progenitor cells (PBPC) by GM-CSF and TNF-α is enhanced by KL and FL individually; combining them results in an additive response.271
KL or FL (in combination with other cytokines) promotes DC formation from uncommitted thymic precursors,272 but the identity and responsiveness to KL or FL of committed lymphoid-derived CFU-DC remains to be determined.
In vivo treatment of mice with FL results in a dramatic increase in the number of myeloid- and lymphoid-derived functional DC in BM, spleen, thymus, peripheral blood, gastrointestinal lymphoid tissues, and other tissues, indicating an absolute increase in functionally mature DC rather than a redistribution.273 In contrast, administration of KL, GM-CSF, or IL-4 to mice does not expand the number of DC in the spleen. A key role of FL in DC generation is further supported by reduced numbers of DC in FL-deficient mice.248
LONG-TERM RECONSTITUTING MURINE STEM CELLS ARE HETEROGENEOUS WITH REGARD TO c-kit AND Flt3 EXPRESSION
Many studies have suggested that most, if not all, pluripotent long-term reconstituting murine stem cells (LTRC; purified by various methods from BM, fetal liver, and the intra-embryonic aorta-gonad-mesonephros) express c-kit.184-188,274-276 Particularly noteworthy was a study in which a single Lin−Sca-1+CD34low/-c-kit+stem cell efficiently long-term reconstituted as much as one of five transplanted mice.277 In addition, cells with the same phenotype isolated from primary recipients were able to reconstitute secondary recipients.277 The corresponding c-kit− population was not investigated. Although these studies have clearly established that a large fraction and probably most LTRC are c-kit+, they do not necessarily rule out the possibility of a coexisting, and probably less frequent c-kit− LTRC, because the reconstitution assays might not have been optimal for detecting the LTRC activity of a (putative) c-kit− stem cell population.
In support of the potential existence of c-kit− stem cells, c-kit− murine BM cells without detectable c-kit expression but with LTRC, but no short-term reconstitution activity, have been identified.278 One study identified a minor but efficient c-kit− LTRC population (0.005% of BM cells).279 The absence of c-kit expression was verified at the cell surface as well as by RT-PCR. As few as 10 of these cells efficiently generated all blood cell lineages for the life span of the mice and showed extensive in vivo self-renewal ability, as assessed through serial transplantation. In contrast, as many as 1,000 of these cells showed no ability to promote radioprotection.279 This is in contrast to most c-kit+ LTRC (with the exception of CD34−/low c-kit+ stem cells277), which in general have been found to also be enriched in short-term reconstituting and radioprotective ability.184-186 188
The existence of an LTRC population with little or no c-kitexpression is also supported by another study280 in which candidate stem cells were subfractionated into c-kitlow and c-kit<low (no detectable cell surface expression but positive for c-kit mRNA) populations, representing 0.006% and 0.008% of the BM cells, respectively. These two populations did not differ in their capacity to provide donor long-term multilineage reconstitution in primary irradiated recipients. However, when BM from primary recipients was transplanted into secondary recipients, multilineage donor reconstitution could only be obtained from cells whose origin was c-kit<low stem cells.280 Tertiary recipients receiving cells derived from c-kit<lowstem cells were also efficiently reconstituted.280
Other investigators have subfractionated murine BM progenitor/stem cells based on different levels of c-kit expression. In one study, murine BM stem cells were isolated by counterflow centrifugal elutriation; subsequently fractionated into c-kitneg, c-kitdull, and c-kitbright subpopulations; and administered to unirradiated W/Wvrecipients.187 One hundred c-kitbrightcells were sufficient to repopulate lympho-hematopoiesis inW/Wv recipients, whereas as many as 2.5 × 104 c-kitdull or 5 × 105 c-kitneg cells had no LTRC activity.
Whereas the majority of BM colony-forming cells in normal mice are c-kitbright, most progenitors from 5-FU-treated mice are c-kitdull.281 Cells resistant to 5-FU represent predominantly dormant progenitor cells; moreover, c-kitdull progenitor cells, unlike c-kitbright progenitor cells, require multiple cytokines to be recruited to proliferate and develop in culture into c-kitbright progenitor cells. This suggests that the most primitive murine progenitors might be c-kitdull.281
The different conclusions reached in these studies might simply reflect that LTRC are heterogeneous with regard to c-kit expression and that differences in purification strategies and reconstitution assays might result in enrichment and detection of different subpopulations of stem cells. For instance, it is possible that the in vitro (cytokine stimulation) and in vivo (5-FU treatment) manipulation of these cells might modulate (up or down) the expression of c-kit. Thus, although a certain level of c-kit expression might prove useful for purification and characterization of LTRC by one specific procedure, it is not necessarily transferable to other methods.
Collectively, these studies suggest that, although most murine LTRC express low or high levels of cell-surface c-kit, they coexist with less frequent subpopulations of LTRC with undetectable c-kit expression. However, cells found to be c-kit− by flow cytometry are not necessarily devoid of cell-surface c-kit expression, because the limit of detection of this method is around 500 molecules per cell. In addition, the finding of c-kit mRNA expression using the much more sensitive RT-PCR method might be due to a minor contaminating c-kit+cell population and does not necessarily reflect cell-surface expression of c-kit. Thus, currently it appears most correct to define apparently c-kit− stem cells as c-kit<low.280 Because these c-kit<low stem cells appear to represent highly quiescent LTRC, they might exclusively promote late, rather than early, engraftment and have a higher self-renewal capacity than most c-kit+ stem cells, as shown through stringent serial transplantation assays.279 280 The inability of c-kit−/c-kit<low murine BM cells to provide long-term reconstitution in other studies might be a direct consequence of such stem cells being present in low numbers and/or not activated when transplanted after standardized myeloablative or nonablative regimens.
In the stem and progenitor cell compartment in mice, the flt3 receptor has been found in Lin−Sca-1+AA4+fetal liver cells,19,166Lin−Sca-1+ BM cells,19,166 and WGA+15-1.1−Rh123 bright and dull cells.282
Virtually all AA4+CD34+ fetal liver cells express c-kit. These, as well as Lin−Sca-1+c-kit+ BM cells, contain distinct flt3+ and flt3−subpopulations, and the long-term repopulating activity appears to be predominantly found in the flt3−subfraction.45 Thus, most murine LTRC appear to be c-kit+ but flt3−/flt3<low. This observation, combined with flt3+ stem cell populations having a lower fraction of cells residing in G0 than flt3− stem cells, has led to the proposal that flt3+ repopulating cells might represent an activated subset of stem cells.45,187 However, note that subpopulations of flt3+ stem cells are quiescent and capable of promoting long-term reconstitution.45Additional long-term serial transplant reconstitution studies using flt3− and flt3+ stem cell populations could provide more definite information regarding the self-renewal capacity of flt3− and flt3+ stem cell populations.
IN VITRO GROWTH-PROMOTING ACTIVITIES OF KL AND FL ON CANDIDATE MURINE STEM CELLS AND PRIMITIVE MYELOID PROGENITOR CELLS: POTENT SYNERGISTIC FACTORS
A characteristic of the most primitive hematopoietic progenitor/stem cells is the requirement for simultaneous activation through multiple cytokine receptors to allow recruitment into active cell cycling.2 4
Based on different patterns of growth-promoting activities on candidate stem cells and their ability to synergistically interact with other factors, cytokines can be grouped into different classes (Table 4). Synergy appears to be most pronounced when cytokines from different classes are combined.2 KL and FL are the only identified members of a distinct group of early acting stem cell factors with unique and potent activities on a variety of candidate murine stem cell populations. Although they have little or no in vitro growth-promoting activity when acting alone, both KL162,197,222,223,281,283-292 and FL45,48,49,166,205,206,223,245,293 can act in combination with most, if not all, other cytokines from the two groups of early acting cytokines to enhance growth of primitive murine progenitor/stem cells through enhanced recruitment of otherwise quiescent progenitor cells and enhanced proliferative activity.
Classification of Early Acting Cytokines
| Class . | Members . |
|---|---|
| I. Stem cell factors | KL, FL |
| II. Colony-stimulating factors | G-CSF, M-CSF, IL-3, TPO |
| III. Purely synergistic factors | IL-1, IL-4, IL-6, IL-11, IL-12, LIF |
| Class . | Members . |
|---|---|
| I. Stem cell factors | KL, FL |
| II. Colony-stimulating factors | G-CSF, M-CSF, IL-3, TPO |
| III. Purely synergistic factors | IL-1, IL-4, IL-6, IL-11, IL-12, LIF |
Classification is based exclusively on the functional ability of various cytokines to promote growth of primitive murine hematopoietic progenitor cells and candidate stem cells in vitro. In principle, this classification holds true for primitive human progenitor cells as well. Recruitment of primitive hematopoietic progenitor/stem cells generally can only occur through combined activation of at least two cytokine receptors, whereas optimal growth usually requires the synergistic interaction between multiple cytokines.2,4 Although usually having little or no growth-promoting activity alone, FL and/or KL can, with few exceptions, synergistically interact with any of the colony-stimulating factors and/or purely synergistic cytokines to enhance growth. Although synergy can occur between cytokines within one class, the most efficient growth-stimulation is obtained by combining cytokines from different classes.2 4
Several studies involving single-cell cloning and delayed addition of cytokines have shown that the effects of KL and FL are mediated directly on the primitive progenitor cells, ruling out indirect effects mediated by other cells. However, the extent of synergy exhibited by KL and FL, both with regard to recruitment and enhanced proliferation, varies considerably, depending in part on the interacting cytokine(s) and the specific target population investigated. Although the magnitude of synergy a specific cytokine exhibits in combination with KL and FL is likely to result from interactions of the distinct signaling pathways involved, it might also be a reflection of the heterogeneity in expression of other cytokine receptors on primary hematopoietic cell populations.2,4 When directly compared and combined with the same cytokine(s), KL often recruits a slightly higher number of primitive murine myeloid progenitor/stem cells into in vitro proliferation than FL does.45,48,49,166,205,206,223,245,293-297 This occurs independently of which cytokine is used as the synergistic factor. In addition, the average size of the resulting colonies is usually significantly larger in KL- than in FL-supplemented cultures. Finally, the progeny of primitive murine progenitor cells usually remain more undifferentiated in FL- than in KL-supported cultures.166,205,206 245
As already described in detail, the expression of flt3 appears more confined to primitive progenitor cells than c-kit, which is also highly expressed on various populations of more committed myeloid progenitor cells (Fig 2). Thus, the smaller clone size and less differentiated progeny observed in FL-supplemented cultures could result from the loss of flt3 expression at an earlier stage than c-kit. In addition, c-kit is expressed on a higher percentage of primitive progenitor/stem cells than flt3,45 166 which may explain the lower cloning frequency of primitive murine progenitor cells cultured/supplemented with FL rather than KL.
The activities of FL on primitive murine progenitor cells may overlap and be redundant with those of KL, as suggested for a number of other cytokines with activity on primitive hematopoietic progenitors.2,4 However, although KL and FL have largely overlapping activities, they can also synergize with each other to promote in vitro growth of primitive murine progenitor/stem cells.205,206,245 This synergistic interaction might help to explain why mice with a combined c-kit and flt3 deficiency have a more severe stem cell defect than mice with a single deficiency in c-kit or flt3.227
c-kit AND Flt3 EXPRESSION ON CANDIDATE HUMAN STEM CELLS
Because no routine and optimal reconstitution assay exists for human LTRC, its status with regard to c-kit and flt3 expression has yet to be established. However, much has been learned from studies of candidate human stem cells in various surrogate assays. c-kitis highly expressed in the CD38− subfraction of CD34+ BM cells,190,298 which, although representing only 0.05% to 0.1% of MNC, contains most, if not all, cells capable of long-term multilineage reconstitution of preimmune fetal sheep and immune-deficient mice.299,300 c-kitis also expressed on all cells in a population of purified quiescent human stem cells that is devoid of progenitors responsive to defined cytokines in vitro but highly enriched in long-term culture-initiating cells (LTC-IC).301 Other studies have shown that most, if not all, LTC-IC are c-kit+.189 191
In one study, CD34+c-kit− cells produced no colony-forming cells (CFC), although more CFC were formed by CD34+c-kitlow than CD34+c-kithigh cells after 9 weeks of culture. In addition, c-kithigh cells emerged from c-kitlow cells after 4 weeks of culture.302
Enrichment of primitive human progenitor cells in the CD34+c-kitlow fraction as compared with the CD34+c-kithigh fraction of BM cells was recently confirmed in long-term engraftment studies in preimmune fetal sheep.303 Although few animals were transplanted in this study, the findings clearly support that CD34+ human BM cells expressing low levels of c-kit are enriched in cells with an ability to provide long-term multilineage reconstitution. In contrast, cells with no or high c-kit expression have less long-term reconstituting ability.303
Subfractionation of CD34+ cord blood into c-kit−, c-kitlow, and c-kithigh populations shows a pattern similar to BM in that c-kitlow cells appear to contain more quiescent and blast cell progenitors.304
There is no evidence yet for a population of c-kit−/c-kit<lowlong-term repopulating human stem cells. However, such a stem cell population is likely to be present at a very low frequency, and current in vivo (and in vitro) reconstitution assays for human cells may be inadequate for detection of such a highly quiescent stem cell population. Therefore, the status of c-kit expression on the earliest human hematopoietic stem cells remains to be elucidated in more detail.
One study has suggested that virtually all BM cells expressing high levels of CD34 and low levels of c-kit are flt3−.57 Because the most primitive human stem cells have been suggested to express low levels of c-kitand high levels of CD34,302,303 this finding would suggest that the earliest human stem cells might not express detectable levels of flt3. However, in another recent study,176 most c-kitlow cells as well as CD34+CD38− cells were found to coexpress flt3 at low levels, and primitive cobblestone area-forming cells appeared to be flt3+ as well as flt3−. However, the flt3 status of human LTRC remains to be investigated.
Our current knowledge regarding c-kit and flt3 expression on hematopoietic stem cells is summarized in Fig 2. Most long-term reconstituting stem cells identified to date in murine reconstitution assays express c-kit.184-188,274-276 The few studies investigating flt3 expression on LTRC suggest that most are flt3− and that these might be more primitive/quiescent than flt3+ LTRC.45 187 However, further studies will be required to dissect the expression of flt3 on the earliest stem cells.
The existence of c-kit<low LTRC has been shown as well278-280 and, depending on the long-term reconstitution assay and stem cell population used, LTRC may predominantly express high, low, or undetectable levels of c-kit.187,278-281 303
It is unclear whether such distinct patterns of c-kit and flt3 expression might help identify subpopulations of LTRC within a hematopoietic hierarchy, although available data indicate the existence of such a hierarchy (Fig 2). The most primitive stem cell is likely to be less frequently and more deeply quiescent than stem cells further down in the hierarchy. These characteristics might make it difficult to purify and subsequently activate this stem cell population in standard reconstitution assays, in which more activated stem cells might have a repopulating advantage. Thus, a minor population of c-kit<low (potentially c-kit−) stem cells that efficiently and exclusively provides long-term reconstitution and has a high self-renewal potential278-280 is likely to represent a highly quiescent stem cell population. The status of flt3 expression on this stem cell population remains to be determined, but some studies indicate that flt3 is predominantly expressed on activated stem cells45,187; thus, the earliest stem cells might also be flt3−. Such c-kit<low/−flt3<low/− stem cells might, upon activation, give rise to long-term repopulating stem cells expressing detectable but low levels of cell-surface c-kit but not flt3.187,281,303 We propose that this stem cell population could next give rise to c-kithighflt3<low stem cells.187,281,302,303 There is also evidence for an activated stem cell population with more restricted long-term repopulating activity that expresses high levels of c-kit as well as flt3.45
It is important to emphasize that this represents a proposed and simplified stem cell hierarchy, exclusively based on expression of c-kit and flt3 and predominantly based on studies in mice. In addition, the information regarding flt3 expression on LTRC is much more limited than for c-kit (in particular for human stem cells). Furthermore, heterogeneity would be expected within each level of the hierarchy based on variable expression of other, potentially important stem cell molecules. Thus, additional studies will be required to confirm or redefine the proposed stem cell hierarchy.
IN VITRO GROWTH PROMOTING ACTIVITIES OF KL AND FL ON PRIMITIVE HUMAN HEMATOPOIETIC PROGENITOR/STEM CELLS
A similar pattern of growth-promoting activities of KL172,191,199,200,224,226,254,302,304-310 and FL48-50,192,207,208,224,293,311,312 is observed on primitive human hematopoietic progenitor cells, as described above for murine progenitors. When stimulated by KL or FL alone, primitive human progenitor cells isolated from fetal liver, cord blood, or BM show little or no growth response, but both ligands in combination with other early acting cytokines synergistically enhance growth in a direct manner. Whereas multiple studies on different populations of primitive murine progenitor cells have found KL more efficient than FL at recruiting primitive progenitor cells into proliferation, several studies on enriched primitive human progenitor cells indicate that FL is at least as efficient as KL at recruiting human cells.192,207,313-315 FL also appears to be more efficient than KL at maintaining primitive human progenitor cells in a less differentiated state.313-316 Again, this might result from the more restricted expression of flt3 on more committed progenitor cells.
ROLE OF c-kit/KL AND Flt3/FL INTERACTIONS IN MAINTAINING STROMA-DEPENDENT LONG-TERM HEMATOPOIESIS IN VITRO
In the mouse, LTRC can be quantified by a competitive repopulation assay; an equivalent assay for human stem cells does not currently exist. Accordingly, the ability of candidate human stem cells to produce committed progenitors over extended periods of culture (minimum of 5 weeks) on established stromal cell layers has been used as a surrogate human stem cell assay, although this should not be considered to represent a true stem cell assay.313,314,317 318
Murine LTC-IC express c-kit and, although their optimal growth and differentiation in stroma-dependent cultures is enhanced by KL, their formation and maintenance appear to be KL-independent.275,319,320 Furthermore, no difference in KL expression is observed between cell clones capable and incapable of maintaining long-term repopulating cells, and the addition of exogenous KL does not reverse the inability of certain clones to support long-term hematopoiesis.320 Similarly, the ability of several stromal cell lines to conserve long-term marrow repopulating stem cells is unaffected by c-kit blocking antibodies, whereas their ability to promote myelopoiesis is virtually eliminated by the same antibody.275,320 Finally, LTC-IC numbers are only marginally reduced in W mutant mice.319
Human LTC-IC, like those of mice, express c-kit but do not depend on c-kit activation for survival; but the addition of c-kit blocking antibodies to long-term cultures inhibits production of mature myeloid and erythroid progenitor cells from human stem cells.189,302,321,322 Although Sl/Slfibroblasts are as efficient as normal murine fibroblasts or irradiated human marrow feeder layers at supporting maintenance and clonogenic cell output of LTC-IC, KL in the absence of feeder layers can also efficiently maintain LTC-IC.322 This suggests that KL, although not required, can support these primitive cells. The superior ability of BM stromal cells to promote long-term hematopoiesis compared with umbilical cord vein endothelial cells or human fibroblasts does not appear to be mediated through c-kit, because these stromal cells do not differ in their expression of soluble or membrane-bound KL.323
Although less is known about the expression and function of flt3 on LTC-IC, several lines of data suggest that LTC-IC (at least in part) express flt3 and that FL, like KL, can enhance their growth and differentiation.17,313,314 Antisense oligonucleotides against flt3 almost completely block the ability of human LTC-IC to form mature myeloid progenitor cells in BM stromal cultures.17 Furthermore, FL on its own has the unique ability to expand human LTC-IC which are reduced in cultures containing KL alone314 and in combination with TPO it maintains LTC-IC over prolonged culture.229
KL PROMOTES ADHESION OF HEMATOPOIETIC PROGENITOR CELLS AND MAY FUNCTION IN ITS MEMBRANE-BOUND FORM AS A HOMING RECEPTOR FOR c-kit+ CELLS
A critical role in hematopoiesis has been implicated for the very late antigen (VLA) family of integrins.324-328 KL is a potent stimulator of the adhesion of mast cells, hematopoietic progenitor cell lines, and CD34+ BM progenitor cells to fibronectin and vascular cell adhesion molecule-1 (VCAM-1) through activation of VLA-4 and VLA-5.329-332 Only one hundredth of the amount of KL is required to induce adhesion compared with the amount needed to induce proliferation.331
The ability of KL to promote adhesion may have physiologic and potential clinical significance, because adhesion molecules are thought (1) to be important regulators of anchoring, migration, and mobilization of stem cells; (2) to affect cell growth and differentiation; and (3) to improve gene transfer into candidate hematopoietic stem cells.333-335
Membrane-bound KL is likely to function in part as an adhesion molecule for mast cells and hematopoietic progenitor cells.336-340The ability of KL to promote adhesion of c-kit+hematopoietic progenitors might explain why progenitor cells exposed to blocking c-kit antibodies show reduced homing efficiency.341 The effect of KL on homing and migration might also result from its chemotactic effect on mast cells and hematopoietic progenitor cells.342-344 Studies have not yet been performed to determine whether FL has a similar ability as KL to promote adhesion of hematopoietic cells.
KL AND FL PROMOTE VIABILITY OF PRIMITIVE HEMATOPOIETIC PROGENITOR/STEM CELLS
Although the primary function of KL and FL in early hematopoiesis might be to induce the growth of quiescent progenitor/stem cells through synergistic interactions with other early acting cytokines, there is also ample evidence that KL345-350 and FL,166,311,351,352 in the absence of other cytokines, selectively promote viability rather than proliferation of primitive murine and human progenitor cells, including the LTRC in the case of KL.345,347 348
INHIBITORS OF KL AND FL ACTIVITY ON PRIMITIVE HEMATOPOIETIC PROGENITOR CELLS
Although the physiologic significance of growth inhibitory cytokines in steady-state hematopoiesis remains to be established, the interactions of transforming growth factor-β (TGF-β) and tumor necrosis factor-α (TNF-α) with KL and FL on primitive hematopoietic progenitor cells are worth mentioning. TGF-β, a potent inhibitor of primitive hematopoietic progenitor cell growth,353 hinders the viability and growth-stimulatory effects of KL and FL on primitive murine and human hematopoietic progenitor cells.224,295,351,354-356 TNF-α, a cytokine that can directly stimulate or inhibit the growth of primitive and committed hematopoietic progenitor cells,357 inhibits KL- and FL-stimulated growth, viability, and expansion of normal primitive murine and human progenitor cells.296,314 358-360
DISTINCT HEMATOPOIETIC ACTIVITIES OF MEMBRANE-BOUND KL
As described above, KL and FL are produced in membrane-bound as well as in soluble forms. In addition to potentially functioning as adhesion molecules by binding to their respective receptors, membrane-bound KL has activities distinct from those of soluble KL.Sl/Sld mutant mice that only produce the secreted form of KL have the same hematopoietic defects characteristic of Sl/Sl mutant mice, suggesting that there is an essential role for membrane-bound KL.88,92 When cDNAs encoding soluble or membrane-bound isoforms of human KL are transfected into stromal cells derived from Sl/Sl mice, membrane-bound KL maintains human hematopoiesis longer than secreted KL.89Membrane-bound KL (or immobilized anti-kit antibodies), when compared with soluble KL, induces (1) more c-kit kinase activity, (2) less rapid downregulation of cell surface c-kitexpression, and (3) enhanced stability of c-kit.361 362 Thus, the difference in activity between soluble and membrane-bound KL might result from the soluble c-kit/KL complex being rapidly internalized and degraded, resulting in transient tyrosine kinase activation of c-kit. In contrast, if the membrane-bound c-kit/KL complex is not internalized and degraded, it could result in a sustained period of enhanced c-kit kinase activity.
HEMATOLOGIC EFFECTS OF KL AND FL IN VIVO
Mutations in the W or Sl loci result in reductions of various primitive hematopoietic progenitor cells,10 but except for erythrocytes, the numbers of other mature blood cells appear normal under steady state conditions. Sl/Sld mice, although severely anemic, survive to adulthood; administration of KL improves their anemia, which reappears when KL treatment is discontinued.36 KL treatment also increases their platelets, granulocytes, monocytes, and lymphocytes above the levels seen in wild-type mice36 and increases CFU-S numbers in their BM and spleen.345
Sl/Sld mice display a dysfunctional regulation of platelet production in response to cytotoxin-induced thrombocytopenia; they do not undergo the rebound thrombocytosis observed in wild-type mice after 5-FU treatment.167 However,Sl/Sld mice treated with 5-FU have a rebound thrombocytotic response after the administration of KL.167Enhanced KL mRNA expression in response to 5-FU-induced thrombocytopenia in the BM of normal mice and c-kit expression on immature megakaryocytes further substantiate the role KL plays in promoting platelet recovery after BM suppression.167 KL also increases the number of megakaryocytes and platelets in normal mice.167
The role of KL in promoting platelet production after hematopoietic injury might be due to its ability to synergize with TPO to enhance megakaryocyte progenitor cell growth.217 Although TPO is the primary regulator of megakaryocytopoiesis and platelet production,217,363 mice deficient in TPO or c-mpl(the TPO receptor) expression do produce functionally mature platelets, albeit at dramatically reduced levels.363 In addition, KL administration to TPO-deficient mice increases platelet counts.364 Thus, it appears that there are TPO-independent mechanisms for platelet production in which KL might also play a role.
Sl/Sl mice lacking functional KL die at day 15 or 16 of gestation.29 However, the total number of fetal liver cells in normal or Sl/Sl mice increase by more than 10-fold between day 13 and 15 of gestation and, although the fetal liver cellularity in the KL-deficient mice is only 20% to 25% of wild-type fetal liver, the increase in fetal liver cells is similar.186 More importantly, the number of cells with a stem cell phenotype (Lin−Sca-1+Thy-1lo) and CFU-S activity also increases in Sl/Sl mice from day 13 to 15.186 This suggests that KL might not be essential for early hematopoietic development in mouse embryos and that fetal hematopoietic progenitor/stem cells can expand/self-renew in the absence of KL.
In mice with viable W mutations, disruption of hematopoiesis appears largely restricted to erythropoiesis and mast cell generation. Specifically, in BM of W41/W41mice (with a partial c-kit signaling deficiency), the number of erythroid, myeloid, pre-B, and multipotent progenitor cells, as well as Lin−Sca-1+ candidate stem cells and LTC-IC, are at near-normal levels.319 However, long-term repopulating units in W41/W41BM are reduced 17-fold.319 Furthermore,W41/W41 fetal liver cells are qualitatively and quantitatively close to normal in their short-term reconstituting ability but promote less long-term reconstitution.365W42 mutant fetal liver cells (completely silent c-kit receptor) show an even more pronounced inability to provide long-term reconstitution. Thus, although c-kit/KL interaction might not be critical for stem cell generation and expansion during early ontogeny, their sustained self-renewal might in fact be KL-dependent. An important role for KL in promoting reconstitution by LTRC is also supported by enhanced expression of KL following myeloablative treatment167,366and the ability of endogenous and exogenous KL to promote survival and hematopoietic reconstitution of mice and dogs after myeloablation.366-370
Other findings indicate that KL plays an important role in steady-state adult hematopoiesis. As early as 2 days after injection of normal mice with c-kit antibodies, most myeloid and erythroid cells disappear, although the BM cellularity remains normal.183The content of in vitro clonogenic myeloid progenitor cells and CFU-S in the BM declines rapidly, whereas a concomitant increase in B-cell precursors is observed.183
KL administration in vivo to normal mice results in an increase in peripheral white blood cells (WBC), predominantly neutrophilic granulocytes, and also a slight increase in lymphocytes.371BM cellularity is not affected, and its content of in vitro clonogenic myeloid progenitor cells and day-8 CFU-S is only slightly enhanced.371 In contrast, the number of myeloid progenitors and CFU-S in the spleen increases dramatically, and KL induces a more rapid and pronounced leukocytosis in splenectomized mice.371
KL administration to mice for 7 days results in depletion of candidate BM stem cells (Lin−Sca-1+Thylo) and a corresponding reduction in radioprotective ability.372 A concomitant increase in both these hematopoietic parameters, as well as multilineage long-term reconstituting activity, is observed in spleen and peripheral blood.372 Because the total number of Lin−Sca-1+Thylo did not significantly change, it was postulated that administration of KL does not result in a net expansion of long-term reconstituting stem cells, but rather redistributes existing stem cell activity to peripheral sites.
The progenitor/stem cell mobilizing ability of KL has been investigated extensively in various animal models. Low doses (25 μg/kg/d) of KL have little or no effect on the number of PBPC in splenectomized mice, but KL synergistically enhances WBC counts and mobilization of PBPC in combination with an optimal dose of G-CSF (200 μg/kg/d).373 The increase includes cells with both short-term and long-term repopulating activity.374Administration of KL to normal mice results in a threefold increase in LTRC that are predominantly redistributed to peripheral blood and the spleen.375 KL in combination with G-CSF also mobilizes progenitor/stem cells to the blood that are capable of engrafting lethally irradiated dogs and baboons.376-379 Although the ability of KL plus G-CSF–mobilized progenitor cells to long-term engraft baboons and dogs remains to be established, it appears that blood count recovery occurs earlier with grafts mobilized with KL plus G-CSF than with G-CSF alone.376-378
In humans, daily administration of KL at dosages of up to 50 μg/kg for 14 days does not increase the number of peripheral blood CD34+ cells, but does increase the absolute number of CD34+ cells and assayable primitive and committed myeloid progenitor cells in BM.380 In a phase I/II study in patients with high-risk breast cancer, mobilization of progenitor cells to peripheral blood by KL plus G-CSF was superior to G-CSF alone.381
The administration of KL plus G-CSF in mice has shown interesting kinetic aspects of distribution/expansion of stem cells.382The most dramatic increase in repopulating ability of peripheral blood stem cells is observed immediately after cytokine treatment, concomitant with a reduction in reconstituting ability of the BM. Subsequently, the repopulating activity of peripheral blood stem cells declines to normal levels within 6 weeks of termination of cytokine treatment, whereas the repopulating activity of BM cells increases by day 14 to levels 10-fold higher than BM cells from untreated mice. The mechanism for this large yet temporary increase in the repopulating activity of BM stem cells after administration of KL and G-CSF is unclear. Increased numbers of primitive (CD34+CD38−) cells are also seen in the BM of rhesus monkeys as long as 2 to 3 weeks after administration of KL and G-CSF.383
In vivo daily administration of recombinant human FL (500 μg/kg/d) to normal mice stimulates an increase in WBC.384 The increase in WBC counts is reflected in an increase in the number of lymphocytes, granulocytes, and especially monocytes.384 A small decrease in hematocrit after 10 days of treatment is reversed upon cessation of treatment. BM cellularity is not affected by FL treatment. The number of CD4+ and CD8+ T cells in the BM is reduced, as are mature (B220+IgM+) B cells.384 In contrast, FL treatment increases the number of immature (B220+IgM−) B cells. The number of monocytes and granulocytes increases as well, as do DC, whereas the number of immature erythroid cells is reduced by 90%.384This decrease may result from the mobilization of erythroid precursors from BM and/or an altered differentiation pathway for progenitors of these erythroid precursors; the exact cause is not known.
Splenic cellularity increases after 10 days of FL treatment, with little effect on CD4+ and CD8+ T cells, but with an increase in NK cells and DC. Most striking is the ninefold increase in B220+IgM− B-cell progenitors, with only a marginal effect on splenic mature B220+IgM+ B cells. As in BM, the number of splenic myeloid cells increases as much as 10-fold. Splenic primitive erythroid cells also increase, although these cells decrease in BM.384
The number of BM GM progenitor cells increases fivefold after 3 days of FL treatment. The number of these cells subsequently decline during the next 12 days of treatment, and decrease to 50% below control levels 1 week after cessation of FL treatment.384 BFU-E numbers in BM increase slightly after 3 days of FL treatment, but decrease subsequently. Colony-forming unit granulocyte, erythrocyte, monocyte, megakaryocyte (CFU-GEMM) numbers also peak early in BM and subsequently return to control values. CFU-GM, BFU-E, and CFU-GEMM increase 123-fold, ninefold, and 108-fold, respectively, in spleen. Maximum levels are seen after 8 to 10 days of treatment, and these numbers return to control levels 1 week after treatment. In peripheral blood, a 537-fold, 113-fold, and 585-fold increase in CFU-GM, BFU-E, and CFU-GEMM, respectively, is observed after 10 days of FL treatment.384 FL also mobilizes primitive, day-13 CFU-S into peripheral blood. Finally, an increase in cells with a stem cell phenotype (Lin−Sca-1+kit+) is observed in the BM, spleen, and peripheral blood of FL-treated mice.384
Cells mobilized to peripheral blood with FL have been shown to have long-term (6 months) reconstituting ability.385 FL also mobilizes progenitor/stem cells into the peripheral blood of nonhuman primates and shows synergy with either G-CSF or GM-CSF with regard to mobilizing ability.385 386
Preliminary results from human clinical trials show that the administration of FL to normal, healthy volunteers is safe and effectively elevates the numbers of CD34+ cells and DC in peripheral blood (Mel Lebsack and Eugene Maraskovsky, Immunex; personal communication). The in vivo hematologic/hematopoietic effects of FL and KL are summarized in Table 5.
In Vivo Hematopoietic Effects of KL and FL
| Cell Type . | Response . | KL . | FL . |
|---|---|---|---|
| LTRC | Expansion | + | + |
| Mobilization | + | + | |
| Primitive/committed progenitors | Expansion Mobilization | + + | + + |
| Red blood cells | Reticulocytes | +/NE | ND |
| Hematocrit | +/NE | Reduced | |
| Platelets | Megakaryocytes | +/NE | ND |
| Platelets | +/NE | ND | |
| White blood cells | Total number | + | + |
| Granulocytes | + | + | |
| Monocytes | +/NE | + | |
| Lymphocytes | +/NE | + | |
| Mast cells | Number | + | NE |
| Activation | + | NE | |
| Dendritic cells | Number | NE | + |
| Cell Type . | Response . | KL . | FL . |
|---|---|---|---|
| LTRC | Expansion | + | + |
| Mobilization | + | + | |
| Primitive/committed progenitors | Expansion Mobilization | + + | + + |
| Red blood cells | Reticulocytes | +/NE | ND |
| Hematocrit | +/NE | Reduced | |
| Platelets | Megakaryocytes | +/NE | ND |
| Platelets | +/NE | ND | |
| White blood cells | Total number | + | + |
| Granulocytes | + | + | |
| Monocytes | +/NE | + | |
| Lymphocytes | +/NE | + | |
| Mast cells | Number | + | NE |
| Activation | + | NE | |
| Dendritic cells | Number | NE | + |
The table is based on the effects of in vivo administration of KL or FL as single agents to normal subjects. The effects of KL are based on studies in mouse, rat, dogs, nonhuman primates, and humans, whereas the effects of FL are predominantly based on studies in mouse and nonhuman primates.
Abbreviations: +, increase; NE, no effect; +/NE, effect found in some but not all species investigated; ND, not determined.
TARGETED DISRUPTION OF THE Flt3 RECEPTOR AND FL GENES
Whether flt3 or FL are required for normal hematopoiesis has been addressed by creating mice that carry a homozygous deletion of most of the gene encoding the flt3 receptor227 or FL.248 Mice in which either the flt3 receptor or ligand have been knocked out are generally healthy, which is in marked contrast to the lethality observed in mice homozygous for the deletion of the gene encoding the c-kit receptor or KL protein.24 The flt3 knockout mice have normal levels of peripheral blood cells.227 However, the loss of a functional flt3 receptor results in a reduced number of early B-cell precursors and a defect in primitive stem cells, as measured in a long-term competitive repopulation assay. Upon adoptive transfer to irradiated secondary recipients, stem cells from flt3 deficient−/− mice have an impaired ability to repopulate myeloid, T-, and B-lymphoid lineages.
Mice bearing targeted disruptions in the flt3 receptor were bred with mice carrying mutations in the c-kit receptor to generate animals of the genotype flt3−/flt3−W/Wv. Offspring had severely reduced numbers of hematopoietic cells and died between 20 and 50 days of age.227 These experiments demonstrated a requirement for both flt3 and c-kit receptors in the development of a normal, functional hematopoietic system.
There is no evidence that FL binds to any other protein in addition to the flt3 receptor. Similarly, no other ligands are known that bind to the flt3 receptor. Thus, one would predict that mice homozygous for a targeted disruption of the FL gene would have an identical phenotype to flt3 receptor knockout mice. FL knockout mice, like the flt3 receptor knockout mice, have a normal, healthy appearance.248 They have a defect in early B-cell development, as do the flt3 receptor knockout mice. However, a couple of significant observations have been made in analyzing the FL knockout mice that were not reported with the flt3 receptor knockout mice. There is a significant reduction in the cellularity in the peripheral blood, spleen, and BM of FL knockout mice, whereas no change in cellularity was reported in the flt3 receptor knockout mice. DC in the spleens of these animals are also significantly reduced. Most notable is a lack of NK cell activity in the spleens of mice treated with either poly IC or IL-15. It is unclear if these unique observations in the FL knockout mice reflect a truly different phenotype or whether strain variations or the depth of analysis account for the observed differences.
HUMAN SERUM/PLASMA LEVELS OF KL AND FL
Levels of KL in human serum from normal individuals are usually found in the range of 2 to 5 ng/mL.387 KL serum levels have also been examined in a wide variety of patients with hematopoietic disorders, and they do not vary much or appear to be of clinical significance.388
In contrast to the relatively high levels seen with KL, serum levels of FL in normal individuals average less than 100 pg/mL, which is the limit of detection of the enzyme-linked immunosorbent assay.389 FL levels are not elevated in a variety of anemias that predominantly affect only the erythroid lineage389 or in patients with rheumatoid arthritis, systemic lupus erythematosus, AML, ALL, or human immunodeficiency virus (Lyman et al, unpublished observations).
In contrast, serum levels of FL are highly elevated in patients with hematopoietic disorders that specifically affect the stem cell compartment. Thus, a majority of patients with anemias affecting multiple hematopoietic lineages (eg, Fanconi anemia, acquired aplastic anemia) have highly elevated levels of FL (up to 10 ng/mL).389 Cancer patients treated with chemotherapy and/or radiation also have highly elevated levels of FL.390
The simplest interpretation of these data is that the loss of functional stem/progenitor cells leads to the loss of a negative regulator of FL production made by the stem/progenitor cells. FL concentrations in blood then become elevated (to a physiologically relevant level) as part of a compensatory hematopoietic response to drive the proliferation of the remaining stem/progenitor cells.
Serum levels of FL returned to normal in a Fanconi anemia patient after a cord blood transplant that cured the pancytopenia.389Similarly, successful treatment of acquired aplastic anemia patients with either BM transplants or immunosuppressive therapy also led to a return to normal of FL serum levels.390 These data suggest that restoration of stem cells in these patients is associated with a return of FL serum levels to those measured in normal, healthy individuals and that FL serum levels may be a surrogate marker for stem cell activity or content in BM.
However, the hypothesis cited above does not explain why about 50% of patients with refractory anemia (RA) have elevated levels of FL,391 because RA is not considered a disease of either stem cell number or activity. FL serum levels are not elevated in any of the other FAB subclasses of myelodysplasia,391 and the reason only some RA patients have elevated serum levels is unknown.
POTENTIAL CLINICAL USES OF KL AND FL
Because both KL and FL have potent effects on primitive hematopoietic cells, the majority of clinical uses envisioned are designed to exploit this activity (Table 6). Both proteins synergize with a wide range of cytokines, and it is possible that they could enhance the effects of other cytokines that function on primitive as well as more differentiated hematopoietic cells.
Some Potential Clinical Uses of KL and FL
| . | Comments . |
|---|---|
| Likely applications | |
| Ex vivo expansion/purging of progenitor/stem cell grafts | In combination with other early acting (stem cells) and lineage-selective cytokines (progenitors) to improve reconstitution and to purge tumor-contaminated progenitor/stem cell grafts. |
| Progenitor/stem cell mobilization | In combinations with other cytokines (GM-CSF, G-CSF, TPO, or others) to improve mobilization of progenitor/stem cells to peripheral blood to be used in transplantation. |
| Gene therapy | (1) In combination with other early acting cytokines to improve gene transfer to stem cells in vitro. (2) Mobilize/expand stem cells in vivo (see above) that might prove better targets for gene transfer. |
| Immunotherapy | (1) Ex vivo (KL and FL) and in vivo (only FL) expansion of DC for use as vaccine adjuvant. (2) In vivo antitumor activity of FL (via effects on DC and NK cells). |
| Additional potential applications | |
| Stem cell deficiencies | Potential diseases include aplastic anemia and myelodysplastic syndromes. |
| Pure erythroid aplasia (Diamond-Blackfan anemia) | KL might prove more efficient than FL due to the wide expression of c-kit and lack of flt3 on primitive erythroid progenitors. |
| Cytopenias after chemotherapy/bone marrow transplantation | G-CSF/GM-CSF are efficient at promoting neutrophil recovery, and TPO may prove efficient at enhancing platelet recovery. However, KL and FL might, in combination with G-CSF and/or TPO, be of benefit when primitive progenitor/stem cells are severely compromised. |
| Immunodeficiencies (HIV) | Adjuvant treatment of cytopenias. |
| . | Comments . |
|---|---|
| Likely applications | |
| Ex vivo expansion/purging of progenitor/stem cell grafts | In combination with other early acting (stem cells) and lineage-selective cytokines (progenitors) to improve reconstitution and to purge tumor-contaminated progenitor/stem cell grafts. |
| Progenitor/stem cell mobilization | In combinations with other cytokines (GM-CSF, G-CSF, TPO, or others) to improve mobilization of progenitor/stem cells to peripheral blood to be used in transplantation. |
| Gene therapy | (1) In combination with other early acting cytokines to improve gene transfer to stem cells in vitro. (2) Mobilize/expand stem cells in vivo (see above) that might prove better targets for gene transfer. |
| Immunotherapy | (1) Ex vivo (KL and FL) and in vivo (only FL) expansion of DC for use as vaccine adjuvant. (2) In vivo antitumor activity of FL (via effects on DC and NK cells). |
| Additional potential applications | |
| Stem cell deficiencies | Potential diseases include aplastic anemia and myelodysplastic syndromes. |
| Pure erythroid aplasia (Diamond-Blackfan anemia) | KL might prove more efficient than FL due to the wide expression of c-kit and lack of flt3 on primitive erythroid progenitors. |
| Cytopenias after chemotherapy/bone marrow transplantation | G-CSF/GM-CSF are efficient at promoting neutrophil recovery, and TPO may prove efficient at enhancing platelet recovery. However, KL and FL might, in combination with G-CSF and/or TPO, be of benefit when primitive progenitor/stem cells are severely compromised. |
| Immunodeficiencies (HIV) | Adjuvant treatment of cytopenias. |
Adverse events associated with KL administration in humans in phase I and phase II trials have been primarily dermatologic reactions (eg, pruitic wheals with erythema at the site of injection) and, more rarely, multisymptom systemic anaphalactoid reactions.8,179,181,182 The most likely cause of these effects is mast cell hyperplasia, activation, and mediator release; as a result, prophylactic antihistamine treatment has been incorporated into clinical protocols.8
Stem cell mobilization.
As described above, KL and FL may prove useful for mobilizing or expanding BM stem cells in vivo. These stem cells can be used in various transplantation settings, in particular autologous and allogeneic stem cell transplantation of cancer patients after high-dose chemotherapy. In addition, mobilized stem cells might be excellent targets for gene therapy383,394-397 (see below). The use of KL and/or FL along with a second cytokine, such as G-CSF or GM-CSF, appears to increase the number of stem cells mobilized (see above). Stem cells mobilized/expanded in vivo by KL plus G-CSF might be better targets for gene therapy than those mobilized with G-CSF alone.366,374,382,383 394 However, qualitative differences in stem cell populations mobilized by different cytokine treatments have not yet been examined in sufficient detail and therefore require further study.
Ex vivo stem/progenitor cell expansion.
Ex vivo expansion of hematopoietic progenitor/stem cells is an area of intense study due to its clinical potential. However, a number of obstacles must be overcome before it can be established whether or not ex vivo-expanded progenitor/stem cells represent an improved therapeutic modality in various settings (for detailed reviews see Williams,398 Lange et al,399 and Emerson400).
Ex vivo–expanded progenitor/stem cells could reduce the need for extensive BM harvests or leukaphereses and enable repetitive cycles of high-dose chemotherapy. Because contaminating tumor cells in autologous stem/progenitor cell grafts can contribute to relapse,401,402 selective ex vivo expansion of progenitor/stem cells may also reduce or eliminate such tumor cells.399 400
Murine in vitro clonogenic progenitor cells as well as CFU-S efficiently expand when stimulated by KL or FL in combination with cytokines such as IL-1, IL-3, IL-6, IL-11, TPO, and G-CSF.205,206,222,287,345,403 Importantly, KL in combination with IL-1, IL-6, or IL-11 promotes efficient expansion of murine (short-term repopulating) progenitor cells without loss of long-term reconstituting ability in the expanded graft.403-406
Because IL-3 has been used extensively in ex vivo expansion protocols, it is noteworthy that IL-3 appears to compromise the long-term reconstituting ability of murine grafts expanded in either KL or FL in combination with other early acting cytokines.404 407
Optimal expansion of human progenitor cells requires the interaction of KL with multiple cytokines, including IL-1, IL-3, IL-6, GM-CSF, G-CSF, and EPO.306-308,408-410 As discussed above, the membrane-bound form of KL is more efficient than the soluble form at maintaining progenitor cell production in stromal cell cultures,89 indicating that membrane-bound KL might be beneficial for maintaining primitive progenitor/stem cells. FL also expands human myeloid progenitor cells in combination with other cytokines.192,208,224,297,311,313,315,316 411
Although KL and FL are efficient at stimulating production of multipotent and lineage-restricted myeloid progenitor cells from candidate human stem cells, the key question of whether ex vivo expansion protocols for human progenitor/stem cells maintain sufficient pluripotent long-term repopulating stem cells remains unanswered. Currently in patients receiving high-dose chemotherapy, the predominant function of progenitor/stem cell grafts might be to provide efficient short-term reconstitution, whereas long-term reconstitution might be provided equally well by endogenous stem cells surviving the high-dose treatment. However, if high-dose chemotherapy is further intensified, it might become crucial to ensure that transplants also contain sufficient LTRC.398-400 In the case of gene therapy, in which the ultimate goal is the introduction of therapeutic genes into LTRC, it is already paramount that such grafts contain LTRC412 (see below). Thus, it will be important to investigate the effects in ex vivo-expansion cultures on the earliest human stem cells using techniques such as gene marking.413
Although not conclusive with regard to LTRC, some recent studies cast light on the ability of FL and KL to maintain/expand candidate human stem cells. In one study, FL alone had the unique ability to slightly expand the number of primitive LTC-IC in CD34+CD38− BM cells, whereas LTC-IC were depleted in cultures containing KL alone.314 Furthermore, in a detailed study of 16 different cytokines, a combination of FL, KL, and IL-3 was both necessary and sufficient to obtain a 30-fold expansion of 6-week LTC-IC.314 In other studies, FL and KL were found to be equally efficient at stimulating the production of progenitor cells for 30 days from CD34+CD38−progenitor cells cultured on stroma,313 whereas progenitor cell output beyond 56 days was significantly higher in FL- than in KL-supplemented cultures.313 In addition, human CD34+ BM cells expanded under stroma-free conditions in KL plus IL-3 plus IL-6 in the presence (but not in the absence) of FL provided long-term reconstitution of immune-deficient mice.316 Other groups have found FL more efficient than KL at expanding human LTC-IC.414 Another promising combination of factors for the ex vivo expansion of stem/progenitor cells from cord blood was the combination of FL and TPO, which allowed continuous expansion of these cells for as much as 5 months.229
Gene therapy.
Hematopoietic stem cells are considered optimal targets for gene therapy, because they display extensive capacity to self-renew and to produce large numbers of progeny that are widely distributed throughout the body. In addition, stem cells can be readily obtained from BM, mobilized peripheral blood, or cord blood and can therefore be easily manipulated in vitro.412,415 416
Gene transfer into mouse long-term repopulating stem cells can be performed with high efficiency and success.417-421 In contrast, gene transfer into stem cells in larger animal models (including studies in humans) has been disappointing.412,415 416
Currently, mouse retroviruses are the only vectors shown to integrate permanently into host DNA, and most gene therapy protocols targeting stem cells use these vectors. One of the caveats with such retroviruses is that they cannot efficiently transduce and integrate into quiescent cells.412,415 416 Therefore, stem cells that normally are highly quiescent must be recruited into active cell cycle to enable successful transduction with such vectors, and FL and KL may be of use through their ability to efficiently trigger cell cycling of candidate stem cells. In addition, it is possible that these early acting cytokines might have a more beneficial effect on preserving the self-renewal, pluripotentiality, and engrafting potential of targeted stem cells than later-acting cytokines.
KL in combination with IL-3 and IL-6 efficiently promotes transduction of mouse stem cells while maintaining their long-term reconstituting ability.419,421 KL plus IL-3 plus IL-6 is also the combination predominantly used to achieve retroviral transduction of human hematopoietic progenitor cells, resulting in high gene transfer efficiency to committed as well as more primitive human progenitor cells (LTC-IC).422-426
Recent studies suggest that FL might be more efficient than KL at promoting gene transfer into human hematopoietic progenitor cells. Specifically, when combined with IL-3, FL is superior to KL at promoting retroviral gene transfer to committed myeloid progenitor cells, and the addition of KL (and other cytokines) to FL plus IL-3 significantly reduces the gene transfer efficiency.315 In the absence of stroma or fibronectin, the combination of IL-3, IL-6, and KL is unable to preserve the capacity of retrovirally transduced human BM CD34+ progenitor cells to sustain long-term hematopoiesis in immune-deficient mice in vivo.316 However, when FL is added to this cytokine combination, the transfected cells support long-term reconstitution of immunodeficient mice,316 although FL cannot fully replace the effect of stromal cells.316 The ability of FL to preserve the capacity of putative human stem cells to sustain long-term hematopoiesis in immune-deficient mice does not necessarily imply that FL enhances gene transfer to long-term repopulating stem cells. It is also possible that FL might have a positive effect on the self-renewal and/or engrafting potential of these cells.
KL and FL might also be used to enhance gene transfer into hematopoietic stem cells through their ability to mobilize stem cells to peripheral sites (described in detail above). Long-term reconstituting mouse stem cells mobilized to peripheral sites in response to administration of KL alone can be as efficiently transduced with retroviral vectors as mice treated with 5-FU.375 In mice treated with a combination of G-CSF and KL, mobilized long-term repopulating stem cells are expanded and transduced 2 to 3 times as efficiently as BM from 5-FU-treated mice, making such cells particularly attractive for gene therapy applications.394
The number of LTRC in the BM of mice and rhesus monkeys is expanded and shows improved gene transfer 1 to 2 weeks after treatment with KL and G-CSF.383 Similar studies of the efficiency of retroviral gene transfer to stem cells mobilized by FL in combination with G-CSF in primates also show an increased efficiency of gene transfer (Harry Malech, NIH, Bethesda, MD; personal communication).
Efficient gene transfer of human c-kit+hematopoietic cell lines has been achieved through targeting of c-kit with a molecular conjugate vector coupled to KL.427 However, whether a similar approach will be successful in normal hematopoietic progenitor/stem cells and whether permanent gene expression can be achieved remains unanswered.
Although these studies imply a role for KL and/or FL in human gene therapy in hematopoietic stem cells, most of these findings have been made in vitro or in immune-deficient mice and do not necessarily reflect true human stem cells. Thus, reproduction of such findings in nonhuman primates and eventually humans is essential.
Immunotherapy.
Immune DC, which may be thought of as professional antigen-presenting cells, have been proposed as cellular vectors for either antitumor or infectious disease vaccines, or as inducers of transplantation tolerance.428-430 The feasibility of using DC as immunotherapy vectors in the clinic has been limited by the small number of DC that can be isolated from the peripheral blood of normal individuals.
Although both KL193,194,431 and FL196,271stimulate the production of DC in vitro (see above), to date only FL has been shown to stimulate DC generation in vivo.273 These DC appear to be both myeloid and lymphoid derived.273Therefore, FL could possibly be used as a vaccine adjuvant: DC subsets would be expanded in vivo by treating individuals with FL, and then antigen-based vaccines would be injected. The goal would be to enhance the magnitude and quality of the immune response generated without the need for chemical adjuvants. Alternatively, larger numbers of circulating DC from FL-treated individuals could be isolated via apheresis for ex vivo manipulation (eg, vaccine or tolerogen exposure), followed by reinfusion of these DC.
Finally, and perhaps most promising, FL may have antitumor effects in vivo that are immune-system mediated. FL administration to mice has been shown to inhibit the growth of a fibrosarcoma cell line in vivo in a dose-dependent manner.432 Administration of FL to mice injected with a breast cancer cell line leads to rejection of these cells in syngeneic mice,433 as does ectopic expression of FL by these breast cancer cells.434 FL may stimulate DC production, which in turn presents tumor antigen(s) to T cells, leading to rejection of the tumors. NK cells are also likely to have a role in this process.
CONCLUDING REMARKS
KL and FL, acting through their respective tyrosine kinase receptors c-kit and flt3, have pleiotropic and potent effects on hematopoiesis in vitro and in vivo. Based on studies of the expression and function of the two receptors, it is now evident that the hematologic actions of these two cytokines are predominantly restricted to the progenitor/stem cell compartment. One important exception is the functional expression of c-kit, but not flt3, on mast cells, which helps explain the adverse events associated with KL administration in humans. The physiologic importance (if any) of the residual expression of c-kit and flt3 on other mature cell types remains unknown.
In the (long-term reconstituting) stem cell compartment, c-kitappears to be expressed on more stem cells than flt3, and, although not yet conclusively documented, c-kit might be expressed on earlier stem cells than flt3. Although recent data suggest that the earliest stem cells might express no or very low levels of c-kit and flt3, the status of c-kit and flt3 expression and function on hematopoietic stem cells needs to be studied in more depth, particularly in the human system.
Most of the hematopoietic activities of KL and FL appear to require a synergistic interaction with other early acting or lineage-selective cytokines. c-kit/KL might be critical for maintenance and self-renewal of long-term reconstituting stem cells, particularly in adult hematopoiesis. In addition, these two ligands appear to be essential for optimal production of mature hematopoietic cells from stem cells. Accordingly, stem cells deficient in c-kit or flt3 expression are defective in their ability to reconstitute hematopoiesis in myeloablated animals.
Interestingly, FL appears more critical for generation of lymphoid progeny than KL. In contrast, multiple lines of data suggest that KL inhibits B-cell development in mice.
The finding that FL plays a less crucial role than KL in the regulation of myelopoiesis and erythropoiesis is not surprising, because flt3 is generally expressed on less myeloid progenitor cells and is not found on erythroid progenitor cells. Thus, both KL and FL appear to have a dual function in hematopoiesis in that they both have activity on stem cells and appear to act as critical early regulators of myelopoiesis/erythropoiesis and lymphopoiesis, respectively.
The activities of FL and KL are distinct, although in some instances they may be complimentary to, synergistic with, or antagonistic to each other. It will be important to further dissect the distinct biological activities of the membrane-bound and soluble forms of KL and to determine whether membrane-bound FL functions differently from soluble FL. Whether these key hematopoietic regulators are involved in diseases or potentially could be used therapeutically remains to be further investigated. In that regard, combination therapy with other cytokines will be of particular interest.
ACKNOWLEDGMENT
The authors acknowledge the extensive and important contributions of colleagues at Immunex, especially Hilary McKenna, Ken Brasel, and Eugene Maraskovsky, and also Doug Williams, Bali Pulendran, Subhashini Srinivasan, Claudia Jochheim, and Dave Lynch for thoughtful discussions and reviewing the manuscript. We also thank members of the Stem Cell Laboratory, University of Lund including Ole Johan Borge, Veslemøy Ramsfjell, Cui Li, and Ole Peter Veiby for valuable input and reviewing the manuscript. We thank Hal Broxmeyer, Hans Drexler, Stefan Karlsson, Jonathan R. Keller, Makio Ogawa, Francis W. Ruscetti, and Alexandra Wodnar-Filipowicz for their critical review of the manuscript. Finally, we thank Anne Bannister and Christine Jones for expert editorial assistance.
Address reprint requests to Stewart D. Lyman, PhD, Department of Molecular Genetics, Immunex Corp, 51 University St, Seattle, WA 98101; or Sten Eirik W. Jacobsen, MD, PhD, Stem Cell Laboratory, Department of Internal Medicine, University Hospital of Lund, S-221 85 Lund, Sweden.

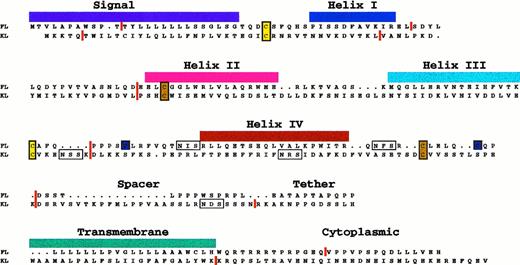
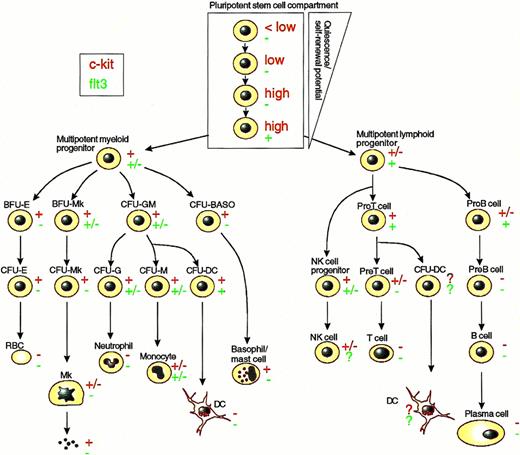
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal