Abstract
CD34 is widely used as a marker in the identification and purification of human hematopoietic stem and progenitor cells; however, its function within hematopoiesis is largely unknown. We have investigated the contribution of cytoplasmic domain of CD34 in cytoadhesion signaling and proliferation signaling in hematopoietic cells. Engagement of particular determinants of CD34 by monoclonal antibodies leads to homotypic adhesiveness of the full-length CD34-transfected BaF3 cells. However, this homotypic adhesiveness is abrogated in BaF3 cells transfected with the truncated CD34 lacking the cytoplasmic domain. Cytoadhesion signaling through the cytoplasmic domain of CD34 cannot be restored through that of erythropoietin receptor (EPOR) or granulocyte colony-stimulating factor receptor (G-CSFR), suggesting that the cytoplasmic domain of CD34 is required for its signal transduction of cellular adhesion. In constrast, we show that replacing the cytoplasmic domain of EPOR or G-CSFR with that of CD34 abolished growth signal transduction in response to EPO or G-CSF in the chimeric receptor-transfected BaF3, 32D, and FDCP1 cells, whereas the wild-type EPOR- or G-CSFR-transfected cells responded to EPO or G-CSF growth signaling well. These results suggest that the cytoplasmic portion of CD34 may not contain the elements necessary to transduce a proliferative signal in hematopoietic cells. Thus, the function of CD34 in hematopoiesis is primarily on hematopoietic cell adhesion.
EXPRESSION OF THE stem cell antigen CD34 is a defining hallmark of human hematopoietic stem and progenitor cells; thus, CD34 is one of the most commonly used markers for isolation, purification, and manipulation of these cells.1-4 The CD34 molecule is an approximately 115-kD type I transmembrane glycoprotein with a protein backbone of approximately 40 kD that shows no significant sequence homology to any other known protein. The extracellular domains of the human and mouse CD34 share approximately 63% amino acid identity and both contain an extensively O-glycosylated mucin-like portion and a cysteine-rich region. The N-terminal regions (1-130 amino acids) of the extracellular domains are the least well-conserved portions of the whole molecule (∼44% amino acid identity), whereas the entire cytoplasmic domains of both species are highly conserved (∼93% amino acid identity), predicting an essential functional importance for this domain.5,6 Studies in humans and baboons have shown that the CD34+ bone marrow (BM) and peripherally mobilized progenitor cells contain stem cells that can reconstitute all of the blood cells after transplantation.7,8 Similarly, the mouse CD34+BM and fetal liver cells also contain stem cells that can repopulate all of the blood lineages in lethally irradiated mice. 9 In addition to its expression on hematopoietic progenitor/stem cells, CD34 is also expressed on all vascular endothelial cells of both adults and embryos.10,11 It has been suggested that the CD34+ endothelial cells lining the yolk sac blood islands interact with the CD34+ hematopoietic progenitor cells of the yolk sac to induce differentiation, proliferation, and, possibly, self-renewal of the stem cells.12 Similar interactions between hematopoietic progenitor/stem cells and endothelial cells have been proposed in the aortic-gonadal-mesonephros region of the embryo as well.13 14 Despite the importance of CD34 as a marker of early hematopoietic progenitor/stem cells in clinical and developmental hematopoiesis, the function and regulation of this stem cell antigen is still unclear.
Studies on the function of CD34 suggest that it may play a role in adhesion and signal transduction on hematopoietic progenitor/stem cells. Ectopic expression of human CD34 in the thymocytes of transgenic mice indicates that CD34 augments the adhesive interactions of CD34+ hematopoietic cells with BM stroma and this CD34-dependent adhesion is enhanced by the engagement of anti-CD34 monoclonal antibodies (MoAbs).15 Similarly, engagement of certain epitopes on CD34 by anti-CD34 MoAbs triggers homotypic adhesion of CD34-expressing KG1a cells, implicating that CD34 has signal transducing capacity to induce cytoadhesiveness.16Furthermore, CD34 may also be involved in the maintenance of the hematopoietic progenitor/stem cell phenotype, ie, downregulation of CD34 may be necessary for differentiation of hematopoietic progenitor/stem cells. For example, the inappropriate or dysregulated expression of the full-length CD34 in leukemic cells may contribute to their undifferentiated phenotype.17 This notion has been supported by an intriguing report that constitutive overexpression of recombinant full-length CD34 protein in murine M1 myeloid leukemia cells blocks differentiation of these cells.18 However, the forced overexpression of the wild-type truncated form of CD34, which lacks a major portion of its cytoplasmic domain, fails to inhibit the cell differentiation of M1 leukemia cells, implying that the cytoplasmic domain of CD34 is required for the negative regulatory role for full-length CD34 in hematopoietic differentiation.18
Emerging evidence suggests that the cytoplasmic domain of CD34 may be involved in transducing a proliferation or differentiation signal in hematopoietic cells. The serine residues within the cytoplasmic domain of CD34 have been found to be phosphorylated upon treatment of hematopoietic cells with protein kinase C (PKC) activators.19 Because PKC is known to be involved in hematopoietic cell proliferation and differentiation,20,21it is possible that the cytoplasmic domain of CD34 may have the capability to transduce a growth or differentiation signal in hematopoietic cells. Furthermore, one recent study of CD34-deficient mice shows that hematopoietic progenitor/stem cells are probably decreased at certain developmental stages in the knockout mice, and this hematopoietic defect can be reversed by ectopic expression of the full-length CD34 in the CD34-deficient embryoid bodies; therefore, it has been proposed that CD34 may be involved in proliferation, survival, or retention of progenitor/stem cells in the hematopoietic compartment.22 However, surprisingly, a wild-type truncated form of CD34 lacking the majority of the cytoplasmic domain can also rescue all hematopoietic phenotypes as effectively as the full-length CD34, suggesting that the crucial functional portion of CD34 is confined to the extracellular domain, and the cytoplasmic domain of CD34 appears to be dispensible in hematopoietic development.22 Thus, the normal functional role of the cytoplasmic domain of CD34 within hematopoiesis has remained elusive.
In this report, we have examined the potential functional importance of the cytoplasmic domain of CD34 in cytoadhesion signaling and proliferation signaling in hematopoietic cells. By comparing the full-length CD34 with the recombinant truncated and chimeric CD34 molecules expressing in the factor-dependent hematopoietic cell lines, we have established that the cytoplasmic domain of CD34 is required for its signal transduction of cellular adhesion. In constrast, we show here for the first time, to our best knowledge, that the cytoplasmic domain of CD34 may not contain the elements necessary to transduce a proliferative signal in hematopoietic cells.
MATERIALS AND METHODS
Reagents.
Chemical reagents, including actinomycin D, cycloheximide, cytochalasin B, EDTA, herbimycin A, H7, sodium azide, sodium fluoride, sodium othovanadate, staurosporine, and other chemicals for buffers, were purchased from Sigma (St Louis, MO). Protease inhibitors, including aprotinin, leupeptin, and Pefabloc, were obtained from Boehringer Mannheim (Indianapolis, IN). Alamar Blue reagent was purchased from Biosource International (Camarillo, CA). The following antihuman CD34 MoAbs were used in this study: QBEND 10 (Immunotech, Inc, Westbrook, ME), ICH-3 and BI-3C5 (Accurate Antibodies, Westbury, NY), HPCA-1 (MY10) and HPCA-2 (8G12) (Becton Dickinson, Mountain View, CA), and VMA27 (Pharmingen, San Diego, CA). The isotype-matched control MoAbs mouse IgG1, IgG2a, and rat IgG2a were purchased from Pharmingen. For homotypic adhesion blocking experiments, the following MoAbs directed against mouse adhesion molecules were used: anti-CD11a M17/4 and 2D7, anti-CD11b M1/70, anti-CD11c HL3, anti-CD18 C71/16 and M18/2, anti-CD29 9EG7, anti-CD31 MEC13.3, anti-CD44 1M7, anti-CD45 30-F11, anti-CD45R RA3-6B2, anti-CD49d R1-2, anti-CD54 3E2, 3E2B and KAT-1, anti-CD62L MEL-14, anti-CD71 C2, and anti-integrin β7. These MoAbs were purchased either from Pharmingen or Serotec Ltd (Indianapolis, IN). L-selectin-IgG/Fc fusion protein was kindly provided by Dr Richard Nelson (Amgen, Inc, Thousand Oaks, CA). Recombinant murine interleukin-3 (IL-3) produced inEscherichia coli, human erythropoietin (EPO) and granulocyte colony-stimulating factor (G-CSF) produced in Chinese hamster ovary cells were prepared at Amgen, Inc.
cDNA constructions.
Full-length human CD34 cDNA was obtained from a human thymus cDNA library (Clontech, Inc, Palo Alto, CA) by the polymerase chain reaction (PCR) technique using Vent DNA polymerase (New England Biolabs, Beverley, MA) with the 5′ primer containing the translational start (underlined) 5′-TATGGTACCAAGCTTGCCACCATGCCGCGGGGCTGGACCGCGCTTTGC-3′ and the 3′ primer containing the end of cytoplasmic domain and two stop codons (underlined) 5′-TATGTCGACATCGATTCATCACAATTCGGTATCAGCCACCACGTGTTG-3′. The PCR-generated product was cloned into the expression vector pEF-BOS23 between the Kpn I and Sal I sites and designated pEF-CD34. The recombinant truncated form of CD34 cDNA, which was truncated after the first amino acid within the intracellular domain (after the N, amino acid 301), was constructed by the same PCR technique with the 5′ primer containing the translational start (underlined) 5′-TATGGTACCGAATTCGCCACCATGCCGCGGGGCTGGACCGCGCTTTGC-3′ and the truncated 3′ primer containing the end of transmembrane domain and the first amino acid of the intracellular domain and two stop codons (underlined) 5′-TATGTCGACATCGATTCATCATTCATCAGGAAATAGCCAGTGATGCCC-3′. The PCR product was cloned into the expression vector pEF-BOS between the Kpn I and Sal I sites and designated pEF-CD34/T. The full-length human EPOR and G-CSFR cDNAs were obtained from Drs Steven Elliott (Amgen, Inc) and Shigekazu Nagata (Osaka, Japan), respectively, and were subcloned into the expression vector pSRα24-neo, which contains the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type I long terminal repeat, and designated pSRα-EPOR and pSRα-G-CSFR, respectively. The cDNA of chimeric receptor CD34/EPOR or CD34/G-CSFR was generated by using a two-step PCR to combine the extracellular domain of CD34 with the transmembrane and cytoplasmic domains of either EPOR or G-CSFR, and the cDNAs were subcloned into the expression vector pEF-BOS between Kpn I and Sal I and designated pEF-CD34/EPOR and pEF-CD34/G-CSFR, respectively. Thus, all of the cDNAs listed in Fig 1 are in the expression vector pEF-BOS that uses a powerful elongation factor promoter to drive transcription of the inserted cDNAs. Similarly, the cDNA of chimeric receptor EPOR/CD34 or G-CSFR/CD34 was generated by using a two-step PCR to combine the extracellular domain of either EPOR or G-CSFR with the transmembrane and cytoplasmic domains of CD34, and the cDNAs were subcloned into the expression vector pSRα-neo between theEcoRI and Cla I, designated pSRα-EPOR/CD34 and pSRα-G-CSFR/CD34, respectively. Therefore, all of the cDNAs listed in Fig 4 are in the expression vector pSRα-neo that uses a strong SR a promoter to drive transcription of the inserted cDNAs. The sequences of all cDNA constructs were confirmed by DNA sequencing on both strands using a PCR procedure employing fluorescent dideoxynucleotides and a model 373A automated sequencer (Applied Biosystems, Foster City, CA).
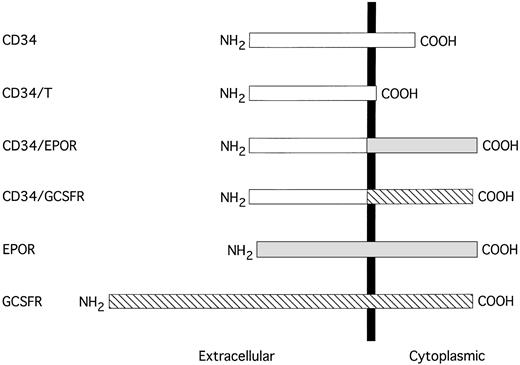
Schematic representation of the truncated CD34 and CD34 chimeric receptors. The sequences are aligned at their transmembrane domains (shown by a solid vertical bar). The sequence of CD34 is represented by an open box, EPOR is shown by a shaded box, and G-CSFR is depicted by a hatched box.
Schematic representation of the truncated CD34 and CD34 chimeric receptors. The sequences are aligned at their transmembrane domains (shown by a solid vertical bar). The sequence of CD34 is represented by an open box, EPOR is shown by a shaded box, and G-CSFR is depicted by a hatched box.
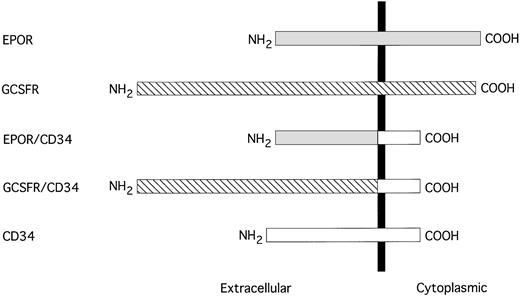
Schematic representation of EPOR/CD34 and G-CSFR/CD34 chimeric receptors. The sequences are aligned at their transmembrane domains, which are depicted by a solid vertical bar. The sequence of EPOR is shown by a shaded box, G-CSFR is denoted by a hatched box, and CD34 is represented by an open box.
Schematic representation of EPOR/CD34 and G-CSFR/CD34 chimeric receptors. The sequences are aligned at their transmembrane domains, which are depicted by a solid vertical bar. The sequence of EPOR is shown by a shaded box, G-CSFR is denoted by a hatched box, and CD34 is represented by an open box.
Cell culture and transfections.
The factor (IL-3)-dependent murine hematopoietic cell lines BaF3,25 32D,26 and FDCP127 used in this study were obtained from Drs Naoki Nakayama and Ian McNiece (Amgen, Inc) and were cultured in RPMI 1640 medium (GIBCO Life Technologies, Inc, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; HyClone Laboratories, Inc, Logan, UT), 5 ng/mL of murine IL-3 (Amgen, Inc), penicillin/streptomycin, and L-glutamine (P/S/G) in a CO2(5%) incubator at 37°C. Similarly, the human hematopoietic cell line KG1a was obtained from Geraldine Trail (Amgen, Inc) and was cultured in Iscove's modified Dulbecco's medium (GIBCO Life Technologies, Inc) supplemented with 20% FBS and P/S/G. Cells to be transfected were washed once with HEPES-buffered saline (HeBS) (21 mmol/L HEPES, pH 7.0, 137 mmol/L NaCl, 5 mmol/L KCl, 0.7 mmol/L Na2HPO4, 5.5 mmol/L dextrose) and resuspended in ice-cold HeBS at a density of 5 × 106 cells/mL. Cells were stably transfected with each expression plasmid (20 μg linearized DNA), which contains the neo gene (G418 resistance), or cotransfected each expression plasmid with pNeo3 (2 μg linearized DNA) by electroporation at 300 V, 960 μF with Gene Pulser (BioRad, Inc, Hercules, CA), and cultured in IL-3–containing medium for 48 hours. Subsequently, the transfected cells were diluted into the selection medium containing G418 (final concentration, 1 mg/mL) at a density of 1 × 103 or 1 × 104 cells/mL and distributed into flat-bottomed 96-well microtiter plates (100 μL/well). G418-resistant colonies were isolated and expanded after 10 to 15 days. Transfectants expressing the exogenous receptors were identified by fluorescence-activated cell sorting (FACS) analyses.
Western blot analysis.
The BaF3 cell transfectants were lysed in WCE lysis buffer (20 mmol/L HEPES, pH 7.4, 2 mmol/L EGTA, 50 mmol/L β-glycerophosphate, 1% Triton X-100, 10% glycerol, 1 mmol/L dithiothreitol, 2 μg/mL leupeptin, 5 μg/mL aprotinin, 1 mmol/L Pefabloc [Boehringer Mannheim], and 1 mmol/L sodium othovanadate). Soluble lysates were prepared by centrifugation at 10,000g for 30 minutes at 4°C, electrophoresed through an 8% sodium dodecyl sulfate (SDS)-polyacrylamide gel, and electroblotted onto polyvinylidene difluoride (PVDF) membranes (Novex, Inc, San Diego, CA). The blot was probed with anti-CD34 MoAb QBEND 10 and visualized by enhanced chemiluminescence (ECL) detection (Amersham, Arlington Heights, IL) using goat antimouse IgG conjugated to horseradish peroxidase as a secondary antibody (Pierce, Rockford, IL).
Homotypic adhesion assay.
The semiquantitative homotypic aggregation assays were performed as described by Rothlein and Springer28 and Majdic et al.16 The individual cell clones of BaF3 transfectants were placed into flat-bottomed 96-well microtiter plates (Falcon, Franklin Lakes, NJ) at a density of 1.3 × 105cells per well in 90 μL of basic medium, and 10 μL of anti-CD34 MoAb QBEND 10 was added into each well (final concentration, 5 μg/mL) and mixed with the cell suspension. The cells were incubated for 90 minutes at 37°C, with mild shaking, and then the degree of cell aggregation was scored under a microscope. Scores ranged from 0+ to 4+. 0+ represented that less than 10% of the cells were in homoaggregates; 1+, 10% to 40%; 2+, 40% to 70%; 3+, 70% to 100%; and 4+ indicated 100% of the cells were in very large homoaggregates. For antibody blocking assays, BaF3 cells were preincubated with inhibitor MoAbs (final concentration, 50 μg/mL) for 30 minutes at 0°C before initiation of the homotypic aggregation assay by adding anti-CD34 MoAb QBEND 10 (final concentration, 5 μg/mL).
Cell proliferation assay.
The individual cell clones of BaF3, 32D, and FDCP1 transfectants were washed extensively with phosphate-buffered saline to remove IL-3 and seeded onto flat-bottomed 96-well microtiter plates at a density of 5 × 103 cells per well in basic medium supplemented with the indicated growth factors (final concentration, 5 μg/mL), including IL-3, EPO, and G-CSF. The cells were incubated for 36 to 48 hours at 37°C in 5% CO2. Subsequently, cell proliferation was measured by adding 10 μL of Alamar Blue reagent into each well and returning the 96-well plates to CO2incubator. After 6 hours of incubation, plates were read with quantitation of fluorescence by excitation at 530 nm and emission at 590 nm by CytoFlor II fluorescence plate reader (PerSeptive Biosystems, Bedford, MA).
RESULTS
The cytoplasmic domain of CD34 is essential for its signal transduction of cellular adhesion.
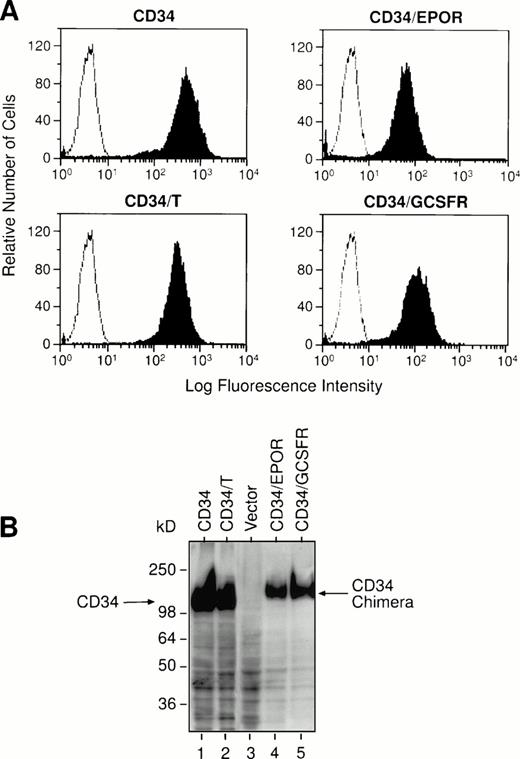
To investigate the functional role of the cytoplasmic domain of CD34 in cytoadhesion signaling, we constructed mammalian cell expression vectors to carry the recombinant truncated human CD34 without the cytoplasmic domain (designated as CD34/T) and two chimeric CD34 receptors consisting of the entire extracellular domain of human CD34 and the transmembrane and cytoplasmic regions of either human EPOR or human G-CSFR, designated as CD34/EPOR and CD34/G-CSFR, respectively (Fig 1). We note that the recombinant truncated CD34 protein (CD34/T) generated in this study was truncated after the first amino acid within the intracellular domain (after the N) and is not the same as wild-type truncated CD34 protein that has the intracellular domain:NRRSWSPTGERLELEP (the underlined amino acids are shared with the full-length CD34). We also constructed an expression vector to express the full-length human CD34. We transfected each of these expression constructs and an empty vector alone into the IL-3–dependent murine lymphoid precursor cell line BaF3, which does not express endogenous CD34. The transfected G418-resistant cell clones were analyzed for expression of the introduced receptors by FACS analysis (Fig 2A), and positive cell clones from each transfection were selected and expanded, and their expression of the introduced receptors was confirmed by Western blot analysis (Fig2B). Analysis of BaF3 cells transfected with vector alone confirmed the lack of any endogenous CD34. Because the majority of molecular mass of sialomucin CD34 is contributed by extensive glycosylation and heavily sialylated glycan chains, there is no significant difference in molecular mass (115 kD) between the full-length and the truncated CD34 molecules.
Expression of the full-length or truncated CD34 and CD34 chimeric receptors on the BaF3 cell transfectants. BaF3 cells were stably transfected with each receptor expression construct as indicated, and individual positive cell clones were selected. (A) Flow cytometry analyses. The vector-transfected BaF3 cells (negative controls, open histograms) or the receptor-transfected BaF3 cells (solid histograms) were stained with the fluorescein isothiocyanate (FITC)-labeled anti-CD34 MoAb HPCA-2. The staining profiles of the BaF3-transfected cells with an isotype-matched control MoAb are the same as those of negative controls (shown as open histograms). (B) Western blot analyses. Cell lysates from the BaF3 cell transfectants were electrophoresed through an 8% SDS-polyacrylamide gel and transferred to a PVDF membrane. The blot was probed with anti-CD34 MoAb QBEND 10 and visualized by ECL detection using goat antimouse IgG conjugated to horseradish peroxidase as a secondary antibody.
Expression of the full-length or truncated CD34 and CD34 chimeric receptors on the BaF3 cell transfectants. BaF3 cells were stably transfected with each receptor expression construct as indicated, and individual positive cell clones were selected. (A) Flow cytometry analyses. The vector-transfected BaF3 cells (negative controls, open histograms) or the receptor-transfected BaF3 cells (solid histograms) were stained with the fluorescein isothiocyanate (FITC)-labeled anti-CD34 MoAb HPCA-2. The staining profiles of the BaF3-transfected cells with an isotype-matched control MoAb are the same as those of negative controls (shown as open histograms). (B) Western blot analyses. Cell lysates from the BaF3 cell transfectants were electrophoresed through an 8% SDS-polyacrylamide gel and transferred to a PVDF membrane. The blot was probed with anti-CD34 MoAb QBEND 10 and visualized by ECL detection using goat antimouse IgG conjugated to horseradish peroxidase as a secondary antibody.
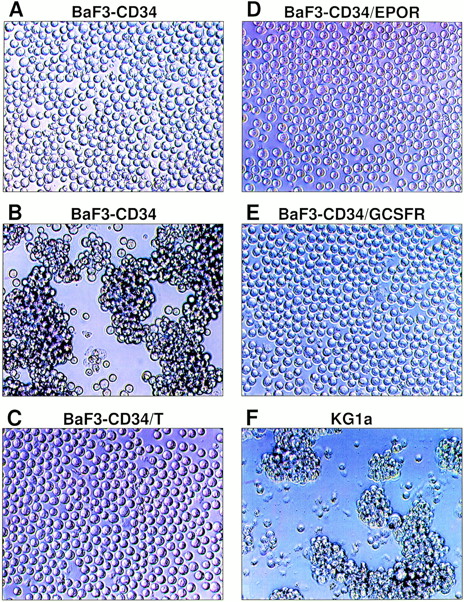
It has been reported that certain MoAbs directed against O-sialoglycoprotease–sensitive epitopes of human CD34 can trigger homotypic aggregation of CD34+ KG1a cells.16 To examine whether these MoAbs can induce homotypic adhesion of BaF3-transfected cells, we incubated BaF3-CD34 cells with several different anti-CD34 MoAbs and examined their effects on homotypic adhesion of these cells at different time points. We found that anti-CD34 MoAb QBEND 10 induced marked homoaggregate formation of BaF3-CD34 cells and MoAb HPCA-1 had a less profound but definite homoaggregation-inducing effect, whereas four other anti-CD34 MoAbs (ICH3, BI-3C5, HPCA-2, and VMA27) were ineffective in this regard. In accordance with the previous findings,16 adhesion induction of BaF3-CD34 cells by MoAb QBEND 10 was temperature-dependent and no homoaggregate formation could be induced at 0°C. The data suggest that the observed homotypic adhesion was not due to antibody-mediated passive aggregation. The homotypic aggregation occurred after 30 minutes and reached maximal at 90 minutes at 37°C. Titration experiments indicated that the optimal concentration of QBEND 10 to induce homoaggregate formation of BaF3-CD34 cells was around 5 μg/mL. A typical example of the observed homoaggregation of BaF3-CD34 cells is shown in Fig 3B.
Homotypic aggregation of the BaF3 cell transfectants upon addition of anti-CD34 MoAb QBEND 10. BaF3-CD34 cells were incubated with the negative control MoAb (A) or anti-CD34 MoAb QBEND 10 (B), which represents the typical morphology of homoaggregate formation of BaF3-CD34 cells (score, 3+). Similarly, BaF3-CD34/T cells (C), BaF3-CD34/EPOR cells (D), and BaF3-CD34/G-CSFR cells (E) were treated with MoAb QBEND 10. As a positive control, KG1a cells were also incubated with MoAb QBEND 10 (F). The final concentration of MoAb is 5 μg/mL. This experiment has been repeated five times with similar results.
Homotypic aggregation of the BaF3 cell transfectants upon addition of anti-CD34 MoAb QBEND 10. BaF3-CD34 cells were incubated with the negative control MoAb (A) or anti-CD34 MoAb QBEND 10 (B), which represents the typical morphology of homoaggregate formation of BaF3-CD34 cells (score, 3+). Similarly, BaF3-CD34/T cells (C), BaF3-CD34/EPOR cells (D), and BaF3-CD34/G-CSFR cells (E) were treated with MoAb QBEND 10. As a positive control, KG1a cells were also incubated with MoAb QBEND 10 (F). The final concentration of MoAb is 5 μg/mL. This experiment has been repeated five times with similar results.
Subsequently, we investigated the contribution of the cytoplasmic domain of CD34 in cytoadhesion signaling based on this induction of homotypic adhesion assay. As expected, treatment of the empty vector-transfected BaF3 cells with MoAb QBEND 10 did not induce any detectable homoaggregate formation, and a similar result was obtained from the untransfected BaF3 cells. Whereas incubation of BaF3-CD34 cells with the control MoAb did not induce any detectable homoaggregate formation (Fig 3A), incubation of these cells with MoAb QBEND 10 caused strong homotypic aggregation (Fig 3B). Strikingly, incubation of BaF3-CD34/T cells with MoAb QBEND 10 failed to induce any detectable homotypic aggregation (Fig 3C), suggesting that the cytoplasmic domain of CD34 is essential for induction of homotypic adhesion via the CD34 molecule. Furthermore, incubation of BaF3-CD34/EPOR and BaF3-CD34/G-CSFR cells with MoAb QBEND 10 also failed to cause any detectable homoaggregate formation (Fig 3D and E), implicating that cytoadhesion signaling through the cytoplasmic domain of CD34 cannot be restored through those domains of EPOR and G-CSFR. Similar results were obtained with two independent BaF3 cell clones from each transfection (Fig 3A through E) in three independent assays. As a positive control, CD34+ KG1a cells were incubated with MoAb QBEND 10 and showed marked homotypic aggregation (Fig 3F). Taken together, these results strongly suggest that the cytoplasmic domain of CD34 is required for its signal transduction of cellular adhesion.
CD34 antibody-induced homotypic adhesion requires cellular adenosine triphosphate (ATP), divalent cations, a functional cytoskeleton, and possibly protein tyrosine kinases.
To define the cellular components crucial for cytoadhesion induction, we next examined the effect of inhibitors on CD34 MoAb-induced homotypic aggregation. BaF3-CD34 cells were preincubated with inhibitors for 30 minutes at 37°C before initiation of the homotypic cell adhesion assay by adding anti-CD34 MoAb QBEND 10. As shown in Table 1, homotypic adhesion induced by MoAb QBEND 10 was significantly inhibited by metabolic depletion of cellular ATP by prior incubation with sodium fluoride and was completely abrogated by chelation of divalent cations with EDTA or inhibition of the cytoskeleton by cytochalasin B. The data suggest that CD34 MoAb-induced homotypic adhesion requires cellular ATP, divalent cations, and a functional cytoskeleton. However, CD34 MoAb-induced homotypic adhesion was not affected by prior incubation with actinomycin D or cycloheximide, suggesting that de novo protein synthesis was not necessary for cytoadhesion induction. Moreover, catalases were not involved in this homotypic adhesion because it was not affected by prior incubation with sodium azide.
Effect of Inhibitors on CD34 MoAb-Induced Homotypic Cell Adhesion
| Inhibitors . | Adhesion Score . |
|---|---|
| None | +++ |
| Sodium flouride | + |
| EDTA | − |
| Cytochalasin B | − |
| Actinomycin D | +++ |
| Cycloheximide | +++ |
| Sodium azide | +++ |
| Herbimycin A | + |
| Staurosporine | ++ |
| H7 | ++ |
| Inhibitors . | Adhesion Score . |
|---|---|
| None | +++ |
| Sodium flouride | + |
| EDTA | − |
| Cytochalasin B | − |
| Actinomycin D | +++ |
| Cycloheximide | +++ |
| Sodium azide | +++ |
| Herbimycin A | + |
| Staurosporine | ++ |
| H7 | ++ |
BaF3-CD34 cells were preincubated with inhibitors for 30 minutes at 37°C before initiation of the homotypic cell adhesion assay by adding anti-CD34 MoAb QBEND 10 (final concentration, 5 μg/mL). Inhibitors were used as follows: 30 mmol/L sodium fluoride, 5 mmol/L EDTA, 10 μg/mL cytochalasin B, 1 μg/mL actinomycin D, 1 μg/mL cycloheximide, 0.02% sodium azide, 5 μmol/L herbimycin A, 1 μmol/L staurosporine, 100 μmol/L H7. Cell aggregation was scored after 90 minutes. Identical results were obtained in three independent experiments.
To further test whether protein tyrosine kinase or PKC activity was involved in cytoadhesion signaling of CD34 MoAb-induced homotypic adhesion, we pretreated BaF3-CD34 cells with the protein tyrosine kinase inhibitor herbimycin A29,30 or the PKC inhibitors staurosporine31 and H732 and analyzed the influence of these agents on CD34 MoAb-induced homoaggregate formation. Herbimycin A significantly reduced homotypic aggregation of BaF3-CD34 cells upon the binding of MoAb QBEND 10. However, staurosporine and H7 only slightly inhibited CD34 MoAb-induced homotypic adhesion. These results suggest that certain protein tyrosine kinases may be involved in CD34 cytoadhesion signaling. All of the substances used at the indicated concentrations did not affect cell viability during the experiments as determined by trypan blue staining.
Lymphocyte function-associated antigen-1 (LFA-1) and intercellular adhesion molecule-1 (ICAM-1) may be involved in CD34 antibody-induced homotypic aggregation.
It has been previously suggested that a concomitant activation of the integrin β2 adhesion pathway may be involved in CD34 MoAb-induced homotypic adhesion.16 To confirm their interesting findings, we performed MoAb inhibition experiments by preincubation of BaF3-CD34 cells at 0°C with blocking MoAbs against integrin β1 (CD49d, CD29), integrin β2(CD11a, CD18), ICAM-1 (CD54), CD11b, CD11c, CD31, CD44, CD45, and other adhesion molecules before the addition of MoAb QBEND 10. As listed in Table 2, one of the anti-CD18 and anti-CD54 MoAbs could indeed significantly but not completely inhibit the CD34 MoAb-induced homotypic adhesion of BaF3-CD34 cells; however, other anti-CD18 and anti-CD54 MoAbs used here only partially reduced the homotypic adhesion of BaF3-CD34 cells. In addition, anti-CD11a MoAbs did not significantly block the homotypic adhesion of BaF3-CD34 cells. Perhaps the observed incomplete inhibition of some MoAbs was due to ineffective blocking activities of those MoAbs. Nevertheless, anti-CD11a, anti-CD18, and anti-CD54 MoAbs were the only MoAbs tested so far that could impede the CD34 MoAb-induced homotypic adhesion of BaF3-CD34 cells. These data agree well with the previous results16 and suggest that LFA-1 (CD11a/CD18) and ICAM-1 (CD54) may be involved in CD34 MoAb-induced homotypic adhesion.
Inhibition of CD34 MoAb-Induced Homotypic Cell Adhesion by MoAbs to Other Adhesion Molecules
| MoAbs . | Adhesion Score . |
|---|---|
| None | +++ |
| Negative control | +++ |
| CD11a (M17/4) | ++ |
| CD11a (2D7) | ++ |
| CD11b (M1/70) | +++ |
| CD11c (HL3) | +++ |
| CD18 (C71/16) | ++ |
| CD18 (M18/2) | + |
| CD29 (9EG7) | +++ |
| CD31 (MEC13.3) | +++ |
| CD44 (1M7) | +++ |
| CD45 (30-F11) | +++ |
| CD45R (RA3-6B2) | +++ |
| CD49d (R1-2) | +++ |
| CD54 (3E2) | ++ |
| CD54 (3E2B) | ++ |
| CD54 (KAT-1) | + |
| CD62L (MEL-14) | +++ |
| CD71 (C2) | +++ |
| Integrin β7 | +++ |
| MoAbs . | Adhesion Score . |
|---|---|
| None | +++ |
| Negative control | +++ |
| CD11a (M17/4) | ++ |
| CD11a (2D7) | ++ |
| CD11b (M1/70) | +++ |
| CD11c (HL3) | +++ |
| CD18 (C71/16) | ++ |
| CD18 (M18/2) | + |
| CD29 (9EG7) | +++ |
| CD31 (MEC13.3) | +++ |
| CD44 (1M7) | +++ |
| CD45 (30-F11) | +++ |
| CD45R (RA3-6B2) | +++ |
| CD49d (R1-2) | +++ |
| CD54 (3E2) | ++ |
| CD54 (3E2B) | ++ |
| CD54 (KAT-1) | + |
| CD62L (MEL-14) | +++ |
| CD71 (C2) | +++ |
| Integrin β7 | +++ |
BaF3-CD34 cells were preincubated with the respective MoAbs (final concentration, 50 μg/mL) for 30 minutes at 0°C before initiation of the homotypic cell adhesion assay by adding anti-CD34 MoAb QBEND 10 (final concentration, 5 μg/mL). Cell aggregation was scored after 90 minutes. Identical results were obtained in three independent experiments.
The cytoplasmic domain of CD34 is not sufficient to transduce growth signal in hematopoietic cells.
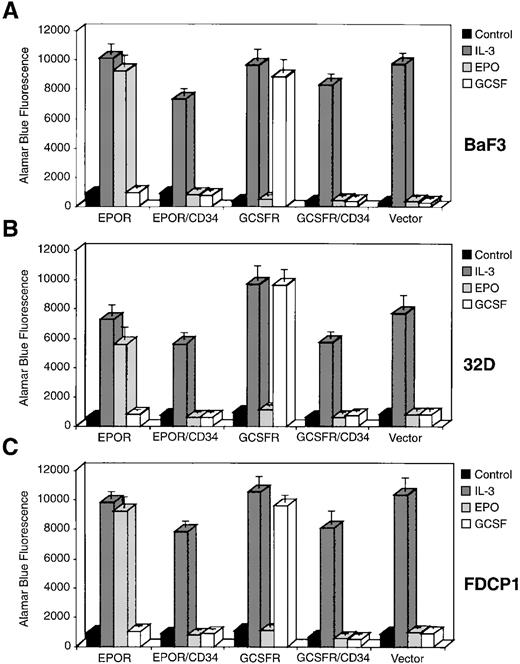
It has been postulated that CD34 may be involved in the maintenance or proliferation of the hematopoietic progenitor/stem cells22; therefore, we wish to use chimeric receptor constructs to investigate whether the cytoplasmic domain of CD34 contains the elements necessary to transduce a proliferative signal in hematopoietic cells. We generated two mammalian cell expression vectors to produce chimeric receptors consisting of the extracellular domain of either human EPOR or human G-CSFR and the cytoplasmic region of human CD34, designated as EPOR/CD34 and G-CSFR/CD34, respectively (Fig 4). We also constructed two expression vectors to express the full-length EPOR or G-CSFR. We transfected each of these four receptor-expressing constructs and an empty vector alone into the IL-3–dependent BaF3 cells, which do not express endogenous EPOR or G-CSFR or CD34. The transfected G418-resistant cell clones were analyzed for expression of the introduced receptors by FACS analysis (Fig 5a through d), and positive cell clones from each transfection were selected and expanded. In general, the expression level of the chimeric receptor EPOR/CD34 or G-CSFR/CD34 was greater than that of the full-length EPOR or G-CSFR. Although we could only obtain low expressing clones of BaF3-EPOR and BaF3-G-CSFR after several times of transfection, these cell clones could respond to the corresponding growth factors well. Subsequently, we have assayed the positive cell clones for proliferation or survival activity in the presence of EPO or G-CSF specifically, using IL-3 as positive control and no factor as negative control. Our results indicated that none of the chimeric receptors (EPOR/CD34, G-CSFR/CD34) could support cell growth or survival, whereas the full-length EPOR or G-CSFR could stimulate cell proliferation in response to EPO or G-CSF, respectively (Fig 6A). Furthermore, to generalize this investigation, we repeated the same DNA transfection and proliferation experiments with IL-3–dependent murine myeloid cell line 32D and hematopoietic precursor cell line FDCP1, which do not express endogenous EPOR or G-CSFR or CD34. Analysis of BaF3 or 32D or FDCP1 cells tranfected with vector alone confirmed the lack of any endogenous EPOR or G-CSFR or CD34. Receptor-expressing clones of 32D and FDCP1 cells (Fig 5e through l) were selected and assayed for proliferation activity in the presence of IL-3 or EPO or G-CSF as described above. Similar results were obtained with 32D and FDCP1 cells (Fig 6B and C), indicating that the observed phenomena are not due to a particular cell type. Taken together, these results imply that the cytoplasmic domain of CD34 may not contain the elements necessary to transmit a growth-stimulatory signal in hematopoietic cells. However, it has not been determined whether the intracellular “NRRSWSPTGERL” domain in wild-type truncated CD34 can signal in this proliferation study. Interestingly, overexpression of the cytoplasmic domain of full-length CD34 through the chimeric receptors (EPOR/CD34, G-CSFR/CD34) diminished approximately 25% of the IL-3 responsiveness of Baf3, 32D, and FDCP1 cells (Fig 6), suggesting that the cytoplasmic domain of CD34 may either have a negative effect on cytokine-mediated signaling or compete with the cytoplasmic domain of IL-3 receptor for vital signaling components in these cells.
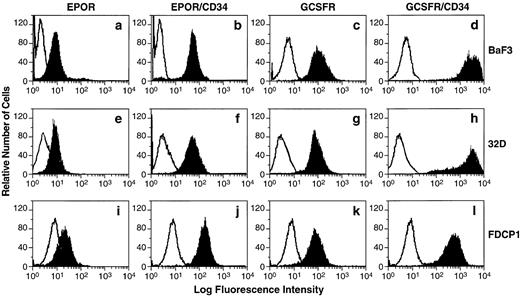
Expression of EPOR, G-CSFR, and their chimeric receptors EPOR/CD34 and G-CSFR/CD34 on the BaF3 or 32D or FDCP1 cell transfectants. BaF3 (a through d), 32D (e through h), or FDCP1 (i through l) cells were stably transfected with each receptor expression construct as indicated, and individual positive cell clones were selected by flow cytometry analyses. The vector-transfected cells (negative controls, open histograms) or the receptor-transfected cells (solid histograms) were stained with either anti-EPOR MoAb or the FITC-labeled G-CSF. The staining profiles of the transfected cells with an isotype-matched control MoAb are the same as those of negative controls, which are depicted as open histograms.
Expression of EPOR, G-CSFR, and their chimeric receptors EPOR/CD34 and G-CSFR/CD34 on the BaF3 or 32D or FDCP1 cell transfectants. BaF3 (a through d), 32D (e through h), or FDCP1 (i through l) cells were stably transfected with each receptor expression construct as indicated, and individual positive cell clones were selected by flow cytometry analyses. The vector-transfected cells (negative controls, open histograms) or the receptor-transfected cells (solid histograms) were stained with either anti-EPOR MoAb or the FITC-labeled G-CSF. The staining profiles of the transfected cells with an isotype-matched control MoAb are the same as those of negative controls, which are depicted as open histograms.
Proliferation of the BaF3, 32D, and FDCP1 cell transfectants in response to IL-3, EPO, or G-CSF. The individual cell clones as indicated were incubated in the absence of any growth factor (strongly shaded box), as a negative control, or in the presence of IL-3 (medianly shaded box) or EPO (lightly shaded box) or G-CSF (unshaded box) for 36 to 48 hours. Proliferation was measured by reduction of Alamar Blue, with quantitation of fluorescence by excitation at 530 nm and emission at 590 nm. Error bars indicate the mean and standard deviation for triplicate assay values.
Proliferation of the BaF3, 32D, and FDCP1 cell transfectants in response to IL-3, EPO, or G-CSF. The individual cell clones as indicated were incubated in the absence of any growth factor (strongly shaded box), as a negative control, or in the presence of IL-3 (medianly shaded box) or EPO (lightly shaded box) or G-CSF (unshaded box) for 36 to 48 hours. Proliferation was measured by reduction of Alamar Blue, with quantitation of fluorescence by excitation at 530 nm and emission at 590 nm. Error bars indicate the mean and standard deviation for triplicate assay values.
DISCUSSION
In the present study, using the truncated and chimeric receptor approaches, we found that the cytoplasmic domain of CD34 is required for its signal transduction of cellular adhesion in hematopoietic cells. This is consistent with the notion that the cytoplasmic domain of CD34 is indispensable for the negative regulatory function of full-length CD34 in hematopoietic differentiation.18 In agreement with the previous findings,16 we showed that CD34 indeed has cytoadhesive signal transducing capability. Although homotypic adhesion of BaF3-CD34 cells depends on molecular engagement of certain epitopes on CD34 molecule, evidence argues against the possibility that the observed homotypic aggregation is merely a passive agglutination of these cells by certain CD34 MoAbs. Firstly, cytoadhesion induction of BaF3-CD34 cells by MoAb QBEND 10 was temperature-dependent and no homoaggregate formation could be induced at 0°C. Secondly, unlike KG1a cells, none of the anti-CD34 MoAbs can induce homotypic aggregation of KG1 cells that also express a high level of CD34 but a very low level of ICAM-1.16 Thirdly, concomitant activation of the LFA-1/ICAM-1 cytoadhesion pathway may be involved in the CD34-dependent cytoadhesion formation.16Fourthly, ectopic expression of the human CD34 molecule in murine hematopoietic cells confers elevated binding of these cells to human BM stromal cells or cell lines but not to their murine counterparts, implying that the cytoadhesive function of CD34 requires specific recognition of counter-receptor or ligand on stromal cells.15 Perhaps the stimulatory anti-CD34 MoAbs act as surrogate ligands mimicking the binding of natural ligands or counter-receptors by interacting with the ligand binding domains, presumably glycosylated regions, of the CD34 molecule.
Recently, one study of CD34-knockout mice shows that hematopoiesis is delayed in developing embryoid bodies; that hematopoietic progenitor cells from yolk sac, fetal liver, and adult BM are reduced twofold to threefold; and that the BM progenitor cells are retarded in their ability to expand ex vivo in response to various hematopoietic growth factors.22 In addition, ectopic expression of the full-length CD34 in the CD34-deficient embryoid bodies results in a reversal of this hematopoietic deficiency and suggests that CD34 may be involved in the proliferation or maintenance of the hematopoietic progenitor/stem cells. However, a wild-type truncated form of CD34, which lacks the majority of the cytoplasmic domain, can also rescue all hematopoietic phenotypes to a similar degree as the full-length CD34, implying that the cytoplasmic domain of CD34 is not necessary for its function in hematopoietic development.22 Evidently, our present results did not accord with their implications. Several possible reasons may explain this difference. Firstly, Cheng et al22 have pointed out that the high level overexpression of either the full-length or the wild-type truncated form of CD34 caused losses of normal CD34 regulation and tissue-specific expression in their transfection system so that the transfected cells may have overcome any need for either PKC-mediated or other intracellular signaling phenomena. Secondly, it is possible that the intracellular “NRRSWSPTGERLELEP” domain of the wild-type truncated CD34 may be able to transduce signals and play an important role in rescuing the phenotype in the CD34-knockout embryoid bodies. Thirdly, other cellular receptors such as the transmembrane glycoprotein CD43 (leukosialin and sialophorin),5 which structurally resemble CD34 and have the characteristic features of cell-associated mucins, may share similar functions as CD34 in hematopoietic development. The expression of these CD43-like redundant receptors could be affected in their CD34-knockout mice. When they rescue the CD34-deficient phenotype with the wild-type truncated form of CD34, the expression of those CD43-like receptors may be restored also and they can compensate the defect of the wild-type truncated form of CD34 in those transfected cells. Fourthly, the human CD34 gene locus has been mapped to chromosome 1q32, a region that contains genes encoding many hematopoietic regulatory and signaling molecules.33 Perhaps some important regulatory or signaling molecules, which are downstream of CD34 signaling, are also turned on by forcibly expressing the wild-type truncated form of CD34 in the transfected cells and achieve the CD34+ functional phenotypes. Finally, another study of CD34-deficient mice indicates decreased eosinophil accumulation after allergen exposure but rather normal hematopoietic development,34 suggesting that the hematopoietic defective phenotypes observed in the former CD34-knockout study may not be a generalized case. Perhaps the expression of other redundant receptors such as CD43 or some vital regulatory and signaling molecules as described above is not affected in the latter CD34-knockout experiment. Therefore, they do not observe the same phenotypes as described by the former study.22Nevertheless, the discrepancy between these two CD34-knockout studies has not been clearly resolved yet.
Using the EPOR/CD34 and G-CSFR/CD34 chimeric receptors, we showed that the cytoplasmic domain of CD34 failed to stimulate cell growth in response to EPO or G-CSF, respectively, implying that the cytoplasmic portion of CD34 may not contain the elements necessary to transduce a proliferative signal in hematopoietic cells. This is consistent with the recent findings obtained by Dr Toshio Suda in Japan (personal communication, November 24, 1996), where they generated the c-Kit/CD34 chimeric receptors to test the proliferative signaling potential of the cytoplasmic region of CD34. In addition, the observed CD34 MoAb-induced adhesion formation could not be significantly blocked by staurosporine or H7, which are potent PKC inhibitors, suggesting that PKC activation may not be involved in this cytoadhesive signaling pathway. In contrast, CD34 MoAb-induced cytoadhesion could be significantly inhibited by herbimycin A, which is a selective inhibitor of several protein tyrosine kinases,16 implicating that some tyrosine phosphorylation event might be involved in the CD34-mediated signal transduction of cellular adhesion. These results agree well with the previous data16 and the new findings observed by Dr Toshio Suda (personal communication, August 26, 1997). However, the cytoadhesive signaling pathways via the cytoplasmic domain of CD34 are not understood yet and remain to be elucidated.
In accordance with the previous findings,16 we found that CD34 MoAb-induced cytoadhesion could be suppressed, but not completely abrogated, by blocking MoAbs directed against some epitopes of LFA-1 (CD11a/CD18) and its counter-receptor ICAM-1 (CD54). This suggests, but does not prove, that a concomitant activation of the LFA-1/ICAM-1 cytoadhesion pathway may play a role in the CD34-mediated cellular adhesion. MoAbs against other adhesion molecules or receptors listed in Table 2 did not indicate any inhibitory effects on the CD34 MoAb-induced homotypic aggregation. However, certain unidentified cellular adhesion molecules may also participate in the CD34-mediated cytoadhesion. The mechanism by which LFA-1/ICAM-1 cooperates with CD34 in cellular adhesion is unknown. Moreover, the natural ligands for CD34 on hematopoietic precursor cells are still enigmatic. It has been found that L-selectin, the lymphocyte homing receptor,35,36 can bind to CD34 expressed on high endothelial venule (HEV) cells in the lymph nodes and that this binding is sialic acid specific and Ca2+-dependent.10,37 However, we were unable to detect any binding of L-selectin-IgG/Fc fusion protein with BaF3-CD34 or KG1a cells (data not shown), both of which express CD34 highly. In fact, thus far, the L-selectin-IgG/Fc chimeric protein has not been reported to bind to CD34 expressed on hematopoietic progenitor cells.4 This finding suggests that the glycosylation patterns of CD34 on hematopoietic cells may be different from those on endothelial cells of HEV so that the adherence of CD34 to L-selectin is abolished. Alternatively, CD34 on hematopoietic cells may primarily interact with other unidentified L-selectin–like ligands on endothelial or stromal cells in the BM compartment.
Cellular adhesion and migration of hematopoietic progenitor/stem cells is likely to be crucial in embryonic or fetal hematopoietic development and the dynamic recapitulation of hematopoiesis that occurs in the adult BM. It has been postulated that CD34+ hematopoietic cells administered intravenously are able to migrate to the BM compartment for their development after BM transplantation. Such a homing mechanism may mimic the multistep process identified for leukocyte-endothelial cell interactions.38 39 When the CD34+ hematopoietic progenitor/stem cells are halted in the blood stream by the interaction of CD34 with L-selectin or certain L-selectin–like adhesion molecules on the endothelial cells of blood vessels in the BM, they may migrate through the endothelial layer, bind to the extracellular matrix of BM stroma, and undergo differentiation or proliferation in the BM. Although this hypothesis requires further investigation, our findings about the essential role of cytoplasmic domain of CD34 in its cytoadhesive signaling open an avenue to delineate the mechanism by which CD34 mediates the cellular adhesion of hematopoietic progenitor/stem cells to BM stroma.
ACKNOWLEDGMENT
The authors thank S. Nagata for providing the full-length human G-CSFR cDNA and pEF-BOS plasmid; S. Elliott for providing the full-length human EPOR cDNA; R. Nelson for providing L-selectin-IgG/Fc fusion protein; L. Antonio for DNA sequencing; T. Boone for mouse IL-3; L. Souza for human G-CSF; V. Gottmer for technical illustration; and W. Boyle, R. Bosselman, and L. Souza for their support.
Address reprint requests to Mickey C.-T. Hu, PhD, Amgen, Inc, 14-1-D, Thousand Oaks, CA 91320.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal