Abstract
To evaluate the incidence, risk factors, and outcome of central nervous system (CNS) recurrence in adult patients with non-Hodgkin's lymphoma, we evaluated 605 newly diagnosed patients with large-cell and immunoblastic lymphoma who participated in prospective chemotherapy studies. The Kaplan-Meier estimate of probability of CNS recurrence at 1 year after diagnosis was 4.5% (95% confidence interval [CI], 4.4 to 4.6). Twenty-four patients developed CNS recurrence after a median of 6 months from diagnosis (range, 0 to 44 months). In univariate analysis, an increased risk for CNS recurrence was associated with an advanced disease stage (P = .0014), an increased LDH (P = .0000), the presence of B-symptoms (P = .0037), involvement of more than one extranodal site (P = .0000), poor performance status (P = .0005), and B-cell phenotype (P = .008). Bone marrow involvement (P = .005), involvement of parenchymal organs (P = .03), and involvement of skin, subcutaneous tissue, and muscle (P = .002) were also associated with an increased risk for CNS disease. Multivariate logistic regression analysis identified only involvement of more than one extranodal site (P = .0005) and an increased LDH (P = .0008) as independent predictors of CNS recurrence. Established CNS recurrence had a poor prognosis. Only 1 of 24 patients remains alive and the Kaplan-Meier estimate of probability of survival at 1 year after the diagnosis of CNS recurrence is only 25.3% (95% CI, 6.9 to 43.7). Intrathecal treatment provided symptomatic benefit in only 1 of 6 patients. Radiation treatment provided symptomatic improvement in 6 of 9 patients treated. However, remissions were short and followed by systemic or CNS recurrence. Serum LDH and involvement of more than one extranodal site are independent risk factors for CNS recurrence in patients with large-cell lymphoma. The presence of both risk factors identifies a patient group at high risk for CNS recurrence. Established CNS recurrence can be rapidly fatal. Transient responses occur after radiation treatment.
CENTRAL NERVOUS SYSTEM (CNS) recurrence is a devastating and almost uniformly fatal complication of intermediate-grade or immunoblastic lymphoma (non-Hodgkin's lymphoma [NHL]).1-10 Its incidence is not sufficiently high to warrant the use of CNS prophylaxis in all patients. The identification of patient subgroups for whom CNS prophylaxis may be of benefit is therefore important.
An increased risk for CNS recurrence in NHL has been associated with features of advanced disease and involvement of extramedullary sites such as blood,8 bone marrow,2-4,7,9testicular,8,11 gastro-intestinal,12 or sinus or orbital13 involvement; with serum lactate dehydrogenase (LDH)10; or with specific histologic subtypes of disease.13 However, the relative importance of any of these risk factors has not been well defined, and data specific for intermediate-grade lymphoma are scarce. Indeed, in the one study that focuses on patients with intermediate-grade lymphoma, no predictive value was assigned to involvement of any specific disease site except for epidural disease.5
We attempted to develop a risk model that would be both sensitive and specific for an increased risk of CNS recurrence in patients with intermediate-grade NHL treated with modern combination chemotherapy. For this purpose, we analyzed the outcome of 605 patients who participated in chemotherapy studies at MD Anderson Cancer Center. We analyzed the incidence, risk factors, treatment, and outcome of patients with CNS recurrence in this large prospectively collected patient cohort. Our results indicate that the use of simple staging parameters such as serum LDH and number of sites of extranodal involvement allows the identification of a subgroup of patients at high risk for CNS recurrence.
In addition, we evaluated the outcome of patients with established CNS recurrence and confirmed their extremely poor outcome. These patients do not appear to benefit from intrathecal treatment and derive only symptomatic benefit from radiation treatment.
PATIENTS AND METHODS
Patient selection and work-up.
The outcome of 605 patients with newly diagnosed intermediate-grade or immunoblastic lymphoma who participated in four consecutive studies of newly diagnosed NHL was analyzed for this study. No prophylactic intrathecal treatment was administered in any of these study protocols.
Protocols DM88-087 and DM90-093 accrued patients with favorable prognostic features. In protocol DM88-087, this was defined as stage A by the MD Anderson staging system,14 and in protocol DM93-003 this was defined as a tumor score of less than 3.15 Patients with disease involving 1 single nodal or extranodal site or 1 single chain of lymph nodes less than 5 cm received 3 cycles of CHOP-Bleo16 (750 mg/m2cyclophosphamide intravenously [IV] on day 1, 50 mg/m2doxorubicin IV on day 1, 2 mg vincristine IV on day 1, 100 mg prednisone orally [PO] on days 1 through 5, and 15 U bleomycin IV on day 1) followed by involved field radiation. All other patients on these protocols received 3 courses of CHOP-Bleo alternating with 3 courses of OPEN (2 mg vincristine IV on day 1, 100 mg prednisone PO on days 1 through 5, 100 mg/m2 etoposide IV on days 1 through 3, and 10 mg/m2 mitoxantrone IV on day 1), followed by involved field radiation to nodal disease that was ≥5 cm in diameter at presentation. Protocols DM88-089 and DM92-054 were designed to treat patients with poor prognostic features. Patients with MD Anderson stages B, C, and D were eligible for protocol DM88-089.14Treatment consisted of alternating triple therapy in which ASHAP, M-BACOS, and MINE were alternated for a total of nine treatment cycles. Details of this treatment have been reported.17 18 In short, ASHAP consists of 50 mg/m2 doxorubicin administered by continuous infusion over 96 hours, 500 mg methylprednisolone IV daily for 5 days, 1.5 gr/m2 cytarabine IV over 2 hours on day 5, and 100 mg/m2 cisplatinum administered by continuous infusion over 96 hours. M-BACOS consists of 10 mg/m2bleomycin IV on day 1, 50 mg/m2 doxorubicin IV administered by continuous infusion over 72 hours on days 1 through 3, 750 mg/m2 cyclophosphamide IV on day 1, 1.4 mg/m2vincristine IV on day 1, 500 mg methylprednisolone IV on days 1 through 3, and 1 g/m2 methotrexate IV on day 10. Methotrexate was followed by leucovorin rescue. MINE consists of 1.5 g/m2ifosfamide IV on days 1 through 3, 10 mg/m2 mitoxantrone IV on day 1, and 80 mg/m2 etoposide IV on days 1 through 3. Patients with a single area of extensive involvement or with a single residual tumor mass less than 5 cm at the end of chemotherapy were referred for involved field radiotherapy.
Patients with a tumor score ≥3 were eligible for protocol DM92-054.15 On this protocol, patients were randomized between idarubicin- and adriamycin-containing regimens. Treatment consisted of 2 cycles of ASHAP (or IDSHAP) alternating with 2 cycles of MBACOS (or MBIDCOS), followed by three cycles of MINE chemotherapy. IDSHAP and MBIDCOS are identical to ASHAP and MBACOS, except that doxorubicin was replaced by 10 mg/m2 idarubicin. Patients with a partial remission after three cycles of chemotherapy were referred for intensification with high-dose thiotepa, busulfan, and cyclophosphamide and autologous stem cell rescue.19
Staging procedures included physical exam; computed tomographic (CT) scan of chest, abdomen, and pelvis; gallium scan; bilateral bone marrow biopsy; and aspirate and lymphangiogram when indicated. A lumbar puncture was not performed unless CNS involvement was suspected. Patients who were seropositive for human immunodeficiency virus or hepatitis B were excluded. Patients with active CNS disease at presentation were eligible for these studies, but are not included in the analysis. At the time of analysis, the median follow-up for patients enrolled on these protocols was 4 years (range, 1 to 8 years).
Diagnosis of CNS disease.
For this analysis, only patients whose initial site of recurrence included symptomatic CNS disease were included. The diagnosis of CNS disease was based on the presence of malignant cells on cytocentrifuge preparations of spinal fluid in 19 cases and on brain biopsy in 2 cases. In 3 cases of parenchymal brain involvement, the diagnosis of CNS disease was based on symptoms and radiologic findings.
Statistical methods.
Probability of CNS recurrence and of survival after CNS recurrence was estimated using Kaplan-Meier plots.20 Exact methods of analysis were applied for further analysis of the data because of the low incidence of CNS recurrence.21 Because CNS recurrence is most likely to occur within the first year after diagnosis, the outcome of interest for the multivariate analysis was CNS recurrence at 1 year. Patients who died without CNS recurrence within 1 year from diagnosis were excluded from the analysis. The Fisher-Freeman-Halton exact test was used to assess the ability of covariates to predict CNS recurrence.22 Those covariates with a P value less than .10 were included in the multivariate analysis. An exact logistic model was built using forward selection.21 Covariates were added as long as the P value of a factor adjusted for all other factors already in the model was less than .05.
RESULTS
Patient characteristics and prognostic factors for CNS recurrence.
Patient-, disease-, and treatment-related characteristics of the 605 patients are listed in Table 1. The median age was 54 years (range, 16 to 84 years). As expected, more than half of the patients were men, half had an elevated LDH, and one third had B-symptoms. Most (88.7%) were classified as having diffuse large-cell lymphoma, 9.5% as follicular large-cell lymphoma and 1% as diffuse mixed-cell lymphoma. Thirty six percent of the patients had stage IV disease, 14% had stage III disease, and 48% had stage I or II disease. Approximately one fifth of the patients had more than one site of extranodal involvement. Involvement of the sinuses and testicular involvement occurred in only 9 patients (1.5%) and 5 patients (1%), respectively. The international index could be calculated for 564 patients. Ten percent had high-risk disease, 20% had high-intermediate risk disease, 22.5% had low-intermediate risk disease and 42.5% had low-risk disease.
Initial Features of 605 Patients With NHL and Kaplan Meier Estimate of Probability of CNS Recurrence at 1 Year After Diagnosis
| . | N (%) . | Probability of CNS Recurrence (95% CI) . | P* . |
|---|---|---|---|
| Age (yr) | |||
| ≤60 | 375 (62) | 4.4 (4.3-4.5) | .46 |
| >60 | 230 (38) | 4.8 (4.6-5.0) | |
| Sex | |||
| Male | 347 (57.3) | 5.2 (5.1-5.3) | .19 |
| Female | 258 (42.6) | 4.5 (4.4-4.6) | |
| Histology | |||
| DLCL | 537 (88.7) | 4.2 (4.1-4.3) | .3 |
| FLCL | 58 (9.5) | 7.7 (7.4-8.0) | |
| DMCL | 8 (1) | 0 | |
| Phenotype | |||
| B | 300 | 7.0 (6.8-7.2) | .008 |
| T | 23 | 4.8 (4.3-5.2) | |
| Unknown | 282 | ||
| LDH | |||
| Elevated | 318 (52.6) | 9.8 (9.7-9.9) | .0000 |
| Normal | 277 (45.7) | 1.1 (1.0-1.2) | |
| Unknown | 10 (1.6) | ||
| B2 microglobulin | |||
| Elevated (>3) | 140 (23.1) | 8.7 (8.4-9.1) | .012 |
| Normal (<3) | 398 (65.7) | 4.7 (4.6-4.8) | |
| Unknown | 67 (11.1) | ||
| Stage | |||
| I-II | 294 (48.6) | 2.2 (2.1-2.3) | .0014 |
| III | 87 (14.3) | 3.0 (2.8-3.2) | |
| IV | 217 (35.9) | 9.0 (8.8-9.2) | |
| Unknown | 7 (1) | ||
| B symptoms | |||
| Yes | 191 (31.6) | 8.6 (8.4-8.8) | .0037 |
| No | 408 (67.4) | 2.8 (2.7-2.9) | |
| Unknown | 6 (1) | ||
| Performance status | |||
| 0-1 | 488 (80.6) | 3.3 (3.2-3.4) | .0005 |
| >1 | 96 (15.8) | 13.2 (12.8-13.6) | |
| Unknown | 21 (3.4) | ||
| Extranodal sites | |||
| 0-1 | 480 (79.3) | 2.8 (2.7-2.9) | .0000 |
| >1 | 119 (19.7) | 13.3 (12.9-13.7) | |
| Unknown | 7 (1) | ||
| International index | |||
| Low (0-1) | 257 (42.4) | 0.8 (0.7-0.9) | .0000 |
| Low intermediate (2) | 141 (22.5) | 6.5 (6.3-6.7) | |
| High intermediate (3) | 121 (20) | 7.8 (7.6-8.0) | |
| High (4-5) | 60 (10.0) | 11.6 (5.7-17.5) | |
| Unknown | 41 (6.7) | ||
| Treatment (for details, see text) | |||
| CHOP-Bleo/OPEN | 217 (35.9) | 1.5 (0.5-2.5) | .003 |
| Alternating triple therapy | 388 (64.1) | 6.5 (6.4-6.6) |
| . | N (%) . | Probability of CNS Recurrence (95% CI) . | P* . |
|---|---|---|---|
| Age (yr) | |||
| ≤60 | 375 (62) | 4.4 (4.3-4.5) | .46 |
| >60 | 230 (38) | 4.8 (4.6-5.0) | |
| Sex | |||
| Male | 347 (57.3) | 5.2 (5.1-5.3) | .19 |
| Female | 258 (42.6) | 4.5 (4.4-4.6) | |
| Histology | |||
| DLCL | 537 (88.7) | 4.2 (4.1-4.3) | .3 |
| FLCL | 58 (9.5) | 7.7 (7.4-8.0) | |
| DMCL | 8 (1) | 0 | |
| Phenotype | |||
| B | 300 | 7.0 (6.8-7.2) | .008 |
| T | 23 | 4.8 (4.3-5.2) | |
| Unknown | 282 | ||
| LDH | |||
| Elevated | 318 (52.6) | 9.8 (9.7-9.9) | .0000 |
| Normal | 277 (45.7) | 1.1 (1.0-1.2) | |
| Unknown | 10 (1.6) | ||
| B2 microglobulin | |||
| Elevated (>3) | 140 (23.1) | 8.7 (8.4-9.1) | .012 |
| Normal (<3) | 398 (65.7) | 4.7 (4.6-4.8) | |
| Unknown | 67 (11.1) | ||
| Stage | |||
| I-II | 294 (48.6) | 2.2 (2.1-2.3) | .0014 |
| III | 87 (14.3) | 3.0 (2.8-3.2) | |
| IV | 217 (35.9) | 9.0 (8.8-9.2) | |
| Unknown | 7 (1) | ||
| B symptoms | |||
| Yes | 191 (31.6) | 8.6 (8.4-8.8) | .0037 |
| No | 408 (67.4) | 2.8 (2.7-2.9) | |
| Unknown | 6 (1) | ||
| Performance status | |||
| 0-1 | 488 (80.6) | 3.3 (3.2-3.4) | .0005 |
| >1 | 96 (15.8) | 13.2 (12.8-13.6) | |
| Unknown | 21 (3.4) | ||
| Extranodal sites | |||
| 0-1 | 480 (79.3) | 2.8 (2.7-2.9) | .0000 |
| >1 | 119 (19.7) | 13.3 (12.9-13.7) | |
| Unknown | 7 (1) | ||
| International index | |||
| Low (0-1) | 257 (42.4) | 0.8 (0.7-0.9) | .0000 |
| Low intermediate (2) | 141 (22.5) | 6.5 (6.3-6.7) | |
| High intermediate (3) | 121 (20) | 7.8 (7.6-8.0) | |
| High (4-5) | 60 (10.0) | 11.6 (5.7-17.5) | |
| Unknown | 41 (6.7) | ||
| Treatment (for details, see text) | |||
| CHOP-Bleo/OPEN | 217 (35.9) | 1.5 (0.5-2.5) | .003 |
| Alternating triple therapy | 388 (64.1) | 6.5 (6.4-6.6) |
*Fisher-Freeman-Halton exact test.
Incidence of CNS recurrence and risk factors.
The Kaplan Meier estimate of probability of CNS recurrence at 1 year after diagnosis is 4.5% (95% confidence interval [CI], 4.4 to 4.6). Twenty-four patients developed CNS recurrence a median of 6 months after diagnosis (range, 0 to 44 months). Only 1 CNS recurrence occurred more than 13 months after initial diagnosis, ie, in a patient with a testicular lymphoma who developed a brain lesion 44 months after diagnosis.
A variety of symptoms led to the diagnosis of CNS recurrence (Table 2). The most common findings were mental status changes, headaches, and cranial nerve palsies. Seizures occurred in only 1 patient. In 18 of 22 cases, lymphoma cells were detected in the spinal fluid. Leptomeningeal disease was documented radiographically in 7 patients and intraparenchymal disease in 8 of 21 patients.
Features of Patients With CNS Recurrence
| Neurologic symptoms at recurrence | |
| Coma | 1 |
| Seizures | 1 |
| Mental status changes, lethargy, confusion | 10 |
| Headache | 7 |
| Cranial nerve palsy | 7 |
| Peripheral sensory or motor symptoms | 5 |
| Gait and balance | 2 |
| Lumbar puncture | |
| Not done | 2 |
| Negative | 1 |
| Lymphoma cells | 19 |
| Other abnormalities/no lymphoma cells | 2 |
| Radiologic findings | |
| Leptomeningeal involvement | 7 |
| Brain parenchymal involvement | 8 |
| Negative radiologic studies | 7 |
| No radiologic studies | 3 |
| Status of systemic disease | |
| Simultaneous CNS and systemic relapse | 5 |
| Systemic relapse with 40-180 days after CNS relapse | 7 |
| Death within 40 days after CNS recurrence without systemic restaging | 7 |
| Survival beyond 40 days after CNS recurrence, no systemic recurrence | 5 |
| Neurologic symptoms at recurrence | |
| Coma | 1 |
| Seizures | 1 |
| Mental status changes, lethargy, confusion | 10 |
| Headache | 7 |
| Cranial nerve palsy | 7 |
| Peripheral sensory or motor symptoms | 5 |
| Gait and balance | 2 |
| Lumbar puncture | |
| Not done | 2 |
| Negative | 1 |
| Lymphoma cells | 19 |
| Other abnormalities/no lymphoma cells | 2 |
| Radiologic findings | |
| Leptomeningeal involvement | 7 |
| Brain parenchymal involvement | 8 |
| Negative radiologic studies | 7 |
| No radiologic studies | 3 |
| Status of systemic disease | |
| Simultaneous CNS and systemic relapse | 5 |
| Systemic relapse with 40-180 days after CNS relapse | 7 |
| Death within 40 days after CNS recurrence without systemic restaging | 7 |
| Survival beyond 40 days after CNS recurrence, no systemic recurrence | 5 |
Five patients had systemic disease documented at the time of recurrence or within 40 days thereafter. Seven other patients developed systemic relapse between 40 days and 6 months after recurrence of CNS disease. In 5 patients who survived from 40 days to 3 years after diagnosis of CNS recurrence, no systemic disease recurrence was ever documented.
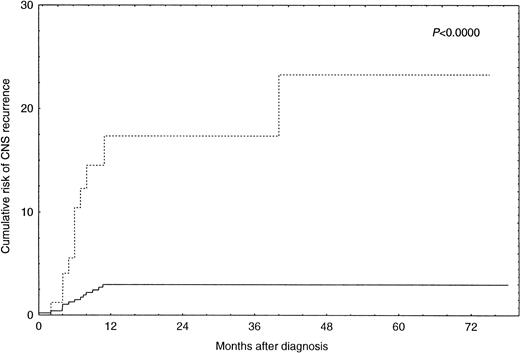
By univariate analysis an increased risk for CNS recurrence was associated with more advanced stage (P = .0014), an increased LDH (P = .00008), the presence of B-symptoms (P= .005), and involvement of more than one extranodal site (P = .00009) (Table 2). There was also an increased risk for CNS recurrence associated with the use of the alternating triple-therapy regimen. However, this association is explained by the fact that this regimen was used exclusively for patients with advanced disease stages. Complete information on disease sites was available for 369 patients. Bone marrow involvement (P = .005), involvement of parenchymal organs (P = .01), and involvement of skin, subcutaneous tissue, and muscle (P = .002) were associated with an increased risk for CNS disease in these patients (Table 3). However, multivariate logistic regression analysis only identified involvement of more than one extranodal site (P = .0005) and an increased LDH (P = .0008) as independent predictors of CNS recurrence (Table 4). The risk of CNS recurrence associated with these covariates is illustrated in Fig 1. Ninety-three patients (15.4%) had both an increased LDH and involvement of more than one extranodal site. For these patients, the Kaplan-Meier estimate of probability of CNS recurrence at 1 year after diagnosis was 17.4% (95% CI, 7.0 to 27.8). For the remaining 512 patients (84.6%), the Kaplan-Meier estimate of probability of CNS recurrence at 1 year after diagnosis was 2.8% (95% CI, 2.7 to 2.9).
Sites of Extranodal Involvement (369 Patients) and Kaplan Meier Estimate of Probability of CNS Recurrence at 1 Year After Diagnosis
| . | N (%) . | Probability of CNS Recurrence (95% CI) . | P* . |
|---|---|---|---|
| Bone marrow | |||
| Yes | 47 (12.7) | 18.5 (12.1-24.9) | .005 |
| No | 322 (87.3) | 5.2 (5.1-5.3) | |
| Bones | |||
| Yes | 36 (9.8) | 9.1 (5.1-13.1) | .61 |
| No | 333 (90.2) | 6.4 (6.3-6.5) | |
| Lungs/pleura | |||
| Yes | 29 (7.9) | 11.9 (5.5-18.3) | .116 |
| No | 340 (92.1) | 6.3 (6.2-6.4) | |
| GI tract | |||
| Yes | 39 (9.2) | 9.3 (4.2-14.4) | .347 |
| No | 330 (90.8) | 6.4 (6.3-6.5) | |
| Sinuses | |||
| Yes | 9 (2.4) | 12.5 (0.9-24.1) | .432 |
| No | 360 (97.6) | 6.6 (6.5-6.7) | |
| Liver/kidney/GU/adrenals/thyroid | |||
| Yes | 33 (8.9) | 19.0 (12.3-26.7) | .03 |
| No | 330 (91.1) | 5.7 (5.6-5.8) | |
| Skin/scalp/subcutaneous tissue/muscle | |||
| Yes | 23 (6.2) | 23.9 (14.5-33.3) | .002 |
| No | 346 (93.8) | 5.6 (5.5-5.7) |
| . | N (%) . | Probability of CNS Recurrence (95% CI) . | P* . |
|---|---|---|---|
| Bone marrow | |||
| Yes | 47 (12.7) | 18.5 (12.1-24.9) | .005 |
| No | 322 (87.3) | 5.2 (5.1-5.3) | |
| Bones | |||
| Yes | 36 (9.8) | 9.1 (5.1-13.1) | .61 |
| No | 333 (90.2) | 6.4 (6.3-6.5) | |
| Lungs/pleura | |||
| Yes | 29 (7.9) | 11.9 (5.5-18.3) | .116 |
| No | 340 (92.1) | 6.3 (6.2-6.4) | |
| GI tract | |||
| Yes | 39 (9.2) | 9.3 (4.2-14.4) | .347 |
| No | 330 (90.8) | 6.4 (6.3-6.5) | |
| Sinuses | |||
| Yes | 9 (2.4) | 12.5 (0.9-24.1) | .432 |
| No | 360 (97.6) | 6.6 (6.5-6.7) | |
| Liver/kidney/GU/adrenals/thyroid | |||
| Yes | 33 (8.9) | 19.0 (12.3-26.7) | .03 |
| No | 330 (91.1) | 5.7 (5.6-5.8) | |
| Skin/scalp/subcutaneous tissue/muscle | |||
| Yes | 23 (6.2) | 23.9 (14.5-33.3) | .002 |
| No | 346 (93.8) | 5.6 (5.5-5.7) |
*Fisher-Freeman-Halton exact test.
Covariates Associated With CNS Recurrence in Final Multivariate Logistic Regression Analysis
| . | P Value . | Relative Risk (95% CI) . |
|---|---|---|
| LDH elevated at diagnosis | .0008 | 7.0 (2.0-38.0) |
| Involvement of more than one extranodal site at diagnosis | .0005 | 5.5 (2.1-14.9) |
| . | P Value . | Relative Risk (95% CI) . |
|---|---|---|
| LDH elevated at diagnosis | .0008 | 7.0 (2.0-38.0) |
| Involvement of more than one extranodal site at diagnosis | .0005 | 5.5 (2.1-14.9) |
Incidence of CNS recurrence in patients with increased LDH and involvement of more than one extranodal site (n = 93; ···) versus all other patients (n = 512; —).
Incidence of CNS recurrence in patients with increased LDH and involvement of more than one extranodal site (n = 93; ···) versus all other patients (n = 512; —).
Treatment of CNS recurrence and outcome.
Patients with leptomeningeal involvement without focal neurologic deficits received intrathecal cytarabine, methotrexate, and hydrocortisone twice a week. An Ommaya reservoir was usually placed. Although intrathecal treatment invariably resulted in a decrease in the percentage of blasts in the spinal fluid, symptomatic improvement occurred in only 1 of 6 patients.
Patients with focal neurologic deficits or intraparenchymal lesions in the brain or spinal cord were offered radiation treatment. This consisted of whole brain radiation for those with brain lesions or cranial nerve palsies and of spinal irradiation or irradiation to segments of the spinal cord for those with lesions of the spinal cord. Radiation treatment resulted in symptomatic neurologic improvement in 6 of 9 patients. However, the responses were transient. The principal cause of treatment failure was progression of CNS disease in 5 patients and systemic recurrence in 4.
Five patients received systemic chemotherapy, combined in 2 cases with intrathecal treatment. The chemotherapy was platinum-based in 4 patients and consisted of MINE in the fifth patient. Responses of the CNS disease were observed in 3 patients and 1 patient has obtained a durable remission.
Of the 24 patients, only 1 is currently alive and in remission at 1,150 days after diagnosis of CNS recurrence. Kaplan-Meier estimate of probability of survival at 1 year after diagnosis of CNS recurrence is 25.3% (95% CI, 6.9% to 43.7%).
DISCUSSION
The identification of risk factors for CNS recurrence in intermediate-grade NHL has been attempted previously. Several large studies containing analysis of potential risk factors are summarized in Table 5. Whereas these and similar studies contributed in important ways to the identification of risk factors for CNS disease, all of them have a number of problems that makes their interpretation difficult. Most studies are based on retrospective analysis of heterogeneously treated patients. In addition, the majority of studies concern patients treated in the 1970s before the widespread use of modern chemotherapeutic regimens and at a time when important entities such as lymphoblastic lymphoma were not reliably recognized. The assessment of risk factors for intermediate-grade NHL is therefore obscured by the inclusion of patients with other histologies. We are aware of only one study that specifically addresses the issue of CNS recurrence in intermediate-grade lymphoma in patients treated with modern chemotherapy.5 This report, like many others, does not differentiate between patients with CNS involvement at presentation, those in which CNS involvement occurs at the time of initial recurrence, and those in which CNS involvement is an expression of terminal disease. In this study, only the presence of epidural disease (P < .001) and age younger than 60 years (P = .046) were found to be significant risk factor for CNS recurrence. Other risk factors, including gender, response to treatment, stage of disease (P = .083 stage I-II v stage III-IV), involvement of more than one extranodal site (P = .077), and involvement of specific disease sites were not found to be significantly correlated with the risk of CNS recurrence. Hence, considerable controversy remains regarding the impact of patient and disease characteristics on the incidence of CNS disease in intermediate-grade lymphoma, preventing a rational approach to preventive strategies.
Incidence of and Risk Factors for CNS Recurrence: Selected Studies With Univariate Analyses of Risk Factors
| Author, Year, Reference . | No. of Patients . | Systemic Treatment Received4-150 . | % CNS Disease4-151 . | % CNS Involvement at Diagnosis . | Subtypes Included . | Risk Factors . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Histology . | Advanced Stage . | B- Symptoms . | No. of Extranodal Sites . | Disease Sites . | Age (yr) . | ||||||
| Litam et al, 19799 | 292 | COP, CHOP-HOP, CHOP-BLeo | 11 | NS | All histologies | Diffuse > nodular DUL and LBL > other diffuse lymphoma | Yes | NE | NE | BM, other, extranodal | <35 |
| Herman et al, 19796 | 1039 | COP, CHOP-HOP, CHOP-BLeo | 5 | 1 | All histologies | Diffuse > nodular DUL and LBL > other diffuse lymphoma | NE | NE | NE | NE | NE |
| Levitt et al, 19807 | 592 | COP, CHOP-BLeo | 9 | 1.7 | All histologies | Diffuse > nodular | Yes | NE | NE | Marrow | NE |
| Liang et al, 199012 | 833 | NS | 6.1 | NS | All histologies | HGL > IGL > LGL | Yes | Yes | NE | Orbit, testis, PB, bone, sinuses, BM | No |
| Bashir et al, 19915 | 277 | CAP-BOP | 5.1 | 1.1 | DMCL, DLCL, IBL | No | No | No | No | Epidural disease‡ | <60 |
| Author, Year, Reference . | No. of Patients . | Systemic Treatment Received4-150 . | % CNS Disease4-151 . | % CNS Involvement at Diagnosis . | Subtypes Included . | Risk Factors . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Histology . | Advanced Stage . | B- Symptoms . | No. of Extranodal Sites . | Disease Sites . | Age (yr) . | ||||||
| Litam et al, 19799 | 292 | COP, CHOP-HOP, CHOP-BLeo | 11 | NS | All histologies | Diffuse > nodular DUL and LBL > other diffuse lymphoma | Yes | NE | NE | BM, other, extranodal | <35 |
| Herman et al, 19796 | 1039 | COP, CHOP-HOP, CHOP-BLeo | 5 | 1 | All histologies | Diffuse > nodular DUL and LBL > other diffuse lymphoma | NE | NE | NE | NE | NE |
| Levitt et al, 19807 | 592 | COP, CHOP-BLeo | 9 | 1.7 | All histologies | Diffuse > nodular | Yes | NE | NE | Marrow | NE |
| Liang et al, 199012 | 833 | NS | 6.1 | NS | All histologies | HGL > IGL > LGL | Yes | Yes | NE | Orbit, testis, PB, bone, sinuses, BM | No |
| Bashir et al, 19915 | 277 | CAP-BOP | 5.1 | 1.1 | DMCL, DLCL, IBL | No | No | No | No | Epidural disease‡ | <60 |
Abbreviations: NS, not specified; NE, not evaluated; HGL, high-grade lymphoma; IGL, intermediate-grade lymphoma; LGL, low-grade lymphoma; DUL, diffuse undifferentiated lymphoma; LBL, lymphoblastic lymphoma; DMCL, diffuse mixed-cell lymphoma; DLCL, diffuse large-cell lymphoma; IBL, immunoblastic lymphoma; PB, peripheral blood; BM, bone marrow.
For details of chemotherapy-regimens, see references.
The percentage of patients with CNS involvement. In most reports, this includes patients with CNS involvement at presentation. When specified, the percentage of patients with CNS involvement at presentation is shown in the next column.
B-symptoms, histology, extranodal involvement, response to therapy and gender are not associated with risk for CNS recurrence.
The current study documents the incidence of CNS recurrence in a cohort of 605 patients with intermediate-grade lymphoma who received their initial treatment at a single institution and who participated in four prospective studies of modern combination chemotherapy. Therefore, the patients received uniform and consistent treatment throughout the course of the study. Our regimen for patients with unfavorable characteristics includes three doses of high-dose cytarabine and three doses of intermediate-dose methotrexate. These drugs penetrate into the CNS and are thought to be useful in the treatment and prophylaxis of CNS leukemia.23 The incidence of CNS recurrence in this patient cohort may therefore be slightly lower than would be anticipated if all patients were treated with CHOP-like regimens. Nevertheless, the cumulative incidence of CNS recurrence of 5.1% (95% CI, 4.9% to 5.3%) is consistent with the incidence reported in one other recent study.5 Almost all the risk occurred in the first year after diagnosis.
It is important to distinguish between patients whose CNS disease is the initial site of recurrence from those where CNS disease occurs long after systemic recurrence. In the former group, CNS recurrence may be potentially treatable. In the latter, CNS recurrence is an expression of end-stage disease, not necessarily predicted by the same risk factors and unlikely to be amenable to therapeutic strategies with curative intent. Our study excluded from analysis the latter patients. Thus defined, the risk for CNS recurrence was associated with indicators of advanced disease such as serum LDH and stage of disease, bone marrow involvement, and skin and parenchymal involvement. For reasons that are unclear to us, B-cell phenotype was also associated with a significantly increased risk of CNS recurrence. The importance of other risk factors, such as sinus involvement, testicular involvement, pulmonary involvement, and gastro-intestinal involvement, could not be confirmed. In cases of testicular and sinus involvement, this may be due to the very low accrual of cases with this particular site of involvement.
A multivariate logistic regression identified only the presence of more than one site of extranodal involvement and an increased LDH as independent risk factors for CNS recurrence. This suggest that, in the majority of cases, the risk for CNS recurrence is determined by disease extent and proliferation rather than by any particular disease localization. The use of these two factors allowed us to identify a group of patients for whom the cumulative risk for CNS recurrence is almost 20%. Serum LDH and involvement of more than one extranodal site are also associated with increased overall risk for treatment failure in patients receiving conventional chemotherapy regimens.24Radically new treatment approaches are needed for such patients, taking into account their risk for CNS recurrence. The initial staging work-up for such patients should include a lumbar puncture. Their treatment should contain CNS prophylaxis similar to what is recommended for patients with acute lymphoblastic leukemia (ALL) or high-grade lymphoma.23,25 26 This should include the use of systemic drugs with activity across the blood-brain barrier as well as frequent intrathecal injections. A similar approach may be necessary for the relatively uncommon patients who present with primary testicular or sinus lymphoma. On the other hand, for the large majority of patients, the risk for CNS recurrence is very low with currently used chemotherapy, and no specific CNS prophylaxis or work-up appears to be necessary.
An ideal risk model would allow identification of all patients at risk for a particular event. The discomfort and side-effects of preventive therapy would be avoided for those not at risk. Because of the low incidence of CNS disease in this patient population, our model is less than optimal. Its use would have resulted in specific CNS-directed treatment for 15% of all patients with intermediate-grade NHL accrued to this study. However, only 11 of the 24 patients destined to develop CNS recurrence would have been identified. Therefore, the sensitivity of our model in predicting CNS recurrence is slightly less than 50%, only marginally better than could be achieved if patients had been selected on the basis of bone marrow, testicular, and sinus involvement. On the other hand, an increased serum LDH correctly identified 21 of the 24 patients destined to develop CNS prophylaxis, but more than 50% of the patients in this study had an elevated LDH.
The median survival after CNS recurrence was only 88 days, consistent with the known poor prognosis of CNS recurrence.27 Prior studies of end-stage lymphoma have emphasized the fact that most patients die from systemic disease rather than from CNS complications. Our study included a more selected patient group; the patients had received adequate systemic treatment and had CNS involvement as their initial site of recurrence. CNS complications were the main cause of death in these patients.
Intrathecal treatment did not result in meaningful responses. Radiation therapy resulted in rapid but transient symptomatic relief in most cases. Five of 9 patients developed recurrences in the CNS. The fact that most patients had lymphoma cells in the spinal fluid is consistent with findings from previous reports1,3,4,6,28 and indicates that CNS involvement is a complication that affects the entire neuraxis. Effective long-term treatment at the time of CNS relapse should be aimed at sites of systemic disease and at the craniospinal axis. Chemotherapy, using agents that are effective across the blood-brain barrier, is theoretically appealing. However, in our series, it was rarely possible to devise such regimens, because most patients had relapse during or shortly after completing programs incorporating most known active agents. Because allogeneic or autologous transplantation can overcome chemotherapy resistance, this approach may prove beneficial for selected patients.29 30
In conclusion, the risk of CNS recurrence in intermediate-grade lymphoma treated with modern chemotherapy regimens is approximately 5%. Our analysis confirms the importance of previously identified risk factors, but in addition establishes the predictive value of increased serum LDH and the involvement of multiple extranodal sites as risk factors for CNS recurrence. In univariate analysis, these two features are stronger predictors than any of the commonly used risk factors. In multivariate analysis, they emerge as the only significant ones. The identification of additional independent risk factors in future studies may allow a more accurate identification of those at risk for CNS recurrence.
Established CNS recurrence is usually rapidly fatal. Intrathecal chemotherapy alone is not effective palliation for intermediate-grade lymphomas, and radiation treatment results in rapid symptomatic improvement but is usually followed by systemic or CNS recurrence. New treatment strategies should be developed for patients at high risk for CNS recurrence and explored in a prospective fashion.
Address reprint requests to Koen van Besien, MD, Hematology/Oncology Section, University of Illinois at Chicago, 840 S Wood St (MC 787), Chicago, IL 60612.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal