Abstract
We have evaluated the susceptibility to human immunodeficiency virus (HIV)-1 infection of in vitro grown megakaryopoietic progenitors/precursors and maturing megakaryocytes (MKs), based on the following approach: (1) human hematopoietic progenitor cells (HPCs), stringently purified from peripheral blood and grown in serum-free liquid suspension culture supplemented with thrombopoietin (Tpo), generated a relatively large number of ≥ 98% to 99% pure megakaryocytic precursors and then mature-terminal MKs; (2) at different days of culture (ie, 0, 5, 8, 10) the cells were inoculated with 0.1 to 1.0 multiplicity of infection (m.o.i.) of the lymphotropic NL4-3 or 0.1 m.o.i. of the monocytotropic BaL-1 HIV-1 strain; (3) finally, the presence of viral mRNA and proteins was analyzed by reverse transcriptase-polymerase chain reaction (RT-PCR)/in situ hybridization and antigen capture assays, respectively, on day 2 to 12 of culture. MKs derived from day 0 and day 5 BaL-1–challenged cells do not support viral replication as assessed by p24 enzyme-linked immunosorbent assay (ELISA) and RT-PCR. On the contrary, HIV transcripts and proteins were clearly detected in all NL4-3 infection experiments by RT-PCR and p24 assay, respectively, with the highest viral expression in day 5 to 8 challenged MKs. In situ hibridization studies indicate that the percentage of HIV+ MKs varies from at least 1% and 5% for day 0 and day 5 infected cells, respectively. Production of an infectious viral progeny, evaluated by the capability of culture supernatants from day 5 NL4-3–challenged MKs to infect C8166 T-lymphoblastoid cell line, was consistently observed (viral titer, ≈ 5 × 103 tissue culture infectious dose50/mL/106 cells). Exposure of MKs to saturating concentration of anti-CD4 OKT4A monoclonal antibody (MoAb), which recognizes the CD4 region binding with the gp120 envelope glycoprotein, markedly inhibited HIV infection, as indicated by a reduction of p24 content in the supernatants: because the inhibitory effect was incomplete, it is apparent that the infection is only partially CD4-dependent, suggesting that an alternative mechanism of viral entry may exist. Morphologic analysis of day 12 MKs derived from HPCs infected at day 0 showed an impaired megakaryocytic differentiation/maturation: the percentage of mature MKs was markedly reduced, in that ≈ 80% of cells showed only one nuclear lobe and a pale cytoplasm with few granules. Conversely, megakaryocytic precursors challenged at day 5 to 8 generated fully mature day 10 to 12 MKs showing multiple nuclear segmentation. Thus, the inhibitory effect of HIV on the megakaryopoietic gene program relates to the differentiation stage of cells subjected to the viral challenge. Finally, HPCs treated with 20 or 200 ng/mL of recombinant Tat protein, analyzed at different days of culture, showed an impaired megakaryocytopoiesis comparable to that observed in HIV-infected cells, thus suggesting that Tat is a major mediator in the above described phenomena. These results shed light on the pathogenesis of HIV-related thrombocytopenia; furthermore, they provide a model to investigate the effects of HIV on megakaryocytic differentiation and function.
THE IMMUNODEFICIENCY IN acquired immunodeficiency syndrome (AIDS) patients is coupled with impaired production of hematopoietic cells and abnormalities of bone marrow (BM) cell morphology.1-4 Recently, we showed that a minority of erythroid and granulomonocytic early hematopoietic progenitor cells (HPCs) is susceptible to in vitro human immunodeficiency virus type 1 (HIV-1) infection: these observations may partially explain the hematopoietic impairment in AIDS patients.5
Peripheral blood (PB) thrombocytopenia, frequently observed in HIV-infected patients and ameliorated by zidovudine therapy,6,7 is generally considered a multifactorial disorder.8 In seropositive patients circulating immune complexes may bind to the surface of platelets and accelerate their destruction.9,10 Ultrastructurally aberrant megakaryocytes (MKs) and nude MK nuclei are observed in BM of all infected individuals, even in the absence of thrombocytopenia.11-13Circumstantial evidence suggests that HIV directly interferes with megakaryocytopoiesis. In vitro studies indicated that megakaryocytic cell lines can be infected by HIV,14-16 while human BM enriched MKs and platelets internalize HIV viral particles.17,18 Furthermore, viral RNA has been detected by in situ hybridization in MKs purified from BM of thrombocytopenic HIV+ patients.19,20 It has been suggested that HIV-1 infects MKs through the CD4 receptor,16 expressed by a significant fraction of these cells,21 but additional routes of viral entry have been also hypothesized.19
Previous studies have been limited by the lack of relatively pure and abundant megakaryopoietic cell populations. Therefore, it has not been possible to establish the in vitro capacity of HIV to infect megakaryocytic progenitors/precursors and the effects of the infection on the cell progeny. Furthermore, a productive viral infection by infected MKs has not been demonstrated.
We have developed a culture system to channel purified PB HPCs into strictly unilineage megakaryocytic differentiation/maturation in serum-free liquid suspension medium.22 Taking advantage of this novel experimental tool, we investigated the susceptibility to HIV-1 infection of megakaryopoietic cells at different stages of differentiation/maturation, and the effects thereof on megakaryocytopoiesis.
MATERIALS AND METHODS
HPC purification and assay.
Adult PB was obtained from 20- to 40-year-old healthy male donors after informed consent. PB HPCs were purified from the buffy-coat by a three-step procedure according to a slight modification22,23 of the method described in Gabbianelli et al.24 Particularly, negative selection of low-density cells was performed with an anti-T-, anti-B-, and antinatural killer lymphocytes, antimonocytes, and antigranulocytes monoclonal antibody (MoAb) cocktail supplemented with anti-CD45, anti-CD11a, and anti-CD71 MoAbs (Becton-Dickinson, Mountain View, CA).
The HPC clonogenetic assay was performed as described in Testa et al.25 Briefly, HPCs were seeded (1 × 102cells/mL/dish, in triplicate) and cultured in 0.9% methylcellulose in the presence or absence of fetal calf serum (FCS). Both FCS+ and FCS- cultures were supplemented with FL (100 ng/mL), KL (100 ng), interleukin (IL)-3 (100 U), granulocyte-macrophage colony-stimulating factor (GM-CSF) (10 ng), Epo (3 U), M-CSF (250 U), and G-CSF (500 U). Colony-forming unit-granulocyte, erythroid, macrophage, megakaryocyte (CFU-GEMM), burst-forming unit-erythroid (BFU-E), and colony-forming unit–granulocyte-macrophage (CFU-GM) colonies were scored on days 14 to 15 and 16 to 18 in FCS+ and FCS- cultures, respectively.
Cell cultures.
Purified HPCs were grown in FCS- liquid culture at 4 × 104 cells/mL22; thus Iscove's modified Dulbecco's medium (IMDM) (GIBCO, Grand Island, NY) was supplemented with bovine serum albumin (BSA) (10 mg/mL), pure human transferrin (0.7 mg/mL), human low-density lipoprotein (40 μg/mL), insulin (10 μg/mL), sodium pyruvate (10-4 mol/L), L-glutamine (2 × 10-3 mol/L), rare inorganic elements supplemented with iron sulphate (4 × 10-8 mol/L), and nucleosides (10 μg/mL of each), in the presence of purified recombinant human thrombopoietin (Tpo) (100 ng/mL), a generous gift from Genetech (San Francisco, CA). Cells were incubated in a fully humidified atmosphere of 5% CO2, 5% O2, 90% N2.
Human megakaryoblastic UT-7 cells were grown in IMDM supplemented with 15% FCS in the presence of 1 ng/mL GM-CSF. CEMss and H9/HTLVIIIB cells were cultured in RPMI medium supplemented with 10% FCS.
HIV-1 Tat recombinant protein (Intracel, Cambridge, MA) was added at a concentration of 20 or 200 ng/mL at day 0 and periodically refed to the culture medium at the starting concentration.
Membrane phenotype and morphological analysis.
The following MoAbs directly conjugated with fluorochrome (fluorescein isothiocyanate [FITC] or phycoerythrin [PE]) were used to characterize the membrane phenotype: anti-CD34 HPCA-2 clone (Becton Dickinson), -HLA-DR, -CD4, -CD61, -CD62 (Becton Dickinson), -CD41b (PharMingen, San Diego, CA). A total of 1 × 104cells were incubated for 60 minutes at 4°C in the presence of an appropriate amount of MoAb. After three washes with cold phosphate-buffered saline (PBS) containing 2 mg/mL BSA, cells were resuspended in 0.2 mL PBS/2.5% formaldehyde and then analyzed by FACScan (Lysis II program, Becton-Dickinson). Cells cytocentrifuged onto glass slides were stained with May-Grünwald Giemsa (Sigma, St Louis, MO) and then identified by morphology analysis.
Virus preparation, infection, and blocking experiments.
HIV-1 BaL-1 virus preparations were obtained as described in Gartner et al26 and titrated in terms of both picograms per milliliter of p24 protein (by quantitative enzyme-linked immunoassorbent assay [ELISA] Abbott, North Chicago, IL) and infectious dose by the end dilution method27 performed on ex vivo monocyte-macrophage culture. HPCs and MK precursors were incubated overnight (4 to 7 × 104 cells/mL) in the presence of Tpo and 0.1 multiplicity of infection (m.o.i.) of BaL-1.
NL4-3 lymphotropic HIV-1 strain was prepared as described in Chelucci et al,5 viral titer ranging from 3 × 106to 107 50% tissue culture infective dose (TCID50)/mL. NL4-3 inactivation was performed at 65°C for 30 minutes and the absence of infectious virus after heat-inactivation was verified on highly HIV susceptible CEM cells. HPCs and MK precursors (4 to 7 × 104) were resuspended in a maximum volume of 50 μL and inoculated with 0.1 or 1 m.o.i. of infectious HIV or with the same volume of heat-inactivated virus. After a 2-hour adsorption at 37°C, complete medium was added and the cells incubated overnight at 37°C. Mock-infected MKs and CD4+ UT-7 human megakaryoblastic cell line resistant to HIV infection28 were used as negative controls.
Infected cells were extensively washed, diluted at 4 × 104/mL and grown in liquid suspension culture for 13 to 14 days as described. Supernatants were periodically removed, stored at -80°C until used and the cells resuspended in fresh medium at 1 × 105/mL. For blocking experiments, cells were preincubated at 4°C for 45 minutes with 1 μg or 5 μg/105 cells of anti-CD4 (OKT4A, Ortho Diagnostic System, Raritan, NJ) or anti HLA-DR (Becton-Dickinson) MoAb in 100 μL final volume and then infected with 0.1 m.o.i. of NL4-3. After extensive washing, cells were resuspended in culture medium supplemented with the same MoAbs. Cells were periodically diluted to 1 × 105 cells/mL and supplemented with OKT4A MoAbs indicated above.
Virus detection.
Detection of p24 antigen in supernatants was performed on serially diluted samples of culture supernatants by ELISA. The HIV infection titer on MK culture supernatants, evaluated as TCID50, was determined as reported by Gartner et al26 by infecting HIV highly susceptible C8166 cells and by measuring the cytopathic effect as syncytia formation 4 to 5 days after infection.
Polymerase chain reaction analysis.
Reverse transcriptase-polymerase chain reaction (RT-PCR) on megakaryocytic cells was performed as previously described.5 Briefly, RNA from 1 to 3 × 104 cells was extracted by cesium chloride (CsCl) gradient technique and reverse transcribed according to the manufacturer's instructions (Boehringer, Mannheim, Germany). PCR was performed in a final volume of 50 μL in the presence of 2.5 U of Taq polymerase (Perkin-Elmer Cetus, Norwalk, CT). The samples were amplified for a Tat and β2-microglobulin gene fragment as described in Chelucci et al,5 and then run on 2% agarose gel. Filters were hybridized using end-labeled probes and autoradiographed at -80°C. The PCR primers and probes were the same as described in Chelucci et al.5
In situ hybridization.
Cytospun cells were fixed in 4% buffered paraformaldehyde at 4°C for 10 minutes, then dehydrated in series graded alcohol up to 70% for storage. Prehybridization was performed in 0.1 mol/L triethanolammine for 2 minutes, acetic anhydride for 10 minutes, 2× saline citrate (SSC) for 10 minutes, and 30%, 50%, 70%, 95%, 100% ethanol for 2 minutes each. Hybridization was performed at 42°C using as a probe 1 × 106 cpm per slide of rev gene of HIV-1 cDNA previously labeled with 35S deoxy adenosine triphosphate (dATP) and 35S dexoy cytidine triphosphate (dCTP) (New England Nuclear, Boston, MA) by Klenow polymerase (Amersham). Both positive and negative controls were performed for each experiment. After washes, the slides were dipped in NTB2 emulsion (Eastman-Kodak, Rochester, NY) and exposed at 4°C for 7 to 10 days in a light proof box, developed in D-19 (Eastman-Kodak), fixed and stained with May-Grünwald Giemsa for microscopic analysis.
RESULTS
Morphologic and phenotypic analysis.
Cells stringently purified from normal PB were ≈ 90% CD34+ as assessed by immunofluorescence analysis: the mean frequency of HPCs was also 90%, as evaluated in clonogenetic assay, coupled with 70% HPC recovery (details in Testa et al25).
Purified HPCs were grown in serum-free liquid suspension culture in the presence of 100 ng/mL Tpo to induce strictly unilineage megakaryocytic differentiation up to terminal maturation (see Guerriero et al22). The differentiation stage was evaluated by morphologic and phenotypic characterization. At day 0, cells became essentially composed of small undifferentiated blasts. At day 5, most cells were larger and mononuclear representing putative MK precursors. At day 8, most cells showed two nuclei and a more dense chromatin. At day 12, the majority of MKs were polyploid and showed platelet formation: the cytoplasm was highly granular and platelets were observed in cytospin preparations of culture supernatants. Direct immunofluorescence for the expression of CD4, CD34, and CD61 antigens (Fig 1, top) showed 10% to 15% up to ≈ 30% CD4+ cells in days 0 to 12 of culture; furthermore, we confirmed (see Guerriero et al22) a gradual decrease of CD34 and an inverse increase of CD61, which is expressed on ≥ 98% to 99% cells in day 12 culture. Double-immunofluorescence analysis (Fig1, bottom) indicated that at day 0, 10% to 20% of quiescent CD34+ cells coexpressed the specific megakaryocytic marker CD61 or the CD4 antigen; double positive CD4/CD61 cells represented 5% to 10% of the total population. At days 4 to 6, ≈ 50% of CD34+ cells coexpressed CD61, while 10% to 15% of cells were double-labeled for CD4/CD61. In late cultures (days 8 to 12), the CD4/CD61 positive population reached ≈ 30%.
Membrane phenotype of Step IIIP HPCs grown in FCS- liquid suspension culture in the presence of 100 ng/mL Tpo. Cells were harvested at various stages of maturation and analyzed by single (top) and double (bottom) fluorescence labeling using anti-CD4, -CD61, -CD34 MoAbs (mean ± standard error of mean [SEM] values from three independent experiments). (▪) CD34+; (▵) CD61+; (▴) CD4+; (○) CD34+/4+; (•) CD34+61+; (□) CD4+/61+.
Membrane phenotype of Step IIIP HPCs grown in FCS- liquid suspension culture in the presence of 100 ng/mL Tpo. Cells were harvested at various stages of maturation and analyzed by single (top) and double (bottom) fluorescence labeling using anti-CD4, -CD61, -CD34 MoAbs (mean ± standard error of mean [SEM] values from three independent experiments). (▪) CD34+; (▵) CD61+; (▴) CD4+; (○) CD34+/4+; (•) CD34+61+; (□) CD4+/61+.
Megakaryocytic progenitors/precursors and maturing megakaryocytes are susceptible to T-tropic, but resistant to M-tropic HIV infection.
The susceptibility to NL4-3 and BaL-1 HIV-1 strains infection of megakaryocytic cells was tested immediately after purification (day 0 HPCs, Fig 2), or after 5, 8, or 10 days of culture (Fig 3 and data not shown). As a negative control, UT-7 cells (a CD4+, HIV-resistant human megakaryocytic cell line) were infected under the same experimental conditions.
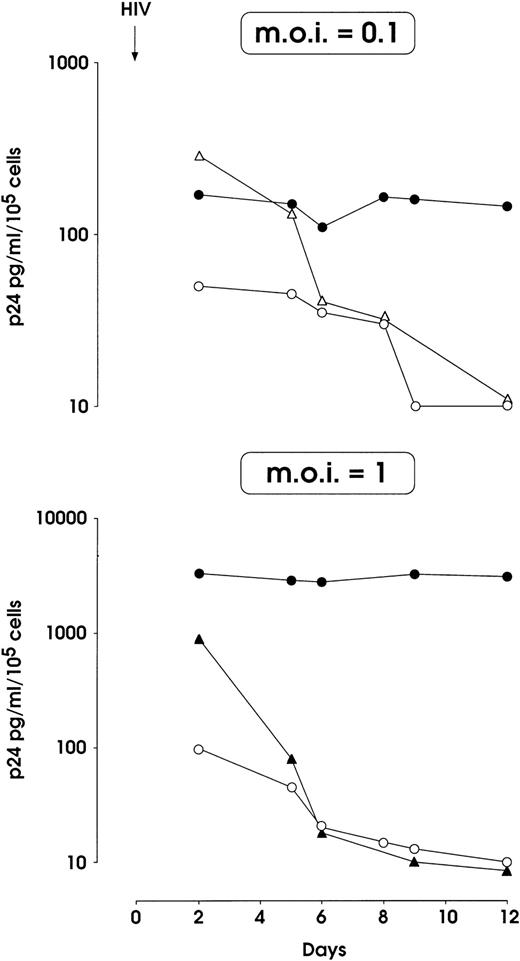
HIV-1 infection of MK-committed HPCs (results from a representative experiment). Cells were infected at day 0 with NL4-3 (m.o.i. 0.1 and 1, top and bottom panels, respectively) or m.o.i. 0.1 of BaL-1 strain (top panel) and p24 release was assayed in culture supernatants at different times. MK and UT-7 cells treated with heat-inactivated and infectious NL4-3 respectively, were used as negative controls. (•) MK and NL4-3; (○) UT-7 and NL4-3; (▴) MK-inactivated NL4-3; (▵) MK BaL-1.
HIV-1 infection of MK-committed HPCs (results from a representative experiment). Cells were infected at day 0 with NL4-3 (m.o.i. 0.1 and 1, top and bottom panels, respectively) or m.o.i. 0.1 of BaL-1 strain (top panel) and p24 release was assayed in culture supernatants at different times. MK and UT-7 cells treated with heat-inactivated and infectious NL4-3 respectively, were used as negative controls. (•) MK and NL4-3; (○) UT-7 and NL4-3; (▴) MK-inactivated NL4-3; (▵) MK BaL-1.
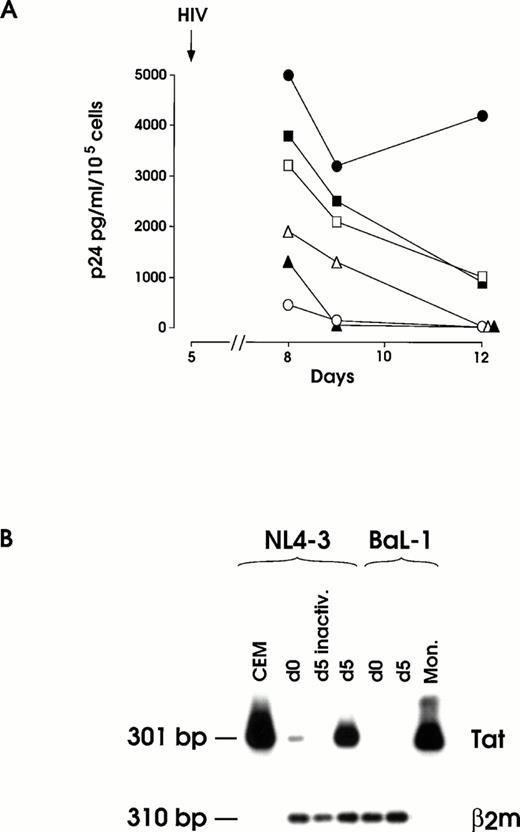
(A) HIV infection and inhibition thereof by OKT4A MoAbs at day 5 of megakaryopoietic cell culture (representative results from the same experiment shown in Fig 2). Cells preincubated or not with 1 to 5 μg OKT4A MoAb were infected with 0.1 m.o.i. NL4-3. After extensive washing, MoAb was added again and cells maintained in culture medium. Results from BaL-1 infection of day 5 MKs are also shown. UT-7 cells challenged with infectious NL4-3 and MKs treated with heat-inactivated virus were used as negative controls. (•) MK and NL4-3; (▪) MK + 1 μg OKT4A + NL4-3; (□) MK + 5 μg OKT4A + NL4-3; (○) UT-7 + NL4-3; (▴) MK-inactivated NL4-3; (▵) MK BaL-1. (B) RT-PCR of total RNA isolated from day 12 harvested cells infected at day 0 or day 5 with infectious or heat-inactivated (inactiv.) NL4-3 or infectious BaL-1 strain. CEM and primary monocytes (Mon.), infected with NL4-3 or BaL-1, respectively, were used as positive controls. β2m RT-PCR was performed on MKs cells for mRNA normalization.
(A) HIV infection and inhibition thereof by OKT4A MoAbs at day 5 of megakaryopoietic cell culture (representative results from the same experiment shown in Fig 2). Cells preincubated or not with 1 to 5 μg OKT4A MoAb were infected with 0.1 m.o.i. NL4-3. After extensive washing, MoAb was added again and cells maintained in culture medium. Results from BaL-1 infection of day 5 MKs are also shown. UT-7 cells challenged with infectious NL4-3 and MKs treated with heat-inactivated virus were used as negative controls. (•) MK and NL4-3; (▪) MK + 1 μg OKT4A + NL4-3; (□) MK + 5 μg OKT4A + NL4-3; (○) UT-7 + NL4-3; (▴) MK-inactivated NL4-3; (▵) MK BaL-1. (B) RT-PCR of total RNA isolated from day 12 harvested cells infected at day 0 or day 5 with infectious or heat-inactivated (inactiv.) NL4-3 or infectious BaL-1 strain. CEM and primary monocytes (Mon.), infected with NL4-3 or BaL-1, respectively, were used as positive controls. β2m RT-PCR was performed on MKs cells for mRNA normalization.
Figure 2 (top) shows that the infection of HPCs with 0.1 m.o.i. of the NL4-3 T-tropic HIV-1 strain resulted in the sustained release of p24, while a rapid decrease of p24 level was observed in (1) supernatants from BaL-1 challenged normal megakaryopoietic and (2) NL4-3–treated HIV-resistant UT-7 cells. A 10-fold higher NL4-3 dose led to a 20-fold increase of p24 levels in megakaryopoietic cell culture up to day 12, which contrasts with the rapid decrease down to an undetectable level in the cultures of control UT-7 cells and in MKs treated with heat-inactivated virus (Fig 2, bottom). No differences were observed in the growth of infected versus uninfected megakaryocytic cells (not shown).
The infection of day 5 megakaryocytic precursors with 0.1 m.o.i. (Fig3A) induced a significant p24 release, which was markedly higher than for day 0 HPCs infected with the same viral dose. Day 5 infection with 1 m.o.i. did not cause a more elevated level of p24 (data not shown). In these experiments a slight reduction of cell proliferation was observed in infected megakaryocytic cells, as compared with mock-infected ones (not shown). Similarly to that observed for HPCs, MKs precursors were resistant to the infection with BaL-1 (Fig 3A).
Expression of viral mRNA was evaluated by RT-PCR analysis on total cellular RNA isolated at days 10 to 12 culture seeded with day 0 and day 5 HIV-treated cells (Fig 3B). As expected, a strong hybridization signal of Tat mRNA was observed for day 5 NL4-3 challenged cells, whereas day 0-infected cells consistently showed a faint specific band. No hybridization signal was observed in day 0 and day 5 BaL-1 challenged cells or in day 0 (not shown) and day 5 cells treated with heat-inactivated NL4-3.
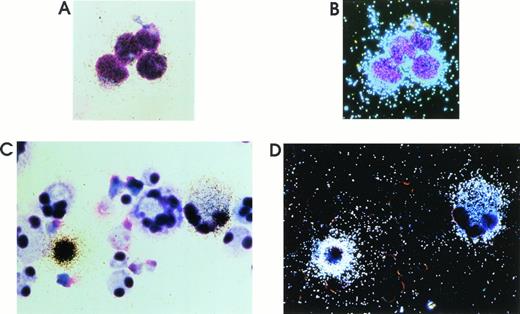
To evaluate the proportion of cells actively expressing the HIV genome, in situ hybridization on both day 0 and day 5 cells infected with 1 m.o.i. was performed on day 12 harvested MKs (Fig 4C and D and data not shown). The percentage of positive HIV cells was at least 1% for day 0 and 5% for day 5 challenged cells. All positive MKs gave a similar strong signal that almost obscured the cell boundary. HIV-infected H9 cells were used as positive control (Fig 4A and B).
Representative results of in situ hybridization on HIV-1 chronically infected H9 cells (A and B) and MKs infected at day 5 and harvested at day 12 of culture (C and D) using a35S-labeled rev DNA probe. Bright (A and C) and dark (B and D) field images are presented (May-Grünwald staining; original magnification × 1,200 for H9; × 500 for MKs). In day 12 culture, 98% to 99% cells are CD61+ megakaryocytic cells with one or more lobes, while ≈ 1% of cells are granulocytes, which are of smaller size (see also Results and Guerriero et al22).
Representative results of in situ hybridization on HIV-1 chronically infected H9 cells (A and B) and MKs infected at day 5 and harvested at day 12 of culture (C and D) using a35S-labeled rev DNA probe. Bright (A and C) and dark (B and D) field images are presented (May-Grünwald staining; original magnification × 1,200 for H9; × 500 for MKs). In day 12 culture, 98% to 99% cells are CD61+ megakaryocytic cells with one or more lobes, while ≈ 1% of cells are granulocytes, which are of smaller size (see also Results and Guerriero et al22).
Viral titration performed on C8166 lymphoblastoid cells demonstrates that day 12 supernatants from day 5 infected MKs contained infectious viral particles at a concentration as high as 5 × 103TCID50/mL/106 cells, thus demonstrating a productive viral infection.
Altogether, these results show that megakaryocytic cells are susceptible to infection with a T-tropic HIV-1 isolate, and the susceptibility appears stronger in megakaryocytic precursors (day 5 cultures) than in quiescent HPCs (day 0 cultures). Furthermore, these experiments exclude the possibility that the observed phenomena were mediated to a significant extent by NL4-3 carryover on HPCs/megakaryopoietic cells: this possibility is incompatible with results on (1) p24 level after treatment with heat inactivated NL4-3 and infectious BAL-1 strain, (2) HIV expression, evaluated by RT-PCR/in situ hybridization, after NL4-3–treatment of day 0 HPCs and day 5 megakaryopoietic cells.
Anti-CD4 antibodies partially inhibit HIV-1 infection of megakaryocytic cells.
In agreement with previous studies5 21 and the present findings, a consistent fraction of both HPCs and maturing MKs express the CD4 molecule, the primary receptor for HIV infection. We, therefore, investigated whether the anti-CD4 OKT4A MoAb, which specifically interacts with the epitope involved in HIV gp120 binding, could inhibit HIV replication into MKs. The experiments were performed on day 5 megakaryocytic cells, which are most susceptible to HIV infection (see above).
Cells were treated with either 1 or 5 μg of OKT4A MoAb/105 cells and then infected with 0.1 m.o.i. of NL4-3 (Fig 3A). As a control, the highly CD4+ HIV susceptible CEMss cells were treated under the same experimental conditions. The amounts of anti-CD4 MoAb able to block HIV replication in CEMss (not shown) induce only a partial reduction (from ≈ 30 to ≈ 75%) of the viral spread in infected MKs. To assess the specificity of the anti-CD4 MoAb inhibitory action, separate samples of MKs were preincubated with anti–HLA-DR MoAb before HIV infection. This treatment, as expected, failed to induce inhibition of HIV replication.
Morphologic abnormalities of HIV-infected MKs.
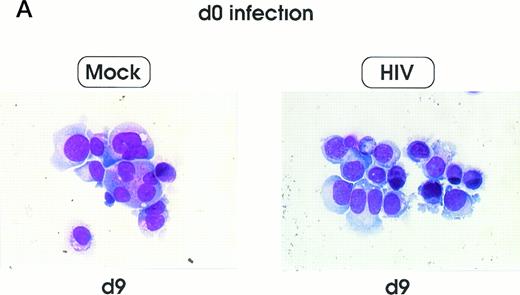
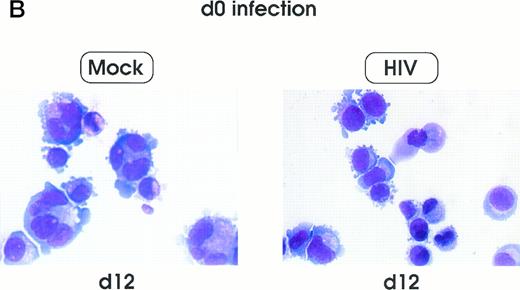
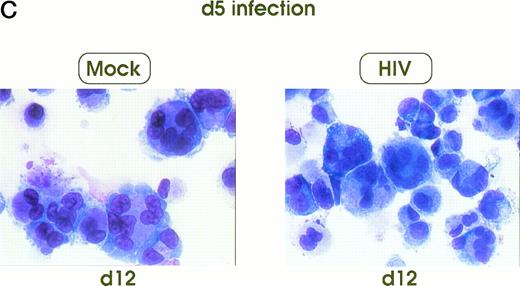
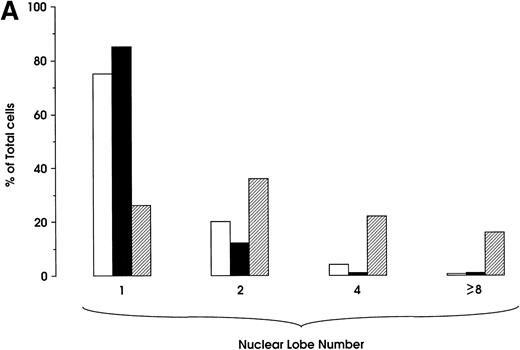
Morphologic analysis of HIV-infected megakaryocytic cells was performed at sequential stages of differentiation/maturation. Relevant alterations were observed for day 0 challenged cells, as compared with the untreated controls (Figs 5A and B and6) and cells challenged with heat-inactivated virus (data not shown). Particularly, a marked delay of MK nuclear segmentation was observed, as shown by the presence of ≈ 90% and ≈ 80% of MKs with one nuclear lobe in day 9 and day 12 cultures, respectively, compared with 60% and 30% of corresponding MKs observed on the same days in mock and untreated cultures. Furthermore, day 0-infected HPCs, analyzed at day 12 of culture, showed the presence of MKs with unusual morphologic features: very large mononuclear MKs displaying a large nucleus and a round intensely blue cytoplasm, as well as large mononuclear MKs with a pale cytoplasm and numerous large membrane protrusions. Conversely, MKs derived from cultures challenged at day 5 were similar to those present in mock and untreated controls (Figs 5C and 6). Furthermore, immunophenotype analysis showed an impaired expression of late MK membrane antigens (eg, CD41b, CD62) in HIV-treated cells, as compared with controls (results not shown).
Cell morphology of mock and HIV-infected MKs (representative results). Cells from day 0 infection harvested at day 9 (A) and at day 12 (B). MKs infected at day 5 and recovered at day 12 (C). May-Grünwald staining; original magnification × 400.
Cell morphology of mock and HIV-infected MKs (representative results). Cells from day 0 infection harvested at day 9 (A) and at day 12 (B). MKs infected at day 5 and recovered at day 12 (C). May-Grünwald staining; original magnification × 400.
Percentage of MKs classified on the basis of nuclear lobes as evaluated by optical microscopy: cells were infected at day 0 (top) and 5 (bottom) and harvested at day 12 (mean ± SEM values from three independent experiments). (□) HIV; (▨) Mock.
Percentage of MKs classified on the basis of nuclear lobes as evaluated by optical microscopy: cells were infected at day 0 (top) and 5 (bottom) and harvested at day 12 (mean ± SEM values from three independent experiments). (□) HIV; (▨) Mock.
Exogenous Tat protein effects on megakaryocytopoiesis mimick NL4-3 action.
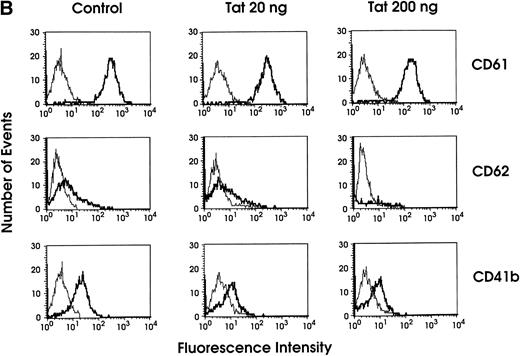
To assess a possible involvement of Tat protein in the NL4-3–induced impairment of megakaryocytic differentiation/maturation, day 0 HPCs were incubated with 20 or 200 ng/mL of the recombinant protein: morphologic and immunophenotype analysis was then performed at different days of megakaryopoietic culture. The morphologic evaluation showed nuclear lobe number abnormalities highly reminiscent of those obtained after HPC NL4-3 infection (Fig7A). Immunophenotypic analysis indicated that after Tat treatment, the expression of late MK membrane markers (eg, CD41b and CD62) is impaired, as compared with uninfected control cells, while the expression of early ones (eg, CD61) is comparable to that in controls (Fig 7B). No growth difference was observed in the control versus treated cell culture (not shown).
(A) Percentage of MKs classified on the basis of nuclear lobes as evaluated by optical microscopy: cells were treated with 20 or 200 ng/mL of recombinant Tat protein starting from day 0 and harvested at day 12 (results from a representative experiment). (□) Tat 20 ng; (▪) Tat 200 ng; (▨) control. (B) Flow cytometry analysis of CD61, CD62, and CD41b expression in day 9 MKs grown in either absence (control) or presence of Tat protein (20 or 200 ng/mL starting from day 0 of culture). The negative control is shown on the far left in each panel (thin line peak).
(A) Percentage of MKs classified on the basis of nuclear lobes as evaluated by optical microscopy: cells were treated with 20 or 200 ng/mL of recombinant Tat protein starting from day 0 and harvested at day 12 (results from a representative experiment). (□) Tat 20 ng; (▪) Tat 200 ng; (▨) control. (B) Flow cytometry analysis of CD61, CD62, and CD41b expression in day 9 MKs grown in either absence (control) or presence of Tat protein (20 or 200 ng/mL starting from day 0 of culture). The negative control is shown on the far left in each panel (thin line peak).
DISCUSSION
Thrombocytopenia is one of the hematologic disorders that may occur during the asymptomatic and clinical stage of HIV-1 infection.3,29 Different mechanisms have been proposed for the thrombocytopenia, including an immune mechanism9,10 and infection of MKs by HIV-1.17-20 However, it has not been possible: (1) to evaluate whether MK progenitors/precursors are susceptible to HIV infection; (2) to establish the effect of HIV infection on megakaryocytic maturation/differentiation process; and (3) to show a productive viral infection by MKs. These limitations are primarily due to the extreme rarity of HPCs and megakaryocytic precursors in human hematopoietic tissues: in an attempt to bypass this hurdle, we have used “pure” and relatively homogenous megakaryopoietic cell populations, generated at sequential times in HPC unilineage differentiation culture yielding 98% to 99% terminal MKs at days 10 to 12.22
It has been previously demonstrated that a minority of human PB HPCs is susceptible to in vitro HIV infection, as indicated by the presence of viral transcripts and proteins in single BFU-E- and CFU-GM–generated colonies.5 In this report, we have analyzed the HIV susceptibility of MK-committed HPCs, MK precursors, and maturing MKs (a series of controls excluded significant HIV carry-over, see Results).
HPCs challenged with NL4-3 at day 0 with a m.o.i. of 0.1 and grown in unilineage MK culture released detectable levels of HIV virions at all stages of differentiation/maturation. A 10-fold increase of m.o.i. led to higher amounts of HIV p24 protein in supernatants, without detectable effects on cell growth. Day 5 megakaryocytic precursors were apparently more susceptible to HIV infection as compared with day 0 HPCs. However, in contrast to HPCs, no differences in the amounts of p24 protein were observed in the supernatants of MK cells infected with 0.1 or 1 m.o.i.: this may indicate that 0.1 m.o.i. is sufficient to infect all susceptible megakaryocytic precursors. The possibility exists that the T-lymphotropic HIV strain used for infection bears a different tropism for HPCs versus MK precursors: accordingly, more viral particles are needed for the infection of day 0 HPC versus day 5 MK precursors to induce a similar p24 release at later culture times.
In situ hybridization, performed at day 12 of culture, indicate that MKs actively expressing HIV RNA were 1% in day 0 and 5% in day 5-infected cells. However, it should be considered that in situ hybridization analysis may underestimate the number of cells infected by HIV. In fact, this technique detects only cells actively expressing the HIV genome at the day of sampling, thus excluding (1) cells infected, but not expressing, the viral genome above the assay threshold and (2) cells not surviving the HIV infection. The lower percentage of infected HPCs versus MK precursors may be attributed to the cycling status of the challenged cells (day 0 HPCs are largely quiescent, while day 5 megakaryocytic precursors actively proliferate). Furthermore, the T-tropic HIV strain CD4 coreceptor, CXCR4 or fusin,30 may play a differential role in viral entry: our preliminary results (not presented) show a sharp increase of CXCR4 mRNA expression during MK differentiation.
MK-committed HPCs and MK precursors were resistant to infection with the M-tropic BaL-1 strain as indicated by RT-PCR and p24 analysis. Preliminary experiments indicate that the major coreceptors for M-tropic strains, CKR5 and CKR3,31 are weakly expressed on HPCs and totally absent on maturing MKs (unpublished results).
CD4, the major portal of entry for HIV, is expressed on 25% to 30% of human mature MKs.21 We report here that CD4 antigen is also expressed on 10% up to ≈ 30% of megakaryocytic progenitors/precursors. Pretreatment of MKs with anti-CD4 MoAb before infection markedly inhibited HIV replication, as evaluated by p24 levels, thus indicating that CD4 is a major membrane molecule mediating the infection of HIV into human megakaryocytic progenitors/precursors, but also suggesting the presence of an alternative pathway(s) for HIV entry.
Morphologic analysis on MKs derived from day 0 challenged HPCs showed a marked delay in the maturation process, particularly in terms of nuclear segmentation, as compared with untreated or mock control groups. While the maturation delay was widely observed in the megakaryocytic progeny, only ≈ 1% MKs were HIV+ by in situ hybridization, although the frequency of HIV-infected cells is presumably more elevated (see above). These observations suggest that the inhibitory effect of HIV infection on megakaryopoietic maturation may be largely indirect, ie, unrelated to HIV replication per se. Results obtained after the treatment of HPCs with Tat protein support this hypothesis. In fact, comparable results were obtained after HIV infection or Tat treatment of HPCs, thus indicating that the HIV inhibitory effect on megakaryocytic differentiation/maturation is possibly, at least in part, mediated by viral Tat protein released by the infected cells. Indeed, Tat can be secreted by infected cells and taken up by neighboring uninfected ones.32
To our knowledge, this is the first report describing an inhibitory effect on cell differentiation/maturation mediated by Tat. It is generally accepted that Tat induces a variety of effects on cell growth and metabolism, ie, it acts as a growth factor for Kaposi's sarcoma cells,33 may induce immunosuppression34 and inhibits antigen-induced lymphocyte proliferation.35Controversial data on the ability of Tat to promote cell growth and survival,36 or conversely, to induce apoptosis37 have been reported: indeed the expression of several cellular genes is either increased or downmodulated by Tat.38
The impaired MK maturation reported here may underlie the thrombocytopenia frequently observed in seropositive patients: indeed, higher ploidy cells produce more platelets than lower ploidy ones, while platelet production and release is more efficient from a single large MK than from several small ones.39 The hypothesis may be considered that, in the BM microenvironment, MKs represent a reservoir for HIV; furthermore, these cells might release inhibitory proteins such as transforming growth factor (TGF)-β and/or Tat.
Megakaryocytic precursors infected at day 5 generated a progeny with normal polyploid morphology. Altogether, our observations suggest that HIV infection impairs the megakaryopoietic program at early, but not late, stages of differentiation.
Our results refer to infection with a T-tropic cell line adapted HIV strain. It will be of interest to extend this study to HIV primary isolates, which with respect to NL4-3, may show interesting differences in the tropism and/or morphologic alterations on HPCs and MK precursors.
The present study reports the novel finding that HIV-1 infects megakaryocytic progenitors/precursors, largely, but not exclusively, through the CD4 receptor. The HPC infection causes an impaired maturation of the generated MKs, which apparently represents a major mechanism in HIV-related thrombocytopenia. The demonstration of HIV viral release by infected MKs suggests that these cells represent a significant reservoir and source of viral spread in the BM of AIDS patients.
Address reprint requests to C. Peschle, MD, Thomas Jefferson Cancer Institute, Thomas Jefferson University, BLSB, Room #528, Locust and 10th St, Philadelphia, PA 19107-5541.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. Membrane phenotype of Step IIIP HPCs grown in FCS- liquid suspension culture in the presence of 100 ng/mL Tpo. Cells were harvested at various stages of maturation and analyzed by single (top) and double (bottom) fluorescence labeling using anti-CD4, -CD61, -CD34 MoAbs (mean ± standard error of mean [SEM] values from three independent experiments). (▪) CD34+; (▵) CD61+; (▴) CD4+; (○) CD34+/4+; (•) CD34+61+; (□) CD4+/61+.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/4/10.1182_blood.v91.4.1225/4/m_blod4040301.jpeg?Expires=1769106530&Signature=Rop-kmhSaPzmSSswygFZXh1cV0NnrybPqDwtTg0R-wQkbwjG0ygh6WHklNTd4~AdDP9WdzhBN90IrPheMdod66kZKvu-CAi4TB-mqXfoEU7Lnenillw126UmeXCiRC40QEJHSkLnsOZkSZX7t3l7SzYO~JiX3rMremAH3Q8lcFC5b65BHcdNnYv-p3AVz9hXxKPCwvscLaDdxH8S~B-nERmzufsJcP~Ht0bPBMdbPqXwhT0crQ~LbaSgzLEmXnf~qOU5XTIKUs~VUF-SaAibcc5h4dCxtJjhNlwZUeLwPGh7diCHmtMuGjbtyJCIFVi0LRwfp7gjZpTsiPJScYGkdw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal