Abstract
Studies on assembly in vitro of α-globin chains with recombinant β16 Gly→Asp, β95 Lys→Glu, β120 Lys→Glu and β16 Gly→Asp, 120 Lys→Glu human β-globin chain variants in addition to human βA- and βS-globin chains were performed to evaluate effects of increased anionic charge in the β chain on hemoglobin assembly using soluble recombinant β-globin chains expressed in bacteria. A β112 Cys→Asp change was also engineered to monitor effects on assembly of increased negative charge at α1β1 interaction sites. Order of tetramer formation in vitro under limiting α-globin chain conditions showed Hb βG16D, K120E = Hb βK120E = Hb βK95E > Hb βG16D > Hb A > Hb S >>> Hb βC112D. In addition, β112 Cys→Asp chains exist as monomers rather than β4tetramers in the absence of α chains, and the β chain in Hb βC112D tetramers was readily exchanged by addition of βs. These results suggest that affinity between α and β chains is promoted by negatively-charged β chains up to a maximum of two additional net negative charges and is independent of location on the surface except at the α1β1 interaction site. In addition, our findings show that β112 Cys on the G helix is critical for facilitating formation of stable αβ dimers, which then form functional hemoglobin tetramers, and that β112 Cys→Asp inhibits formation of stable α1β1 and β1β2 interactions in α2β2 and β4 tetramers, respectively.
HEMOGLOBIN IS COMPOSED of two α- and two non–α-polypeptide chains and has served as a model macromolecule to study various aspects of structure, synthesis, and assembly of multisubunit proteins.1,2 The human α- and β-like globin genes are located on different chromosomes and give rise to the two different chains involved in hemoglobin biosynthesis.2A number of factors can influence relative levels of human hemoglobin variants, which are produced in vivo.3-8 Formation of hemoglobin requires balanced production of α-and β-polypeptide chains. The α- and β-globin mRNAs are first translated into their respective polypeptide chains, and the two hemoglobin chains diffuse into the cytoplasm and assemble into αβ dimers, which then form stable, functional α2β2 tetramers.
The rate-limiting step in hemoglobin assembly is the bimolecular reaction involving α + β → αβ, which is thought to be governed by electrostatic attraction between monomeric partner subunits.2,3 The higher proportion of Hb A than Hb S in AS heterozygotes has been explained by assembly rate differences with α chains of βA and βS chains, which have Glu and Val at the β6 position, respectively.2,3 In addition, extensive work by Bunn et al3,6 7 showed using naturally-occurring hemoglobin variants that an additional negative charge in the β chain promotes αβ assembly of hemoglobin. Although the additional negatively-charged β chains promote electrostatic attraction between partner subunits, the maximum enhancement by increased negative charge, as well as the role of direct interaction sites in promoting stable αβ assembly, is not clear. Recent studies using site-directed mutagenesis should provide further elucidation of the mechanism of subunit assembly of hemoglobin.
Studies of hemoglobin assembly in vitro require isolation of large amounts of individual α- and β-chain variants from their tetramers, and the isolated chains are then reconstituted in vitro to form hemoglobin tetramers. To facilitate assembly studies and further our understanding of this process, production of soluble α- and β-chain variants is critical. We recently succeeded in producing authentic human, soluble β-globin chains in bacteria using an expression vector containing cDNAs for methionine aminopeptidase and human β globin.9 Of interest, the β-globin chain fraction contained monomers and disulfide cross-linked dimers. The dimers, which are formed by oxidation of cysteine residues, could be reduced to monomers by addition of dithiothreitol (DTT). Furthermore, dimers were unable to form tetramers in vitro on addition of exogenous α chains, while monomeric β chains, which are in the reduced form as a result of DTT addition, were able to form tetramers.9 Our results indicate that α- and β-globin chains fold independently and that conditions for efficient dimer-tetramer assembly are now available. We are now able to produce soluble β-chain variants to systematically evaluate factors affecting hemoglobin assembly by engineering various mutations at predetermined sites. In this report, we expressed five variant β-globin chains to confirm the role of subunit surface charge using soluble β-globin chains expressed in bacteria and to assess the role of α1β1 direct interaction sites on assembly of hemoglobin. Three of the variants, β16 Gly→Asp, β95 Lys→Glu, and β120 Lys→Glu, are found in J-Baltimore, N-Baltimore, and Hb Hijiyama, respectively, and contain an additional one or two net negatively-charged amino acid substitutions on the surface of the β chain. Heterozygotes express more than 50% of these variant hemoglobins.3 In addition to these variants, we engineered a three net negatively-charged β-chain variant, β16 Gly→Asp, 120 Lys→Glu, to assess the maximum effect of negative charge addition on promotion of αβ assembly. A fifth variant, β112 Cys→Asp, was also made, which contains a negatively-charged amino acid substitution at an α1β1 interaction site, to clarify the role of this site in stable αβ assembly.
MATERIALS AND METHODS
Expression of soluble recombinant human β-globin chain variants in Escherichia coli.
β16 Gly→Asp, β95 Lys→Glu, β120 Lys→Glu, β16 Gly→Asp, 120 Lys→Glu and β112 Cys→Asp chains were expressed as described previously9 using the vector pHE2β, which contains cDNAs coding for the human β-chain variant and methionine aminopeptidase. The basic strategy for generation of these variants by site-specific mutagenesis of the normal β chain involves recombination/polymerase chain reaction as described previously.10 Clones were subjected to DNA sequence analysis of the entire β-globin cDNA region using site-specific primers and fluorescently-tagged terminators in a cycle sequencing reaction in which extension products were analyzed on an automated DNA sequencer.11 Plasmids were transfected into E. coli(JM 109) (Promega Co, Madison, WI), bacteria were grown at 30°C, and soluble β-globin chain variants were isolated and purified as described.9
Authentic human α chain was purified from tetrameric Hb A isolated from erythrocyte lysates according to previously described methods.12 Removal of p-mercuribenzoate was accomplished using 20 mmol/L DTT, and globin chains were isolated by gel filtration on a fast protein liquid chromatography (FPLC) Superose 12 column (Pharmacia Biotech, Uppsala, Sweden).
Biochemical characterization of purified β-globin chains.
Molecular mass and sample purity were assessed by sodium dodecyl sulfate- polyacrylamide gel electrophoresis (SDS-PAGE) as described.13 Electrospray ionization mass spectrometry (ESMS) was performed on a VG BioQ triple quadrapole mass spectrometer (Micromass, Altrincham, Cheshire, UK).14 The multiply-charged ions derived from α globin (Mr: 15,126.4) served as internal and external standards for mass scale calibration. Data analysis used the MassLynx software package (Micromass, Altrincham, Cheshire, UK).
Purified β-globin chains were also analyzed by cellulose acetate electrophoresis on Titan III membranes at pH 8.6 with Super-Heme buffer (Helena Laboratories, Beaumont, TX). Isoelectric focusing of purified β-chain variants, βA and βS was performed on an Ampholine PAG plate (pH 5.5 to pH 8.5) using a Multiphor II system (Pharmacia Biotech, Piscataway, NJ). After focusing for 2 hours at constant 25 W at 4°C, the gel plate was stained with Coomassie Brilliant Blue R-250 to detect proteins. Isoelectric point of each β-globin variant was estimated from a calibration curve prepared with isoelectric focusing (IEF) standards (Bio-Rad, Hercules, CA). Absorption spectra of purified β globins in the CO forms were recorded using a Hitachi U-2000 spectrophotometer (Hitachi Instruments Inc, Danbury, CT). Circular dichroism (CD) spectra of β-globin variants were recorded using an Aviv model 62 DS instrument (Varian Analytical Instruments, San Fernand, CA) employing a 0.1-cm light path cuvette at 10 μmol/L globin concentration. CD spectra of β-globin variants compared with normal βA were monitored in the wavelength range from 190 to 260 nm. Oxygen dissociation curves were determined in 50 mmol/L Bis-Tris buffer containing 0.1 mol/L NaCl, pH 7.1 at 20°C using a Hemox Analyzer (TCS Med Co, Huntington Valley, PA).15
Hemoglobin concentration was determined spectrophotometrically on a Hitachi U-2000 spectrophotometer using a millimolar extinction coefficient of 13.4 at 540 nm for carbon-monoxy hemoglobin.16 Assembly studies of purified β-chain variants (75 μmol/L) were performed after addition of varying amounts of α-globin chain in the CO form in 0.1 mol/L phosphate buffer, pH 7.0 at 25°C,9 and tetramer formation was assessed by FPLC (fast protein liquid chromatography) using Mono S and Superose 12 gel-filtration chromatography.
RESULTS
Expression and characterization of β-chain variants.
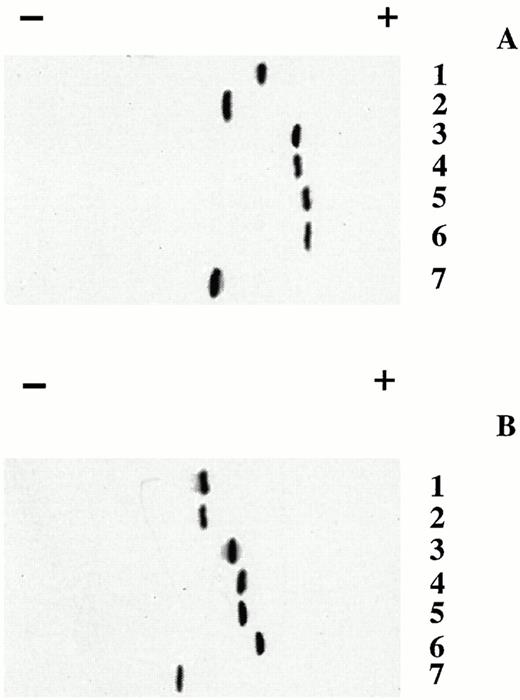
After DNA sequence confirmation, the five β-chain variant cDNAs were expressed in bacteria. All five purified variants migrated as single bands following cellulose acetate electrophoresis at pH 8.6 (Fig 1). As expected, addition of negative charges in β16 Gly→Asp, β95 Lys→Glu, β120 Lys→Glu and β16 Gly→Asp, 120 Lys→Glu compared with βA chains resulted in increased anodic mobility on electrophoresis. These results suggest that these negatively-charged β chains, like βA chains, exist as β4 tetramers in solution. In contrast, electrophoretic mobility of β112 Cys→Asp was similar to that of βS chains, indicating that β112 Cys→Asp chains exist as monomers (charge of -2) and not β4 tetramers (charge of -4) like the βA chain.17, 18Isoelectric focusing (IEF) of the purified β-chain variants, βA and βS was also performed on an Ampholine PAG plate (pH 5.5 to pH 8.5) to assess effects of additional negative charges on the surface charge and pI (isoelectric point) of the β-chain variants (Table 1). Our results show that the pIs for four of the five variants were lower than that of the βA chain and that the lowest pI was associated with the variant with three net additional negative charges compared with the βA chain (β16 Gly→Asp, 120 Lys→Clu). In addition, the pI of β95 Lys→Glu was slightly higher than that of β120 Lys→Glu (5.84 v5.73) even though both variants have the same two net negative charge changes. These findings indicate that β120 is more exposed on the surface compared with β95. The only variant with a pI > the βA chain was the β112 Cys→Asp chain, indicating again that the β112 Cys→Asp chains migrate as monomers rather than tetramers like β4Achains as shown on cellulose acetate electrophoresis.
Electrophoresis of purified β-globin chain variants β16 Gly→Asp, β95 Lys→Glu, β120 Lys→Glu, β16 Gly→Asp, 120 Lys→Glu and β112 Cys→Asp and in vitro assembled tetramers. Purified β-globin chain variants β16 Gly→Asp, β95 Lys→Glu, β120 Lys→Glu, β16 Gly→Asp, 120 Lys→Glu and β112 Cys→Asp (A) and in vitro assembled tetramers (B) formed by mixing with α-globin chains isolated from human red blood cells and incubating in 10 mmol/L potassium phosphate buffer pH 7.0 at 25°C were analyzed by electrophoresis on cellulose acetate membranes at pH 8.6 using Supre-Heme buffer (Helena Lab, Beaumont, TX). (A) Lane 1, βA chain (purified from human red blood cells); lane 2, β112 Cys→Asp chain; lane 3, β16 Gly→Asp chain; lane 4, β95 Lys→Glu chain; lane 5, β120 Lys→Glu chain; lane 6, β16 Gly→Asp, 120 Lys→Glu chain; and lane 7, βS chain (purified from human red blood cells). (B) Lane 1, Hb A (α2β2 purified from human red blood cells); lane 2, in vitro assembled α2β2 (β112 Cys→Asp); lane 3, in vitro assembled α2β2 (β16 Gly→Asp); lane 4, in vitro assembled α2β2 (β95 Lys→Glu); lane 5, in vitro assembled α2β2 (β120 Lys→Glu); lane 6, in vitro assembled α2β2 (β16 Gly→Asp, 120 Lys→Glu); and, lane 7, Hb S (α2β2S purified from human sickle red blood cells).
Electrophoresis of purified β-globin chain variants β16 Gly→Asp, β95 Lys→Glu, β120 Lys→Glu, β16 Gly→Asp, 120 Lys→Glu and β112 Cys→Asp and in vitro assembled tetramers. Purified β-globin chain variants β16 Gly→Asp, β95 Lys→Glu, β120 Lys→Glu, β16 Gly→Asp, 120 Lys→Glu and β112 Cys→Asp (A) and in vitro assembled tetramers (B) formed by mixing with α-globin chains isolated from human red blood cells and incubating in 10 mmol/L potassium phosphate buffer pH 7.0 at 25°C were analyzed by electrophoresis on cellulose acetate membranes at pH 8.6 using Supre-Heme buffer (Helena Lab, Beaumont, TX). (A) Lane 1, βA chain (purified from human red blood cells); lane 2, β112 Cys→Asp chain; lane 3, β16 Gly→Asp chain; lane 4, β95 Lys→Glu chain; lane 5, β120 Lys→Glu chain; lane 6, β16 Gly→Asp, 120 Lys→Glu chain; and lane 7, βS chain (purified from human red blood cells). (B) Lane 1, Hb A (α2β2 purified from human red blood cells); lane 2, in vitro assembled α2β2 (β112 Cys→Asp); lane 3, in vitro assembled α2β2 (β16 Gly→Asp); lane 4, in vitro assembled α2β2 (β95 Lys→Glu); lane 5, in vitro assembled α2β2 (β120 Lys→Glu); lane 6, in vitro assembled α2β2 (β16 Gly→Asp, 120 Lys→Glu); and, lane 7, Hb S (α2β2S purified from human sickle red blood cells).
Molecular Masses and Isoelectric Points of Purified β-Globin Variants
| β-Chain Variant . | Mass Analysis . | Isoelectric Point . | |
|---|---|---|---|
| Expected . | Obtained . | ||
| βA | 15,867.2 | 15,867.9 | 6.25 |
| β16 Gly → Asp | 15,925.2 | 15,924.2 | 5.97 |
| β95 Lys → Glu | 15,868.2 | 15,868.0 | 5.84 |
| β120 Lys → Glu | 15,868.4 | 15,867.2 | 5.73 |
| β16 Gly → Asp, 120 Lys → Glu | 15,926.3 | 15,925.2 | 5.48 |
| β112 Cys → Asp | 15,879.2 | 15,878.9 | 6.33 |
| β-Chain Variant . | Mass Analysis . | Isoelectric Point . | |
|---|---|---|---|
| Expected . | Obtained . | ||
| βA | 15,867.2 | 15,867.9 | 6.25 |
| β16 Gly → Asp | 15,925.2 | 15,924.2 | 5.97 |
| β95 Lys → Glu | 15,868.2 | 15,868.0 | 5.84 |
| β120 Lys → Glu | 15,868.4 | 15,867.2 | 5.73 |
| β16 Gly → Asp, 120 Lys → Glu | 15,926.3 | 15,925.2 | 5.48 |
| β112 Cys → Asp | 15,879.2 | 15,878.9 | 6.33 |
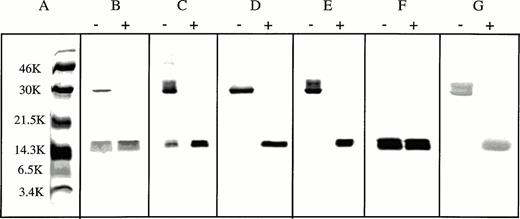
We previously reported that the βA-globin chain fraction isolated after expression in bacteria contained monomers and disulfide cross-linked dimers.9 The dimers formed by oxidation of cysteine residues and were reduced to monomers by addition of DTT. The purified negatively-charged β-chain variants, except for the β112 Cys→Asp chain, also contained a mixture of monomers and disulfide-linked dimers like the βA-chain fraction.9 In addition, dimer formation of the variants and βA chain was apparent during purification. In contrast, β112 Cys→Asp chains migrate as monomers on SDS-PAGE, suggesting that disulfide bond formation generating β-β dimers is caused by cross-linking of β112 Cys residues (Fig 2). Mass spectral analysis of the five variants using ESMS showed the expected β-globin chain molecular masses (Table 1).
SDS-PAGE of purified β-globin variants. β-chain variants (2 to 5 μg) expressed in bacteria were purified and subjected to SDS-PAGE after treatment with (+) or without (-) 200 mmol/L DTT for 30 minutes at 25°C. After heating for 3 minutes in the presence of 3% (wt/vol) SDS in a boiling water bath, samples were electrophoresed on a 12.5% (wt/vol) polyacrylamide gel at a constant voltage of 100 V. Gels were stained with Coomassie Brilliant Blue R-250 to detect proteins. (A) Molecular weight standards (Amersham Life Science, Arlington Heights, IL); (B) β16 Gly→Asp chain; (C) β95 Lys→Glu chain; (D) β120 Lys→Glu chain; (E) β16 Gly→Asp, 120 Lys→Glu chain; (F) β112 Cys→Asp chain; and (G) β▵ chain.
SDS-PAGE of purified β-globin variants. β-chain variants (2 to 5 μg) expressed in bacteria were purified and subjected to SDS-PAGE after treatment with (+) or without (-) 200 mmol/L DTT for 30 minutes at 25°C. After heating for 3 minutes in the presence of 3% (wt/vol) SDS in a boiling water bath, samples were electrophoresed on a 12.5% (wt/vol) polyacrylamide gel at a constant voltage of 100 V. Gels were stained with Coomassie Brilliant Blue R-250 to detect proteins. (A) Molecular weight standards (Amersham Life Science, Arlington Heights, IL); (B) β16 Gly→Asp chain; (C) β95 Lys→Glu chain; (D) β120 Lys→Glu chain; (E) β16 Gly→Asp, 120 Lys→Glu chain; (F) β112 Cys→Asp chain; and (G) β▵ chain.
Characterization of assembled α2β2tetramers containing the five β-chain variants.
The negatively-charged β chains were assembled in vitro with α chains to form tetrameric hemoglobins, and tetramers were then purified by Mono S-FPLC chromatography. Electrophoretic mobility (Fig 1B) and FPLC elution profile of assembled tetrameric α2β2 (β112 Cys→Asp) were similar to Hb A (α2β2). In contrast, surface charges of assembled tetramers of α2β2 (β16 Gly→Asp), α2β2 (β95 Lys→Glu), α2β2 (β120 Lys→Glu), and α2β2 (β16 Gly→Asp, 120 Lys→Glu), as assessed by cellulose acetate electrophoresis were more negative than that of Hb A, and their elution from Mono S-cation FPLC occurred just before that of Hb A. These results indicate that β16 Gly→Asp, β95 Lys→Glu, β120 Lys→Glu and β16 Gly→Asp, 120 Lys→Glu are exposed on the surface, while β112 Cys→Asp is located at an internal position in the α2β2tetramer.1 Absorption spectra of the CO forms of these tetrameric variants containing negatively-charged β chains were the same as those of native tetrameric Hb A.
Naturally-occurring variants J-Baltimore (Hb βG16D) and N-Baltimore (Hb βK95E) and reconstituted hemoglobin tetramers containing β16 Gly→Asp or β95 Lys→Glu have the same oxygen affinity and cooperativity as normal Hb A.19 We performed functional studies of Hb βG16D, K120E, and Hb βC112D in 50 mmol/L Bis Tris buffer, pH 7.1 containing 0.1 mol/L NaCl at 20°C in the presence and absence of 2,3-biphosphoglycerate (BPG) and compared results with those of Hb A (Table 2). Reconstituted Hb βG16D, K120E exhibited the same oxygen affinity and cooperativity as those of normal Hb A tetramers. These results indicate that these recombinant hemoglobins are correctly folded and assembled, and that changes in amino acids on the surface, which do not involve direct α1β1 and α1β2 interaction sites of hemoglobin do not affect functional properties of dimeric and tetrameric hemoglobins. In contrast, oxygen affinity of Hb βC112D, which has a substitution at an α1β1 interaction site, was slightly higher (P50 of 2.3 v 3.7 for Hb A); and its cooperativity was slightly lower than that of normal Hb A tetramers (2.60 v 2.74).
Oxygen-Binding Properties for Hb βG16D, K120E, and Hb βC112D
| Hemoglobin . | P50 . | n50 2, 3-BPG (−) . | |
|---|---|---|---|
| 2, 3-BPG (−) . | 2, 3-BPG (+) . | ||
| Hb A | 3.7 | 13.5 | 2.74 |
| Hb βG16D, K120E | 3.6 | 11.4 | 2.74 |
| Hb βC112D | 2.2 | 10.3 | 2.60 |
| Hemoglobin . | P50 . | n50 2, 3-BPG (−) . | |
|---|---|---|---|
| 2, 3-BPG (−) . | 2, 3-BPG (+) . | ||
| Hb A | 3.7 | 13.5 | 2.74 |
| Hb βG16D, K120E | 3.6 | 11.4 | 2.74 |
| Hb βC112D | 2.2 | 10.3 | 2.60 |
Oxygen equilibrium curves of Hb A, Hb βG16D, K120E, and Hb βC112D were determined in 50 mmol/L Bis Tris buffer, pH 7.1 containing 0.1 mol/L NaCl at 20°C. P50 is the partial oxygen pressure required to give 50% oxygen saturation of hemoglobin. n50 values were calculated from the Hill plot of oxygen equilibrium curves. Concentration of 2, 3-BPG when present (+) is 2 mmol/L.
Tetramer formation in vitro.
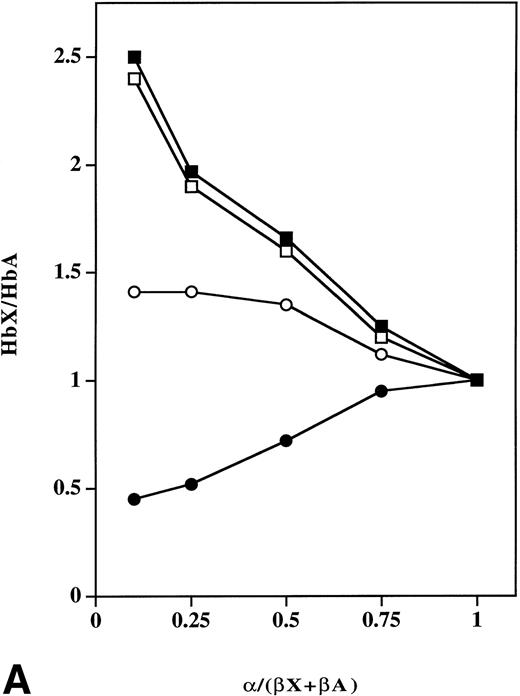
Previous studies in vitro in the presence of limiting amounts of α chains and mixtures of equal amounts of purified normal and mutant subunits showed that mutant hemoglobin percentages were higher when using more negatively-charged β chains like J-Baltimore (β16 Gly→Asp) and N-Baltimore (β95 Lys→Glu).3,6,7 These results suggest that more negatively-charged β chains bind positively-charged α chains more readily than βA chains.3,6-8 To confirm and extend those studies, we produced β16 Gly→Asp, β95 Lys→Glu, β120 Lys→Glu and β16 Gly→Asp, 120 Lys→Glu chains using a bacterial expression system and then performed assembly studies in vitro with purified α chains and compared results with those using βA and βSchains. Results of assembly in vitro with α chains and equimolar mixtures of βA and β16 Gly→Asp or β95 Lys→Glu chains were the same as those reported previously.3,6,7 In addition, assembly results for β120 Lys→Glu chains were similar to those of β95 Lys→Glu chains. The ratio of Hb X/Hb A as the α-chain concentration approached zero was 2.7, 2.5, 1.5, and 0.4 for Hb βK120E, Hb βK95E, Hb βG16D, and Hb S, respectively (Fig 3A). These results confirm results from earlier studies3 6-8 showing that subunit surface charge plays a critical role in relative affinity of α chains for β chains before formation of tetrameric hemoglobin.
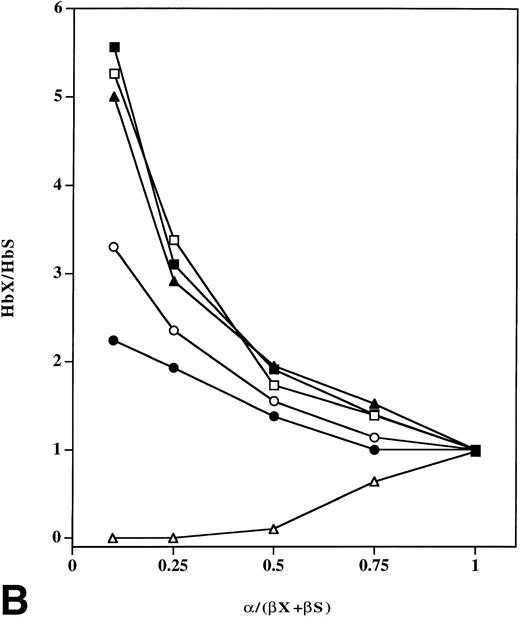
Effect of β-chain surface charge on relative amounts of in vitro assembled tetramers as a function of varying amounts of input α chains. (A) Equimolar mixtures of normal (βA), sickle (βS), β16 Gly→Asp, β95 Lys→Glu or β120 Lys→Glu chains (75 μmol/L) were added to varying amounts of α-globin chain in 0.1 mol/L phosphate buffer, pH 7.0 at 25°C, and assembled tetramers were analyzed by FPLC. The relative ratio (y axis) of Hb S to Hb A (•) in (βS + βA)/α mixtures, Hb βG16D to Hb A (○) in (β16 Asp +βA)/α mixtures, Hb βK95E to Hb A (□) in (β95 Glu +βA)/α mixtures and Hb βK120E to Hb A (▪) in (β120 Glu +βA)/α mixtures was calculated as a function of varying amounts of input α-globin chain. (B) Equimolar mixtures of βs with β16 Gly→Asp, β95 Lys→Glu, β120 Lys→Glu, β16 Gly→Asp, 120 Lys→Glu or β112 Cys→Asp chains (75 μmol/L) were added to increasing amounts of α-globin chain in 0.1 mol/L phosphate buffer, pH 7.0 at 25°C. Tetramer formation was analyzed by FPLC, and the relative ratio of Hb A to Hb S (•), Hb βC112D to Hb S (▵), Hb βG16D to Hb S (o), Hb βK95E to Hb S (□), Hb βK120E to Hb S (▪), and Hb βG16D, K120E to Hb S (▴) was calculated (y axis).
Effect of β-chain surface charge on relative amounts of in vitro assembled tetramers as a function of varying amounts of input α chains. (A) Equimolar mixtures of normal (βA), sickle (βS), β16 Gly→Asp, β95 Lys→Glu or β120 Lys→Glu chains (75 μmol/L) were added to varying amounts of α-globin chain in 0.1 mol/L phosphate buffer, pH 7.0 at 25°C, and assembled tetramers were analyzed by FPLC. The relative ratio (y axis) of Hb S to Hb A (•) in (βS + βA)/α mixtures, Hb βG16D to Hb A (○) in (β16 Asp +βA)/α mixtures, Hb βK95E to Hb A (□) in (β95 Glu +βA)/α mixtures and Hb βK120E to Hb A (▪) in (β120 Glu +βA)/α mixtures was calculated as a function of varying amounts of input α-globin chain. (B) Equimolar mixtures of βs with β16 Gly→Asp, β95 Lys→Glu, β120 Lys→Glu, β16 Gly→Asp, 120 Lys→Glu or β112 Cys→Asp chains (75 μmol/L) were added to increasing amounts of α-globin chain in 0.1 mol/L phosphate buffer, pH 7.0 at 25°C. Tetramer formation was analyzed by FPLC, and the relative ratio of Hb A to Hb S (•), Hb βC112D to Hb S (▵), Hb βG16D to Hb S (o), Hb βK95E to Hb S (□), Hb βK120E to Hb S (▪), and Hb βG16D, K120E to Hb S (▴) was calculated (y axis).
Previous competition experiments in vitro using mixtures of purified α and β chains showed that αβA dimers form about twice as readily as αβs dimers when the concentration of α chains becomes limiting.3 8 This results in assembly of less Hb S relative to Hb A when equimolar amounts of βA and βS chains compete for limiting amounts of α globin. Our results also show that tetramer formation occurs efficiently in vitro; and, that under limiting α-chain conditions, less Hb S compared with Hb A formed in mixtures containing equal amounts of βS and βA chains (Fig 3). In addition, we performed subunit competition experiments in which varying amounts of α chains were added to equimolar mixtures of βS and either β16 Gly→Asp, β95 Lys→Glu, β120 Lys→Glu, β16 Gly→Asp, 120 Lys→Glu or βA chains to further assess the effect of β6 Glu→Val on assembly. Under limiting α-chain conditions, percentages of hemoglobin tetramers containing negatively-charged β chains were much higher in mixtures containing βS instead of βA chains (Fig 3B). Total amounts of Hb βK120E and Hb βK95E were always more than that of Hb βG16D, while values for the double mutant Hb βG16D, K120E were similar to those of Hb βK120E and Hb βK95E. The order of tetramer formation in vitro under limiting α-globin chain conditions was Hb βG16D, K120E = Hb βK120E =Hb βK95E > Hb βG16D > Hb A. Ratios of Hb X/Hb S as α-chain concentration approached zero were 6.2, 6.3, 6.0, 4.0, and 2.5 for Hb βG16D, K120E, Hb βK120E, Hb βK95E, Hb βG16D, and Hb A, respectively (Fig 3B). These results also indicate that promotion of assembly by additional negative charges in the β chain is independent of location on the surface, and that two net additional negative charges compared with βAchains appear to be the maximum charge for facilitating formation of α+ ----β- electrostatic intermediates. Furthermore, the ratios of (Hb βK120E/Hb S)/(Hb βK120E/Hb A), (Hb βK95E/Hb S)/(Hb βK95E/Hb A) and (Hb βG16D/Hb S)/(Hb βG16D/Hb A) approached 2.5, which is the same value obtained for the ratio of Hb A/Hb S under limiting α-chain conditions.
Subunit competition studies were also performed in which varying amounts of α chains were added to equimolar mixtures of βS and β112 Cys→Asp chains to assess the role of α1β1 interaction sites on assembly. Competition experiments with βA, βS, and the β112 Asp chain variant were difficult to do, as Hb A tetramers were not readily separated from Hb βC112D tetramers by FPLC. Our results show under limiting α-chain conditions that relatively much less Hb βC112D compared with Hb βK95E, Hb βG16D, and Hb A tetramers formed in these mixtures containing equimolar amounts of the βS chain (Fig 3B). Furthermore, Hb βC112D levels in mixtures containing βS and β112 Cys→Asp chains were almost zero when the ratio of α chain to total β chains was less than 0.5. Tetramer formation for the β112 Cys→Asp variant after addition of α chains was also monitored by cellulose acetate electrophoresis (Fig 1) and FPLC (Superose 12 gel-filtration). Gel-filtration patterns of mixtures containing β112 Cys→Asp or the other β-chain variants and α chains were similar to those of βA and α chains (Hb A), indicating that β112 Cys→Asp chains, just like native βA chains, form hemoglobin tetramers with exogenously-added α chains (data not shown). These results indicate that the relative affinity of α for β chains is dependent on direct α1β1 interaction sites, even though surface charge of the chains plays a critical role in the initial stage of assembly. After initial electrostatic interactions of the two chains, stable α1β1 interactions may then occur between the two subunits.
Dissociation of Hb βC112D tetramers.
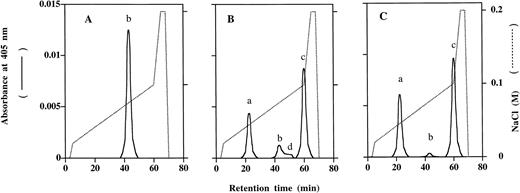
Tetrameric Hb readily dissociates in solution and exists in equilibrium with αβ dimers.17,20 In contrast, native αβ dimers dissociate slowly to monomers with a first-order dissociation rate constant of about 3 × 10-3h-1.21 This indicates that over a 72-hour period, only 33% of the total single chains are exchangeable from normal tetrameric hemoglobin by a competing chain. In contrast, Hb βC112D tetramers can be readily exchanged by addition of βS chains (Fig 4), as shown in timed FPLC chromatography experiments of mixtures of Hb βC112D tetramers and βS chains. Over a period of only 30 minutes (Fig 4C), almost all of the βS chains were incorporated into tetrameric Hb S (peak “c” in Fig 4), generating free β112 Cys→Asp chains (peak “a” in Fig 4). In contrast, Hb βG16D, K120E, Hb βK120E, Hb βK95E, Hb βG16D, or Hb A tetramers do not exchange with βS chains during these same time intervals.
βS-chain exchange as a function of incubation time with Hb β112 Cys→Asp tetramers. Tetrameric Hb βC112D was incubated with βs chains in 0.1 mol/L phosphate buffer, pH 7.0 at 25°C, and Hb S tetramer formation, as well as generation of β112 Cys→Asp chains, were analyzed by FPLC. (A), (B), and (C) correspond to chromatographic analyses before (zero time point) and after 15 and 30 minutes incubations in the presence of βs chains, respectively. Peaks a, b, c, and d represent β112 Cys→Asp, Hb βC112D, Hb S and βS, respectively. The dotted line is a trace of the gradient profile for NaCl.
βS-chain exchange as a function of incubation time with Hb β112 Cys→Asp tetramers. Tetrameric Hb βC112D was incubated with βs chains in 0.1 mol/L phosphate buffer, pH 7.0 at 25°C, and Hb S tetramer formation, as well as generation of β112 Cys→Asp chains, were analyzed by FPLC. (A), (B), and (C) correspond to chromatographic analyses before (zero time point) and after 15 and 30 minutes incubations in the presence of βs chains, respectively. Peaks a, b, c, and d represent β112 Cys→Asp, Hb βC112D, Hb S and βS, respectively. The dotted line is a trace of the gradient profile for NaCl.
Gel filtration of β112 Cys chain.
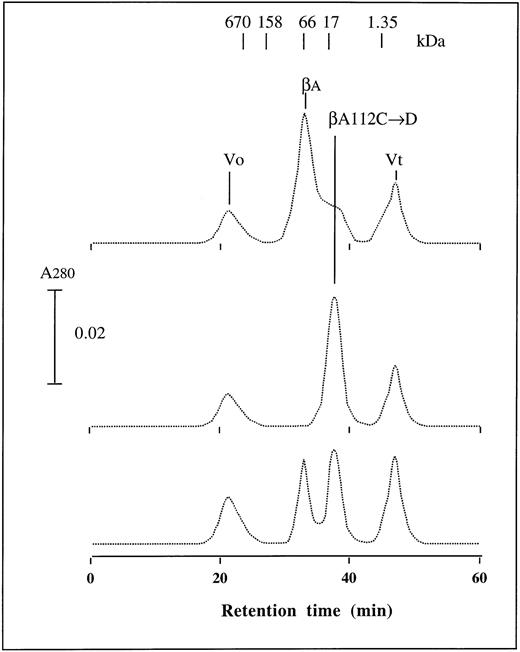
It is known that isolated β-globin chains aggregate to form β4 homotetramers.18 Our gel-filtration results show that βA and the four negatively-charged β chains, containing β16 Gly→Asp, β95 Lys→Glu, β120 Lys→Glu and β16 Gly→Asp, or 120 Lys→Glu, exist almost totally as β4 tetramers, which depends on concentration; while β112 Asp chains exist only as monomers (Fig 5). Mixtures of βA and β112 Asp chains show high and low molecular weight forms, indicating that β112 Asp chains exist as monomers and inhibit formation of β4 tetramers as observed in our electrophoretic studies. These results suggest that β112 Cys is a key amino acid in formation of β4 tetramers and that loss of β112 Cys inhibits tetramer formation. It is also important to note that the circular dichroism spectrum measured from 190 to 260 nm for β112 Asp chains in the CO form was identical to that of βA-globin chains. These results indicate that β112 Asp chains made in the Escherichia coli expression system are properly folded like authentic β globin. Furthermore, our findings suggest that altered properties of assembly of the β112 Asp chain with α chain are caused by the Cys to Asp change at β112 rather than by incorrect folding.
Gel-filtration chromatography of β112 Cys→Asp chains. Gel-filtration chromatography of purified (≈ 70 μmol/L in 200 μL) βA chains (A), β112 Cys→Asp chains (B), and a mixture of the two chains (C) was performed at a flow rate of 0.5 mL/minute in 0.1 mol/L phosphate buffer, pH 7.0. Vo and Vt refer to void and total column volumes, respectively.
Gel-filtration chromatography of β112 Cys→Asp chains. Gel-filtration chromatography of purified (≈ 70 μmol/L in 200 μL) βA chains (A), β112 Cys→Asp chains (B), and a mixture of the two chains (C) was performed at a flow rate of 0.5 mL/minute in 0.1 mol/L phosphate buffer, pH 7.0. Vo and Vt refer to void and total column volumes, respectively.
DISCUSSION
The kinetics of human hemoglobin assembly in vitro were studied previously using naturally-occurring variants, and three intermediate steps were proposed.3,6-8 The first involves dissociation of oligomers to monomers; the second, interaction of α and β monomers to form essentially irreversible αβ dimers; and the third, aggregation of αβ dimer to form functional α2β2 tetramers. Formation of monomers and tetramers of β chains depends on experimental conditions. Even though our experimental conditions for assembly favor formation of predominantly β4 tetramers rather than β monomers due to the relatively high concentration of β chains used (eg, 75 μmol/L in 0.1 mol/L phosphate buffer at 25°C), our results on effects of charge were similar to those previously reported.7 In the previous study, conditions favored formation of predominantly β-chain monomers because of lower hemoglobin concentrations (1.25 to 12.5 μmol/L), low ionic strenth (10 mmol/L) and low temperature (0°C). These results suggest that the different amounts of hemoglobin variants and Hb A formed in these earlier studies and in our studies are caused by differences in affinities of the individual β-chain variants for α chains.3,7,8 In addition, our findings demonstrate that both β95 Lys→Glu and β120 Lys→Glu promoted assembly with α chain more than that of β16 Gly→Asp, while addition of another negative charge in β16 Gly→Asp, 120 Lys→Glu chains did not influence assembly compared with β120 Lys→Glu. These results suggest that surface charge effects of the β chain on assembly are independent of position and are dependent on total surface net amino acid charge up to a maximum of two additional net negative charges compared with βA chains. These results also support the previously proposed electrostatic model of assembly.3 7
Cys β112 is located at the interface of α1β1 in α2β2 hemoglobin tetramers, and interacts with Val α107 (G14) and Ala α110 (G11), which is critical for stabilization of the αβ interface.1,20 Our present studies show that Hb βC112D levels in equimolar mixtures containing βS and β112 Cys→Asp chains were almost zero when the ratio of α chain to total β chains was less than 0.5. Furthermore, the order of tetramer formation was Hb βG16D, K120E = Hb βK120E = Hb βK95E > Hb βG16D > Hb A > Hb S >>> Hb βC112D, and dissociation of α2β2 (β112 Cys→Asp) into monomers was much faster than that of Hb A tetramers. In addition, oxygen affinity of Hb βC112D was slightly higher than that of Hb A with slightly less cooperativity than Hb A, which is comparable to results showing lack of cooperativity (n = 1) and higher oxygen affinity for recombinant Hb βR40D (P50of 1.2 v 5.1 for Hb A).22 This β-chain variant also has a negatively-charged β-chain substitution at an α1β2 interaction site of tetrameric hemoglobin and dissociates into monomers more readily than βA chains; however, complete dissociation to monomers did not occur under similar hemoglobin concentrations used for our experiments.22 These results reinforce the notion that oxygen affinity of tetrameric hemoglobin is affected mainly by α1β2 interaction sites.1,2 It is also interesting to note that recombinant β chains containing Gly instead of Cys at β112 on the α1β1 interface appear to stabilize α1β2 interactions and affect the allosteric equilibrium of hemoglobin.23 Even though assembly of this variant with α chains was not studied, the change to Gly at β112 should affect α1β1 assembly and the α1β2 interface differently compared with the Cys to Asp change we engineered at β112. The small differences in oxygen-binding properties of α2β2 (β112 Cys→Asp) compared with those of Hb A may be caused by propagation of changes induced at the α1β1 site to the α1β2 interface by this substitution. Further studies are required to evaluate effects of this change at the α1β1 site on the α1β2 interface. These studies should facilitate further understanding of the allosteric transition of hemoglobin. Furthermore, our results indicate that relative affinity of α for β chains is dependent on direct α1β1 interaction sites, even though surface charge of the chains affects interactions at the initial stage of assembly.
Analysis of α2β2 and β4subunit interfaces by x-ray diffraction showed a high degree of similarity between the quaternary structures of CO β4 and CO Hb (α2β2).20 Unlike the α2β2 tetramer, the β4tetramer has high oxygen affinity, does not bind oxygen cooperatively, and is influenced much less by allosteric effectors of native hemoglobin oxygen affinity.18,20 In addition, the α and β subunits of hemoglobin assemble to form tetramer through a stable αβ dimer intermediate, whereas β4 assembles from monomeric β chains with relatively little dimer formation.17 Recent x-ray analysis of β4hemoglobin at 1.8 Å resolution indicated that β112 Cys (G14) is located at the β-chain interface, and the side chains of β112 Cys at β1 and β2 in the β4 tetramer are very close to the molecular dyad at the β1β2 interface.20 These two residues exist on the surface of the β chains and may be involved in weak interactions with other residues. Our present results also show the absence of disulfide dimer formation for β112 Asp (G14), which normally occurs in negatively-charged β chains like the βA chain.9 These results clearly indicate that β112 Cys residues in the β1 and β2 globin chains are close together and that disulfide β-chain dimer formation is governed by these two cysteine residues, and not by Cys β93.9 In addition, β112 Asp chains do not form β4 tetramers and these chains in α2β2 (β112 Cys→Asp) tetramers exchange readily with other β chains, probably because of unstable interactions between β112 Asp (G14) and α107 Val (G14) at the α1β1 interaction sites. This finding suggests that β112 Cys (G14) is a critical amino acid in formation of stable β4 tetramers, as well as αβ dimers.
Studies aimed at production of more efficient hemoglobin variants are now critical for development of gene therapy approaches to sickle cell disease and thalassemia. There are limitations on expression levels of Hb A or Hb F with viral vectors, and it is critical to design the most efficient hemoglobin variants.24 Design and testing of more efficient Hb A or Hb F variants for gene therapy and the growing knowledge regarding hematopoietic stem cell biology will facilitate future efforts to enhance expression levels and gene transfer efficiency of vectors containing β- or γ-chain variants. In addition to changing oxygen affinity of the hemoglobin variant for potential use in gene therapy,24 we can now apply results from studies of subunit assembly to increase Hb A or Hb F levels by engineering more efficient β- or γ-chain variants, which will be more stable and promote assembly. Modification of surface charge of hemoglobin variants is now expected to facilitate increases in total hemoglobin variant levels in red blood cells, as the assembly rate of α and non-α chains to form αβ or αγ dimers depends on electrostatic attraction. More negatively-charged β or γ chains in addition to stabilization of α1β1 or α1γ1 interaction sites would be expected to promote higher affinity of the variant β-like chain for α rather than βA or βschains. Information gained from these studies can be applied to produce better hemoglobin variants for gene therapy in the future, as well as to facilitate our understanding of the mechanism of assembly of a number of other multisubunit proteins.
ACKNOWLEDGMENT
We thank Dr Eric Rappaport and members of the Nucleic Acid/Protein Core at The Children's Hospital of Philadelphia for automated DNA sequence analysis. We are grateful to Dr H.E. Witkowska for mass spectral analysis of the β-chain variants performed at the Children's Hospital Mass Spectrometry Facility in Oakland, CA (Dr C. Shackleton, Director), which is supported in part by National Institutes of Health Grant No. HL20985 and a Shared Instrumentation Grant No. RR06505.
Supported by Grants No. P60 HL38632 and DK 16691 from the National Institutes of Health, Bethesda, MD; the March of Dimes Birth Defects Foundation (FY95); American Heart Association; the Nemours Foundation; and UNICO National Inc.
Address reprint requests to Kazuhiko Adachi, PhD, Division of Hematology, The Children's Hospital of Philadelphia, 34th St & Civic Center Blvd, Philadelphia, PA 19104.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal