Abstract
Acute bleeding after bone marrow transplantation (BMT) was investigated in 1,402 patients receiving transplants at Johns Hopkins Hospital between January 1, 1986 and June 30, 1995. Bleeding categorization was based on daily scores of intensity used by the blood transfusion service. Moderate and severe episodes were analyzed for bleeding sites. Analysis of the cause of death and the interval of the bleeding episode to outcome endpoints was recorded. Survival estimates were computed for 1,353 BMT patients. The overall incidence was 34%. Minor bleeding was seen in 10.6%, moderate bleeding was seen in 11.3%, and severe bleeding was seen in 12% of all patients. Fourteen percent of patients had moderate or severe gastrointestinal hemorrhage, 6.4% had moderate or severe hemorrhagic cystitis, 2.8% had pulmonary hemorrhage, and 2% had intracranial hemorrhage. Sixty-one percent had 1 bleeding site and 34.4% had more than 1 site. Moderate and severe bleeding was more prevalent in allogeneic (31%) and unrelated patients (62.5%) compared with autologous patients (18.5%). Significant distribution of incidence was found among the different diagnoses, but not by disease status in acute myeloid leukemia, acute lymphoblastic leukemia, chronic myelogenous leukemia, Hodgkin's disease, and non-Hodgkin's lymphoma. Bleeding was associated with significantly reduced survival in allogeneic, autologous, and unrelated BMT and in each disease category except multiple myeloma. Survival was correlated with the bleeding intensity, bleeding site, and the number of sites. Although close temporal association was evident to mortality, bleeding was recorded as the cause of death in only the minority of cases compared with other toxicities after BMT (graft-versus-host disease, infections, and preparative regimen toxicity). Acute bleeding is a common complication after BMT that is profoundly associated with morbidity and mortality. Although bleeding was not a direct cause of death in the majority of cases, it has a potential prognostic implication as a predictor of poor outcome in clinical assessment of patients after BMT.
BLEEDING AFTER bone marrow transplantation (BMT) is a daily clinical concern for BMT units. Previous studies have reported the incidence, risk factors, and outcome of bleeding after BMT; however, in most of these studies, bleeding was investigated by the specific site, eg, gastrointestinal bleeding,1-5 urinary tract bleeding,6-14pulmonary hemorrhage,15-26 or intracranial hemorrhage.27-30 Yet, because patients may experience bleeding from multiple sites either simultaneously or sequentially, the extent and significance of overall acute bleeding is unknown.
Bleeding research has been hampered by the lack of uniform methodology used in bleeding classification, which resulted in significant variations in the reported incidence by site. Gastrointestinal (GI) hemorrhage was found in 5% to 15%1-3 of patients after transplantation, hemorrhagic cystitis (HC) was found in 4% to 52%,6-13 less than 1% to 21% of diffuse alveolar hemorrhage (DAH) was found in autologous patients,15-20 and 2.5% to 5% of subdural hematomas were found in leukemic patients after transplantation.27 30
Limited data are available regarding the association of bleeding with early mortality. High mortality has been reported with DAH in as many as 50% to 100% of cases15,16,24 and with intracerebral hemorrhages27,29; however, GI bleeding, HC, and subdural hematomas, although capable of causing significant morbidity, have not usually been associated with decreased outcome.1,7,8,13 27
Bleeding associated with mortality was investigated by Tornebohm et al31 in a retrospective analysis of 83 leukemic patients with terminal course after transplantation. Bleeding was defined according to the classification suggested by Gmur et al.32Hemorrhage was found in 38 of 83 (46%) patients. Two of 38 had grade 1 bleeding, 16 of 38 had grade 2 bleeding, 13 of 38 had grade 3 bleeding, and 7 of 38 had grade 4 bleeding. Fatal bleeding was identified in 16 of 83 (19%) patients from intracranial (IC) hemorrhage (5 patients), gastrointestinal (5 patients), and generalized bleeding (6 patients). The overall hemorrhagic incidence was similar in allogeneic and autologous BMT populations (18% and 19%, respectively). Findings of terminal hemorrhage in autopsies of patients after BMT identified DAH33,34 and, less frequently, intracranial hemorrhage.35 36
The goals of this study were to determine the extent and significance of bleeding complications in a large cohort of BMT patients in the acute posttransplantation period. We included clinically significant bleeding from all sites, whether sole or overlapping, occurring simultaneously or sequentially within 100 days after BMT. Based on a concurrent evaluation of bleeding, the entire bleeding episode was retrospectively graded by severity to apply a quantitative analysis for incidence and outcome.
MATERIALS AND METHODS
Study Group
One thousand four hundred two patients received transplants at Johns Hopkins Hospital for hematologic diseases or solid tumors from January 1, 1986 to June 30, 1995. All patients were screened for bleeding during the first 100 days after BMT. The patients demographics, including diagnosis, stage of disease, type of BMT, preparative regimens, age, and sex, are given in Table1. The majority of patients had hematologic malignancies and breast cancer. The remaining diseases, mainly solid tumors in pediatric patients, were grouped as others. BMT for breast cancer was first performed in 1988, BMT for multiple myeloma (MM) was first performed in 1989. Patients were divided into good- and poor-risk populations based on the stage of disease. Good-risk prognosis included acute leukemia in first complete remission, chronic myelogenous leukemia (CML) in chronic phase, chemotherapy sensitive Hodgkin's disease (HD) and non-Hodgkin's lymphoma (NHL), and patients with aplastic anemia (AA). All other patients were considered poor risk. Breast cancer patients were transplanted with recurrent disease. Most allogeneic patients had matched siblings, 28 patients were transplanted with 1 major antigen mismatch from family member. Analyses applied to allogeneic patients included both matched and 1 antigen mismatched transplants. Twenty two transplants grouped as others included: 12 peripheral blood stem cells transfusions, 7 syngeneic, 2 cases had haploidentical BMT, and 1 patient had transfusion of cord blood hematopoteic stem cells. The preparative regimens used included busulfan plus cyclophosphamide (Bu-Cy) for hematologic malignancy, Cy plus total body irradiation (Cy-TBI) for hematologic malignancy, Bu-Cy plus etoposide (BU-VP-Cy) for hematologic malignancy, Cy plus Cyclosporine A (Cy-CSA) for AA, Cy plus Thiotepa (Cy-Thio) for breast cancer, and etoposide-carboplatin Cy (VP-CBDCA-Cy) for solid tumors. Indications and dosage were previously published.37-40
Demographics of 1,402 BMT Patients Receiving Transplants at Johns Hopkins Hospital from January 1, 1986 to June 30, 1995
| . | No. of Patients . | % . |
|---|---|---|
| Diagnosis | ||
| AA | 20 | 1.4 |
| AML | 289 (140, 149) | 20.6 |
| ALL | 124 (51, 73) | 8.8 |
| Breast cancer | 199 | 14.2 |
| CML | 181 (148, 33) | 12.9 |
| HD | 112 (62, 48) | 8.0 |
| NHL | 279 (216, 59) | 19.9 |
| MM | 32 | 2.3 |
| Others | 166 | 11.8 |
| Type of BMT | ||
| Autologous | 887 | 63.3 |
| Allogeneic | 469 | 33.5 |
| Unrelated | 24 | 1.7 |
| Others | 22 | 1.5 |
| Preparative regimen | ||
| Bu-Cy | 520 | 37.1 |
| Bu-VP-Cy | 122 | 8.7 |
| Cy-CSA | 19 | 1.4 |
| Cy-TBI | 410 | 29.2 |
| Cy-Thio | 199 | 14.2 |
| VP-CBDCA-Cy | 90 | 6.4 |
| Others | 42 | 3.0 |
| Age | ||
| Pediatric | 294 | 21.0 |
| Adults | 1,108 | 79.0 |
| Sex | ||
| Females | 676 | 48.2 |
| Males | 726 | 51.8 |
| . | No. of Patients . | % . |
|---|---|---|
| Diagnosis | ||
| AA | 20 | 1.4 |
| AML | 289 (140, 149) | 20.6 |
| ALL | 124 (51, 73) | 8.8 |
| Breast cancer | 199 | 14.2 |
| CML | 181 (148, 33) | 12.9 |
| HD | 112 (62, 48) | 8.0 |
| NHL | 279 (216, 59) | 19.9 |
| MM | 32 | 2.3 |
| Others | 166 | 11.8 |
| Type of BMT | ||
| Autologous | 887 | 63.3 |
| Allogeneic | 469 | 33.5 |
| Unrelated | 24 | 1.7 |
| Others | 22 | 1.5 |
| Preparative regimen | ||
| Bu-Cy | 520 | 37.1 |
| Bu-VP-Cy | 122 | 8.7 |
| Cy-CSA | 19 | 1.4 |
| Cy-TBI | 410 | 29.2 |
| Cy-Thio | 199 | 14.2 |
| VP-CBDCA-Cy | 90 | 6.4 |
| Others | 42 | 3.0 |
| Age | ||
| Pediatric | 294 | 21.0 |
| Adults | 1,108 | 79.0 |
| Sex | ||
| Females | 676 | 48.2 |
| Males | 726 | 51.8 |
In parenthesis (after the number of patients) are numbered the good- and poor-risk patients for each diagnosis.
Bleeding Evaluation
Bleeding identification.
Bleeding was identified and evaluated based on a daily score of intensity that is assigned by the blood transfusion service for every hospitalized BMT patient. The scoring system integrates the patient's clinical assessment (rounds and chart review) with daily hematologic laboratory values and daily red blood cell (RBC) usage. The frequency of scoring is twice a week up to every weekday, depending on the clinical status. Bleeding score is not assigned during the weekends; however, it is retrospectively assigned in the next scoring evaluation. This scoring method has been successfully used in the same format since the early 1980s to manage individual blood product requirements.
The daily bleeding scores were defined as the following. Score 0 signified no bleeding. Score 1 was given for occult blood in body secretions detected by heme positive in dipstick (Hemoccult/Gastroccult, SmithKline Diagnostics Inc; Bili-Labstix, Bayer Corp), which is performed as a routine screen. Mild petechiae or minimal vaginal spotting were also considered as score 1. A score 2 was assigned for minor bleeding that did not require RBC transfusion over the routine transfusion needs (eg, ecchymosis, epistaxis, line oozing, vaginal bleeding, skin bleeding, mild hematemesis, melena, and mild hematuria). A slowly decreasing hematocrit level in a BMT patient is expected secondary to treatment induced aplasia in combination with multiple blood draws. Hemorrhage causing rapid decreases in hematocrit level necessitating 1 or more units of RBC transfusions per day over the expected rate of an individual or failure to obtain a posttransfusion increment while actively bleeding resulted in a score 3. Life-threatening hemorrhage defined as either massive bleeding causing severe hemodynamic compromise or bleeding into a vital organ (eg, intracranial hemorrhage, pericardial, and DAH) resulted in score 4.
The daily scores are recorded with a brief clinical description in the transfusion history report of each patient. These reports were reviewed for all 1,402 patients for primary identification of bleeding within 100 days after BMT.
The policy of transfusions along the study period has been as follows. Prophylactic platelet transfusions were administered to patients when the morning platelet count was equal to or less than 20,000. Patients with daily score 2, were usually transfused to keep their platelets level greater than 30,000. In cases of active bleeding (score 3), the threshold was usually set on 50,000. Higher thresholds could be set in life-threatening hemorrhage. Packed RBC were transfused prophylactically to maintain a hematocrit level equal to or greater than 25% in nonbleeding patients. Patients with active bleeding received RBC transfusions as needed, based on the intensity and rate of bleeding and the hemodynamic situation.
Preference for apheresis single donor products was always given to the BMT patients. During the years 1986 through 1988, 60% of transfusions were apheresis products. The yearly percentage of apheresis products has constantly grown; by 1995, 96% of platelet transfusions were single donor apheresis, whereas only 4% were from random donors.
Defining a bleeding episode and inclusion criteria.
The bleeding episodes were defined by assembling the daily scores as continuous events within 100 days from marrow transfusion (which was considered day 0). The minimal episode to be included in the study was a daily score 2 for at least 4 of 7 days (4/7). Any score 3 or 4 was included. The definition of minimal event was also used for determining the episode boundaries (initiation and cessation). Because of fluctuating daily intensity of bleeding, a major episode (moderate or severe bleeding, see below) was permitted to have up to a 1-week gap of no bleeding within its boundaries.
Grading the bleeding episodes severity.
Episodes fitting the criteria described above were then retrospectively categorized into minor, moderate, and severe. Minor bleeding included daily score 2 for up to 7 days. Moderate bleeding was defined as more than a week of daily score 2 or 1 to 2 days of score 3 (within 7 days). Severe bleeding included score 3 for 3 or more days (within 7 days) or any score 4. This categorization characterizes the pattern of bleeding episode by the maximal score, bleeding frequency, and duration.
The daily bleeding score is regularly assigned by a transfusion coordinator assigned to the BMT unit for rotational period. To reduce differences due to personal subjectivity in daily score selection, moderate and severe episodes (328 cases) were reviewed with clinical reports (discharge summaries, patients charts, clinical notes etc) and compared with the original scores. In cases of discrepancy, reevaluation of the daily scores was performed by one experienced member of the transfusion unit (V.S.). After this review, 15 of 328 cases (4.6%) had a change in the original category of intensity, and 22 of 328 cases (6.7%) had completion of daily scores around terminal course.
The method described above was used to identify and quantify bleeding from any site with the exception of pulmonary hemorrhage, for which definition excluded evidence of infection, as broadly used.15,17,24,26 33 Most cases of pulmonary hemorrhage were confirmed by histology and were retrospectively reviewed by a pathologist.
Specification of the Bleeding Site
Moderate and severe episodes were grouped to learn about the effect of sites (source and number of sites) on survival. Exclusion of minor bleeding enabled us to focus the analysis on the range of clinically significant bleeding.
The bleeding sites were detailed as GI, urinary, pulmonary, IC, and bleeding relating to procedure (eg, liver biopsy, chest tube insertion, etc). Other was termed for epistaxis, vaginal, skin, cardiac, abdominal, and several sites (representing an overall moderate episode from several sites, each minor by intensity).
The number of sites was counted as 1, 2, 3 and more (3+) in cases in which the episodes could clearly be separated by initiation dates. Multiple bleeding was defined as simultaneous initiation of 3 or more sites. A site was counted only if the bleeding intensity at that site was moderate or severe.
Survival Assessment
Survival after BMT was calculated for 1,353 patients with outcome endpoints of death or follow-up ending in December 1995. Forty-nine cases were not included in the survival analysis: 6 patients who had nonspontaneous (procedural) bleeding and 43 patients for whom there was no follow-up data. Overall survival in patients who experienced bleeding within 100 days after transplantation (BLD) was compared with survival of patients who did not bleed (NBLD) to learn about the long-term consequences of bleeding.
The timing relation of the bleeding event to survival endpoints (temporal association) was investigated by the interval between the last day of bleeding to outcome endpoints. The temporal analysis was assessed by three outcome intervals: death within 0 to 5 days (from the last day of bleeding), death within 6 to 30 days, and any outcome (live or death) occurring within more than 30 days. It should be noted that this interval refers to the latest significant bleeding event, however this event may not consist of the maximal scores.
Analysis of Cause of Death (COD)
All patients who died were assigned a cause of death by the BMT staff and a pathologist. Two hundred sixty-two patients (18.7%) died within 100 days after BMT (BLD and NBLD); a COD was assigned in 244 cases. In 53 cases in which the COD was not available, retrospective assessment of COD was performed by one senior physician of the BMT staff, and 258 patients were finally included in the analysis. (The 4 patients who were not included were 2 in whom data was not available and 2 for whom the COD could not be determined.) The analysis of COD was performed to learn about fatal bleeding in early mortality and about the frequency of other COD in BLD versus NBLD patients.
Clinical Data Extraction and Processing
Patients' demographics, including referring diagnosis, stage of disease, BMT type, preparative regimen, age, and sex, were obtained from the computed database of the oncology center. Bleeding was identified by the daily scores in the transfusion report of each patient and was verified by RBC transfusion history reports. In addition to the daily bleeding scores, bleeding was screened in quality assurance reports of diagnostic discharge codes. Moderate and severe episodes were reviewed for site specification by discharge summaries, relevant radiology and pathology notes, postmortem reports, discharge summary codes, and patients' charts. Outcome data for survival analysis were updated by recent follow-up, day of last contact, or death dates.
Statistical Analysis
Patients were categorized into groups based on factors including diagnosis, stage of disease, and BMT type. The group of BLD was compared with NBLD using the χ2 test. Kaplan-Meier estimates of survival41 were computed from the time of BMT, for which marrow infusion was considered day 0. Survival curves for different groups were compared using the log-rank test.42
RESULTS
Incidence
Acute bleeding was identified in 476 patients, defining an overall incidence of 34% (476/1,402). Six of the 476 patients had procedure-related bleeding without other spontaneous bleeding, and therefore were excluded from further analysis. Minor bleeding was seen in 10.6%, moderate bleeding was seen in 11.3%, and severe bleeding was seen in 12% of all BMT patients. The yearly incidence (considering 1986 through 1994) ranged from 31.8% to 42.4%, with similar yearly distribution of intensity (P = .43).
In 328 patients who experienced spontaneous moderate and severe episodes, GI bleeding was found in 61.3% (201/328), HC bleeding in 27.4% (90/328), IC bleeding in 8.8% (29/328), and pulmonary hemorrhage in 10% (33/328). DAH (29 cases) was found in 3% of allogeneic (14/469), 1.2% of autologous (11/887), and 16.7% of unrelated transplants (4/24). Multiple bleeding affected 8.8% (29/328), bleeding related to procedure (additional to other spontaneous bleeding) was found in 3.4% (11/328), other sites accounted for 14% (46/328), and in 4.6% (15 cases) the site was not identified.
Sixty-one percent (200/328) of moderate and severe episodes had bleeding from 1 site, 17.4% (57/328) had bleeding from 2 sites, 3% (10/328) had bleeding from 3 or more sites, and 6.4% (21/328) had simultaneous multiple bleeding (initiating simultaneously from multiple sites). In 40 cases the number of sites was not counted (11 cases had several sites, 14 cases could not be well described by this categorization, and the site was not identified in 15 cases). The number of sites detailed by the bleeding severity is shown in Table 2. The bleeding sites are detailed by severity (in cases of 1 site only) are shown in Table 3.
The Number of Sites Detailed by the Bleeding Severity for 288 Patients
| . | 1 Site . | 2 Sites . | 3+ Sites . | Multiple . |
|---|---|---|---|---|
| Moderate | 104 | 17 | 1 | 8 |
| Severe | 96 | 40 | 9 | 13 |
| Total | 200 | 57 | 10 | 21 |
| . | 1 Site . | 2 Sites . | 3+ Sites . | Multiple . |
|---|---|---|---|---|
| Moderate | 104 | 17 | 1 | 8 |
| Severe | 96 | 40 | 9 | 13 |
| Total | 200 | 57 | 10 | 21 |
Sixty-one percent (200/328) of moderate and severe bleeding episodes within the first 100 days after BMT had bleeding from 1 site, 17.4% (57/328) had 2 bleeding sites, 3% (10/328) had 3 or more sites, and 6.4% (21/328) had multiple bleeding (initiating simultaneously from multiple sites). Significant correlation was found between the number of sites and the bleeding severity (P = .0022).
The Main Bleeding Sites Detailed by the Bleeding Severity
| . | GI . | HC . | IC . | Pulmonary . |
|---|---|---|---|---|
| Moderate | 61 | 29 | 0 | 3 |
| Severe | 63 | 9 | 14 | 8 |
| Total | 124 | 38 | 14 | 11 |
| . | GI . | HC . | IC . | Pulmonary . |
|---|---|---|---|---|
| Moderate | 61 | 29 | 0 | 3 |
| Severe | 63 | 9 | 14 | 8 |
| Total | 124 | 38 | 14 | 11 |
For 187 cases with 1 bleeding site, significant association was found between the sites and the bleeding severity (P < .0001).
Moderate and severe bleeding was more common in allogeneic (31.0%,P < .0001) and unrelated transplants (62.5%, P < .0001) compared with autologous BMT (18.5%). Minor bleeding was found in 9.3% of allogeneic, 11.8% autologous, and 0% of unrelated patients.
Although the incidence of moderate and severe bleeding significantly varied among diagnoses (higher incidence in AML and CML [32.0% and 38.2%, respectively] and lower incidence in breast cancer [6.5%] when compared with the rest of the diseases), bleeding incidence in good- and poor-risk prognosis groups was similar within each hematologic diagnosis. Incidence of moderate and severe bleeding in good- versus poor-risk groups was, for acute lymphoblastic leukemia (ALL), 17.6% and 23.3%; for acute myeloid leukemia (AML), 32.1% and 31.8%; for CML, 37.2% and 42.4%; for HD, 30.6% and 30.4%; and for NHL, 20.1% and 27.6% respectively (in all, no significant difference).
Outcome
Bleeding was associated with significantly reduced survival (P< .0001) in an analysis of the entire BMT population. In allogeneic BMT, the median survival in NBLD (n = 260) was greater than 33 months, whereas the median in BLD (n = 184) was 3.5 months (P < .0001; Fig 1). In autologous BMT, the median survival in NBLD (n = 612) was 42.9 months, whereas in BLD (n = 270) the median was 11.0 months (P < .0001; Fig 1). The BLD allogeneic survival curve was found to be significantly different from the BLD autologous survival curve (P = .0002). In unrelated BMT, the median in NBLD (n = 9) was 12.3 months versus 1.9 months in BLD (n = 15; P = .04).
Survival estimates for allogeneic (Allo) and autologous (Auto) transplants comparing patients who bled (BLD) with patients who did not bleed within 100 days after transplantation (NBLD). In allogeneic patients, the median survival in 260 NBLD was greater than 33 months, compared with 3.5 months in 184 BLD (P < .0001). In autologous patients, median survival in 612 NBLD was 42.9 months, compared with 11 months in 270 BLD (P < .0001).
Survival estimates for allogeneic (Allo) and autologous (Auto) transplants comparing patients who bled (BLD) with patients who did not bleed within 100 days after transplantation (NBLD). In allogeneic patients, the median survival in 260 NBLD was greater than 33 months, compared with 3.5 months in 184 BLD (P < .0001). In autologous patients, median survival in 612 NBLD was 42.9 months, compared with 11 months in 270 BLD (P < .0001).
Significantly decreased survival in BLD compared with NBLD was found in each disease category, except MM. The median values of BLD and NBLD in ALL were 5.0 and 26.6 months, respectively (P = .0007); in AML, 8.3 versus 16.3 (P = .0097); and in NHL, 7.1 versus 83.3 (P < .0001). In CML, the median survival in BLD was 3.0 months, whereas 60% of NBLD lived more than 32.8 months (P < .0001). In HD, the median survival in BLD was 3.8 months, whereas 61% of NBLD lived more than 42.9 months (P < .0001). In AA, the median survival in BLD was 2.0 months, whereas 84% of NBLD lived more than 6 months (P = .0054). In breast cancer, the median survival in BLD was 12.0 months, whereas 74% of NBLD lived more than 23.1 months (P < .0001). In MM, the median in BLD was 2.4 compared with 22.0 in NBLD (P = .64); however, interpretation should consider the sample size.
The adverse effect of bleeding was shown in both good- and poor-risk prognosis within each diagnosis. Table 4summarizes the median values for each disease category for good- and poor-risk, BLD versus NBLD groups.
Median Survival for Diseases by Risk Prognosis Status in Bleeding Versus Nonbleeding Patients
| . | Good-Risk Prognosis . | Poor-Risk Prognosis . | ||
|---|---|---|---|---|
| NBLD . | BLD . | NBLD . | BLD . | |
| ALL | 17.9 | 10.9 (P = .33) | 30.5 | 3.5 (P < .0001) |
| AML | 20.6 | 11.8 (P = .055) | 13.9 | 7.8 (P = .091) |
| CML | >39 | 3.8 (P < .0001) | 7.2 | 1.8 (P = .0002) |
| HD | >87.8 | 4.0 (P < .0001) | 22.0 | 3.6 (P = .006) |
| NHL | >58.8 | 19.6 (P = .0013) | 4.1 | 2.5 (P = .0063) |
| . | Good-Risk Prognosis . | Poor-Risk Prognosis . | ||
|---|---|---|---|---|
| NBLD . | BLD . | NBLD . | BLD . | |
| ALL | 17.9 | 10.9 (P = .33) | 30.5 | 3.5 (P < .0001) |
| AML | 20.6 | 11.8 (P = .055) | 13.9 | 7.8 (P = .091) |
| CML | >39 | 3.8 (P < .0001) | 7.2 | 1.8 (P = .0002) |
| HD | >87.8 | 4.0 (P < .0001) | 22.0 | 3.6 (P = .006) |
| NHL | >58.8 | 19.6 (P = .0013) | 4.1 | 2.5 (P = .0063) |
The adverse effect of bleeding was shown in both good- and poor-risk prognosis within each diagnosis. The median is given in months. P values compare BLD and NBLD groups in each risk group.
Characteristics of Bleeding as Predictors of Survival
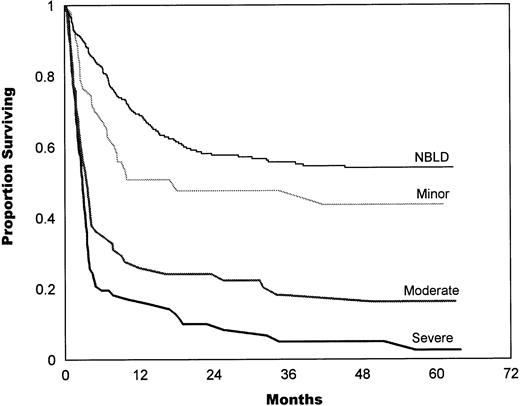
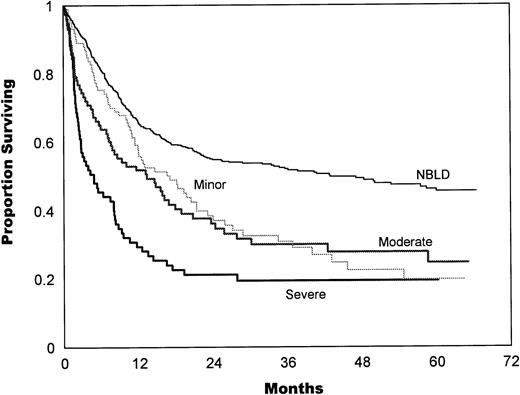
The bleeding intensity closely correlated with survival estimates for both allogeneic (Fig 2) and autologous (Fig 3) patients. The median survival in allogeneic minor BLD was 10 months, 3.5 months in moderate, and 2.8 months in severe BLD. The difference between NBLD and minor bleeding was not significant statistically (P = .089). Significant difference was found between minor versus moderate bleeding (P< .001) and between moderate and severe bleeding (P = .025). Median survival in autologous minor bleeding was 17.3 months, 13.5 months in moderate, and 5.2 months in severe bleeding. A significant difference was found between NBLD and minor bleeding (P = .0004). Minor versus moderate did not differ significantly (P = .59), but moderate versus severe bleeding was significantly different (P = .023).
In allogeneic patients, survival estimates highly correlated with bleeding intensity. The median survival in allogeneic minor bleeding was 10 months, 3.5 months in moderate, and 2.8 months in severe bleeding. The difference between NBLD and minor bleeding was not significant statistically (P = .089); a significant difference was found between minor versus moderate (P< .001) and between moderate versus severe bleeding (P= .025). NBLD, patients who did not bleed within 100 days after transplantation.
In allogeneic patients, survival estimates highly correlated with bleeding intensity. The median survival in allogeneic minor bleeding was 10 months, 3.5 months in moderate, and 2.8 months in severe bleeding. The difference between NBLD and minor bleeding was not significant statistically (P = .089); a significant difference was found between minor versus moderate (P< .001) and between moderate versus severe bleeding (P= .025). NBLD, patients who did not bleed within 100 days after transplantation.
Survival estimates by bleeding intensity in autologous patients. Median survival in minor bleeding was 17.3 months, 13.5 months in moderate, and 5.2 months in severe bleeding. Significant differences were found between NBLD and minor bleeding (P = .0004) and between moderate versus severe bleeding (P = .023). Minor versus moderate bleeding was not statistically significant (P = .59). NBLD, patients who did not bleed within 100 days after transplantation.
Survival estimates by bleeding intensity in autologous patients. Median survival in minor bleeding was 17.3 months, 13.5 months in moderate, and 5.2 months in severe bleeding. Significant differences were found between NBLD and minor bleeding (P = .0004) and between moderate versus severe bleeding (P = .023). Minor versus moderate bleeding was not statistically significant (P = .59). NBLD, patients who did not bleed within 100 days after transplantation.
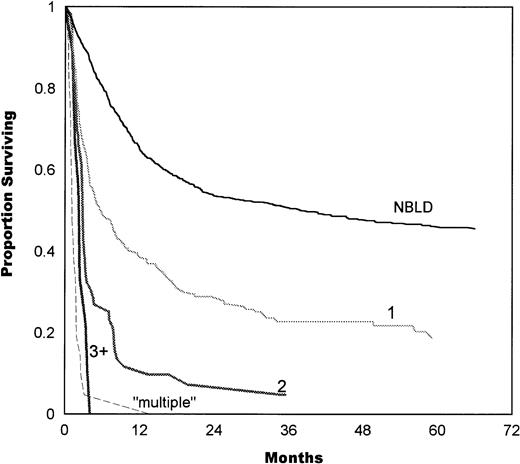
The number of bleeding sites also correlated with survival, as shown in Fig 4. Median survival in patients with 1 bleeding site was 5.7 months, compared with 2.8 months in 2 sites and 2.3 months in 3+ sites. Simultaneous multiple bleeding had a median of 1.1 months. A significant difference was found between 1 and 2 bleeding sites (P = .0001) and between 2 and multiple sites (P = .0001). The difference between 2 and 3+ sites was not significant (P = .1).
Survival estimates as a function of the number of bleeding sites. Median survival in patients with 1 bleeding site was 5.7 months, 2.8 months in 2 sites, and 2.3 months in 3+ sites. Simultaneous initiation of multiple bleeding had a median of 1.1 months. A significant difference was found between 1 and 2 bleeding sites (P = .0001) and between 2 and multiple sites (P= .0001). The difference between 2 and 3+ sites was not significant (P = .1). NBLD, patients who did not bleed within 100 days after transplantation.
Survival estimates as a function of the number of bleeding sites. Median survival in patients with 1 bleeding site was 5.7 months, 2.8 months in 2 sites, and 2.3 months in 3+ sites. Simultaneous initiation of multiple bleeding had a median of 1.1 months. A significant difference was found between 1 and 2 bleeding sites (P = .0001) and between 2 and multiple sites (P= .0001). The difference between 2 and 3+ sites was not significant (P = .1). NBLD, patients who did not bleed within 100 days after transplantation.
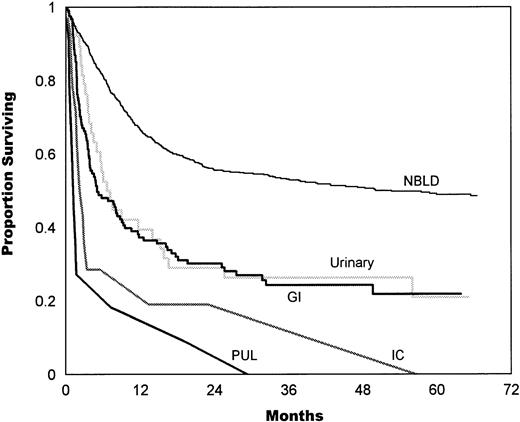
Analysis by site (in episodes of 1 site) showed that the outcome of GI bleeding and HC was not statistically different (P = .54), with median values of 5.2 and 7.2 months, respectively. Median survival in IC hemorrhage was 2.5 months and in pulmonary hemorrhage was 1.3 months (P = .18; Fig 5).
Survival estimates given by the bleeding site (in episodes of 1 site only). The median survival in GI bleeding was 5.2 months. The median survival in urinary bleeding (HC) was 7.2 months, which is not statistically different from GI bleeding (P = .54). The median survival in IC hemorrhage was 2.5 months, which is not statistically different from pulmonary (PUL) hemorrhage (1.3 months,P = .18). NBLD, patients who did not bleed within 100 days after transplantation.
Survival estimates given by the bleeding site (in episodes of 1 site only). The median survival in GI bleeding was 5.2 months. The median survival in urinary bleeding (HC) was 7.2 months, which is not statistically different from GI bleeding (P = .54). The median survival in IC hemorrhage was 2.5 months, which is not statistically different from pulmonary (PUL) hemorrhage (1.3 months,P = .18). NBLD, patients who did not bleed within 100 days after transplantation.
Analysis of the temporal association of bleeding to outcome endpoints showed that, the more severe the overall bleeding episode was, the stronger was the association to death in both autologous and allogeneic patients (Table 5A and B).
Temporal Association of Bleeding to Survival
| . | Outcome by Intervals From the Last Day of BLD Episode . | ||
|---|---|---|---|
| Death Within 0-5 d . | Death Within 6-30 d . | Alive >30 d . | |
| A. Autologous BMT | |||
| Minor BLD | 5 (4.8%) | 4 (3.8%) | 95 (91.4%) |
| Moderate BLD | 15 (17.4%) | 6 (7%) | 65 (75.6%) |
| Severe BLD | 34 (45.3%) | 2 (2.7%) | 39 (52%) |
| B. Allogeneic BMT | |||
| Minor BLD | 5 (11.9%) | 2 (4.8%) | 35 (83.3%) |
| Moderate BLD | 29 (47.5%) | 5 (8.2%) | 27 (44.3%) |
| Severe BLD | 61 (79.2%) | 4 (5.2%) | 12 (15.6%) |
| . | Outcome by Intervals From the Last Day of BLD Episode . | ||
|---|---|---|---|
| Death Within 0-5 d . | Death Within 6-30 d . | Alive >30 d . | |
| A. Autologous BMT | |||
| Minor BLD | 5 (4.8%) | 4 (3.8%) | 95 (91.4%) |
| Moderate BLD | 15 (17.4%) | 6 (7%) | 65 (75.6%) |
| Severe BLD | 34 (45.3%) | 2 (2.7%) | 39 (52%) |
| B. Allogeneic BMT | |||
| Minor BLD | 5 (11.9%) | 2 (4.8%) | 35 (83.3%) |
| Moderate BLD | 29 (47.5%) | 5 (8.2%) | 27 (44.3%) |
| Severe BLD | 61 (79.2%) | 4 (5.2%) | 12 (15.6%) |
The intervals between the last day of bleeding to outcome estimates (death within 30 days or any outcome longer than 30 days) showed that, the more severe the overall bleeding episode was, the stronger was the timing association to death, as analyzed for 275 autologous and 180 allogeneic patients.
Of 258 patients who died within 100 days after transplantation, 175 were BLD (21 minor, 59 moderate, and 92 severe) and 87 patients were NBLD. Bleeding was listed as the COD in 13 of 258 (5%): 12 patients who were identified as BLD and 1 patient who was classified as NBLD (a patient who relapsed and died at home). Death was commonly attributed to infections (26.3% of BLD and 18.4% of NBLD), interstitial pneumonia (12% BLD and 11.4% NBLD), and regimen-related toxicity (14.3% BLD and 19.3% NBLD) in both autologous and allogeneic patients. Early relapse was seen in 31.8% of NBLD and in 8.6% of BLD patients. Graft-versus-host disease (GVHD) as main COD was significantly higher in allogeneic BLD (35.6%) compared with allogeneic NBLD (11.1%).
DISCUSSION
Investigation of bleeding after BMT has been usually approached by site-specific studies. The current study summarizes the extent and clinical significance of acute bleeding of all sites as studied in a population of 1,402 patients receiving transplants during the last 10 years at a single institution. Acute bleeding within 100 days after BMT was found to be a common complication with profound association to reduced survival.
Bleeding Incidence
The incidence by site of moderate and severe bleeding of 14.4% GI bleeding, 6.4% HC, 2.8% pulmonary hemorrhage, and 2% IC hemorrhage is within the range of reports found in the literature,2,6,15,18,19,29,30 although comparison should consider different methodologies in bleeding definitions (especially with HC), time span from transplantation, and the structure of populations. The report by Tornebohm et al31 of a 19% incidence of terminal hemorrhages (considering all bleeding sites) is close to the 24% of moderate and severe bleeding in our study.
Although the analysis of the number of sites showed that 60% of moderate and severe episodes had a solitary bleeding source, in some of these cases additional minor bleeding was noticed in sites other than the main bleeding site. These bleeding events were not represented in the count of sites. Hence, it is possible that bleeding in many cases is a symptom of systemic underlying disorder rather than a strictly local complication, reflecting a correlation between the extent of underlying damage and outcome after transplantation. Epithelial and endothelial cell damage in BMT patients was previously suggested through different mechanisms.26,33,34 43-46 Further investigation considering all bleeding sites is needed to explore this direction in the pathophysiology of bleeding.
Conflicting association of bleeding with marrow source was previously reported.1,6-10,15,19-21,24,25,27,30,31 The higher incidence in allogeneic and unrelated BMT compared with autologous transplants should be further investigated in relation to GVHD and infectious complications during profound immunosuppression. GVHD in association with bleeding was reported with conflicting conclusions,2,3,8,9,33 47-50 and this is an area we study further.
Within each hematologic diagnosis, no association was noticed between bleeding incidence and status of disease. These findings suggest that bleeding does not simply represent the high-risk individuals on the basis of disease status. Therefore, the association of bleeding with reduced survival, as observed in this study, cannot be attributed to the poor prognosis patients groups.
Outcome of Bleeding After BMT
The association of bleeding to reduced outcome was evident by the analysis of overall survival in BLD compared with NBLD and by the strong association to mortality in the temporal analysis. Nevertheless, hemorrhage was listed as a primary COD in only the minority of BLD. The relatively low incidence of fatal bleeding compared with the incidence of clinically moderate and severe bleeding may reflect that bleeding in most cases was considered secondary to other major complication. In these cases, the association to mortality is probably combined with influences of coexisting acute complications. This hypothesis is compatible with our clinical impression that most deaths cannot be simply attributed to the bleeding episode. Nevertheless, a major consequence of this study is that bleeding is a potential indicator of patients at risk and a prognostic factor for poor survival.
The differentiative pattern of reduced survival defined by bleeding was shown with survival analysis by BMT type, disease, and stage of disease. The significant difference between BLD allogeneic and autologous survival curves may reflect the influence of acute GVHD,51-54 which was found frequent in BLD in the COD analysis, and may correlate with differences in overall early toxicity between allogeneic and autologous transplantations.55-58
Bleeding occurrence had changed patients initial categorization of risk in each disease category. For example, in AML, BLD good-risk patients had similar outcome as NBLD poor-risk patients. In CML and HD, BLD good-risk patients had even worse outcome compared with NBLD poor-risk patients. Only in NHL, good-risk patients (BLD and NBLD) had consistently better survival than poor-risk patients, although bleeding significantly reduced survival in both groups. ALL poor-risk NBLD patients had better survival than good-risk patients (BLD and NBLD), which may reflect the influence of age on survival (48 of 74 poor-risk patients were pediatric, compared with 6 of 51 good-risk patients). The bleeding poor-risk patients in all diseases had the worst survival. These results suggest that bleeding, and possibly risk factors for bleeding, once identified, should be considered in clinical evaluation of patient risk after BMT.
The adverse effect of bleeding on survival was predicted by the bleeding intensity, the number of sites, and the bleeding source, which were found to be highly correlated variables. The differences in early and long-term survival between minor, moderate, and severe episodes indicate the clinical relevance of bleeding grading, as was defined in this study. Whereas moderate and severe bleeding were associated with early mortality, it is of interest that minor bleeding was also an indicator of poor prognosis.
Analysis by the number of sites suggests that outcome assessment should account for all expressions of bleeding, because significant differences in survival were found between 1, more than 1 site, and simultaneous multiple bleeding.
Because most outcome assessments of bleeding in the literature relate to the immediate course after the cessation of bleeding episode, the effect on overall survival is not always clear. In the cases of DAH and IC parenchymal hemorrhage, grave outcome immediately affected survival, as reported earlier.15,16,24 However, this study associates GI bleeding and HC (the last rarely terminates with death) with reduced survival. This association with HC was previously observed by Yang et al.10 Thus, we conclude that bleeding from all sites is associated with reduced survival.
Bleeding was infrequently discussed in reviews of acute complications after BMT.59-62 Possible etiologies and risk factors, such as preparative regimen toxicity, marrow source, thrombocytopenia, coagulation disorders, infections, GVHD (in part previously mentioned), and more, were previously discussed.1,3,8,9,17,18,24,26,30,32,49 63-66 The spectrum of bleeding that is included in this study probably represents many of these causes. The COD analysis shows some of the diverse complications coexisting in BLD; however, this study has initially concentrated on defining the extent and significance of acute bleeding. Further exploration of potential mechanisms is essential to the understanding of whether bleeding is a specific or nonspecific process in clinical deterioration after transplantation and to the study of the association with early mortality.
The association of bleeding to reduced survival may also be tested in relation to adverse effects of allogeneic blood transfusions. Transfusion reactions, associated infections, and alloimmunization are among complications that potentially can contribute to reduced outcome. Transfusion-induced immunomodulation after perioperative transfusions has been suggested in association with reduced survival and recurrence of malignancy.67-70 However, the role of transfusion-related complications in the course of BLD necessitates careful analysis, because all patients after transplantation are heavily transfused.
Study Limitations
The scoring methodology, although assigned by different personnel, is based on well-defined criteria and was practiced consistently over the study period. The final categorization into mild, moderate, and severe episodes was based on the pattern of intensity in accordance with clinical reports. Consequently, a different grading scheme may result in somewhat different incidence, yet the profound differences in survival of the three bleeding grades, compared with NBLD and with each other, strongly support the adequacy of our grading scheme. Bleeding categorization did not include parameters of patients outcome; hence, the association with reduced survival was not biased by our definition methodology.
Because the daily scores are assigned to hospitalized patients, outpatients may have potentially been underdetected for minor bleeding. However, outpatients experiencing significant bleeding were hospitalized, re-entering the scoring follow-up. Therefore, we believe that scores 3 and 4, generally described as moderate and severe bleeding, are accurately reported. Fatal bleeding may be underestimated if bleeding occurred in nonhospitalized patients or when the terminal bleeding event occurred after the last score was recorded. In this case, autopsy study in NBLD would review the extent of terminal hemorrhage and may result in possibly higher association of bleeding to death.
The importance of our results primarily relates to patients' care. If bleeding is a marker of multifactorial clinical deterioration, protracted supportive care to prevent bleeding would be futile. However, if bleeding has a direct role in deterioration, aggressive treatment against underlying mechanisms of bleeding may yield a significant improvement in survival after BMT.
ACKNOWLEDGMENT
The authors thank Dr Michael Grever, Kongyan Pan, Adriane Hill, Henri Hammond, Regina White, Joyce Kane, Vanessa Williams, Dorothy Williams, Felicia Reeder, and the members of the blood transfusion service for their own individual contributions for this study.
Supported in part by National Cancer Institute Grant No. CA15386.
Address reprint requests to G.B. Vogelsang, MD, Johns Hopkins Oncology Center, 600 N Wolfe St, Room 3-121, Baltimore, MD 21287-8985.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal