Abstract
Defining how the stromal requirements of hematopoietic progenitors change during leukemia progression is an important topic that is not well understood at present. The murine ELM erythroleukemia is an interesting model because the erythroid progenitors retain dependence on bone marrow-derived stromal cells for long-term growth in vitro, and they also undergo erythroid differentiation in the presence of erythropoietin (EPO) and interleukin-3 (IL-3). In this report, we have shown using neutralizing antibodies that stem cell factor (SCF), insulin-like growth factor (IGF)-1, and integrin signaling pathways are all involved. We then determined whether ELM cells can be maintained long-term without stroma in various combinations of growth factors produced by stroma cells or growth factors for which ELM cells have receptors. This showed that ELM cells could be maintained with high efficiency in SCF alone; furthermore, the cells remained absolutely SCF-dependent and did not become more tumorigenic than cells maintained on stroma. In contrast, ELM cells underwent clonal extinction when serially cloned in IGF1; any cells that survived long-term growth in IGF-1 were found to be IGF1-independent. One important difference between maintaining ELM cells on stroma and growth in SCF is that stroma reversibly inhibits their differentiation in response to EPO and IL-3, whereas SCF does not.
STROMAL CELL interactions are known to be important for maintenance and growth of hematopoietic stem cells and progenitors in vivo.1,2 Defining the stromal signals involved is therefore important both for understanding the regulation of hematopoietic cell homeostasis and, practically, for devising methods for ex vivo amplification of the different hematopoietic cell compartments for transplantation and protection against hematopoietic failure after chemotherapy. Perhaps the best characterized system is that of T-cell production, where activation of T cells by antigen-presenting cells requires activation of the T-cell receptor in conjunction with integrin signaling.3-5 In the erythroid lineage, the importance of the stem cell factor (SCF) receptor/SCF interaction has been demonstrated by the anemia-inducing effects of theW and Sl mutations in mice, affecting SCF receptor6 and SCF7 expression, respectively. In contrast, many leukemic cells, in particular, most murine retrovirus-induced erythroleukemias, have lost the stromal requirement of their normal counterparts and grow readily in suspension culture, often without added growth factors other than those present in serum; they are also usually arrested in differentiation and no longer responsive to erythropoietin (EPO) due to activation of the EPO receptor by the retrovirus-encoded gp55 glycoprotein.8-10
The murine ELM erythroleukemia model is unusual in having a less abnormal leukemic phenotype. We have shown, for example, that the cells will not grow in the regular suspension culture medium suitable for other erythroleukemia cells, but require direct contact with marrow-derived stromal cells for long-term growth in vitro.11-14 ELM cells also undergo erythroid differentiation in response to EPO and interleukin-3 (IL-3).15 Moreover, stroma independent variants can be obtained and we have shown that these have elevated expression of members of the ets family of transcription factors/oncogenes.16 The aim of the present work was to identify the stroma signaling processes that are critical for long-term ELM cell growth and determine whether these could be substituted by a combination of soluble growth factors for which ELM cells have receptors in conjunction with extracellular matrix (ECM) components.
MATERIALS AND METHODS
Cells.
ELM cells12 were grown on unirradiated or irradiated (60 Gy) MS5 cells13 or the Sl-/Sl- stromal cell line, UNC,17kindly provided by Dr C. Eaves (Terry Fox Laboratory, British Columbia Cancer Agency, Vancouver, Canada). Stroma and ELM cells were routinely passaged in alpha minimal essential medium (α-MEM; GIBCO, Paisley, Scotland) containing 8% horse serum (Sigma, Poole, Dorset, UK) and 4 mmol/L glutamine. In some cases, cells were grown in protein-free hybridoma medium (GIBCO; cat. No. 12040-051).
Growth factors.
All murine (SCF, IL-3, granulocyte-macrophage colony-stimulating factor [GM-CSF], colony-stimulating factor [CSF]-1, and IL-6) and human (IGF-1, IGF-2, and IL-11) recombinant growth factors were obtained from R & D Systems (Abingdon, UK), except for insulin, which was obtained from Sigma. Optimal concentrations of growth factors were determined in short-term proliferation assays. A total of 104 ELM cells were freshly removed from stroma and incubated in microtiter wells with increasing concentrations of growth factor and 5 days later proliferation was assayed using the Promega MTT kit (Promega, Madison, WI). The concentrations of growth factors used in all other experiments were the minimum found to give the optimal stimulation of growth in these assays (SCF, 10 ng/mL; IGF-1, 100 ng/mL; insulin, 10 μg/mL; IGF-2, 100 ng/mL; IL-3, 120 U/mL). Other growth factors were used at the following concentrations: GM-CSF, 1 ng/mL; CSF-1, 10 ng/mL; IL-6, 1 ng/mL; IL-11, 1 ng/mL.
Removal of ELM cells from MS5 stromal cells.
Fresh medium was added to flasks containing ELM cells growing on MS5 feeder layers and the flasks were shaken vigorously for a few seconds. Most of the ELM cells became detached from the stromal layer and were decanted into a fresh flask. To remove any contaminating MS5 cells, the cells were allowed to settle for 30 to 60 minutes, when any residual MS5 cells adhered to the plastic, whereas the ELM cells remained unattached. To ensure removal of all MS5 cells, this procedure was repeated two to three times. To check for contamination of ELM cells with MS5 stromal cells, purified ELM cells were plated out in a flask and the numbers of stromal cell colonies counted 2 weeks later. Stromal cell colonies are unmistakably different from ELM cells in morphology. Typically, 106 purified ELM cells gave 10 to 20 stromal cell colonies in this assay. In some experiments, ELM cells were passaged on MS5 cells that had been lethally irradiated (60 Gy Co60) to ensure that pure populations of ELM cells had been obtained.
Clone-serial transfer experiments.
ELM cells were cloned on irradiated MS5 cells by end-point dilution in 96-well plates and after 2 to 3 weeks when the colonies contained about 105 cells, they were serially passaged 1:10 in microtiter plates at weekly intervals in medium containing various growth factors.
Clone-clone transfer experiments.
ELM cells growing in culture were removed from stroma as described above, centrifuged, and resuspended in fresh medium. The cells were cloned by end-point dilution every 21 days on irradiated stroma or in medium containing growth factors.
Antibody inhibition experiments.
The anti–IGF-1 and anti-integrin antibodies were obtained from Pharmingen (San Diego, CA). The anti–SCF-receptor antibody (ACK2) has been described previously.18 ELM cells were plated out on MS5 cells in wells in Terasaki plates (100 ELM and 200 MS5 in 20 μL medium) and incubated with antibody (30 μg/mL). Plates were incubated for 5 days at 37°C, 5% CO2 in a humidified incubator and the number of ELM cells counted under a light microscope. Cell counts were averaged from at least eight wells for each sample.
Preparation of extracellular matrix (ECM) in situ.
ECM was prepared using the method described by Scott-Burden et al.19 Briefly, MS5 cells were plated out in flasks or microtiter plates and allowed to grow for 3 to 5 days until they became confluent. The cells were then washed twice with phosphate-buffered saline (PBS), once with water, and the cells were then lysed in 25 mmol/L NH4OH for 5 minutes at 4°C. The plates were washed three times with PBS to remove any cell debris and washed once with culture medium before plating of cells.
Tumorigenicity experiments.
ELM cells were injected via the tail vein into groups of 15 previously irradiated (3 Gy Co60) C3H mice. Mice were checked twice daily and the experiment terminated (usually after about 15 days) when the first signs of ill health were apparent due to enlarged spleens. Spleens were then dissected and individually weighed.
Detection of IGF-1 receptors by IGF-1 binding.
The IGF-1 binding assay was performed as described previously,20 with modifications. Binding of IGF-1 to cells was performed in suspension. A total of 5 × 105 cells were incubated with 50,000 cpm 125I IGF-1 (Amersham, Little Chalfont, UK) and increasing concentrations of unlabeled IGF-1 (0 to 1,000 ng/mL) in a final volume of 0.25 mL of binding medium (α-MEM, 25 mmol/L [4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid] pH8.0, 0.1% bovine serum albumin) at 4°C for 18 hours. Cells were washed twice in binding medium and cell-associated125I IGF-1 counted in a gamma counter. Nonspecific binding was assessed in the presence of unlabeled IGF-1 at 1,000 ng/mL. Scatchard analysis was performed using the Cricket Graph computer program (Computer Associates International, Islandia, NY).
Northern analysis.
RNA was extracted using Trizol (GIBCO) and analyzed by Northern transfer and hydridization with radioactive probes as described previously.16
Western blotting.
Activation of mitogen-activated protein (MAP) kinases was assayed by Western blotting of whole cell lysates using a polyclonal antibody (Promega) with specificity for active, dually phosphorylated forms of MAP kinases (p44/p42 [ERK1/ERK2]). IGF-1–receptor levels were determined by Western blotting with a polyclonal antibody against the IGF-1 receptor β subunit (Santa Cruz, Santa Cruz, CA). The manufacturer's instructions were followed for preparation of lysates and Western blotting. Cells were lysed in 50 mmol/L Tris pH 6.8, 100 mmol/L dithiothreitol, 2% sodium dodecyl sulfate (SDS), 0.1% bromophenol blue, 10% glycerol, fractionated by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred onto ECL nitrocellulose membrane (Amersham). Immunodetection was performed by enhanced chemiluminescence (ECL, Amersham).
RESULTS
Our previous studies have shown that the murine stromal cell line, MS5, will support long-term growth of the stroma-dependent ELM cells, whereas in the absence of stromal cells, ELM cells die within 7 to 14 days.14 To ensure that pure populations of ELM cells could be obtained from the cocultures, the MS5 cells were normally lethally irradiated before use. To determine which growth factor signaling pathways might be involved in growth of ELM cells on stroma, we tried to grow ELM cells without stroma in the presence of growth factors for which ELM cells have receptors, or with growth factors produced by the stromal cells, as judged by semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR) experiments (summarized in Table 1). Because the interaction of ELM cells with extracellular matrix components may also be involved, we determined by histocytochemistry which integrins are expressed on the surface of the cells. Integrin α4β1 was present on ELM cells and α5β1 was detected on MS5 stroma cells, whereas αM, αL, and β2 were absent (data not shown).
Expression of Growth Factors and Their Receptors by ELM Cells and MS5 Stroma Cells
| ELM cells | |
| Growth factor receptors | |
| Expressed | SCF, IGF-1, IL-6, EPO, insulin, IL-3/GM-CSF/IL-5 (α + β) chain |
| Not expressed | PDGF (α + β) chain, CSF-1, IGF-2 |
| MS5 stroma cells | |
| Growth factors | |
| Expressed | IL-6, CSF-1, IGF-1, SCF |
| Not expressed | GM-CSF, IL-3, IGF-2 |
| ELM cells | |
| Growth factor receptors | |
| Expressed | SCF, IGF-1, IL-6, EPO, insulin, IL-3/GM-CSF/IL-5 (α + β) chain |
| Not expressed | PDGF (α + β) chain, CSF-1, IGF-2 |
| MS5 stroma cells | |
| Growth factors | |
| Expressed | IL-6, CSF-1, IGF-1, SCF |
| Not expressed | GM-CSF, IL-3, IGF-2 |
Short-term growth of ELM cells in growth factors and extracellular matrix.
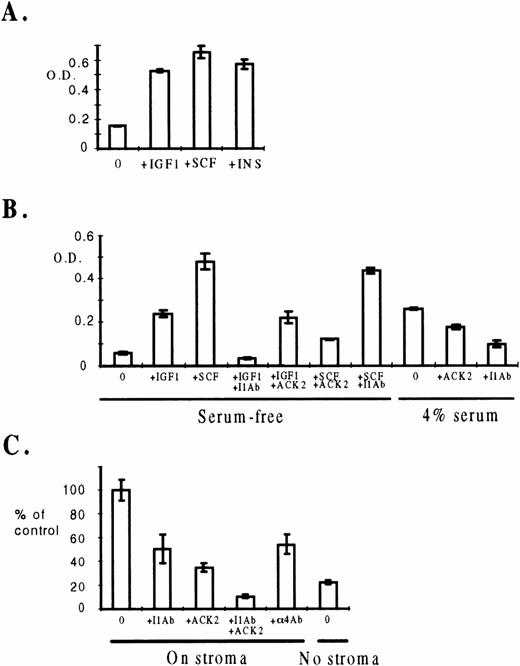
We then measured the growth of ELM cells, after removal from stroma cells, in each of the candidate growth factors (IL-6, CSF-1, IGF-1, IGF-2, SCF, insulin, IL-3, GM-CSF, IL-11, and EPO), using a range of growth factor concentrations in short-term proliferation (MTT) assays. In serum-containing medium, the only growth factors that allowed ELM cells to proliferate over a 5-day period were SCF, IGF-1, and insulin (Fig 1A) (and to a lesser extent IGF-2 and IL-3, data not shown). However, it should be noted that the level of insulin required to support ELM growth was 100-fold higher than the level of IGF-1 required, suggesting that the IGF-1 receptor is more important than the insulin receptor in supporting ELM growth by IGF-1 or insulin. It is well established that insulin can bind to the IGF-1 receptor with low-affinity.20 21 The same growth factors also stimulated ELM growth in serum-free medium for 5 days (Fig 1B, data for insulin not shown). Addition of 4% serum without extra growth factors stimulated ELM growth to a limited extent (Fig 1B); this is probably mainly due to IGF-1 because neutralizing antibodies to IGF-1 (I1Ab) reduced growth of ELM cells in serum alone, whereas antibodies against the SCF-R (ACK2) had minimal effect (Fig 1B). The antibodies were specific as shown by the fact that each antibody only reduced the growth of ELM cells in serum-free medium stimulated by the growth factor against which the antibody was raised (Fig 1B). The effect of ECM was tested by preparing ECM in situ from stroma cells growing in the assay wells before adding the ELM cells. However, ECM did not stimulate proliferation or enhance proliferation of ELM cells by any of the growth factors (data not shown).
Growth factor requirements for short-term growth of ELM cells in the presence or absence of stroma. (A) Effect of growth factors on the growth of ELM cells in serum-containing medium after removal from MS5 stroma cells. A total of 104 ELM cells were plated in 96-well plates in medium containing 8% horse serum in the presence of increasing amounts of IGF-1, insulin, or SCF. Proliferation under these conditions was assayed by the MTT method and data shown are mean values from three independent experiments (12 cultures) with standard errors. The data shown are for the minimum amounts of growth factors found to give the maximal stimulation (SCF, 10 ng/mL; IGF-1, 100 ng/mL; insulin, 10 μg/mL). (B) Effect of growth factors and neutralizing antibodies on the growth of ELM cells in serum-free medium or 4% serum. A total of 104 ELM cells were plated in 96-well plates in serum-free medium in the presence of 100 ng/mL IGF-1 or 10 ng/mL SCF and 30 μg of neutralizing antibodies to IGF-1 (I1Ab) or the SCF-R (ACK2). Proliferation under these conditions was assayed by the MTT method and data shown are mean values from three independent experiments (12 cultures) with standard errors. (C) Effect of antibodies against IGF-1, SCF, or integrins on the growth of ELM cells on MS5 stroma cells. A total of 100 ELM cells were plated on 200 MS5 cells in wells in Terasaki plates and incubated for 5 days in the presence of 30 μg/mL neutralizing antibodies to SCF-R (ACK-2), IGF-1 (I1Ab), or α4 integrin (α4Ab). The right-hand panel shows the growth of ELM cells without stroma. The results are the number of cells in the wells relative to the number on stroma alone and represent the mean of three independent experiments (24 cultures) with standard errors.
Growth factor requirements for short-term growth of ELM cells in the presence or absence of stroma. (A) Effect of growth factors on the growth of ELM cells in serum-containing medium after removal from MS5 stroma cells. A total of 104 ELM cells were plated in 96-well plates in medium containing 8% horse serum in the presence of increasing amounts of IGF-1, insulin, or SCF. Proliferation under these conditions was assayed by the MTT method and data shown are mean values from three independent experiments (12 cultures) with standard errors. The data shown are for the minimum amounts of growth factors found to give the maximal stimulation (SCF, 10 ng/mL; IGF-1, 100 ng/mL; insulin, 10 μg/mL). (B) Effect of growth factors and neutralizing antibodies on the growth of ELM cells in serum-free medium or 4% serum. A total of 104 ELM cells were plated in 96-well plates in serum-free medium in the presence of 100 ng/mL IGF-1 or 10 ng/mL SCF and 30 μg of neutralizing antibodies to IGF-1 (I1Ab) or the SCF-R (ACK2). Proliferation under these conditions was assayed by the MTT method and data shown are mean values from three independent experiments (12 cultures) with standard errors. (C) Effect of antibodies against IGF-1, SCF, or integrins on the growth of ELM cells on MS5 stroma cells. A total of 100 ELM cells were plated on 200 MS5 cells in wells in Terasaki plates and incubated for 5 days in the presence of 30 μg/mL neutralizing antibodies to SCF-R (ACK-2), IGF-1 (I1Ab), or α4 integrin (α4Ab). The right-hand panel shows the growth of ELM cells without stroma. The results are the number of cells in the wells relative to the number on stroma alone and represent the mean of three independent experiments (24 cultures) with standard errors.
Signaling pathways required for proliferation of ELM cells on stromal cells.
To determine whether any of the growth factors shown to stimulate short-term proliferation of ELM cells in the absence of stroma are important for their growth on stroma, the effects of neutralizing antibodies to the SCF-R, IGF-1, or integrin α4β1 were determined on ELM growth in cocultures with stroma cells. Antibodies against IGF-1 or α4 integrin each reduced the growth of ELM cells after 5 days in culture on stroma cells by about 50%. Antibodies against the SCF-R had a slightly greater effect (about 60% reduction) (Fig 1C), as we have previously shown.14 The use of antibodies to IGF-1 and SCF-R together reduced the growth of ELM cells below the level found in the absence of stroma cells (Fig 1C). These results imply that integrin, SCF, and IGF-1 signaling pathways are required for optimal growth of ELM cells on stromal cells.
Long-term growth of ELM cells in the absence of stromal cells.
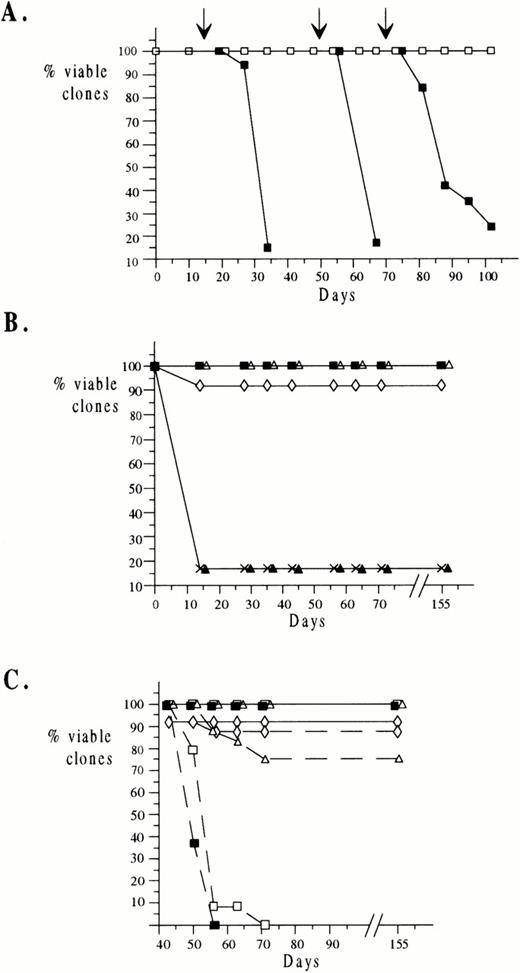
To try to determine whether any combination of growth factors could substitute for stroma in maintaining long-term growth of ELM cells, we first grew ELM cells on extracellular matrix prepared from stroma cells in a complex mixture of growth factors. To allow for any synergistic interactions between growth factors, the nine growth factors selected (IGF-1, IGF-2, insulin, SCF, GM-CSF, CSF-1, IL-3, IL-6, IL-11) were those to which ELM cells had shown a proliferative response in short-term assays plus any other growth factors for which ELM cells have receptors, even if they did not respond to the growth factor on its own. The concentrations of growth factors used were the minimum amounts found to give the optimal stimulation of growth in short-term assays when used individually (see Materials and Methods) or, in the case of growth factors that did not stimulate ELM growth individually, the concentrations shown to be optimal for growth of other cell types (details in the Materials and Methods). Initially, 30 clones of ELM cells were isolated on irradiated stroma cells by end-point dilution in microtiter plates. When the clones had reached 104 to 105 cells in size, they were diluted and one tenth of each clone was tested for growth under different conditions by weekly 1:10 serial passage, either on irradiated stromal cells or in the mixture of the nine growth factors in wells containing extracellular matrix from MS5 cells. We found that ELM cells could be maintained indefinitely (>200 days) under both of these conditions. During this period, the cells remained dependent on the growth factor/ECM mix for long-term growth, as removal of the growth factors at day 15, 50, and 70 resulted in the rapid death of the cells (Fig 2A). We also attempted to grow ELM cells long-term in the mixture of growth factors in serum-free medium (protein-free hybridoma medium), but the cells died after about 5 weeks (data not shown).
Long-term growth of ELM cells in growth factors. A total of 30 clones of ELM cells were isolated as described in Materials and Methods and transferred to wells in 96-well microtiter plates containing various growth factors and/or ECM and passaged 1:10 weekly. The viability of the clones was recorded before each passage. (A) ELM clones were transferred to wells preplated with MS5 ECM and a cocktail of growth factors added (IGF-1, IGF-2, insulin, SCF, IL-3, IL-6, IL-11, GM-CSF, and CSF1) (□). At 15 days, 50 days, and 70 days (arrows), growth factors and ECM were removed and the effect on viability of the clones monitored (▪). (B) A total of 30 ELM clones were passaged in a single growth factor (IGF-1 (◊), IGF-2 (▴), insulin (▵), SCF (▪), and IL-3 (crosses)) and clone viability monitored. (C) After clones had been maintained for 45 days in IGF-1 (◊), insulin (▵), SCF (▪), and IGF-1+SCF (□) as described in (B), they were either passaged 1:10 weekly in the same growth factors until day 155 (solid lines) or in their absence (broken lines).
Long-term growth of ELM cells in growth factors. A total of 30 clones of ELM cells were isolated as described in Materials and Methods and transferred to wells in 96-well microtiter plates containing various growth factors and/or ECM and passaged 1:10 weekly. The viability of the clones was recorded before each passage. (A) ELM clones were transferred to wells preplated with MS5 ECM and a cocktail of growth factors added (IGF-1, IGF-2, insulin, SCF, IL-3, IL-6, IL-11, GM-CSF, and CSF1) (□). At 15 days, 50 days, and 70 days (arrows), growth factors and ECM were removed and the effect on viability of the clones monitored (▪). (B) A total of 30 ELM clones were passaged in a single growth factor (IGF-1 (◊), IGF-2 (▴), insulin (▵), SCF (▪), and IL-3 (crosses)) and clone viability monitored. (C) After clones had been maintained for 45 days in IGF-1 (◊), insulin (▵), SCF (▪), and IGF-1+SCF (□) as described in (B), they were either passaged 1:10 weekly in the same growth factors until day 155 (solid lines) or in their absence (broken lines).
We then repeated the experiment using a less complex mixture of growth factors comprising only the five growth factors to which ELM cells respond in short-term proliferative assays (IGF-1, IGF-2, insulin, SCF, and IL-3). In fact, the ELM clones maintained in the growth factors and ECM grew almost as well as those maintained on stromal cells, and the removal of ECM made little difference (data not shown).
To determine whether any of the growth factors used alone would permit long-term growth of the cells in the absence of stroma, another set of 30 ELM clones was serially passaged for 155 days individually in IGF-1, IGF-2, insulin, SCF, or IL-3 (Fig 2B). A total of 90% to 100% of the clones passaged in SCF, insulin, or IGF-1 survived long-term (Fig 2B, filled squares, open triangles, and open diamonds, respectively). However, clones passaged in IL-3 (Fig 2B, crosses) or IGF-2 (Fig 2B, filled triangles) died within 2 weeks, as did clones in medium containing no growth factors (data not shown). To test whether the ELM cells grown in SCF, IGF-1, or both remained dependent on these growth factors, after ELM cell clones had been grown for 45 days in a particular growth factor, the growth factor was removed and the cells were serially passaged in its absence. This indicated that ELM cells previously grown for 45 days in SCF (Fig 2C, filled squares) and SCF+IGF-1 (Fig 2C, open squares) died rapidly upon removal of growth factor(s) (broken lines). However, surprisingly, most of the clones grown in IGF-1 (Fig 2C, open diamonds/broken line) or insulin (Fig 2C, open triangles/broken line) continued to proliferate after growth factor removal.
Cloning efficiency of ELM cells in SCF and IGF-1 compared with stroma.
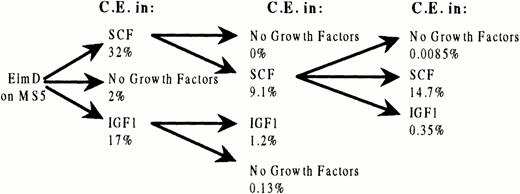
To evaluate more rigorously the ability of SCF to maintain ELM cells and to elucidate how IGF-1–independent cells arise after long-term growth in IGF1, we measured the cloning efficiencies of ELM cells were serially recloned in either IGF-1 or SCF. ELM cells serially cloned in SCF with high efficiency and remained SCF-dependent through three rounds of cloning (Fig 3). However, ELM cells from the third round of serial cloning in SCF were found to have lost responsiveness to IGF-1, in contrast to ELM cells freshly removed from stroma (Fig 3). The same change was also found in ELM cells that were serially passaged in mass culture for several months in SCF or SCF plus IGF-1 (data not shown). The reasons for this change in IGF-1 responsiveness are unknown, but it is not due to downregulation of IGF-1–receptor levels or affinities (see below). As far as serial cloning in IGF-1 is concerned, although ELM cells cloned with about half the cloning efficiency as in SCF in the first round of cloning (Fig 3), the cloning efficiency of the cells after two rounds of cloning in IGF-1 declined dramatically (Fig 3). These results imply that SCF is the only growth factor that, by itself, can support indefinite growth or serial recloning of ELM cells.
Cloning efficiency of ELM cells repeatedly cloned in SCF or IGF-1. ELM cells cloned on stroma (30 clones) were cloned in 10 ng/mL SCF, 100 ng/mL IGF-1, or no growth factors and the cloning efficiency measured. Five clones surviving in each condition were then picked and recloned again in the same growth factors. In the case of cells cloned twice in SCF, the cloning in SCF or IGF-1 or no growth factors was repeated a third time.
Cloning efficiency of ELM cells repeatedly cloned in SCF or IGF-1. ELM cells cloned on stroma (30 clones) were cloned in 10 ng/mL SCF, 100 ng/mL IGF-1, or no growth factors and the cloning efficiency measured. Five clones surviving in each condition were then picked and recloned again in the same growth factors. In the case of cells cloned twice in SCF, the cloning in SCF or IGF-1 or no growth factors was repeated a third time.
The importance of SCF for long-term maintenance of ELM cells was confirmed by cloning on SCF-deficient Sl/Sl stroma. Any clones that formed tended to be very small and overall cloning efficiency was much lower than on normal stroma, unless SCF was added (Table 2). Moreover, ELM cells surviving long-term on Sl/Sl stroma became stroma-independent, and yet they did not clone in SCF (Table 2). These altered properties resemble those of ELM cells that survived long-term selection in IGF-1. ELM cells also clone with only very low efficiency on Sl/Sld stroma, which expresses only the soluble form of SCF (data not shown). We presume this is because the cells do not produce sufficient SCF to permit optimal growth/cloning of ELM cells.
Cloning of ELM Cells on NormalSl/Sl Stroma
| . | % Cloning Efficiency . |
|---|---|
| Cells transferred from MS5 stroma to: | |
| MS5 | 36.5 |
| MS5 + SCF | 44.5 |
| Sl/Sl stroma | 0.1 |
| Sl/Sl stroma + SCF | 49 |
| SCF | 32.5 |
| No growth factors | 2 |
| Cells transferred from Sl/Sl stroma to: | |
| MS5 | 30.5 |
| Sl/Sl stroma | 41 |
| Sl/Sl stroma + SCF | 54 |
| SCF | 0.4 |
| No growth factors | 5.5 |
| . | % Cloning Efficiency . |
|---|---|
| Cells transferred from MS5 stroma to: | |
| MS5 | 36.5 |
| MS5 + SCF | 44.5 |
| Sl/Sl stroma | 0.1 |
| Sl/Sl stroma + SCF | 49 |
| SCF | 32.5 |
| No growth factors | 2 |
| Cells transferred from Sl/Sl stroma to: | |
| MS5 | 30.5 |
| Sl/Sl stroma | 41 |
| Sl/Sl stroma + SCF | 54 |
| SCF | 0.4 |
| No growth factors | 5.5 |
ELM cells grown on MS5 stroma were cloned on MS5 cells or SCF-deficient Sl/Sl stroma (UNC) either in the presence or absence of 10 ng/mL SCF. ELM cells grown on Sl/Sl stroma were cloned in SCF, IGF-1, or no growth factors.
Mechanisms of IGF-1–independent growth.
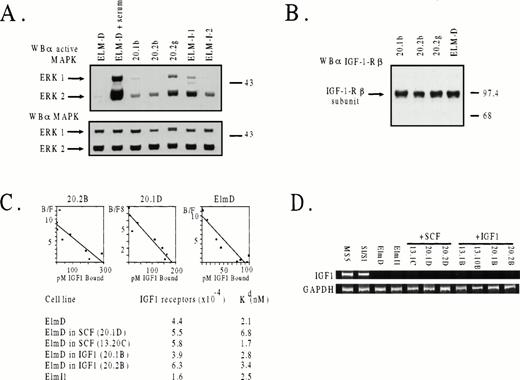
To determine whether constitutive activation of a mitogenic signaling pathway was responsible for the IGF-1–independence of ELM clones obtained by continuous growth in IGF-1, we determined whether MAP kinases were activated in serum-treated or serum-starved cells using antibodies that recognize the activated forms of ERK1 and ERK2. The results (Fig 4A) indicated that three IGF-1–independent ELM clones (like two stroma-independent clones, I/1 and I/2, isolated previously16) contained readily detectable levels of activated ERK2 (and to a lesser extent ERK1) when assayed in the absence of serum, in contrast to ELM cells freshly removed from stroma.
Levels of activated MAP kinase, IGF-1 receptors, and IGF-1 receptor mRNA in ELM cells grown long-term on stroma or in SCF or IGF-1. (A) Phosphorylation status of MAP kinase (ERK1) in three IGF-1–selected clones (20.1b, 20.2b, and 20.2g) or two stroma-independent variants isolated previously.16 Cells were starved in 0.5% serum medium for 6 hours before analysis. As a positive control, serum-starved ELM cells were stimulated with 20% serum for 10 minutes. Activated ERK1 and ERK2 were determined by Western blotting with antibodies against the activated form of MAP kinase (top panel) or with antibody against total MAP kinase as a loading control (bottom panel). (B) Levels of IGF-1 receptors in ELM clones isolated by long-term growth in IGF-1, determined by Western blotting. (C) Numbers and affinities of IGF receptors in ELM clones selected by long-term growth in SCF or IGF-1, compared with ELM cells growing on stroma, as determined by IGF-1–binding studies. Top panel, typical Scatchard plots for ELM cells freshly removed from stroma, and two ELM clones maintained long-term in either IGF-1 (20.2B) or SCF (20.1D). Bottom panel, average numbers of receptors/cell and affinities (Kd) calculated from the Scatchard plots of two separate experiments. (D) Levels of IGF mRNA by RT-PCR.
Levels of activated MAP kinase, IGF-1 receptors, and IGF-1 receptor mRNA in ELM cells grown long-term on stroma or in SCF or IGF-1. (A) Phosphorylation status of MAP kinase (ERK1) in three IGF-1–selected clones (20.1b, 20.2b, and 20.2g) or two stroma-independent variants isolated previously.16 Cells were starved in 0.5% serum medium for 6 hours before analysis. As a positive control, serum-starved ELM cells were stimulated with 20% serum for 10 minutes. Activated ERK1 and ERK2 were determined by Western blotting with antibodies against the activated form of MAP kinase (top panel) or with antibody against total MAP kinase as a loading control (bottom panel). (B) Levels of IGF-1 receptors in ELM clones isolated by long-term growth in IGF-1, determined by Western blotting. (C) Numbers and affinities of IGF receptors in ELM clones selected by long-term growth in SCF or IGF-1, compared with ELM cells growing on stroma, as determined by IGF-1–binding studies. Top panel, typical Scatchard plots for ELM cells freshly removed from stroma, and two ELM clones maintained long-term in either IGF-1 (20.2B) or SCF (20.1D). Bottom panel, average numbers of receptors/cell and affinities (Kd) calculated from the Scatchard plots of two separate experiments. (D) Levels of IGF mRNA by RT-PCR.
One obvious mechanism whereby growth of ELM cells in IGF-1 could result in outgrowth of IGF-1–independent cells might be overexpression of IGF-1 receptors or autocrine production of IGF-1. However, IGF-1–selected ELM cells have the same levels of receptors, whether assayed by Western blotting (Fig 4B) or IGF-1–binding studies (Fig4C). Scatchard analysis of the binding studies also shows that the affinities of binding of IGF-1 to its receptor is approximately the same in ELM clones isolated on stroma, in SCF, or in IGF-1 (Fig 4C). This suggests that IGF-1–independent clones do not survive because the IGF-1 receptor has become more sensitive to traces of IGF-1 in the serum. Another explanation for the generation of IGF-1–independent cells might be that the ELM cells grown long-term in IGF-1 are induced to produce IGF-1 constitutively. However, this does not appear to be the case, as judged by measurement of IGF-1 mRNA levels by RT-PCR (Fig4D) or Northern analysis (data not shown). Other experiments have shown that IGF-1– or stroma-independent clones do not produce SCF (data not shown). At present, we have been unable to identify the molecular mechanism(s) whereby such cells have a constitutively active MAP kinase pathway, except in one variant, which has an activating mutation in the SCF receptor (N. Leslie, unpublished data).
Differentiation capacity of ELM cells maintained long-term in SCF or IGF-1.
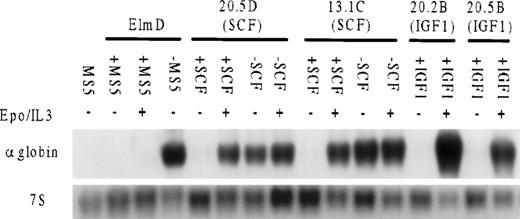
To test whether long-term growth in SCF or IGF-1 affected the ability of ELM cells to undergo erythroid differentiation, growth factors were removed from several clones of ELM cells grown long-term in SCF and the levels of globin mRNA were measured by Northern analysis. ELM cells freshly removed from stroma were used for comparison. The response of ELM cells to EPO and IL-3 was also determined in the presence of stroma or SCF. The results show several interesting features (Fig 5). First, ELM cells removed from stroma or SCF differentiate spontaneously. As described earlier, ELM cells grown long-term in IGF-1 become independent of IGF-1 for growth and do not differentiate if IGF-1 is removed. Second, EPO/IL-3 can induce differentiation of ELM cells in the presence of SCF or IGF-1, whereas growth on stroma prevents EPO/IL-3–induced differentiation. This suggests that one mechanism whereby stroma maintains ELM cells in vivo or in vitro is by preventing their differentiation. This is not due to integrin signaling because ELM cells still differentiate in response to EPO and IL-3, even if grown in SCF on ECM-coated plates (data not shown). In contrast, the differentiation arrest by SCF can be overridden by EPO/IL-3.
Inducibility of globin mRNA by EPO and IL-3 in ELM cells grown with or without stroma or ELM clones grown continuously in SCF (20.5D, 13.1C) or IGF-1 (20.2B, 20.5B). ELM cells were grown for 4 days in the growth factors shown, RNA extracted and 10 μg analyzed by Northern transfer for globin mRNA expression by probing with an α-globin cDNA probe or 7S ribosomal RNA as a loading control. The amounts of growth factors used were: SCF, 10 ng/mL; IGF-1, 100 ng/mL; EPO, 5 U/mL; IL-3, 10 U/mL.
Inducibility of globin mRNA by EPO and IL-3 in ELM cells grown with or without stroma or ELM clones grown continuously in SCF (20.5D, 13.1C) or IGF-1 (20.2B, 20.5B). ELM cells were grown for 4 days in the growth factors shown, RNA extracted and 10 μg analyzed by Northern transfer for globin mRNA expression by probing with an α-globin cDNA probe or 7S ribosomal RNA as a loading control. The amounts of growth factors used were: SCF, 10 ng/mL; IGF-1, 100 ng/mL; EPO, 5 U/mL; IL-3, 10 U/mL.
Tumorigenicity of ELM cells grown under different conditions.
To test whether growing ELM cells long-term in SCF or IGF-1, as opposed to normal stroma, affected their tumorigenicity, several ELM clones selected in SCF or IGF-1 cells were injected via the tail vein into syngeneic C3H mice and the growth of the cells intrasplenically measured by the increase in spleen weight. Our previous studies have shown that the increase in spleen size is due to growth of the injected ELM cells.16 However, maintaining ELM cells long-term in SCF or IGF-1 did not affect their tumorigenicity compared with injection of cells maintained on stroma (data not shown).
DISCUSSION
Bone marrow-, spleen- or fetal liver-derived stromal cells have been shown to support the growth of various normal hematopoietic stem cells and progenitors,22,23 including erythroid progenitors,24-26 which are often found in association with macrophages in bone marrow27,28 or in long-term Dexter cultures set up to favor erythropoiesis.29 There is increasing evidence that stromal derangements can contribute to the evolution of leukemias (reviewed by Duhrsen and Hossfeld30). Some primary leukemic cells still maintain this stroma-dependence,23,31-33 but there is evidence that different types of stroma can favor the growth of normal versus leukemic progenitors.34-38 However, most in vitro cultures of leukemic cells, including retrovirus-induced murine erythroleukemias,8-10 have lost their dependence on stroma and grow readily in suspension culture with or without added growth factors. The ELM erythroleukemia model is one of the few examples where bone marrow–derived stromal cells are required for long-term maintenance of the cell in culture,11-13 whereas clonal extinction occurs after a limited period of growth after stroma withdrawal, even in the presence of conditioned medium from stromal cells.14
Therefore, it is of considerable interest to define the cell interactions and signaling events responsible for this long-term maintenance of ELM cells by stroma. We have previously shown that one component of this is the interaction between SCF receptor on ELM cells and membrane-presented SCF by the stroma cells (Friel et al, manuscript submitted). Our present results extend this analysis further and define other components of the ELM-stroma interaction. Our antibody blocking experiments show that growth of ELM cells on stroma is mediated by a combination of SCF, IGF-1, and integrin signaling. The involvement of the SCF receptor is also supported by the fact thatSl/Sl stroma, which does not express any form of SCF, is less efficient than normal stroma at supporting ELM cell growth or cloning long-term. SCF and integrin signaling in cooperation with CD44 have been shown to be involved in the interactions of stromal cells with normal erythroid progenitors.39-42 IGF-1 is also required for growth and differentiation of normal erythroid progenitors in the absence of accessory cells.43 Thus, the ELM erythroleukemia model is unusual in retaining many of the signaling pathways of normal erythroid progenitors.
It is obviously of practical importance for basic research and clinical applications to find ways of maintaining erythroid progenitors long-term under defined conditions and to determine whether such conditions affect the progression of erythroleukemic cells to a more aggressive phenotype. We therefore investigated whether combinations of growth factors could support the proliferation of ELM cells, either in normal serum medium or serum-free media without generating abnormal phenotypes. The outcome of all of these experiments showed that SCF alone is capable of maintaining ELM cells for many months, without generating SCF- or stroma-independent variants and without increasing their tumorigenicity in vivo compared with ELM cells maintained on stroma. The main phenotypic difference we have found between ELM cells grown long-term in SCF and stroma is that growth on stroma blocks their differentiation in response to EPO/IL-3, whereas growth in SCF does not. This is somewhat different to the situation with normal human erythroid cells and K562 cells where SCF has been reported to block erythroid differentiation.44 45 ELM cells maintained in SCF also differ from cells maintained on stroma in that they lose their responsiveness to IGF-1.
Normal stroma produce two forms of SCF, a membrane inserted form and a soluble, secreted form.46 Because we found that ELM cells can be grown indefinitely in soluble SCF alone, we were initially surprised that neither conditioned medium from normal stroma, norSl/Sld stroma, which expresses soluble SCF, was able to support long-term maintenance or cloning of ELM cells. We believe the most likely reason for this is that the amounts of SCF produced by stroma may be suboptimal, or SCF may diffuse away from the ELM cell/stroma monolayer into the medium too quickly. Another factor may be that the signal produced by the interaction of SCF receptor with the membrane form of SCF is reported to be longer-lived than the signal from the interaction with soluble SCF.47 This may be important, as differentiation or proliferation of, for example, pheochromocytoma PC12 cells in response to growth factors is believed to be governed by the duration of the period of activation of the MAP kinase pathway they induce.48
In contrast to SCF, IGF-1 does not maintain ELM cells long-term, and after forced adaptation to growth in IGF-1, they, in fact, become IGF-1–independent for growth. It is presently unclear how these IGF-1–independent ELM cells arise. Two different explanations could be envisaged. One explanation might be that growth in IGF-1 induces permanent changes in growth factor or growth factor receptor expression, which allows the cells to grow autonomously. Beug et al49,50 have provided evidence that relatively late chicken erythroid progenitors can be converted into more immature progenitors with high proliferative capacity by growth in SCF, transforming growth factor (TGF)α, estradiol, and an unknown serum factor and this is associated with upregulation of the TGFα receptor. Kamai et al51 have also reported that long-term growth of preadipocyte cells in IGF-1 and growth hormone permanently altered the splicing pattern of IGF-1 transcripts to give an IGF-1 mRNA encoding secreted IGF-1, which then supported cell growth in a paracrine/autocrine manner. However, in the ELM system, we have been unable to find changes in the expression of IGF-1 or SCF or their receptors in IGF-1–independent variants, although some change resulting in constitutive activation of MAP kinases does occur. The alternative explanation we favor for how the IGF-1–independent ELM variants arise is that they are mutants generated spontaneously during culture. This is consistent with the fact that the cloning efficiency of ELM cells in IGF-1 is very low and with our previous estimate that stroma-independent variants arise at a frequency of about 10-5.14 16 In contrast, we did not isolate a single independent variant in all of the SCF cloning experiments performed in the course of this work, and the reason for this is probably that the growth rate/cloning efficiency of ELM cells in SCF is sufficiently high to prevent outgrowth of any independent cells in the population; whereas in IGF-1, ELM cells die out, leaving any independent cells to overgrow.
ACKNOWLEDGMENT
We thank Prof J. Wyke and Dr I. Pragnell for their critical reading of the manuscript.
Supported by the Cancer Research Campaign, UK, and the Deutsche Forschungsgemeinschaft, Germany.
Address reprint requests to Paul R. Harrison, PhD, The Beatson Institute for Cancer Research, CRC Beatson Laboratories, Garscube Estate, Switchback Rd, Bearsden, Glasgow G61 1BD, Scotland.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal