Abstract
Hereditary macrothrombocytopenia is a hallmark of Wistar Furth (WF) rats. In addition, a platelet/megakaryocyte alpha granule defect, similar to that of patients with gray platelet syndrome, is present. Several observations indicate cytoskeletal abnormalities in WF platelets and megakaryocytes, suggesting the potential for functional defects in hemostatic processes requiring cytoskeletal reorganization, such as platelet adhesion and spreading. However, no bleeding abnormality has been noted. Here, we report a prolonged bleeding time (>30 minutes in 10 of 11 rats tested) with defective clot formation in the WF strain. Prolonged bleeding time can result from defects in platelet adhesion, aggregation, or the release reaction. Because aggregation to collagen and adenosine diphosphate were reported to be normal, we determined whether WF rat platelets are defective in their ability to adhere to substrates. Platelet adherence and spreading was evaluated from 30 seconds to 30 minutes on Formvar-coated, carbon-stabilized grids or poly-L-lysine–coated glass coverslips by transmission electron microscopy or immunofluorescence, respectively, and scanning electron microscopy. We classified the adhered platelets according to their pattern of spreading, ie, rounded, rounded or spreading with short filopodia, spindle-shaped, spreading with long filopodia, spreading with lamellipodia, and fully spread. Adherent normal rat platelets displayed all stages of spreading within 30 seconds to 2 minutes, including many spindle-shaped forms, and forms with multiple, long filopodia. In contrast, adhered WF platelets at these early time points rarely developed long filopodia or were spindle shaped. The majority of adherent WF platelets at these early time points were either round, spread with a few short filopodia, or extensively spread with wide lamellipodial skirts. By 15 to 30 minutes, most platelets in both Wistar and WF samples were fully spread. These data show abnormal WF platelet spreading. The paucity of spindle-shaped forms and forms with long filopodia may reflect an inability of WF platelets to undergo the early stages of spreading, or, alternatively, their more rapid than normal progression through these stages. We hypothesize that this failure to spread normally may relate to prolonged bleeding times in vivo and defective clot formation in WF rats.
WISTAR FURTH (WF) rat platelets and megakaryocytes exhibit several abnormalities suggestive of cytoskeletal defects, including a spherical platelet shape, megakaryocyte plasma membrane blebbing, a haphazard distribution of megakaryocyte and platelet membranes and organelles, and a marked difference in the subcellular distribution of platelet myosin and talin.1-5However, no hemostatic defect has been reported, despite well-documented evidence of the importance of the cytoskeleton in platelet responses to vascular injury, including adhesion, aggregation, and contractility. Aggregation in response to adenosine diphosphate and collagen was reported to be normal1; however, a preliminary report suggested an abnormality in the rate of WF platelet adhesion and spreading on glass.6 Here, we have examined bleeding time and clot formation at the bleeding time wound site, and report that WF bleeding times are markedly prolonged and WF rat clot formation is defective.
Prolonged bleeding time can result from defects in platelet aggregation, adhesion, or release of granule contents. Discoid platelets become spherical with irregular protrusions and extend fine filopodia after activation in suspension.7 In contrast, platelet adhesion and spreading involve the formation of two different actin-based structures, filopodia and lamellipodia.8Filopodia are long, thin extensions containing elongated bundles of actin filaments that terminate at the filopodial tips, whereas lamellipodia, or spreading cytoplasmic veils, contain orthogonally arranged actin networks at platelet peripheries.8 These different morphological structures have been suggested to play important, distinct roles in the adhesive process: filopodia bind to fibrin and other platelets to form a three-dimensional clot, and lamellipodia arrest vascular leakage by adhering to wounded surfaces.9
To determine whether the bleeding time and clot formation abnormalities in this rat strain may be related to defects in the formation of these structures by WF rat platelets, we conducted a detailed in vitro temporal study of the sequence and rate of platelet adhesion and spreading. Normal platelets, which circulate as flat discs, proceed through well-defined morphological stages after adhesion and spreading in vitro,8,10 11 as follows: rounded spheres; round or spreading cells with short filopodia and spindle-shaped (tapered) forms; spreading cells with long filopodia; spreading cells with lamellipodia; and finally, a “fried-egg” (fully spread) platelet morphology. Although lamellipodia formation appeared normal in WF rat platelets, and they became fully spread, they showed a near absence of spindle-shaped forms, and absent or only short, stubby filopodia. We hypothesize that the failure to develop spindle-shaped forms and long filopodia after platelet adhesion results in fragile clots and prolonged bleeding times in the WF rat.
MATERIALS AND METHODS
Bleeding Time Determination
Retired breeder male Wistar and WF rats were purchased from Harlan Industries (Indianapolis, IN). The terminal 1-mm tip of the tail of Wistar and WF rats was removed with a sterile razor blade under general anesthesia according to institutional guidelines. The resultant wound was gently blotted with filter paper at time 0 and at 30-second time intervals thereafter until bleeding stopped. The time when no blood could be blotted on the filter paper was defined as the bleeding time. Pressure was applied to wounds still bleeding at 30 minutes to achieve hemostasis, and the bleeding time from such wounds was recorded as greater than 30 minutes.
Platelet Isolation for In Vitro Adhesion and Spreading Studies
Blood was collected from the abdominal aorta of anesthetized rats into syringes containing acid-citrate-dextrose (0.13 mol/L citric acid, 0.15 mol/L sodium citrate, 0.1 mol/L dextrose) anticoagulant (1:9; anticoagulant:blood). Platelets were separated from other blood cells by differential centrifugation. Platelets were pelleted from platelet-rich plasma (PRP) and washed three times in EDTA-HEPES-saline buffer (0.001 mol/L Na2 EDTA, 0.01 mol/L HEPES buffer [Sigma Chemical Co, St Louis, MO], and 0.15 mol/L NaCl).12 The final platelet pellet was resuspended in 1 mL of Hanks' Buffered Salt Solution (HBSS) with or without calcium (0.14 g/L) and magnesium (0.10 g/L) (Life Technologies, Grand Island, NY). The platelet suspension was incubated at 37°C for 30 minutes before the adherence studies were performed to allow platelets to return to their discoid morphology.13
Transmission Electron Microscopy
Negatively stained preparations of adherent platelets.
Resting platelets were prepared by fixing platelet suspensions in 1.5% glutaraldehyde fixative for 10 minutes, washing the platelets twice in 100 mmol/L sodium cacodylate buffer, and then adhering them to poly-L-lysine– (Sigma) coated grids. Adherent platelets were prepared by micropipetting 10 μL of suspended platelets onto the surfaces of Formvar-coated, carbon-stabilized grids, and allowing them to settle for either 30 seconds, 1 minute, 2 minutes, 5 minutes, 10 minutes, 15 minutes, or 30 minutes. Excess fluid was then removed from the edge of the grids with filter paper. The grids were immediately inverted sequentially onto two droplets of phosphate-buffered saline (PBS) and then a droplet of 1.5% glutaraldehyde fixative. The grids were fixed for 1 to 2 minutes. Excess fluid was removed as before, after which the grids were rinsed twice in droplets of double-distilled water. The water was removed with filter paper and the grids inverted onto droplets of 1% uranyl acetate in double-distilled water that had been microfiltered immediately before use. Staining was performed for 30 seconds to 1 minute, after which excess fluid was removed with filter paper and the grids were allowed to air dry. The platelets were viewed and counted on a Philips 301 transmission electron microscope (Philips Electronics, Mahwah, NJ).
Quantitation of adherent platelet shapes at each time point was performed using the following method and criteria. A whole grid square was located in one corner of the grid meshwork, and counting of the first 100 adherent platelets for each rat was performed by moving linearly from this starting point. This analysis was performed on five rats of each strain at each time point except the 2-minute time point in which four Wistar rats were examined. Platelets were assigned to the following shape categories: round (no filopodia), round or spreading with short filopodia, spindle-shaped (tapered), spreading with long filopodia, spreading with lamellipodia, or fully spread (“fried egg” morphology).
The plots of the observed means suggested a quadratic trend over time; therefore, a quadratic growth curve model with random intercept was fitted for each of the morphological forms.14 The independent factors (predictor variables) included in the model were group (G), linear effect of time (T), first order interaction of group and time (G*T), quadratic effect of time (T2), and second order interaction of group and time (G*T2). A factor in the model was considered to be a significant predictor of the proportion of a particular morphological stage if the P value corresponding to that factor was less than α (=.05). The analysis was conducted using PROC MIXED in SAS (1996) statistical software package (SAS Institute, Inc, Cary, NC).
Unstained Adherent Platelets Collected Onto Grids by Cytocentrifugation
The method of preparation of adherent platelets for transmission electron microscopic examination used above is dependent on both the sedimentation rate of platelets in the suspension buffer and their rate of spreading once they contact the adherence surface. To increase the number of adherent platelets at an early time point after contact with the adherence surface, platelets were collected by centrifugation in a cytocentrifuge directly onto parlodion-coated, carbon-stabilized grids that had been mounted onto microscope slides. Specifically, three grids were placed on each glass slide and the slide then was covered with water. A drop of 3% parlodion was placed on top of the water and the slides were allowed to dry overnight. The slides were carbon-coated to stabilize the adherence of the grids onto the slides. Blood was collected from the dorsal aorta of metofane-anesthetized WF and Wistar rats into acid citrate-dextrose anticoagulant (9:1; blood:anticoagulant). PRP was prepared by differential centrifugation, and the PRP diluted 1:100 in PBS. Then 100 μL of diluted PRP was placed in each cytocentrifuge chamber, and the platelets collected onto the parlodion-coated, carbon-stabilized grids by centrifugation for 5 minutes at 1,500 rpm in a Shandon cytocentrifuge (Shandon, Inc, Pittsburgh, PA). Immediately thereafter, the slides were placed in a Coplin jar containing 2.5% paraformaldehyde in 100 mmol/L PIPES buffer (pH 7.2), and fixed for 10 minutes. The slides were rinsed in 100 mmol/L PIPES buffer and stored flat in PIPES buffer at 4°C until examination by transmission electron microscopy.
Immunofluorescence Microscopy of Adherent Platelets
To determine the kinetics and morphology of adherent platelets using a different technique and substrate, we evaluated platelet adherence to poly-L-lysine–coated glass coverslips by immunofluorescence.
Glass coverslips cleaned in 70% ethanol with 1% hydrochloric acid were air-dried, coated with poly-L-lysine for 5 minutes, and allowed to dry in a 37°C oven. Additional coverslips were coated with either fibronectin (Calbiochem, La Jolla, CA), according to manufacturer's instructions, or 1 mg/mL rat fibrinogen (Sigma) 45 minutes before use. Fifty microliters of platelet suspension was pipetted onto the coverslips and allowed to adhere for the same time periods as above. After each time interval, the coverslips were dipped sequentially into two beakers containing PBS to remove nonadherent platelets and then blotted briefly on the edge of filter paper to remove excess fluid. They were placed immediately into Coplin jars containing paraformaldehyde/picric acid fixative (2% paraformaldehyde/15% picric acid in 100 mmol/L phosphate buffer), fixed for 30 minutes at room temperature, washed five times in PBS, and stored in the same buffer before immunostaining.
Coverslips were incubated in PBS containing 0.05% saponin (“Immunofluorescence Buffer”) as a permeabilizing agent. This solution was used for all antibody incubations and washes. The coverslips were blocked for 15 minutes in PBS/saponin solution containing 0.1% bovine serum albumin, incubated with primary antibody for 40 minutes at room temperature, and washed three times for 5 minutes each in immunofluorescence buffer. This was followed by incubation with the appropriate fluorescein or rhodamine-conjugated secondary antibody for 40 minutes at room temperature. Subsequently, they were washed five times for 5 minutes each in immunofluorescence buffer, followed by a PBS wash. Excess buffer was blotted away and coverslips were mounted with n-propyl gallate (Sigma) in buffered glycerol and sealed with nail polish.
Rhodamine phalloidin was purchased from Molecular Probes (Eugene, OR) and fluorescein and rhodamine conjugated goat anti-rabbit immunoglobulin-G probes were purchased from Cappell Laboratories (Organon Teknika Corp, Durham, NC). Polyclonal antibodies to platelet membrane glycoprotein (GP) IIb were the kind gift of Dr Peter Newman (Blood Center of SE Wisconsin, Milwaukee) and polyclonal antibodies to talin were generously provided by Dr Mary Beckerle (University of Utah, Salt Lake City).
Scanning Electron Microscopy of Adherent Platelets
Poly-L-lysine–coated coverslips containing platelets which were allowed to adhere for the same time intervals as above were dipped sequentially into two beakers containing PBS to remove nonadherent platelets, and then blotted briefly on the edge of filter paper to remove excess fluid. They were placed in Coplin jars containing glutaraldehyde/lysine fixative prepared immediately before use. The fixative was prepared by combining 4% glutaraldehyde in 60 mmol/L Na Cacodylate buffer, pH 7.4; and 80 mmol/L lysine (L-lysine; Sigma) in 60 mmol/L Na Cacodylate buffer, pH 7.4 (1:1). The adherent platelets were fixed in this solution for 1 hour, fixed an additional hour in 1.5% glutaraldehyde in 100 mmol/L Na Cacodylate buffer, pH 7.4, and then rinsed in 100 mmol/L Na Cacodylate buffer. They were incubated in 1% osmium tetroxide in 100 mmol/L Na Cacodylate buffer for 1 hour, dehydrated in a graded series of ethanols, and stored in 100% ethanol. The platelets were critical point dried, coated with gold-palladium, and viewed with an Amray scanning electron microscope (Amray, Inc, Bedford, MA).
Quantitation of Platelet Adhesion to Poly-L-lysine–Coated Glass Beads
The platelets were prepared as for the other adhesion experiments. The percentage of adherent platelets was determined by counting by phase microscopy the number of platelets in suspension after a 2-minute incubation on a column of polylysine-coated glass beads divided by the number of platelets in suspension after a 2-minute incubation on a siliconized glass bead column. The beads were Superbrite glass beads Type 100-5005 from The 3M Company (St Paul, MN).
RESULTS
Bleeding Times
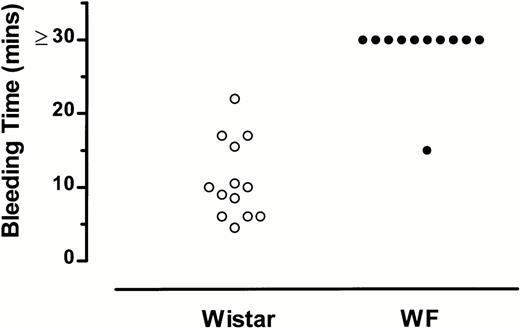
Bleeding time in 13 Wistar rats averaged 10.9 minutes, with a median of 10 minutes (Fig 1). Bleeding times were markedly prolonged (>30 minutes) in 10 of 11 WF rats examined (Fig1). A comparison of the bleeding times of Wistar and WF rats15 indicated that WF rats have a significantly prolonged bleeding time as compared with Wistar rats (P < .0001).
Bleeding times of WF rats are prolonged compared with those of normal (Wistar) rats. Each point represents the bleeding time for an individual rat.
Bleeding times of WF rats are prolonged compared with those of normal (Wistar) rats. Each point represents the bleeding time for an individual rat.
WF rat platelet clots that formed at the cut ends of tails were large, loose, gelatinous clots that extended from the wound. The clots were more easily dislodged from the wound site than Wistar rat clots.
Morphology of Grid-Adherent Platelets
Uranyl acetate-stained Wistar and WF rat platelets adherent to Formvar-coated, carbon-stabilized grids displayed the complete range of morphological forms at all time points examined. However, the proportions of the different forms varied markedly between WF and Wistar rats (Figs 2-4). Thepredominant forms of Wistar platelets allowed to adhere for 30 seconds to 5 minutes were round (with no filopodia or only stubby filopodia), spindle-shaped forms, and round or spreading platelets with long filopodia. From 10 to 30 minutes, spreading platelets with lamellipodia and fully spread cells were the most abundant forms of adhered Wistar rat platelets (Figs 2 and 3).
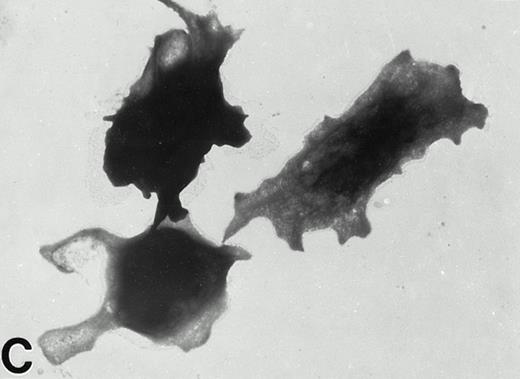
Wistar rat platelets allowed to adhere for 30 seconds (A) or 1 minute (B) onto Formvar-coated, carbon-stabilized grids, and subsequently fixed and stained with uranyl acetate. WF rat platelets allowed to adhere for 30 seconds (C) or 1 minute (D) onto Formvar-coated, carbon-stabilized grids. (A through D, original magnification ×3,700).
Wistar rat platelets allowed to adhere for 30 seconds (A) or 1 minute (B) onto Formvar-coated, carbon-stabilized grids, and subsequently fixed and stained with uranyl acetate. WF rat platelets allowed to adhere for 30 seconds (C) or 1 minute (D) onto Formvar-coated, carbon-stabilized grids. (A through D, original magnification ×3,700).
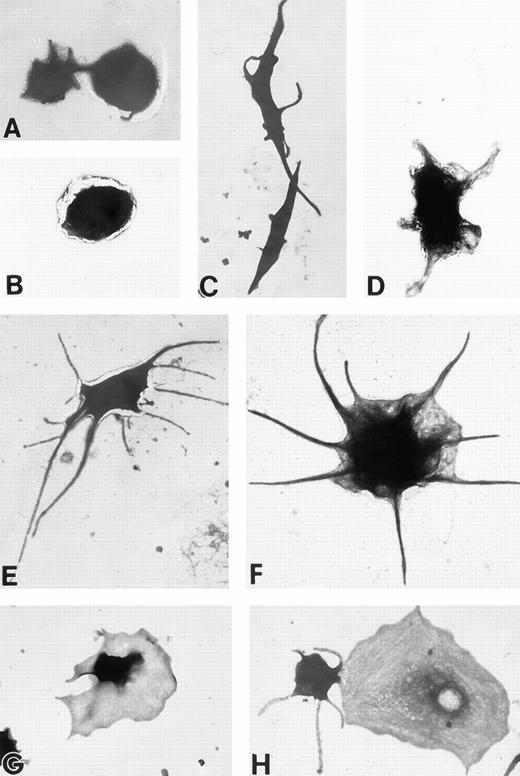
(A) Representative resting Wistar rat platelets fixed, adhered to poly-L-lysine–coated grids, and stained with uranyl acetate. (B through H) Wistar rat platelets allowed to adhere to Formvar-coated, carbon-stabilized grids from 30 seconds to 30 minutes, and subsequently fixed and stained with uranyl acetate. (B through H) illustrate the forms characteristic of Wistar rat platelet spreading, including rounded shapes (B), followed by the development of spindle-shaped, or tapered, forms (C) and stubby filopodia (D), the extension of elongated, multiple filopodia (E and F), expansion of lamellipodial “skirts” which fill in the filopodia ribs (G), and the final “fried-egg,” or fully spread form (H). (B through E) 30-second adherent samples, (F) 1-minute adherent sample, (G and H) 30-minute adherent samples. (A through H, original magnification ×7,000).
(A) Representative resting Wistar rat platelets fixed, adhered to poly-L-lysine–coated grids, and stained with uranyl acetate. (B through H) Wistar rat platelets allowed to adhere to Formvar-coated, carbon-stabilized grids from 30 seconds to 30 minutes, and subsequently fixed and stained with uranyl acetate. (B through H) illustrate the forms characteristic of Wistar rat platelet spreading, including rounded shapes (B), followed by the development of spindle-shaped, or tapered, forms (C) and stubby filopodia (D), the extension of elongated, multiple filopodia (E and F), expansion of lamellipodial “skirts” which fill in the filopodia ribs (G), and the final “fried-egg,” or fully spread form (H). (B through E) 30-second adherent samples, (F) 1-minute adherent sample, (G and H) 30-minute adherent samples. (A through H, original magnification ×7,000).
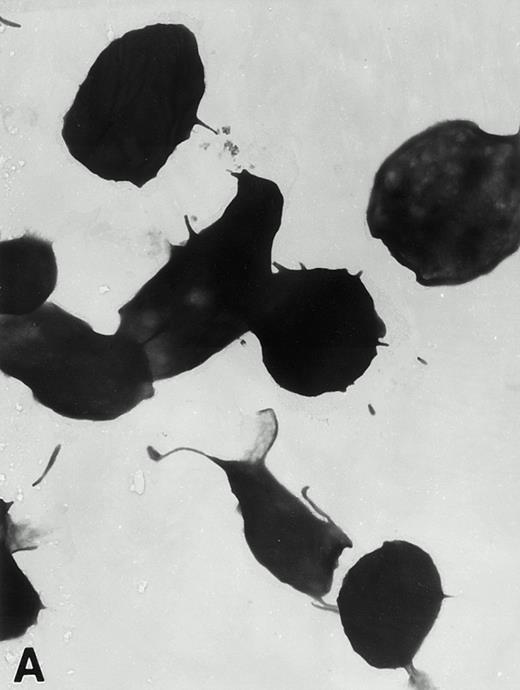
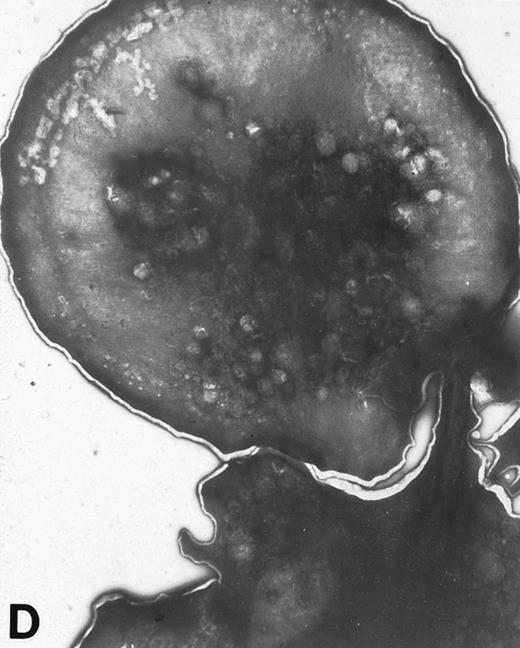
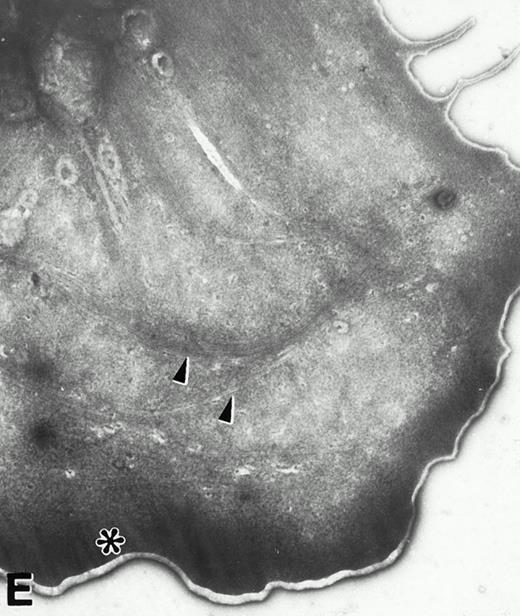
(A) Representative resting WF rat platelets prepared identically to resting Wistar platelets in Fig 2. (B through E) WF rat platelets allowed to adhere to Formvar-coated, carbon-stabilized grids from 30 seconds to 30 minutes, and subsequently fixed and stained with uranyl acetate. (B through E) illustrate the forms characteristic of WF rat platelet spreading, including platelets with stubby filopodia (B), lamellipodia (C), or fully spread forms (D and E). (E) shows the spread margin of a WF platelet at higher power. The arrowheads indicate microtubules and the asterisk shows the dense peripheral weave at the platelet margin. (B) 30-second adherent sample, (C) 1-minute adherent sample, (D and E) 30-minute adherent sample. (A through D, original magnification ×7,000; E, original magnification ×19,000).
(A) Representative resting WF rat platelets prepared identically to resting Wistar platelets in Fig 2. (B through E) WF rat platelets allowed to adhere to Formvar-coated, carbon-stabilized grids from 30 seconds to 30 minutes, and subsequently fixed and stained with uranyl acetate. (B through E) illustrate the forms characteristic of WF rat platelet spreading, including platelets with stubby filopodia (B), lamellipodia (C), or fully spread forms (D and E). (E) shows the spread margin of a WF platelet at higher power. The arrowheads indicate microtubules and the asterisk shows the dense peripheral weave at the platelet margin. (B) 30-second adherent sample, (C) 1-minute adherent sample, (D and E) 30-minute adherent sample. (A through D, original magnification ×7,000; E, original magnification ×19,000).
In contrast, only a small percentage of adherent WF rat platelets were spindle shaped or spread with long filopodia after 30 seconds to 5 minutes. The majority of adhered WF platelets were round (with or without stubby filopodia) or spread with lamellipodia. From 10 to 30 minutes, the predominant forms were spread with lamellipodia and fully spread WF platelets (Figs 2 and 4).
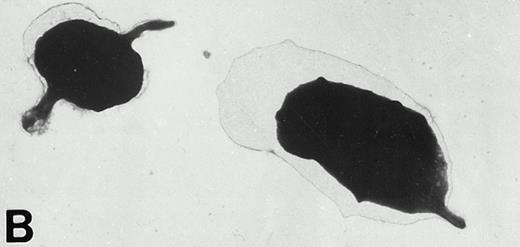
Resting Wistar and WF rat platelets fixed and then adhered to poly-L-lysine–coated grids did not exhibit the morphological forms of adherent platelets, but rather, were either discoid or rounded with few filopodia (Figs 3A and 4A).
Unstained platelet cytospins showed similar results to uranyl acetate-stained grid-adherent platelets. Wistar platelets exhibited filopodia, whereas WF platelets were round, with no filopodia (data not shown).
Kinetics of Grid-Adherent Platelets
Wistar and WF rat platelets adherent to coated grids exhibited quantitative differences in the numbers of morphological forms at each time point examined (Fig 5).
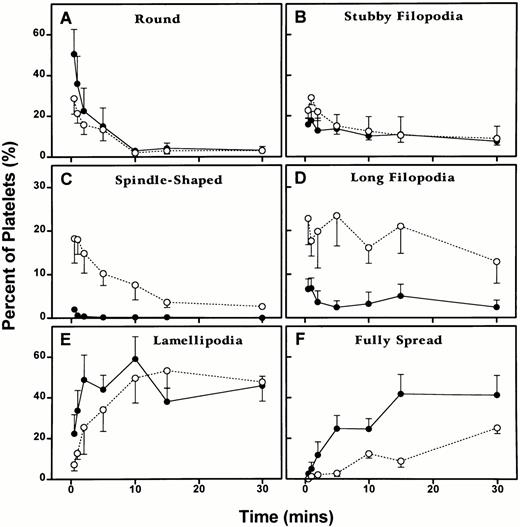
Differences in the kinetics of the morphological changes of Wistar and WF rat platelets on adhesion to Formvar-coated, carbon-stabilized grids as described in Materials and Methods. Platelets were allowed to adhere for 30 seconds, 1 minute, 2 minutes, 5 minutes, 10 minutes, 15 minutes, or 30 minutes. One hundred platelets were counted for each rat at each time point and assigned to the following shape categories: round, round or spreading with stubby filopodia, spindle shaped, round or spreading with long filopodia, spreading with lamellipodia, and fully spread. One hundred platelets were evaluated for each of the five rats of each strain at each of the seven time points, except for the 2-minute time point for Wistar rat platelets, in which data was collected from 100 platelets from four rats, rather than five rats. Hence, the experimental data for this adhesion analysis represent a total of 3,500 WF rat platelets, and 3,400 Wistar rat platelets for five experiments conducted at each of the seven time points. Each point represents the mean ± 1 SEM for five rats of each strain. (○) and (---), Wistar rats; (•) and (—), WF rats.
Differences in the kinetics of the morphological changes of Wistar and WF rat platelets on adhesion to Formvar-coated, carbon-stabilized grids as described in Materials and Methods. Platelets were allowed to adhere for 30 seconds, 1 minute, 2 minutes, 5 minutes, 10 minutes, 15 minutes, or 30 minutes. One hundred platelets were counted for each rat at each time point and assigned to the following shape categories: round, round or spreading with stubby filopodia, spindle shaped, round or spreading with long filopodia, spreading with lamellipodia, and fully spread. One hundred platelets were evaluated for each of the five rats of each strain at each of the seven time points, except for the 2-minute time point for Wistar rat platelets, in which data was collected from 100 platelets from four rats, rather than five rats. Hence, the experimental data for this adhesion analysis represent a total of 3,500 WF rat platelets, and 3,400 Wistar rat platelets for five experiments conducted at each of the seven time points. Each point represents the mean ± 1 SEM for five rats of each strain. (○) and (---), Wistar rats; (•) and (—), WF rats.
Quantitation of grid-adherent platelets.
No significant difference was observed between the two groups for the rounded and stubby filopodia stages, and the pattern of change was similar for the two groups. However, a significant difference was observed between the two groups for spindle-shaped forms (P = .0001), long filopodia forms (P = .0226), and spreading with lamellipodia forms (P = .0434). The Wistar platelet group had much higher levels of spindle-shaped and long filopodia forms and the WF platelet group had slightly higher levels of forms with lamellipodia. The pattern of change between the two groups was significantly different in the formation of spindle-shaped platelets (P = .005). Finally, no difference was observed in the proportion of fully spread platelets, although the pattern of change in the two groups was significantly different (P = .0033).
No significant differences were present in the proportions of adherent Wistar or WF platelet forms in HBSS lacking calcium or magnesium, or HBSS containing these divalent cations, at 30 seconds, 1 minute, or 30 minutes.
Platelet Adherence to Poly-L-Lysine Coated Coverslips
Immunofluorescence of adherent platelets.
Qualitatively, WF rat platelets adhering to poly-L-lysine–coated coverslips exhibited significantly fewer numbers of spindle-shaped forms and forms extending long filopodia at early time points (30 seconds to 1 minute), compared with Wistar rat platelets (Fig6). These data were similar for preparations labeled with rhodamine-phalloidin (Fig 6), GP IIb (not shown), or talin (not shown). Significantly fewer Wistar and WF rat platelets were adherent to either fibronectin or fibrinogen than to poly-L-lysine–coated coverslips. Nevertheless, for WF rat platelets, a decrease of spindle-shaped and long filopodial forms was present as quantitated on poly-L-lysine–coated coverslips (not shown).
Absence of spindle-shaped forms among adhered WF rat platelets. Immunofluorescence staining of Wistar (a, c, and e) and WF (b, d, and f) rat platelets with rhodamine-phalloidin after 30 seconds (a and b), 1 minute (c and d), or 30 minutes (e and f) of adherence to poly-L-lysine coated glass coverslips. Spindle-shaped Wistar platelet forms are indicated by arrows (a and c); these forms are absent in WF rat platelet preparations (b and d), which contain rounded or spreading forms. By 30 minutes, the majority of Wistar and WF rat platelets are fully spread. Original magnification ×630.
Absence of spindle-shaped forms among adhered WF rat platelets. Immunofluorescence staining of Wistar (a, c, and e) and WF (b, d, and f) rat platelets with rhodamine-phalloidin after 30 seconds (a and b), 1 minute (c and d), or 30 minutes (e and f) of adherence to poly-L-lysine coated glass coverslips. Spindle-shaped Wistar platelet forms are indicated by arrows (a and c); these forms are absent in WF rat platelet preparations (b and d), which contain rounded or spreading forms. By 30 minutes, the majority of Wistar and WF rat platelets are fully spread. Original magnification ×630.
Scanning electron microscopy of adherent platelets.
Wistar rat platelets adherent to poly-L-lysine–coated coverslips exhibited greater numbers of spindle-shaped forms and forms with long filopodia than their WF counterparts at all time points examined (Fig7). By 30 minutes, large numbers of both Wistar and WF rat platelets were fully spread (not shown).
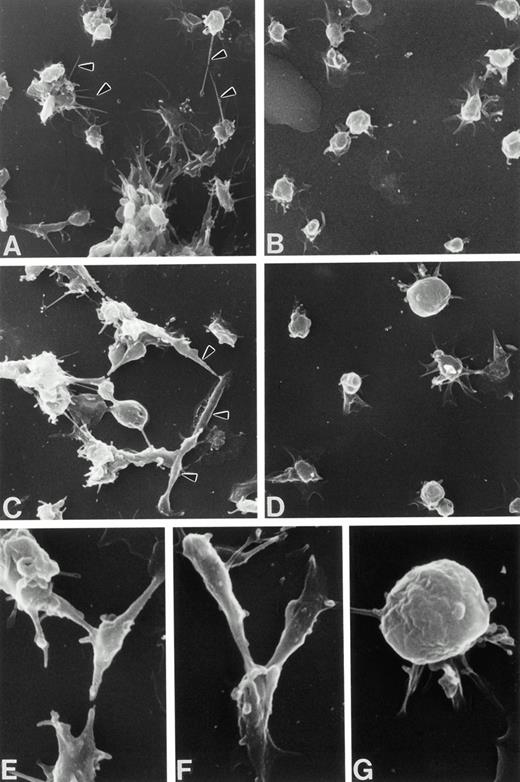
Scanning electron micrographs of adherent Wistar (A, C, E, and F) and WF (B, D, and G) rat platelets from 1 to 2 minutes postadherence on poly-L-lysine–coated coverslips. Wistar platelets extend fine filopodia from round or spreading bodies (A, arrowheads) or exhibit spindle-shaped forms (C, arrowheads; higher magnification, E and F), unlike WF platelets, the majority of which are rounded, spreading with lamellipodial skirts, or fully spread. (A through D, original magnification ×2,800; E through G, original magnification ×7,100).
Scanning electron micrographs of adherent Wistar (A, C, E, and F) and WF (B, D, and G) rat platelets from 1 to 2 minutes postadherence on poly-L-lysine–coated coverslips. Wistar platelets extend fine filopodia from round or spreading bodies (A, arrowheads) or exhibit spindle-shaped forms (C, arrowheads; higher magnification, E and F), unlike WF platelets, the majority of which are rounded, spreading with lamellipodial skirts, or fully spread. (A through D, original magnification ×2,800; E through G, original magnification ×7,100).
Platelet Adhesion to Glass Bead Columns
Approximately the same proportion of Wistar and WF rat platelets adhered to the glass bead columns after a 2-minute incubation. The percentages for adhered Wistar platelets were 67, 88, and 82, as compared with 52, 75, and 80 for WF platelets, respectively, in the three experiments.
DISCUSSION
We report prolonged bleeding time with defective clot formation in the WF rat, a strain with inherited macrothrombocytopenia, α granule protein content deficiencies, and cytoskeletal defects.1,2,4,16 Prolonged bleeding time is a variable characteristic of patients with gray platelet syndrome, a platelet abnormality closely resembling that of the WF rat.16
In addition to these in vivo findings, using a combined structural approach of electron microscopy and immunofluorescence, we show differences in postadherence morphological changes and kinetics of Wistar and WF rat platelets. Our results suggest that WF rat platelets eventually proceed to the fully spread stage, but do not become spindle shaped or extend fine, elongated filopodia as do normal Wistar rat platelets. We hypothesize that these abnormalities may be related to defects in the cytoskeletal system of the WF rat.
Unstimulated platelets in suspension contain a complex actin filament cytoskeleton coursing through the cytoplasm which is connected to both a membrane skeleton, a spectrin-rich lamina underlying the plasma membrane, and a peripheral microtubule coil.10,13,17-21Activation of suspended platelets results in a rapid increase in actin filament content, from a resting level of 30% to 40% of total actin to 60% to 70%22 and cytoskeletal reorganization,7,23,24 including filopodia formation. Fewer studies have examined the composition and structure of the adherent platelet cytoskeleton, despite the importance of platelet adhesion in normal hemostasis, ie, after injury, platelets adhere to exposed subendothelium, spread, and release storage granule glycoprotein contents that function locally. Biochemical differences have been reported between spreading and suspension-activated platelet cytoskeletons6; in that study, total protein, talin, and vinculin were more extensively incorporated into adhering platelet cytoskeletons than suspension-activated platelet cytoskeletons.6
Morphological studies of adherent platelets suggest that circulating discoid platelets progress through sequential stages of shape change after adhesion to a substrate10,11,25: rounding; extension of short filopodia and the development of spindle-shaped, tapered forms; extension of long filopodia by round and partially spreading platelets; expansion of lamellipodia which fill in the filopodial “ribs” with cytoplasm; and finally, the fully spread or “fried-egg” form. Evagination of the surface-connected canalicular system is a major component contributing to platelet spreading on surfaces.26 Although this sequence of platelet shape change after adhesion is generally accepted, the idea also has been proposed that spindle-shaped (“fusiform”) platelets are actually the form produced by megakaryocytes, and these forms are transformed to discoid platelets in the circulation.27
The newly polymerized actin filaments formed after adhesion are present in two new structures: bundles of long filaments within newly formed filopodia that terminate at filopodial tips, and lamellipodial networks in platelet peripheries, consisting of orthogonally arranged short actin filaments.7,8,28 Filopodia elongate from barbed ends of pre-existing filaments and lamellipodia form from calcium-dependent severing of peripheral actin filaments by gelsolin, followed by the uncapping of severed oligomers.8 Both of these structures are important for physiological hemostasis and play a role in thrombotic conditions such as stroke, myocardial infarction, and peripheral vascular insufficiency. However, their specific functions appear to differ. Lamellipodia plug vascular leaks by adhering at wounded surfaces, whereas filopodia bind fibrin strands and other platelets to form a three-dimensional blood clot.9 Our results indicate that filopodia are not a necessary prerequisite for lamellipodia formation.
Data reported here strongly support the specific functions of filopodia and lamellipodia. WF rat platelets are capable of forming lamellipodia after adhesion, consistent with their ability to eventually plug vascular leaks at sites of injury. In contrast, the lack of normal filopodia formation suggests that these platelets are not as effective in binding to adjacent platelets and fibrin strands and forming an effective clot. This is consistent with our observation of defective clot formation of WF rats. Although the significance of spindle-shaped platelet forms is not clearly understood, their tapered shape may facilitate binding of platelets to one another, thereby contributing to effective clot formation. A greater proportion of WF rat platelets rapidly progress to the lamellipodial and fully spread stages than do normal rat platelets. This suggests that WF rats do not develop spindle-shaped forms or platelets with elongated filopodia, but immediately progress from rounded forms with and without stubby filopodia to spreading forms with expanding lamellipodia. We conclude that the absence of specific adherent platelet forms may be responsible for the increased bleeding time and clot fragility in the WF rat.
ACKNOWLEDGMENT
The authors acknowledge the expertise provided by Deepthi Jayawardene and Deo Kumar Srivastava in the statistical analysis of adherent platelets.
Supported in part by R01 Grant HL 51546 (C.W.J. and P.E.S.) from the National Heart, Lung, and Blood Institute, P30 CA21765 Cancer Center Support Grant and P01 CA20180 from the National Cancer Institute, Public Health Service, Department of Health and Human Services, and American Lebanese Syrian Associated Charities.
Address reprint requests to Paula E. Stenberg, PhD, Dept of Pathology L113, Oregon Health Sciences University, 3181 SW Sam Jackson Park Rd, Portland, OR 97201.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal