Abstract
In many different cells, glycosylphosphatidylinositol (GPI)-anchored molecules are clustered in membrane microdomains that resist extraction by detergents at 4°C. In this report, we identified the presence of such domains in human erythrocytes and examined the ability of exogenously-added GPI-anchored molecules to colocalize with the endogenous GPI-anchored proteins in these detergent-insoluble complexes. We found that the addition to human erythrocytes of three purified GPI-anchored proteins having different GPI lipid moieties resulted in their efficient and correct incorporation into the membrane. The extent of membrane insertion was dependent on the intactness of the GPI lipid moiety. However, unlike the endogenous GPI-anchored proteins, the in vitro incorporated GPI molecules were not resistant to membrane extraction by Triton X-100 at 4°C. In addition, in contrast to the endogenous GPI-anchored proteins, they were not preferentially released from erythrocytes during vesiculation induced by calcium loading of the cells. These results suggest that in vitro incorporated GPI-linked molecules are excluded from pre-existing GPI-enriched membrane areas in human erythrocytes and that these microdomains may represent the sites of membrane vesicle formation.
GLYCOSYLPHOSPHATIDYLINOSITOLS (GPIs) serve as an important alternative mechanism for anchoring proteins to cell membranes. They are synthesized by all eukaryotic cells examined to date and anchor a wide variety of functionally diverse proteins to cell surfaces.1,2 In mammalian cells GPI-anchored proteins have been found to be involved in intracellular targeting,3potocytosis,4 and signal transduction.5,6 All these functions may relate to the reported preferential association of GPI-anchored proteins with specialized membrane microdomains. These microdomains are rich in glycolipids, sphingolipids, and cholesterol, and resist solubilization by neutral detergents, such as Triton X-100.7,8 However, whether such specialized GPI-enriched microdomains involved in transmembrane signaling indeed exist or are artifacts induced by the extraction procedure has remained controversial.9-11
A composition reminiscent of the GPI membrane microdomains has been reported for vesicles released from mammalian cells. Plasma membrane vesiculation has been described to occur spontaneously in normal and tumor cells in culture.12-18 The released vesicles seem to be enriched in sphingomyelin and cholesterol,13,15,18gangliosides,19 and several proteins such as 5′-nucleotidase13,15 and alkaline phosphatase,17,18 which in most cells are now known to be GPI-anchored. Similarly, release of vesicles can also be induced in normal and pathological human erythrocytes.20-24 Although in these cases the vesicles have a similar lipid composition as the erythrocytes, they are highly enriched in the GPI-anchored proteins acetylcholinesterase (AChE) and CD55 (decay accelerating factor).25
Recent evidence suggests that mammalian and parasite GPI-anchored molecules may transfer spontaneously from donor to acceptor membranes. In addition, it has been shown that purified GPI-linked molecules readily incorporate in vitro into mammalian cells. The transferred molecules seem to be stably inserted into the outer leaflet of the plasma membrane via their acyl/alkyl chains on the GPI moiety.26,27 In some cases, the in vivo transferred or in vitro inserted GPI-anchored proteins displayed biological activity28-33 indicating that they integrated correctly into the target cell membrane. Efficient incorporation of purified GPI-anchored proteins into target membranes was shown to require the intact GPI lipid moiety.34
The aim of our work was to study if GPI-anchored molecules inserted into a target cell would colocalize with endogenous GPI-anchored proteins in detergent-insoluble microdomains. For this purpose we incorporated three purified GPI-anchored proteins, having different GPI lipid moieties, plus a set of molecules derived from them into human erythrocytes and studied their fate during extraction with Triton X-100. In addition, we followed their release from erythrocytes during vesiculation to study if the in vitro incorporated GPI-anchored molecules would be enriched in the released vesicles similar to the endogenous GPI-anchored proteins.
MATERIALS AND METHODS
All reagents were of analytical grade and from Boehringer (Mannheim, Germany), Fluka (Buchs, Switzerland), Sigma (St Louis, MO), or Merck (Darmstadt, Germany). [1-3H]Ethan-1-ol-2-amine hydrochloride ([3H]ethanolamine) was purchased from Amersham (Buckinghamshire, UK) and [3H]diisopropylfluorophosphate ([3H]DFP) from Du Pont NEN (Regensdorf, Switzerland). GPI-PLD was purified from bovine serum as described elsewhere.35Phosphatidylinositol-specific phospholipase C (PI-PLC) fromBacillus cereus was from Boehringer.
Erythrocytes.
Concentrated erythrocytes in standard anticoagulant buffer were obtained from the ZLB Central Laboratory, Swiss Red Cross Blood Transfusion Service (Bern, Switzerland). Erythrocytes were pelleted by centrifugation and washed twice with 0.9% (wt/vol) NaCl.
GPI-anchored proteins.
Human erythrocyte AChE was purified by affinity chromatography as described before.36 The final suspension contained purified AChE in 10 mmol/L Tris-HCl, pH 7.4; 144 mmol/L NaCl; and 0.05% (wt/vol) Triton X-100 (Fluka). The absolute amounts of AChE in the assays were calculated based on its enzymatic activity with 1 IU equaling 2.5 pmol of protein. The AChE activity was measured according to Ellman et al.37 Purified AChE was then labeled covalently in the active site by incubation in the presence of [3H]DFP for 16 hours at room temperature. Unreacted DFP was removed by extensive dialysis.
[3H]Ethanolamine-labeled variant surface glycoprotein (VSG) from Trypanosoma brucei bloodstream forms was prepared following the procedure of Hereld et al.38 The resulting suspension contained radiochemically pure VSG in 1% (wt/vol) sodium dodecyl sulfate (SDS).
[3H]Ethanolamine-labeled procyclin from Trypanosoma brucei brucei 427 insect forms was prepared exactly as described by Bütikofer et al.39 The surface of insect form trypanosomes is covered by an invariant protein coat consisting of GPI-anchored procyclins. T b brucei 427 cells express two different forms of procyclin, the so-called EP and GPEET procyclins consisting either of extensive tandem repeat units of glutamic acid (E) and proline (P) or internal pentapeptide (GPEET) repeats. Thus, the [3H]thanolamine-labeled extract from T b brucei427 cells contains a mixture of radiochemically pure EP and GPEET procyclin in 9% (vol/vol) butan-1-ol in water.39
All proteins were subsequently purified by octyl-Sepharose chromatography exactly as described before.39 The resulting specific radioactivities of the three protein preparations were 66,265 cpm/μg AChE, 737 cpm/μg VSG, and 15,416 cpm/μg procyclin.
Enzymatic and chemical treatment of GPI-anchored proteins.
The purified [3H]-labeled proteins were modified in the GPI or protein portion by the following procedures: (1) GPI-PLD treatment: [3H]-labeled AChE, VSG, and procyclin were incubated with GPI-PLD in 50 mmol/L 2-morpholino-ethanesulfonic acid monohydrate (MES), pH 6.5, containing 0.5 mmol/L CaCl2 and 0.02% (wt/vol) Triton X-100, for 16 to 24 hours at 37°C40; (2) mild base treatment: [3H]-labeled AChE, VSG, and procyclin were treated with mild base (50 mmol/L NaOH in 90% [vol/vol] ethanol) for 2 hours at 37°C to remove ester-linked fatty acids41; (3) Pronase treatment: [3H]-labeled VSG and procyclin were treated with Pronase (5 mg/mL final concentration) in 50 mmol/L Tris-HCl, pH 7.5, containing 5 mmol/L CaCl2, for 16 hours at 37°C.
The radiolabeled reaction products were repurified by octyl-Sepharose chromatography as described above. Fractions containing radioactivity were pooled and dried in a speed vacuum system.
Incorporation of [3H]-labeled AChE, VSG, and procyclin into human erythrocytes.
Human erythrocytes were washed twice with incubation buffer (10 mmol/L Tris-HCl, pH 7.4, containing 144 mmol/L NaCl, 0.54 mmol/L adenine, 12.7 mmol/L inosine, and 2 g/L glucose) and resuspended in the same buffer at a hematocrit level of 16%. Subsequently, [3H]-labeled AChE (2,000 cpm/mL), VSG (2,000 cpm/mL), procyclin (2,500 cpm/mL), or the [3H]-labeled products derived from these proteins (1,000 cpm/mL), were added to the erythrocytes and the suspension was incubated for 1 hour at 37°C. After centrifugation for 5 minutes at 800g and at 10°C, the erythrocytes were washed twice with cold incubation buffer and the radioactivity in the supernatants was counted.
Treatment of erythrocytes with GPI-hydrolyzing phospholipases.
After incorporation of [3H]-labeled GPI-anchored proteins, erythrocytes were treated with 4.5 IU/mL GPI-PLD or 0.1 IU/mL PI-PLC for 3 hours at 37°C40 and the release of radioactivity from erythrocytes was determined in the supernatant after pelleting the cells.
Preparation of ghosts.
Erythrocyte ghost membranes were prepared according to the method of Dodge et al.42
Detergent extraction of erythrocyte ghosts.
Erythrocyte ghost membranes (0.5 mL) were incubated with 9.5 mL of extraction buffer (25 mmol/L HEPES, pH 7.5; 150 mmol/L NaCl; and 1% [wt/vol] Triton X-100) at 4°C or 37°C for 20 minutes followed by centrifugation at 12,000 rpm for 10 minutes at 4°C using a Sorvall SS-34 rotor (Du Pont Instruments, Wilmington, DE). The supernatant was saved and the pellet was resuspended in 200 μL water by sonication.
Density gradient centrifugation.
Detergent-insoluble fractions were layered on top of 20% to 40% (wt/vol) sucrose gradients (12-mL gradients with a 0.5-mL cushion of 60% [wt/vol] sucrose at the bottom) and centrifuged at 36,000 rpm for 15 hours at 4°C using a Centricon TST 41.14 rotor (Kontron Ltd, Zurich, Switzerland). After centrifugation, fractions of 0.4 mL were collected from the bottom and aliquots were removed for determination of protein, AChE activity, and radioactivity.
Vesiculation of human erythrocytes.
Human erythrocytes can be induced to vesiculate by loading the cells with calcium21 or by depletion of their intracellular adenosine triphosphate (ATP) stores.20 The release of membrane vesicles from erythrocytes can be monitored by following the increase in AChE activity in the cell-free supernatant after pelleting the cells.20,21,25 Calcium-induced and ATP depletion–induced vesiculation of human erythrocytes was performed exactly as described by Bütikofer et al.25 Briefly, for calcium loading, washed cells (16% hematocrit) were equilibrated in buffer (10 mmol/L Tris-HCl, pH 7.4; 144 mmol/L NaCl; and 2 g/L glucose) containing 1 mmol/L CaCl2 for 3 minutes at 37°C. Subsequently, the calcium ionophore A23187 was added to the suspension from a 10 mmol/L stock solution in ethanol to give a final concentration of 4 μmol/L, and the incubation was carried out for 1 hour at 37°C. Vesicle release was stopped by the addition of EDTA (12 mmol/L, final concentration) to the suspension. ATP depletion of erythrocytes was performed by incubating washed cells at a hematocrit level of 20% in buffer (10 mmol/L Tris-HCl, pH 7.4; 144 mmol/L NaCl; 1 mmol/L EDTA; 0.5 mmol/L adenine; 2 × 105 IU/L penicillin; and 1.5 × 105 IU/L streptomycin) at 37°C. After 4 to 6 hours of incubation, the erythrocytes were pelleted and the pH in the supernatant was readjusted to pH 7.4. Then the cells were resuspended and the incubation was continued for 40 hours under constant gentle shaking.
Isolation of vesicles.
At the end of the incubation, erythrocytes were pelleted by centrifugation for 5 minutes at 1,800 rpm and at 10°C and the vesicle-containing supernatant was collected. Vesicles were pelleted by centrifugation in a centrifuge (Micro Centaur, Loughborough, UK) for 20 minutes at 18,000 rpm and at 4°C and washed three times with large volumes of buffer (10 mmol/L Tris-HCl, pH 7.5; and 2 mmol/L EDTA).
Purification and iodination of monoclonal anti-human CD59 antibody.
YTH 53.1 rat monoclonal anti-human CD59 hybridoma cells were kindly provided by Dr Ethan Shevach (National Institutes of Health, Bethesda, MD). The antibody was purified from tissue culture supernatants by ammonium sulfate precipitation followed by chromatography on DEAE-Sephacel (Pharmacia Biotech, Uppsala, Sweden). The purified antibody was iodinated with 125I as NaI (Amersham Corp, Arlington Heights, IL) by using Iodogen (Pierce Chemical Co, Rockford, IL). The specific activity of the final preparation was 7.25 × 105 cpm/μg and 98.4% of the 125I was protein-bound as determined by precipitation with 10% trichloroacetic acid.
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotting of detergent-extracted erythrocyte membranes.
SDS-PAGE and electroblotting were performed using a Mini-PROTEAN II electrophoresis system and Mini Trans-Blot Cell (Bio-Rad Laboratories, Hercules, CA). Aliquots of detergent-extracted erythrocyte membranes containing 20 μg of protein were run on 12% SDS-PAGE gels under nonreducing conditions and transferred electrophoretically to polyvinylidene fluoride (PVDF) membranes (Bio-Rad Laboratories) at 100 V (0.25 A limit) for 1 hour in transfer buffer (25 mmol/L Tris, pH 8.5; 192 mmol/L glycine; and 20% [vol/vol] methanol). Completeness of transfer was assessed by silver staining the gels. No bands were visible at the predicted apparent molecular mass of CD59 (18 to 20 kD).
Quantitation of CD59 on electroblots of detergent-extracted erythrocyte membranes.
After transfer of protein onto PVDF membranes, the blots were incubated twice with blotting buffer (25 mmol/L Tris, pH 7.5; 137 mmol/L NaCl; 2.7 mmol/L KCl; 0.05% [vol/vol] Tween-20) containing 5% instant nonfat milk powder for 1 hour at room temperature. The blot then was incubated with 100 μg of 125I-labeled anti-CD59 in 8 mL of blotting buffer for 1 hour at room temperature, washed six times with the same buffer, and air dried. 125I bound to the blot was quantitated by exposing a storage phosphor screen (Molecular Dynamics, Sunnyvale, CA) to the blot for 16 hours at room temperature, imaging the exposed screen with a PhosphorImager SF (Molecular Dynamics), and analyzing the CD59 bands on the image of the blot using Molecular Dynamics ImageQuant software. To determine the linearity of transfer of CD59, aliquots of untreated erythrocyte membranes were run on gels simultaneously with the detergent-extracted membranes. Transfer of CD59 (as determined by probing blots with125I-labeled anti-CD59) was linear for membrane aliquots containing from 1.25 to 40 μg of protein.
Protein determination.
Protein was determined using the BCA reagent kit (Pierce Chemical Co) with bovine serum albumin as standard. Because erythrocyte-derived vesicles contain high amounts of hemoglobin and are resistant to hypotonic lysis, the amount of membrane protein in vesicles was determined by subtracting the amount of hemoglobin43 from the total amount of protein.
RESULTS
Preparation of GPI-anchored proteins and derivatives.
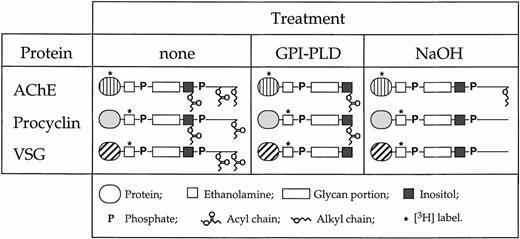
To study the incorporation of GPI-anchored proteins into human erythrocytes, we purified several GPI-anchored proteins from different sources and modified their GPI lipid moieties and protein parts by enzymatic and chemical treatment (Fig 1).[3H]DFP-labeled AChE from human erythrocytes, and [3H]ethanolamine-labeled VSG and procyclin from T brucei bloodstream and insect forms, respectively, were treated with GPI-PLD to remove the glycerol-bound lipid components from the GPI anchors. The GPI-PLD reaction products were separated from unreacted material by octyl-Sepharose chromatography. Intact GPI-anchored AChE, VSG, and procyclin bound to the column material and eluted after increasing the 1-propanol concentration from 5% to 40% (vol/vol)39,44 (Fig 2A). In contrast, the GPI-PLD–treated proteins no longer bound to the octyl-Sepharose and were recovered in the flow-through of the column (Fig 2A). The removal of the glycerol-bound lipid components by GPI-PLD treatment renders the GPI anchor of VSG completely hydrophilic, whereas the GPI moieties of AChE and procyclin still contain a fatty acid attached to the inositol (Fig 1). Treatment of GPI-anchored proteins with mild base results in the removal of ester-bound fatty acids from the GPI moiety.41 In the case of VSG and procyclin, base treatment removed all hydrophobic components from the GPI structures, whereas in the case of AChE, the long chain alkyl group remained attached to the glycerol (Fig 1). To generate radiolabeled GPI structures containing a small peptide or a single amino acid attached to an intact GPI moiety, purified [3H]ethanolamine-labeled VSG and procyclin were treated with large amounts of Pronase to digest the protein portion attached to the GPI anchor. We found that treatment of procyclin with Pronase decreased the apparent molecular masses of the EP and GPEET forms of procyclin from 42 kD and 22 to 32 kD, respectively, to a single broad band of 17 to 22 kD (Fig 2B). This band has been shown to represent the procyclin GPI anchor with only a single amino acid attached to it.39 Similarly, treatment of VSG with Pronase resulted in a significant reduction of its apparent molecular mass from 55 kD to < 6 kD (Fig 2B), indicating that most of the protein part was removed. Because of the limited supply of material, the exact extent of degradation by Pronase (ie, the N-terminal amino acid of the VSG fragment after Pronase treatment) was not determined. The [3H]-labeled material obtained after Pronase treatment of VSG and procyclin was rechromatographed on an octyl-Sepharose column. The GPI-linked structures were found to bind to the column material and eluted after increasing the 1-propanol concentration from 5% to a concentration of 25% to 30% (vol/vol) (results not shown).39 44
Schematic representation of the GPI anchors of AChE from human erythrocytes, VSG from T brucei bloodstream forms, and procyclin from T brucei insect forms. The purified proteins were incorporated into human erythrocytes with their intact GPI anchors, or after (partial) removal of the lipid moieties by GPI-PLD or NaOH treatment.
Schematic representation of the GPI anchors of AChE from human erythrocytes, VSG from T brucei bloodstream forms, and procyclin from T brucei insect forms. The purified proteins were incorporated into human erythrocytes with their intact GPI anchors, or after (partial) removal of the lipid moieties by GPI-PLD or NaOH treatment.
Purification and characterization of GPI-anchored proteins. (A) Delipidated SDS extracts from bloodstream form trypanosomes containing [3H]-labeled VSG before (▪) and after (□) treatment with GPI-PLD were applied to an octyl-Sepharose column (2 mL bed volume) previously equilibrated in 100 mmol/L ammonium acetate containing 5% (vol/vol) 1-propanol. The column was washed with 5 mL of the same buffer (fractions 1 through 10) followed by 5 mL of 100 mmol/L ammonium acetate containing 20% (vol/vol) 1-propanol (fractions 11 through 20), and was eluted with a linear gradient of 20% to 40% (vol/vol) 1-propanol in 100 mmol/L ammonium acetate (fractions 21 through 40). Fractions of 0.5 mL were collected and aliquots were counted for radioactivity. Intact VSG eluted at 31% 1-propanol. After treatment with GPI-PLD, VSG eluted in the column flow-through. Intact procyclin (P) and AChE (A) eluted at 25% and 35% 1-propanol, respectively (as indicated by the arrows), whereas GPI-PLD–treated procyclin and AChE eluted in the same fractions as GPI-PLD–treated VSG. The actual 1-propanol concentration in an individual fraction was determined by measuring the refractive index. (B) Purified radiolabeled VSG and procyclin were treated with large amounts of Pronase to completely digest the protein portions of the molecules followed by repurification by octyl-Sepharose chromatography. Control untreated VSG (lane a) and procyclin (lane c) and the corresponding Pronase reaction products (lanes b and d, respectively) were analyzed by SDS-PAGE and autoradiography.
Purification and characterization of GPI-anchored proteins. (A) Delipidated SDS extracts from bloodstream form trypanosomes containing [3H]-labeled VSG before (▪) and after (□) treatment with GPI-PLD were applied to an octyl-Sepharose column (2 mL bed volume) previously equilibrated in 100 mmol/L ammonium acetate containing 5% (vol/vol) 1-propanol. The column was washed with 5 mL of the same buffer (fractions 1 through 10) followed by 5 mL of 100 mmol/L ammonium acetate containing 20% (vol/vol) 1-propanol (fractions 11 through 20), and was eluted with a linear gradient of 20% to 40% (vol/vol) 1-propanol in 100 mmol/L ammonium acetate (fractions 21 through 40). Fractions of 0.5 mL were collected and aliquots were counted for radioactivity. Intact VSG eluted at 31% 1-propanol. After treatment with GPI-PLD, VSG eluted in the column flow-through. Intact procyclin (P) and AChE (A) eluted at 25% and 35% 1-propanol, respectively (as indicated by the arrows), whereas GPI-PLD–treated procyclin and AChE eluted in the same fractions as GPI-PLD–treated VSG. The actual 1-propanol concentration in an individual fraction was determined by measuring the refractive index. (B) Purified radiolabeled VSG and procyclin were treated with large amounts of Pronase to completely digest the protein portions of the molecules followed by repurification by octyl-Sepharose chromatography. Control untreated VSG (lane a) and procyclin (lane c) and the corresponding Pronase reaction products (lanes b and d, respectively) were analyzed by SDS-PAGE and autoradiography.
Incorporation of GPI-anchored proteins and derivatives into human erythrocytes.
Several purified GPI-anchored proteins have been shown to spontaneously incorporate in vitro into mammalian cells. However, their rate and extent of incorporation differs considerably depending on the system used.26,27 In addition, there is inconsistency on whether the exogenously added proteins become functional within the target cell membrane.33 45 In the present work, the availability of three different purified GPI-anchored proteins plus a set of well-defined structures derived from them, allowed us to systematically study the influence of different GPI anchor and protein components on the incorporation of GPI-anchored proteins into erythrocytes.
In a first set of experiments, erythrocytes were incubated in the presence of the intact [3H]-labeled GPI-anchored proteins AChE, procyclin, and VSG. Our results showed that after 1 hour of incubation at 37°C, 44% ± 14% of AChE, 68% ± 5% of procyclin, and 67% ± 3% of VSG added to the suspension could not be removed from erythrocytes by repetitive washing (Fig 3A, B, and C). In contrast, when the [3H]-labeled GPI-anchored proteins were incubated with the cells at 4°C, less than 4% of radioactivity was incorporated. Longer incubation times did not result in increased amounts of radioactivity in the erythrocyte pellets (results not shown). When the washed [3H]-labeled cells were subsequently incubated for 7 hours at 37°C, less than 2% of radioactivity was released into the cell-free supernatant after pelleting the cells, indicating that the radiolabeled proteins were stably associated with the erythrocytes.
Incorporation of exogenously added GPI-anchored proteins into human erythrocytes. Erythrocytes were incubated for 1 hour at 37°C in the presence of purified [3H]-labeled AChE (A), procyclin (B), and VSG (C), and thoroughly washed to remove nonincorporated material. The total radiolabel in the wash supernatants was counted and used to calculate the amounts of label incorporated into erythrocytes. The figure shows the relative incorporation of intact (bars a), GPI-PLD–treated (bars b), mild base-treated (bars c), and Pronase-treated (bars d) GPI-anchored proteins. The numbers represent the mean values ± SD from n independent experiments with the number of experiments shown in parentheses. *, Not determined.
Incorporation of exogenously added GPI-anchored proteins into human erythrocytes. Erythrocytes were incubated for 1 hour at 37°C in the presence of purified [3H]-labeled AChE (A), procyclin (B), and VSG (C), and thoroughly washed to remove nonincorporated material. The total radiolabel in the wash supernatants was counted and used to calculate the amounts of label incorporated into erythrocytes. The figure shows the relative incorporation of intact (bars a), GPI-PLD–treated (bars b), mild base-treated (bars c), and Pronase-treated (bars d) GPI-anchored proteins. The numbers represent the mean values ± SD from n independent experiments with the number of experiments shown in parentheses. *, Not determined.
When we treated the GPI-anchored proteins with GPI-PLD before their addition to erythrocytes, we found that they no longer incorporated into the cells (Fig 3A, B, and C). This result indicates that the removal of the glycerol moiety with one (procyclin) or two (AChE and VSG) fatty acyl or alcohol chains prevents incorporation of the protein into erythrocytes. Furthermore, the results show that the presence of a single fatty acid attached to the inositol (AChE and procyclin) is not sufficient to stably incorporate the protein into the cells. Similarly, we found that when all hydrophobic components of the GPI anchors of procyclin and VSG were removed by mild base treatment, the proteins no longer incorporated into erythrocytes (Fig 3B and C). In contrast, mild base-treated AChE still bound to the cells, although much less efficiently than the intact protein (Fig 3A). Because the ether-bound alkyl chain of AChE is not removed by base treatment, this finding indicates that a single long chain alkyl substituent on the glycerol moiety is sufficient to mediate incorporation of the protein into cells. When the protein parts of procyclin and VSG were removed by extensive proteolysis, the residual GPI-anchored structures were found to readily incorporate into erythrocytes (Fig 3B and C). However, while the extent of incorporation of the procyclin GPI anchor was only slightly decreased compared with the intact protein, incorporation of the VSG anchor was reduced by greater than 85%. It is possible that the different levels of incorporation of the two GPI anchor structures may relate to the different carbohydrate substituents present on the conserved GPI glycan backbones. VSG GPI anchors have been shown to contain very few extra carbohydrates attached to the GPI tetrasaccharide core46 and thus may form micellar structures when added to aqueous media and not readily incorporate into acceptor membranes. In contrast, the procyclin GPI anchors contain very large and complex glycosylated side chains44 47 that may prevent self-association and thus facilitate insertion into membranes.
Treatment of erythrocytes with GPI-hydrolyzing phospholipases.
After incorporation of [3H]-labeled GPI-anchored proteins, when the erythrocytes were treated with GPI-PLD for 3 hours at 37°C, less than 3% of incorporated radioactivity was released from the cells irrespective of the type of incorporated protein. Similarly, erythrocytes loaded with [3H]-labeled AChE or procyclin showed no release of radioactivity after treatment with bacterial PI-PLC. In contrast, PI-PLC treatment of erythrocytes loaded with [3H]-labeled VSG resulted in the release of greater than 50% of radioactivity after 3 hours of incubation at 37°C, whereas mock-treated erythrocytes released less than 2% of radioactivity. Hemolysis under these conditions was less than 1%.
Isolation of Triton X-100–insoluble fractions from human erythrocytes and partitioning of endogenous proteins.
In many different cell types GPI-anchored proteins have been found to partition preferentially into Triton X-100–insoluble fractions.8 To determine if detergent-resistant complexes are also present in human erythrocytes and if endogenous GPI-anchored proteins are enriched in these fractions, we analyzed the Triton X-100–insoluble fractions from erythrocyte ghosts for the presence of the endogenous GPI-anchored proteins AChE and CD59 (also known as membrane inhibitor of reactive lysis). We found that 47.2% ± 2.5% of total membrane protein (mean ± SD from six independent experiments) resisted extraction by Triton X-100 at 4°C. This number is in good agreement with a previous report showing that after extraction of human erythrocyte ghost membranes with cold 0.1% Triton X-100, approximately 45% of total protein was recovered in the detergent-insoluble residue.48 Protein analysis by SDS-PAGE showed that the Triton X-100–insoluble fraction from erythrocytes contained most of the major skeletal proteins, ie, spectrin, ankyrin, protein 4.1, and actin; whereas band 3, ie, the major integral membrane protein, together with protein 4.2 and band 6, were preferentially present in the soluble fraction (results not shown).48 In addition, we found that the endogenous GPI-anchored proteins, AChE and CD59, were enriched 1.69- and 1.55-fold, respectively, in the Triton X-100–insoluble fractions relative to bulk protein (Table1). The relative enrichment of AChE in the Triton X-100–insoluble residue was unchanged after purification by sucrose density gradient centrifugation, after which the material was found to band at 31% (wt/vol) sucrose (result not shown) as compared with the detergent-insoluble material from other membranes which has been found to band at 15% to 25% (wt/vol) sucrose.6 7 In contrast, when the detergent extraction was carried out at 37°C, very little AChE and CD59 were recovered in the Triton X-100–insoluble residue which represented 20.3% ± 2.8% of total membrane protein (mean ± SD from three independent experiments) (Table 1).
Partitioning of Endogenous and Exogenous GPI-Anchored Proteins in Triton X-100–Insoluble Fractions of Erythrocyte Ghost Membranes
| Protein . | Enrichment (Relative to Bulk Protein)* . | n . |
|---|---|---|
| Endogenous AChE | 1.69 ± 0.10 | 9 |
| Endogenous AChE (37°C) | 0.016 ± 0.003 | 9 |
| Endogenous CD59 | 1.55 ± 0.05 | 2 |
| Endogenous CD59 (37°C) | 0.019 ± 0.012 | 2 |
| Exogenous AChE | 0.59 ± 0.04 | 3 |
| VSG | 0.88 ± 0.06 | 8 |
| VSG GPI anchor | 0.20 | 1 |
| Procyclin | 0.90 ± 0.09 | 3 |
| Procyclin GPI anchor | 0.20 ± 0.05 | 3 |
| Procyclin after incubation-151 | 0.94 | 1 |
| Procyclin GPI anchor after incubation-151 | 0.20 | 1 |
| Protein . | Enrichment (Relative to Bulk Protein)* . | n . |
|---|---|---|
| Endogenous AChE | 1.69 ± 0.10 | 9 |
| Endogenous AChE (37°C) | 0.016 ± 0.003 | 9 |
| Endogenous CD59 | 1.55 ± 0.05 | 2 |
| Endogenous CD59 (37°C) | 0.019 ± 0.012 | 2 |
| Exogenous AChE | 0.59 ± 0.04 | 3 |
| VSG | 0.88 ± 0.06 | 8 |
| VSG GPI anchor | 0.20 | 1 |
| Procyclin | 0.90 ± 0.09 | 3 |
| Procyclin GPI anchor | 0.20 ± 0.05 | 3 |
| Procyclin after incubation-151 | 0.94 | 1 |
| Procyclin GPI anchor after incubation-151 | 0.20 | 1 |
*The numbers represent mean values ± SD from n independent experiments. Except where indicated, the extractions were carried out at 4°C.
Detergent extraction was performed after incubation of erythrocytes for 24 hours at 37°C.
Partitioning of in vitro incorporated GPI-anchored proteins in Triton X-100–insoluble fractions.
To study the partitioning of exogenously added GPI-anchored proteins in detergent-resistant fractions, [3H]-labeled AChE, procyclin and VSG, as well as the procyclin and VSG GPI anchor structures, were incorporated into erythrocytes and their distribution in Triton X-100–resistant fractions at 4°C was measured. Our results show that the specific radioactivity of all exogenously added radiolabeled GPI-anchored proteins in the detergent-resistant complexes was decreased compared with ghost membranes (Table 1). Thus, in contrast to endogenous AChE and CD59, the incorporated GPI-anchored structures were depleted from Triton X-100–insoluble complexes. Interestingly, the procyclin and VSG GPI anchors were depleted more severely (fivefold) from the detergent-resistant complexes than the intact proteins (<1.7-fold). Because it has been reported that exogenous GPI-anchored proteins may redistribute into detergent-resistant fractions after equilibration in the membrane,33 we incubated erythrocytes preloaded with radiolabeled procyclin or procyclin GPI anchor for 24 hours at 37°C and determined the distribution of radioactivity in the Triton X-100–insoluble fractions. Our results showed that the specific radioactivity in the detergent-resistant fractions from these equilibrated erythrocytes was unchanged compared with extracts from freshly labeled cells (Table 1).
Release of GPI-anchored proteins during vesiculation of erythrocytes.
It has been shown before that the endogenous GPI-anchored proteins, AChE, CD55, and CD59, are preferentially released from human erythrocytes by vesiculation.20,21 25 As a result of this process, GPI-anchored proteins are depleted in the remnant cells and highly enriched in the released vesicles. To study whether GPI-anchored proteins incorporated in vitro would behave similarly, we followed the release of incorporated [3H]-labeled proteins from erythrocytes during Ca2+-induced vesiculation. Our results showed that during 1 hour of Ca2+-loading, the in vitro incorporated VSG was progressively released from erythrocytes together with the endogenous AChE (Fig 4). However, while we typically observed a release of 30% to 40% of total endogenous AChE, only 10% to 15% of incorporated VSG was shed from erythrocytes. A similar release was also observed for incorporated AChE and procyclin (results not shown). When vesiculation was induced 2 hours or 7 hours after incorporation of GPI-anchored proteins into erythrocytes, the amounts of radiolabel released into the cell-free supernatant after pelleting the cells were similar (results not shown). In contrast, less than 1% of the radiolabel was released from erythrocytes loaded with the radiolabeled GPI anchors of VSG (Fig 4) and procyclin (result not shown). Our observation that greater than 94% of radioactivity and greater than 96% of AChE activity in the vesicle-containing supernatants could be pelleted by high speed centrifugation shows that the GPI-anchored proteins were firmly associated with the vesicles.
Release of endogenous AChE and in vitro incorporated VSG from human erythrocytes during Ca2+-induced vesiculation. [3H]-labeled GPI-anchored molecules were incorporated into erythrocytes as described in Fig 3 and the release of membrane vesicles was induced by loading the cells with calcium using the ionophore A23187. At designated times, erythrocytes were pelleted and the AChE activity (▪) and the radioactivity (○, ◊) were measured in the vesicle-containing supernatants of cells labeled with in vitro incorporated VSG (○) and Pronase-treated VSG (◊). The values represent single determinations from a typical experiment; corresponding results were obtained with in vitro incorporated AChE, procyclin, and Pronase-treated procyclin.
Release of endogenous AChE and in vitro incorporated VSG from human erythrocytes during Ca2+-induced vesiculation. [3H]-labeled GPI-anchored molecules were incorporated into erythrocytes as described in Fig 3 and the release of membrane vesicles was induced by loading the cells with calcium using the ionophore A23187. At designated times, erythrocytes were pelleted and the AChE activity (▪) and the radioactivity (○, ◊) were measured in the vesicle-containing supernatants of cells labeled with in vitro incorporated VSG (○) and Pronase-treated VSG (◊). The values represent single determinations from a typical experiment; corresponding results were obtained with in vitro incorporated AChE, procyclin, and Pronase-treated procyclin.
Furthermore, in agreement with a previous report,25 we found that the endogenous AChE was highly enriched (4.9-fold relative to bulk membrane protein) in the released vesicles compared with untreated cells (Table 2). In contrast, the relative enrichment of [3H]-labeled AChE, VSG, and procyclin in the vesicles was 0.9- to 1.7-fold compared with untreated ghost membranes (Table 2). Similar results were obtained when the release of vesicles was induced by ATP-depletion of erythrocytes (results not shown). These findings show that in contrast to endogenous AChE and CD55,25 and CD59,49 the exogenously added GPI-anchored proteins are not preferentially released from erythrocytes during vesiculation.
Enrichment of GPI-Anchored Proteins in Ca2+-Induced Vesicles
| Protein Incorporated Into Erythrocytes . | Enrichment in Released Vesicles (Relative to Ghost Membranes) . |
|---|---|
| Endogenous AChE | 4.90 ± 0.73* |
| Exogenous AChE | 0.90/1.60† |
| VSG | 1.20/1.68† |
| Procyclin | 1.40/1.42† |
| Protein Incorporated Into Erythrocytes . | Enrichment in Released Vesicles (Relative to Ghost Membranes) . |
|---|---|
| Endogenous AChE | 4.90 ± 0.73* |
| Exogenous AChE | 0.90/1.60† |
| VSG | 1.20/1.68† |
| Procyclin | 1.40/1.42† |
*The numbers represent the mean value ± SD from 10 independent experiments.
The numbers are from two independent experiments.
DISCUSSION
Incorporation of purified GPI-anchored molecules into plasma membranes in vitro provides a general means for modifying cell surfaces with exogenously added proteins.26,27 It has been reported that GPI-anchored molecules may incorporate directly into GPI-enriched microdomains50 or alternatively, insert randomly and diffuse laterally in the plane of the plasma membrane until they reach microdomains rich in GPI-linked structures.33 The reported biological activity of some of the incorporated GPI-linked molecules is taken as evidence for their correct integration into the target cell membrane. However, it is not clear if the correct localization and functionality of an inserted GPI-anchored molecule in the membrane is typical for certain proteins or if different exogenously added GPI-anchored proteins would colocalize in the same microdomains.
In the present work we incorporated the GPI-anchored proteins AChE, VSG, and procyclin into human erythrocytes and studied their fate in the membrane. The use of a series of structures derived from these proteins having different GPI lipid moieties allowed us to study the roles of the lipid and protein parts of the molecules during incorporation into target cells. We found that efficient incorporation of GPI-anchored proteins into human erythrocytes only occurred when the GPI lipid moiety was intact. These results are in good agreement with a previous study using modified forms of CD55 incorporated into sheep erythrocytes.34 The reasons for the considerably higher extent of incorporation of GPI-anchored proteins into erythrocytes observed in our work (40% to 60%) compared with the earlier study (1% to 3%)34 are not known. In addition, our results showed that the incorporated proteins could not be released from the cells by GPI-PLD treatment which is consistent with the reported inability of the enzyme to cleave membrane-bound GPI-anchored proteins.51 Similarly, because PI-PLC is known to be inactive on inositol-acylated GPI-linked structures,52incorporated AChE and procyclin could not be released from the erythrocytes by PI-PLC treatment, whereas the in vitro incorporated VSG with its PI-PLC–sensitive GPI anchor was readily released. Together with the observation that the incorporated proteins remained associated with the cells during prolonged incubation at 37°C, these results show that the exogenously added GPI-anchored proteins inserted stably and in a correct orientation into erythrocyte membranes. The view that membrane insertion is mediated primarily by the GPI lipid moiety is further supported by our finding that the isolated procyclin and VSG GPI anchors (obtained by Pronase treatment of procyclin and VSG, respectively) also readily incorporated into erythrocytes. The observation that the free GPI anchors were less efficiently inserted into the membrane than the intact proteins suggests that the protein portions may play an important role in facilitating incorporation of GPI-linked molecules into membranes, possibly by decreasing their ability to form micellar structures.
Interestingly, we found that a single fatty acyl chain attached to the inositol was unable to insert the protein into the target cell membrane, whereas a single alkyl chain on the glycerol was sufficient to mediate incorporation. This finding contradicts an earlier report showing that inositol-acylated CD55 after GPI-PLD treatment was able to insert into sheep erythrocytes, although to a much smaller extent than the intact protein.34 This apparent discrepancy may be explained by the purity of the protein preparation. While in our study the GPI-PLD–treated proteins were separated from nonreacted material by octyl-Sepharose chromatography, the GPI-PLD–treated CD55 in the earlier study may have been contaminated with residual amounts of intact protein.
Treatment of human erythrocytes with Triton X-100 at 4°C has been reported to result in the solubilization of most of the major integral and cell surface proteins.48 In contrast, most skeletal proteins (ie, >40% to 50% of total protein) together with greater than 80% of sphingomyelin were retained in the detergent-insoluble residue. In the present report we found that under the same conditions the majority of the endogenous GPI-anchored proteins, AChE (>75%) and CD59 (>55%), also resisted extraction by Triton X-100. The two proteins were enriched 1.5- to 1.7-fold relative to bulk protein in the detergent-resistant complexes compared with untreated ghost membranes. In contrast, when erythrocytes loaded with the exogenously added radiolabeled AChE, VSG, or procyclin were extracted with Triton X-100 at 4°C, the incorporated proteins were found not to be enriched in the detergent-resistant complexes. In fact, the relative specific radioactivities of the incorporated proteins in Triton X-100–insoluble complexes were lower than in intact membranes showing that, in contrast to the endogenous GPI-anchored proteins, the inserted proteins were mostly solubilized by Triton X-100 at 4°C. In addition, the solubility in Triton X-100 of the incorporated GPI-anchored molecules did not change during incubation of erythrocytes at 37°C, indicating that the proteins did not undergo time-dependent redistribution into detergent-insoluble domains, as has been shown to occur for CD59 incorporated into U937 monocytic cells.33
Vesicles released from human erythrocytes have been found to be enriched in several endogenous GPI-anchored proteins, as we and others20-22,25 have shown. Although the process of membrane vesiculation has been shown to involve the detachment of the skeletal protein network from the membrane,23,53 the exact mechanism leading to a release of GPI-enriched vesicles has not been elucidated. It has been proposed that the reported increased lateral mobility of some GPI-anchored proteins compared with transmembrane proteins54-56 may allow them to (rapidly) move into the membrane spicules that are formed before vesicle release.53 However, we now found that the incorporated [3H]-labeled GPI-anchored proteins, AChE, VSG, and procyclin, were not enriched in the released vesicles, indicating that an increased lateral mobility of GPI-anchored proteins per se cannot account for their enrichment in the vesicles. Alternatively, our results suggest that GPI-enriched microdomains may pre-exist in the erythrocyte membrane and represent the sites of membrane vesicle formation from which exogenously added GPI-anchored proteins are excluded. Similar to the situation in other cells,8 such GPI-enriched microdomains in human erythrocytes may also contain other non–GPI-linked membrane proteins. One of these proteins may be the complement receptor 1 (C3b receptor, CD35), a transmembrane glycoprotein, which has been shown to be preferentially lost from human erythrocytes during vesiculation induced by ATP-depletion.57 The unique composition of the microdomains released from human erythrocytes during vesiculation may have important physiological consequences because vesiculation has been reported to be involved in the aging process of human erythrocytes both in vivo and in vitro.23 58-60
Interestingly, when we incorporated the VSG and procyclin GPI anchors instead of the intact proteins into erythrocytes, we found that they were almost completely absent from the detergent-resistant complexes. This indicates that the described insolubility of membrane-bound GPI-anchored proteins in Triton X-100 at 4°C results from interactions of the GPI-linked molecules with detergent-insoluble membrane components and not from a (partial) insolubility of the GPI anchor. In addition, we found that the incorporated VSG and procyclin GPI anchors were not released from erythrocytes during vesiculation, indicating that the GPI anchor by itself does not incorporate, or relocate, into the GPI-rich membrane microdomains that are involved in vesiculation.
In summary, our results show that purified GPI-anchored proteins added to human erythrocytes readily incorporate into the plasma membrane in a correct orientation. However, unlike the endogenous GPI-anchored proteins, the exogenously added GPI molecules are readily solubilized by Triton X-100 at 4°C. In addition, they are not released from erythrocytes together with the endogenous GPI-anchored proteins during vesiculation. Thus, although in some cases exogenously added GPI-linked molecules have been shown to be biologically functional within the acceptor cell membrane,28,33 61 they may not necessarily colocalize with the endogenous GPI-anchored proteins in specific microdomains. Alternatively, it is possible that only a small fraction of incorporated GPI-anchored molecules partitions into the pre-existing GPI-rich domains but that this fraction may be sufficient to infer a biological activity.
The recent development of novel methods to study the lateral mobility of membrane proteins has led to a revision of the fluid mosaic model initially proposed by Singer and Nicolson.62The ‘transient interaction model’45,63 and the ‘membrane-skeleton fence model’64 propose that the observed confinement of a protein in the plane of a membrane may result from it being transiently trapped by the membrane-apposed skeletal protein network or by direct interactions with proteins bound to the skeleton. In addition, the lateral mobility of a GPI-anchored protein may be restricted by its association with specific lipid microdomains.63-65 Our observation that exogenously added GPI-anchored molecules are unable to redistribute into pre-existing GPI-rich membrane microdomains may be explained by either model. Of course, these models are not mutually exclusive and it is likely that combinations of them exist. In addition, these models also offer a possible mechanism for the shedding of vesicles from erythrocytes. Because the released vesicles are enriched in GPI-anchored proteins and devoid of skeletal proteins, vesicle formation likely occurs at specific sites in the membrane. Such a proposed initiation point for vesiculation may be identical with the above mentioned ‘fenced’ areas rich in GPI-anchored proteins and explain why exogenously added GPI-anchored molecules are not enriched in the released vesicles.
ACKNOWLEDGMENT
We thank the ZLB Central Laboratory, Swiss Red Cross Blood Transfusion Service (Bern, Switzerland) for supplying fresh human erythrocytes. We also thank Joyce Mitsuyoshi and Monika Boschung for excellent technical assistance during parts of the study and Tracy Chapman for stimulation.
Supported by Swiss National Science Foundation Grants No. 31-039209.93 and 31-049458.96 and by National Institutes of Health Grant No. HL 20985.
Address reprint requests to Peter Bütikofer, PhD, Institute of Biochemistry and Molecular Biology, University of Bern, Bühlstrasse 28, CH-3012 Bern, Switzerland.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 2. Purification and characterization of GPI-anchored proteins. (A) Delipidated SDS extracts from bloodstream form trypanosomes containing [3H]-labeled VSG before (▪) and after (□) treatment with GPI-PLD were applied to an octyl-Sepharose column (2 mL bed volume) previously equilibrated in 100 mmol/L ammonium acetate containing 5% (vol/vol) 1-propanol. The column was washed with 5 mL of the same buffer (fractions 1 through 10) followed by 5 mL of 100 mmol/L ammonium acetate containing 20% (vol/vol) 1-propanol (fractions 11 through 20), and was eluted with a linear gradient of 20% to 40% (vol/vol) 1-propanol in 100 mmol/L ammonium acetate (fractions 21 through 40). Fractions of 0.5 mL were collected and aliquots were counted for radioactivity. Intact VSG eluted at 31% 1-propanol. After treatment with GPI-PLD, VSG eluted in the column flow-through. Intact procyclin (P) and AChE (A) eluted at 25% and 35% 1-propanol, respectively (as indicated by the arrows), whereas GPI-PLD–treated procyclin and AChE eluted in the same fractions as GPI-PLD–treated VSG. The actual 1-propanol concentration in an individual fraction was determined by measuring the refractive index. (B) Purified radiolabeled VSG and procyclin were treated with large amounts of Pronase to completely digest the protein portions of the molecules followed by repurification by octyl-Sepharose chromatography. Control untreated VSG (lane a) and procyclin (lane c) and the corresponding Pronase reaction products (lanes b and d, respectively) were analyzed by SDS-PAGE and autoradiography.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1784/3/m_blod4052602.jpeg?Expires=1769101756&Signature=e~f0B6JJ1QPwhbLFvKxne-OCHaURc4znIbEESddTjBGUf5opJJcHf46YIQiw4LGloYGVXtOPp4qIPFWeEpj9G3BPEpfnyOxtQDEvKOVFiIiIuMif4ZsylLGEDWw-xiOhaFPx4VPcYYFTx~5CmEOHA3ZHhKxQDnHq0bzh9ybE~ypScI-b5xgeM9RBZU~eRkF0qu7wgrkjRAu3oXl17YxBznu33pUSFOTdRISYxf7HASxx65RQgZq2hHUjIPYwyYEAfKFSyh7USxBm66fGovQa8oHk8LoCgorhU0dT4riuLIu5n4tlHelenOAdSay6FZY0xvxGuX0jMlDphPCMA-Xz~g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Incorporation of exogenously added GPI-anchored proteins into human erythrocytes. Erythrocytes were incubated for 1 hour at 37°C in the presence of purified [3H]-labeled AChE (A), procyclin (B), and VSG (C), and thoroughly washed to remove nonincorporated material. The total radiolabel in the wash supernatants was counted and used to calculate the amounts of label incorporated into erythrocytes. The figure shows the relative incorporation of intact (bars a), GPI-PLD–treated (bars b), mild base-treated (bars c), and Pronase-treated (bars d) GPI-anchored proteins. The numbers represent the mean values ± SD from n independent experiments with the number of experiments shown in parentheses. *, Not determined.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1784/3/m_blod4052603.jpeg?Expires=1769101756&Signature=UqDVov-7TR0lZvUTjsHnUbcKzHqcn9Tc7HpNQSvRcjjX8li~HlQ-HkKg3S7M3CxLOMSf1YfkG50jLJBVqo2PcZZHMGRup0zTR57QfBxQBSf9~B3P6xMppLskQe3xN6JuBNDEcpI0Vh6ZkTTYHxaNOtw6xUve3ObtEw8HxE1Z25qUXsBqP3RJtflamlzl~wzKO0SZc40yOChke43YNAt68hRZZPIRuJZHivBZ3M-5Igf8SOgZbqJZgIT9Cbc9HK~11EcNBcf6L0VsvWciKcfKjesi~xNb7w8jwOLBs~-rUVkubLztfhtfxuwfLB2Z7uwHmfwEiuhswlqYDrBhJKzGmg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Release of endogenous AChE and in vitro incorporated VSG from human erythrocytes during Ca2+-induced vesiculation. [3H]-labeled GPI-anchored molecules were incorporated into erythrocytes as described in Fig 3 and the release of membrane vesicles was induced by loading the cells with calcium using the ionophore A23187. At designated times, erythrocytes were pelleted and the AChE activity (▪) and the radioactivity (○, ◊) were measured in the vesicle-containing supernatants of cells labeled with in vitro incorporated VSG (○) and Pronase-treated VSG (◊). The values represent single determinations from a typical experiment; corresponding results were obtained with in vitro incorporated AChE, procyclin, and Pronase-treated procyclin.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1784/3/m_blod4052604.jpeg?Expires=1769101756&Signature=eOrNVJ0~nWgBBbrOCyLpjngiJYJ5U~jLtX1y6Iuy9qqvWqg3nr03rDSgiqw4QuUwlMgsB-L~2Y3HYx8BY0qIaYAHGMsZ1NYWoC7VE2SDi1RbHeO0YYdfxeGwD8NgzJrPfvulChP6EMQTjoqhqgqR862MpIoZp4aKfI32G36nLl5Y7TKRizYapczp0NbmQuuE12m5CPFJm-2yfzIjLJV6Og4pclPHzYKwHS~VFO~VosuSSm8~TJilwXM0TCNAnmTaC84TuIFOeTPqXyxsZWlQcEXipewK3MRwgNpGeMvbrOraU3IQYh7U4ZM5uhkBSFkzrJc5v-5pBbGI2OVsJxU-zg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal