Abstract
Tumor cells have been found in autologous hematopoietic cell transplants used after high-dose chemotherapy. To specifically eliminate contaminating mammary tumor cells during ex vivo expansion of CD34+ hematopoietic progenitor cells, we used recombinant immunotoxins (ITs) directed against cell-surface antigens expressed on mammary carcinoma cells. ITs were expressed from fusion cDNAs combining a single-chain antibody fragment (scFv) directed against the Erb-B2 or epidermal growth factor (EGF) receptors with a truncatedPseudomonas exotoxin A fragment devoid of its cell-binding domain. CD34+ hematopoietic progenitor cells did not express Erb-B2 and EGF receptors as detected by Western blotting. Ex vivo expansion of total hematopoietic cells or of colony-forming cells from CD34+ progenitors in the presence of stem-cell factor (SCF), interleukin-1 (IL-1), IL-3, IL-6, and erythropoietin (Epo) was not affected when ITs were added to the cultures. In contrast, MDA-MB 453 and MCF-7 mammary carcinoma cells were depleted in a dose- and time-dependent manner by more than 3 log in coculture with CD34+ cells over a period of 7 days in the presence of 100 to 1,000 ng/mL of anti–Erb-B2 IT. This included elimination of the subpopulations with regrowth potential. Similarly, addition of either anti–Erb-B2 or anti-EGF receptor ITs to primary breast cancer cells isolated from patients with metastatic disease resulted in elimination of cytokeratin-positive cells in seven of seven samples. ITs are highly efficient and convenient to use for the depletion of mammary tumor cells during ex vivo expansion of hematopoietic progenitor-cell autografts.
HIGH-DOSE CHEMOTHERAPY in combination with the transplantation of autologous hematopoietic progenitor cells is being used for the treatment of hematologic malignancies and a variety of solid tumors.1 Clinical trials using high-dose chemotherapy protocols in patients with breast cancer have suggested an increased therapeutic benefit when compared with conventional chemotherapy regimens. This includes increased quality of life, improved response rates, and prolonged survival.2,3However, tumor-cell contamination of autologous grafts has been demonstrated in various malignancies and could contribute to disease relapse. This was shown by genetic marking of tumor cells within the graft.4-6 Tumor cells have been found at an incidence of up to one per 104 cells in bone marrow, and at approximately one per 105 cells within mobilized peripheral blood harvests of breast cancer patients.7,8 An indication that reinfused contaminating breast cancer cells may contribute to disease relapse in breast cancer comes from experiments showing that mammary tumor cells residing in bone marrow are capable of in vitro growth.7,9 In addition, reports on breast cancer patients and patients with gynecologic cancer suggest a positive association between numbers of tumor cells reinfused after transplant and adverse clinical outcome.10-13
Enrichment of CD34+ hematopoietic progenitor cells from leukapheresis products has been shown to reduce tumor-cell numbers in transplants.14 However, large-scale selection of CD34+ cells has been found less efficient compared with that found in laboratory scale experiments, with purities of 50% to 85%. Residual tumor cells have been demonstrated in autologous grafts after CD34+ selection15 (and our own unpublished results). Therefore, additional methods have been investigated to achieve tumor-cell–free grafts, such as ex vivo culture and expansion of hematopoietic progenitor cells.16For ex vivo expansion, hematopoietic cells are seeded into culture medium in the presence of a combination of hematopoietic growth factors, resulting in 100- to 1,000-fold amplification of total cell numbers and up to 100-fold amplification of lineage-committed colony-forming progenitors.16-19 More primitive hematopoietic stem-cell populations, eg, long-term bone marrow culture-initiating cells (LTCIC) have been found to be maintained at input numbers or to amplify to a limited degree during ex vivo expansion.19,20 CD34+ blood progenitor cells expanded ex vivo in the presence of stem-cell factor (SCF), interleukin (IL)-1β, IL-3, IL-6, and erythropoeitin (Epo) have been used to mediate hematopoietic reconstitution after high-dose chemotherapy in patients with solid tumors.21 However, tumor cells from epithelial malignancies have been found to survive during ex vivo expansion cultures.22
Immunotoxins (ITs) were initially developed for the elimination of lymphocytes from allogeneic bone marrow transplants23,24 by conjugating potent toxins of plant or bacterial origin to monoclonal antibodies, or recombinant antibody variant chain domains, which specifically target surface antigens. ITs that target epithelial cells of certain tumor-cell types have subsequently been constructed.25-27 In this study, we used single-chain antibody fragment (scFv) directed against the Erb-B2 receptor or against the epidermal growth factor (EGF) receptor genetically fused to a modified Pseudomonas exotoxin A.27,28Pseudomonas exotoxin A consists of several functional domains that are responsible for the binding to the surface of mammalian cells, uptake and translocation to the cytosol, and catalytic activity. Upon cell binding and internalization, exotoxin A is cleaved by a cellular protease within its domain II and a N-terminal 28-kD and a C-terminal 37-kD fragment are generated. After reduction of a disulfide bond, the C-terminal enzymatically active fragment translocates to the cytoplasm. The catalytic domain of exotoxin A adenosine diphosphate (ADP)-ribosylates and inactivates eukaryotic elongation factor 2, an essential component in protein synthesis.29 Both the Erb-B2 and EGF receptor have been found to be overexpressed in a high percentage of mammary-cell carcinomas.30 This study investigated the potential of these ITs to deplete autologous stem-cell grafts from contaminating tumor cells during ex vivo culture. We found that ITs directed against Erb-B2 and EGF receptors spare hematopoietic cells, and at the same time deplete tumor cells from cocultures with CD34+ cells by 3 log within a period of 5 to 7 days. ITs are potent tools for purging breast cancer cells from hematopoietic cell populations.
MATERIALS AND METHODS
Breast cancer cells.
Breast cancer cell lines MDA-MB-453 and MCF-7 were obtained from the American Type Culture Collection (ATCC, Rockville, MD) and grown in Dulbecco's modified minimal essential medium (DMEM; BioWhittaker, Verviers, Belgium) supplemented withl-glutamine and 10% fetal calf serum (FCS; Pan Systems, Passau, Germany). Primary breast cancer cells were isolated from bone marrow samples, ascites, or pleural effusions from patients with metastatic disease after informed consent. Cells were washed in phosphate-buffered saline (PBS) and subsequently cultured in α-MEM (GIBCO-BRL, Paisley, Scotland) and 10% FCS for up to 7 days before use. Cell populations isolated with this method contained greater than 60% cytokeratin-positive cells, as identified by immunostaining, and were used as a source of cells for the evaluation of IT treatment on primary breast cancer cells. Cells were detached with trypsin-ethylenediamine tetraacetic acid (EDTA) solution (Sigma, Munich, Germany) immediately before use.
Expression and purification of ITs.
The genes that encode the ErbB2 and the EGF receptor specific recombinant single-chain antibody toxins scFv(FRP5)-ETA and scFv(14E1)-ETA were constructed by fusing a truncated Pseudomonas aeruginosa exotoxin A gene (ETA) to the respective scFv gene derived from the genes that encode the light- and heavy-chain variable domains of the monoclonal antibodies FRP5 and 14E1, respectively, as described previously.27 28 Briefly, the resulting plasmids were grown in Escherichia coli strain HB 101 and fusion proteins were purified from inclusion bodies on Ni2+ loaded chelating sepharose columns (Pharmacia, Uppsala, Sweden), eluted in two steps with 50 mmol/L and 150 mmol/L imidazole in solubilization buffer, respectively, and dialyzed overnight against PBS containing 400 mmol/L arginine followed by dialysis against PBS. Purified protein was analyzed by sodium dodecyl sulfate–polyacrylamide gel electropheresis (SDS-PAGE) and quantitated by densitometry after Coomassie-blue staining in comparison to bovine serum albumin (BSA) standards.
Detection of Erb-B2 and EGF receptor expression by immunoblotting.
Human tumor cells and purified human CD34+ cells were extracted in lysis buffer (50 mmol/L Tris-Cl, pH 7; 5 mmol/L EDTA; 1% Triton X-100; 150 mmol/L NaCl; 1 mmol/L phenylmethylsulfonyl fluoride [PMSF]; 80 μg/mL aprotinin, 50 μg/mL leupeptin; 4 μg/mL pepstatin). A431 and SKBR3 cells from ATCC were used as a positive control. Extracts were subjected to a 7.5% SDS-PAGE and proteins were electroblotted onto nylon membranes. The p185Erb-B2 and p170 EGF receptor proteins were detected using the 21N and 12E antisera, respectively27 28 and an enhanced chemiluminescence detection kit (Amersham, Braunschweig, Germany).
CD34+ blood progenitor-cell selection and expansion.
Peripheral blood progenitor cells were obtained from patients with solid tumors (two with bronchial carcinoma, two with mammary carcinoma) or lymphomas (two) after informed consent. Mobilization of progenitors into blood was induced by a 1-day course of standard-dose chemotherapy that included etoposide, ifosfamide, cisplatin, and epirubicin, and subsequent daily application of granulocyte colony-stimulating factor (G-CSF). Aphereses were performed using Baxter CS3000 cell separators (Baxter, Round Lake, IL) on day 10 to 12 after the start of mobilization. CD34+ cells were selected using Ceprate LC columns (CellPro, Bothell, WA) according to the manufacturer's instructions. The purity of the CD34+-enriched cells was analyzed by flow cytometry on a FACScan (Becton Dickinson, Heidelberg, Germany) using phycoerythrin-conjugated monoclonal HPCA-2 anti-CD34 antibody (Becton Dickinson). Expansion cultures were performed in 24-well plates at 37°C in a 5% CO2 atmosphere using RPMI 1640 medium (BioWhittaker, Brussels, Belgium) that contained 10% FCS and 3 ng/mL IL-1β (Genzyme, Darmstadt, Germany), 100 ng/mL IL-3 and IL-6 (Novartis, Basel, Switzerland), 10 ng/mL SCF (Genzyme), and 1 U/mL Epo (Cilag, Sulzbach, Germany). CD34+ cells (purity, 50% to 90%) were seeded at 3 × 104 total cells/mL in a culture volume of 1 mL. Various concentrations of the ITs were added to duplicate cultures immediately after seeding the CD34+cells into the 24-well plates. Nucleated cell counts were performed in a hemocytometer using trypan blue exclusion of the dead cells. Colony-forming cells in the expansion cultures were determined in semisolid cultures as previously described.19 Briefly, 3,000 CD34+ cells (day 0) or 50,000 expanded cells were suspended in duplicate in 1-mL culture dishes supplemented with 0.9% methylcellulose in Iscove's modified Dulbecco's medium (IMDM), 30% FCS, 100 ng/mL IL-3, 100 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Novartis), and 1 U/mL Epo. Colonies with greater than 50 cells were scored under a light microscope on day 14 after plating. Analogous clonogenic assays of the CD34+fraction (day 0) were performed in the presence of various concentrations of the ITs.
Coculture of CD34+ progenitor cells and breast cancer cells.
Tumor cells (MDA-MB-453 and MCF-7) were mixed with 3 × 104 CD34+ blood progenitor cells at a ratio of tumor cells to CD34+ cells of 1:10, cultured in 1 mL IMDM, 10% FCS, 3 ng/mL IL-1β, 100 ng/mL IL-3 and IL-6, 10 ng/mL SCF, and 1 U/mL Epo, and treated with the ITs at various concentrations. All experiments were performed in duplicate in 24-well plates. The efficacy of ITs to eliminate the tumor cells during ex vivo expansion of CD34+ blood progenitor cells was calculated after immunocytochemical staining of untreated and treated cell suspensions using anticytokeratin monoclonal antibodies.
Effect of ITs on primary breast cancer cells.
Enriched primary breast cancer cells (>60% cytokeratin-positive cells) isolated from the patient samples as described earlier were seeded into 24-well plates in FCS-supplemented α-MEM with the indicated concentrations of ITs. The starting cell density was 20,000 cells/cm2 and the culture volume was 1 mL. Following a 7-day incubation with no medium changes, cells were trypsinized, counted, and processed for immunocytologic tumor-cell quantitation analyses. IT-mediated tumor-cell depletion was expressed relative to tumor-cell numbers of untreated control cultures. Experiments were performed in duplicate. Control untreated cultures showed that the cytokeratin-positive cells were essentially nondividing over the 7-day culture period.
Tumor-cell clonogenic assay.
The number of viable clonogenic tumor cells after IT treatment was assessed in limiting dilution clonogenic assays. MDA-MB-453 and MCF-7 cells were seeded into 24-well plates (2 × 104/well) and treated with different concentrations (up to 1,000 ng/mL) of ITs for a 4-day period. The supernatant was then removed and the adherent cells were detached with trypsin-EDTA and subsequently reseeded in 100 μL of normal growth medium without addition of ITs in 96-well plates via the automatic cell deposition unit of a MoFlo flow cytometer-cell sorter (Cytomation, Fort Collins, CO). Series of 1, 10, and 30 cells/well were prepared. Only events residing in the viable cell gates, as assessed by the forward and 90° light scatter of the cells, were sorted. The microtiter plates were placed in a humidified incubator with 5% CO2 at 37°C for 30 days. To avoid a change in the osmolarity of the medium due to the 4-week incubation time, the plates were sealed in gas-permeable polyethylene bags and only the 60 inside wells of each plate were used, while the outer wells were filled with H2O. Wells that contained at least one cluster of greater than 30 cells, as assessed by visual inspection under an inverted microscope, were scored as positive.
Cytokeratin staining.
Cells from the mixed cultures and the primary cultures were washed twice in hydroxyethyl-piperazine-ethane sulfonic acid (HEPES) buffer and attached to Poly-l-lysine–coated adhesion slides (BioRad, Munich, Germany) consisting of 12 spots of 5 mm diameter. Duplicate analyses were performed from each culture. Cells were anchored and dried on the spots according to the manufacturer's recommendations and slides were stored in sealed plastic bags at −20°C until use. For cytokeratin staining, cells were fixed in serial dilutions of aceton in ethanol for 5 minutes at 4°C and immunostained in an alkaline phosphatase–antialkaline phosphatase assay (DAKO, Hamburg, Germany).8 A mixture of two immunoglobulin G1 (IgG1) anticytokeratin antibodies (AE1/AE3, Boehringer Mannheim, Mannheim, Germany; and KL1, Dianova, Hamburg, Germany), which specifically recognize members of the acidic as well as of the basic subfamily of cytokeratins, was used. The sensitivity of this assay is 1:100,000 to 1:500,000 as previously described.8 Cytokeratin-positive cells stained bright red, while hematopoietic cells remained unstained. MCF-7 cells were used as a positive control in each assay, and one spot with an IgG1 isotype served as a negative control. A total of 2,000 cells per spot were examined microscopically, and the incidence of the tumor cells, defined as cytokeratin-positive cells, was determined. If five or fewer than five tumor cells per 2,000 total cells was detected, then all cells contained on each spot (3 to 5 × 104, enumerated as described previously8) were screened. The absolute number of tumor cells per culture was calculated from the incidences and the total cell counts at the end of the culture period.
RESULTS
Expression of Erb-B2 and EGF receptor on CD34+ blood progenitor cells and mammary carcinoma cells.
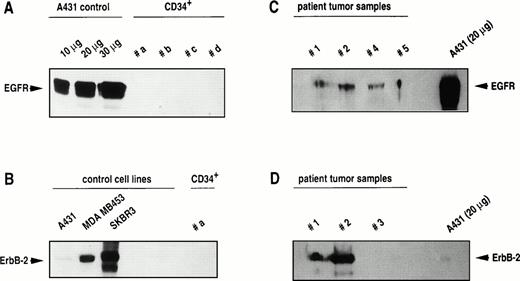
The basis of specific tumor-cell targeting is the selective recognition of cell-surface epitopes that mediate binding of ITs and their transfer into malignant cells. Strong expression of EGF receptor was detected in A431 control cells, but EGF receptor was undetectable in CD34+ cells (five of five patient samples). Similarly, Erb-B2 receptor was found to be expressed at various levels in cell lines as a 185-kD protein, but was undetectable in CD34+hematopoietic cells (four of four patient samples). In patient tumor samples, EGF receptor was found to be expressed in five of seven specimens, and Erb-B2 receptor in five of six samples. Representative analyses of CD34+ cell samples and patient tumor samples for Erb-B2 and EGF receptor expression are shown in Fig1. These data demonstrate that target proteins for ITs are selectively expressed on mammary tumor cells.
Western blot analysis of Erb-B2 and EGF receptor expression in breast cancer cell lines, in CD34+ blood progenitor cells, and in primary tumor cells isolated from breast cancer patients. Total protein was extracted and 100 μg was run on a polyacrylamide gel unless otherwise indicated. After blotting, expression of Erb-B2 and EGF receptor was determined by staining with anti–Erb-B2 and anti–EGF receptor antisera as described in Materials and Methods.
Western blot analysis of Erb-B2 and EGF receptor expression in breast cancer cell lines, in CD34+ blood progenitor cells, and in primary tumor cells isolated from breast cancer patients. Total protein was extracted and 100 μg was run on a polyacrylamide gel unless otherwise indicated. After blotting, expression of Erb-B2 and EGF receptor was determined by staining with anti–Erb-B2 and anti–EGF receptor antisera as described in Materials and Methods.
Effect of ITs on development and ex vivo expansion of CD34+ blood progenitor cells.
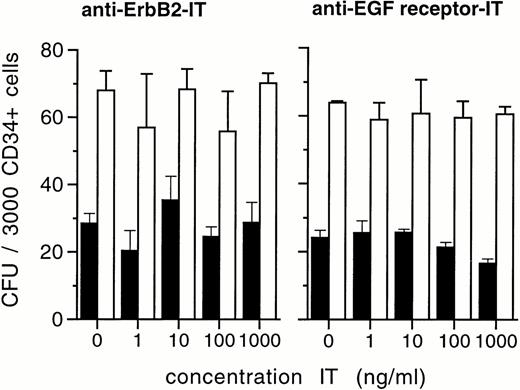
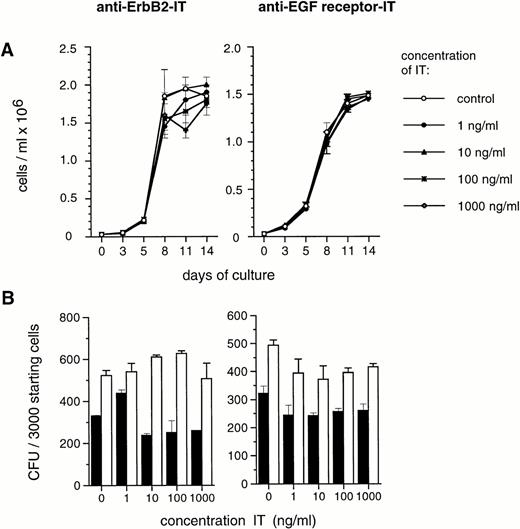
Ex vivo culture of hematopoietic progenitors aims at growth factor–mediated cell amplification, and involves the generation of colony-forming progenitor cells. In direct methylcellulose cultures of CD34+ cells, ITs showed no detectable direct effects on the development of both colony-forming units granulocyte-macrophage (CFU-GM) and erythroid burst-forming units (BFU-E) at IT concentrations as high as 1,000 ng/mL (Fig 2). Moreover, ex vivo expansion of total nucleated cells and of colony-forming cells from CD34+ progenitor cells in the presence of SCF, IL-1β, IL-3, IL-6, and Epo remained unchanged when anti–Erb-B2 or anti-EGF receptor ITs were added to the cultures at concentrations of up to 1,000 ng/mL (Fig 3). This indicates that ITs have insignificant toxicity to blood progenitor cells and do not cause loss of hematopoietic proliferative potential in ex vivo expansion cultures of CD34+ cells.
Development of lineage-specific colonies from CD34+ blood progenitor cells in the presence of anti–Erb-B2 and anti–EGF receptor IT. A total of 3,000 CD34+ cells were inoculated into IL-3, GM-CSF, and EPO supported soft-gel assays and evaluated for GM-CFU (▪) and BFU-E numbers (□) in a light microscope on day 14. Values are the means ± SD of duplicate determinations. Results shown are representative of at least four experiments in each case (ie, anti–Erb-B2 and anti–EGF receptor IT) with CD34+ cells derived from different patients.
Development of lineage-specific colonies from CD34+ blood progenitor cells in the presence of anti–Erb-B2 and anti–EGF receptor IT. A total of 3,000 CD34+ cells were inoculated into IL-3, GM-CSF, and EPO supported soft-gel assays and evaluated for GM-CFU (▪) and BFU-E numbers (□) in a light microscope on day 14. Values are the means ± SD of duplicate determinations. Results shown are representative of at least four experiments in each case (ie, anti–Erb-B2 and anti–EGF receptor IT) with CD34+ cells derived from different patients.
Effect of ITs on ex vivo expansion of CD34+blood progenitor cells. Varying concentrations of anti–Erb-B2 and anti–EGF receptor IT were added as indicated to ex vivo expansion cultures of CD34+ progenitor cells in the presence of SCF, IL-1β, IL-3, IL-6, and EPO. (A) Influence on generation of total nucleated cells at various time points, and (B) on de novo generation of GM-CFU (▪) and BFU-E (□) on day 7 of ex vivo expansion, expressed as numbers of CFU per 3,000 CD34+ cells seeded on day 0. Colony assays were conducted in the absence of ITs as described in the Methods. Values are the means ± SD of duplicate determinations. The results shown are representative of at least 4 experiments for each IT with CD34+ cells derived from different patients.
Effect of ITs on ex vivo expansion of CD34+blood progenitor cells. Varying concentrations of anti–Erb-B2 and anti–EGF receptor IT were added as indicated to ex vivo expansion cultures of CD34+ progenitor cells in the presence of SCF, IL-1β, IL-3, IL-6, and EPO. (A) Influence on generation of total nucleated cells at various time points, and (B) on de novo generation of GM-CFU (▪) and BFU-E (□) on day 7 of ex vivo expansion, expressed as numbers of CFU per 3,000 CD34+ cells seeded on day 0. Colony assays were conducted in the absence of ITs as described in the Methods. Values are the means ± SD of duplicate determinations. The results shown are representative of at least 4 experiments for each IT with CD34+ cells derived from different patients.
Effect of ITs on tumor-cell elimination during ex vivo expansion of CD34+ progenitor cells.
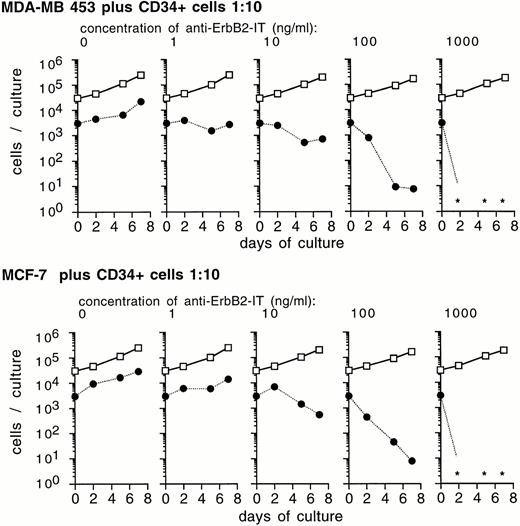
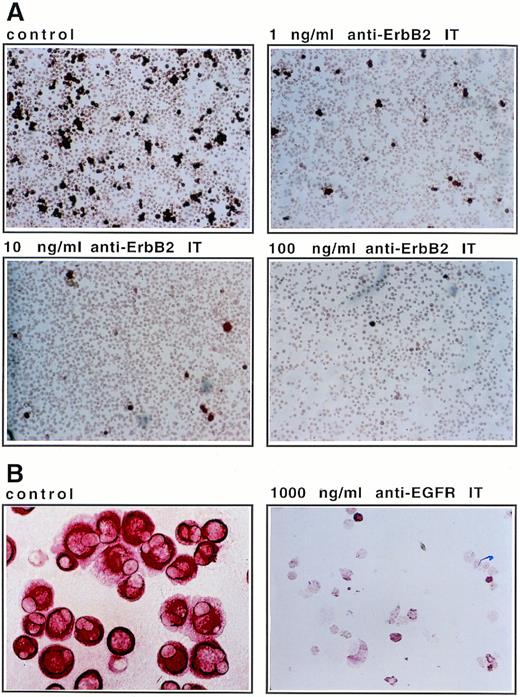
There are only few breast cancer cells within CD34+-mobilized blood specimens.15 Therefore, to assess the optimal conditions for an effective ex vivo purging procedure, we performed mixing experiments with tumor-cell lines added to CD34+ progenitor cell cultures. MDA-MB 453 or MCF-7 cells and CD34+ cells were cocultured at a ratio of 1:10. The cells were kept in medium that corresponded to established ex vivo expansion conditions in the presence of SCF, IL-1β, IL-3, IL-6, and Epo, and exposed to anti–Erb-B2 IT. The extent of tumor-cell elimination depends on IT concentrations and on the period of treatment (Fig 4). Cells were investigated at various time points after IT addition and IT concentrations of up to 1,000 ng/mL were applied. After 2 days of exposure of the cultures to anti–Erb-B2 IT, a moderate effect on the elimination of tumor cells was observed. An exposure period of 5 to 7 days resulted in a complete depletion of tumor cells (2 to 3 log at 100 ng/mL and below detection limits at 1,000 ng/mL; Fig 4). The cytotoxic effect for tumor cells of anti–Erb-B2 IT during a 7-day mixed culture is shown in Fig5A.
Efficacy of anti–Erb-B2 IT to eliminate tumor cells during ex vivo expansion of CD34+ cells. MDA-MB-453 and MCF-7 cells were mixed with CD34+ cells at a ratio of tumor cells to CD34+ cells of 1:10 as indicated, cultured in medium containing SCF, IL-1β, IL-3, IL-6, Epo, and 10% FCS, and treated with the anti–Erb-B2 IT at several concentrations and for various time periods as indicated. Numbers of cytokeratin-positive cells (•) and cytokeratin-negative cells (□) were determined after immunocytochemical analysis as described in the Methods. Data shown are a representative experiment of 3 independent experiments and show mean values of duplicate cultures. *No cytokeratin-positive cells were detected in at least 3 × 104 total cells evaluated.
Efficacy of anti–Erb-B2 IT to eliminate tumor cells during ex vivo expansion of CD34+ cells. MDA-MB-453 and MCF-7 cells were mixed with CD34+ cells at a ratio of tumor cells to CD34+ cells of 1:10 as indicated, cultured in medium containing SCF, IL-1β, IL-3, IL-6, Epo, and 10% FCS, and treated with the anti–Erb-B2 IT at several concentrations and for various time periods as indicated. Numbers of cytokeratin-positive cells (•) and cytokeratin-negative cells (□) were determined after immunocytochemical analysis as described in the Methods. Data shown are a representative experiment of 3 independent experiments and show mean values of duplicate cultures. *No cytokeratin-positive cells were detected in at least 3 × 104 total cells evaluated.
Elimination of mammary carcinoma cells during ex vivo culture in the presence of IT. (A) The 7-day time point of a 1:10 mixture of MDA-MB-453 breast cancer cells and CD34+progenitor cells as shown in Fig 4A was immunostained with anticytokeratin antibodies and photographed at 100× magnification. Anti–Erb-B2 IT was present in the cultures at the indicated concentrations. (B) Tumor cells from patient no. 5 (Table 2) were cultured in α-MEM/10% FCS without (control) and with 1,000 ng/mL anti–EGF receptor (EGFR) IT for 7 days. Cells were fixed on glass slides, stained with anticytokeratin antibodies, and photographed at identical (1,000×) magnification.
Elimination of mammary carcinoma cells during ex vivo culture in the presence of IT. (A) The 7-day time point of a 1:10 mixture of MDA-MB-453 breast cancer cells and CD34+progenitor cells as shown in Fig 4A was immunostained with anticytokeratin antibodies and photographed at 100× magnification. Anti–Erb-B2 IT was present in the cultures at the indicated concentrations. (B) Tumor cells from patient no. 5 (Table 2) were cultured in α-MEM/10% FCS without (control) and with 1,000 ng/mL anti–EGF receptor (EGFR) IT for 7 days. Cells were fixed on glass slides, stained with anticytokeratin antibodies, and photographed at identical (1,000×) magnification.
Elimination of clonogenic tumor cells by ITs.
It is possible that the sensitivity of detection of our immunohistochemical assay misses small amounts of tumor cells. Tumor cells may include resting cells with high proliferative potential, which may preferentially survive exposure against ITs. For these reasons, we performed tumor-cell regrowth assays. After exposure of MCF-7 and MDA-MB 453 cells to anti–Erb-B2 IT for 4 days, surviving cells were reseeded into optimal tumor-cell growth medium. Table1 shows that treatment with anti–Erb-B2 IT decreased the subpopulations of MCF-7 and MDA-MB 453 cells with regrowth potential. This indicates that the elimination of tumor cells by ITs during ex vivo expansion includes the clonogenic populations.
Regrowth Potential of Mammary Carcinoma Cells After 4-Day Exposure to IT
| IT Concentration (ng/mL) . | Cell Line (no. of cells seeded per well) . | ||
|---|---|---|---|
| MDA-MB-453 . | MCF-7 . | ||
| 10 . | 30 . | 30 . | |
| 0 | 27/60-150 | 44/60 | 28/60 |
| 10 | 5/60 | 24/60 | 0/60 |
| 100 | 4/60 | 8/60 | 0/60 |
| IT Concentration (ng/mL) . | Cell Line (no. of cells seeded per well) . | ||
|---|---|---|---|
| MDA-MB-453 . | MCF-7 . | ||
| 10 . | 30 . | 30 . | |
| 0 | 27/60-150 | 44/60 | 28/60 |
| 10 | 5/60 | 24/60 | 0/60 |
| 100 | 4/60 | 8/60 | 0/60 |
Cell lines were cultured in the presence or absence of anti–Erb-B2 IT at the indicated concentrations for 4 days. Subsequently, cells were washed twice in PBS, reseeded into 96-well plates at 10 or 30 events per well by the automatic cell deposition unit of a flow cytometer as described in Materials and Methods, and incubated for 30 days in optimal growth medium (DMEM containing 10% FCS).
Data are represented as the number of positive wells, ie, wells containing at least 1 cluster of >30 cells per total number of wells seeded.
Effect of IT on primary breast cancer cells isolated from patients.
Having established time periods and IT concentrations needed for effective tumor-cell purging by ITs, we investigated the effect of ITs against primary breast cancer cells. Pleural effusion, ascites, and bone marrow aspirates, containing numerous tumor cells, were exposed to 1,000 ng/mL of ITs. The effect of ITs on enriched primary tumor-cell populations is shown in Table 2. Within a period of 7 days, viable tumor cells were depleted in three of five samples expressing Erb-B2 receptor by anti–Erb-B2 IT, or in five of five samples expressing EGF receptor by anti–EGF receptor IT. In one case with no detectable expression of EGF receptor, anti–EGF receptor IT-mediated killing activity was also recorded. Identical results were observed in the additional presence of hematopoietic growth factors in the cultures. Therefore, these factors do not influence the efficacy of ITs in eliminating tumor cells (data not shown). An example of primary mammary tumor-cell elimination by anti–EGF receptor IT is shown in Fig5B. These results indicate that ITs can exert their cytotoxic activities on primary tumor-cell material.
Elimination of Primary Tumor Cells Isolated From Patients With Breast Cancer During Culture in the Presence of IT
| Patient No. . | Age (yr) . | Cell Source . | Receptor Expression . | Tumor Cell Depletion . | ||
|---|---|---|---|---|---|---|
| Erb-B2 . | EGFR . | Anti–Erb-B2 IT . | Anti–EGFR-IT . | |||
| 1 | 61 | Ascites | + | + | —* | >99.2% |
| 2 | 48 | Pleural effusion | + | + | >99.2% | >99.2% |
| 3 | 73 | Pleural effusion | − | + | — | >99.2% |
| 4 | 74 | Bone marrow | ND | + | — | >99.2% |
| 5 | 84 | Pleural effusion | + | + | — | 75% |
| 6 | 49 | Ascites | + | − | 66% | ND |
| 7 | 68 | Ascites | + | − | 74% | 73% |
| Patient No. . | Age (yr) . | Cell Source . | Receptor Expression . | Tumor Cell Depletion . | ||
|---|---|---|---|---|---|---|
| Erb-B2 . | EGFR . | Anti–Erb-B2 IT . | Anti–EGFR-IT . | |||
| 1 | 61 | Ascites | + | + | —* | >99.2% |
| 2 | 48 | Pleural effusion | + | + | >99.2% | >99.2% |
| 3 | 73 | Pleural effusion | − | + | — | >99.2% |
| 4 | 74 | Bone marrow | ND | + | — | >99.2% |
| 5 | 84 | Pleural effusion | + | + | — | 75% |
| 6 | 49 | Ascites | + | − | 66% | ND |
| 7 | 68 | Ascites | + | − | 74% | 73% |
Tumor cells (>60% purity) were cultured in a-MEM/10% FCS for 7 days in the presence of 1,000 ng/mL ITs as described in Materials and Methods. Tumor cells were quantitated by immunohistochemical staining with anticytokeratin antibodies.
Abbreviations: EGFR, EGF receptor; +, receptor expression detectable by Western blot; −, receptor expression undetectable by Western blot; ND, not determined.
—, No response detected. Percent values represent the percent depletion of cytokeratin-positive cells in treated cultures compared with untreated control cultures.
DISCUSSION
Breast cancer is one of the most common disease entities in medical oncology. The proportion of cases diagnosed early and treated at a state of limited disease has increased in recent years. In addition, the treatment of breast cancer patients with high-dose chemotherapy has resulted in improved survival rates compared with conventional treatment regimens.2,3 Contamination of autologous hematopoietic cell transplants with tumor cells is of clinical disadvantage.4-6 Cell-separation methods have been investigated for the ex vivo purging of autologous transplants, exploiting cell-surface markers and monoclonal antibody technology.14 In contrast to these selection protocols, which last up to several hours, longer-term purging procedures require specific culture conditions to ensure the viability or expansion of hematopoietic progenitor cells. To this end, recombinant hematopoietic cytokines have been used to maintain and expand hematopoietic progenitors in culture.16 A minimum number of tumor cells within a transplant that could affect the course of the disease has not been defined. Current concepts aim at maximal depletion of transplants from contaminating tumor cells. A specific molecular targeting of solid tumor cells, eg, by ITs directed specifically against epithelial or breast cancer cell antigens, is therefore highly desirable for clinical practice. ITs could be useful in tumor cell purging of hematopoietic stem cells used as autologous grafts after high dose chemotherapy.
ITs are effective during ex vivo expansion of CD34+progenitor cells for tumor-cell purging.
The specific activity of ITs may depend on properties related to the antibodies used, the choice of toxins, the method by which the toxins are linked to the antibodies, the concentration of the ITs, and the treatment period. Our results indicate that the antibodyPseudomonas exotoxin A fusion proteins specific for Erb-B2 and EGF receptor are not cytotoxic for hematopoietic cells at concentrations of up to 1,000 ng/mL, including CD34+hematopoietic progenitors. A number of ITs containing thePseudomonas exotoxin,25,26 the toxin ricin,31 or the recombinant ricin A chain32 and directed against epithelial antigens have been used to purge bone marrow from breast cancer cells. Only insignificant toxicity to hematopoietic progenitors was shown, but a reduction of colony-forming cells by up to 50% was reported during short incubation times (up to 2 hours).26 We show that treatment periods up to 14 days with recombinant ITs do not alter the survival and the proliferative potential of the CD34+ progenitor cells in ex vivo expansion cultures. We also show that whereas prolonged exposure times do not result in increasing toxicity to hematopoietic cells, they may increase the efficiency to eliminate mammary carcinoma cells from autologous grafts. The extended culture of hematopoietic grafts may offer advantages of more efficient tumor-cell elimination at a given IT concentration with no detectable side effects on hematopoietic cells. Short-term treatments with ITs at concentrations noncytotoxic for hematopoietic cells have been shown to effectively purge autologous grafts in previous studies.25,26,33 34 However, these results must be interpreted with caution, since they refer to removal of cell lines that show high target antigen expression and rapid IT internalization. To our knowledge, this is the first report to demonstrate potent in vitro cytotoxic activity of immunotoxins not only towards cell lines, but also towards primary breast cancer cells.
No tumor-cell kill was observed in primary samples no. 1 and 5 with anti–Erb-B2 IT, although expression of the receptor was evident by Western blot. In previous studies, we have observed significant differences in the anti–Erb-B2 immunotoxin sensitivity among established tumor-cell lines expressing similar amounts of the target receptor27; this may also account for the observed differences in the primary cell samples. Receptor density on the cell surface, and the rate of receptor internalization will determine the efficiency of tumor-cell elimination by ITs.27 This can explain discrepancies between positive receptor expression and impaired elimination by ITs of selected tumor-cell samples. Conversely, in one patient in whom no expression of EGF receptor was detectable by Western blot, we still observed efficient tumor-cell kill after IT addition. This may be due to very low receptor expression, which was missed by the Western blot technique.
By the analytical means used in this study, we cannot formally determine if there is a coexpression of IT receptors and cytokeratin in the target cells of the IT. However, as the expression levels of IT receptors are likely to be homogeneous,30 and as the calculations of tumor-cell depletion were based on the enumeration of cytokeratin-positive cells, ITs target the cytokeratin-positive cell populations with high efficiency. In this study, we used primary cells in an essentially nondividing state. This may be of relevance in the situation of clinical tumor-cell purging, since micrometastatic tumor cells from bone marrow have been described to reside in the G0 phase of the cell cycle.34
Erb-B2 and EGF receptor as targets for purging protocols in breast cancer.
For antibody-depletion systems to be effective, the antigens recognized by the ITs must be expressed on the target cells. The antigenic phenotype of contaminating mammary tumor cells in autologous hematopoietic transplants is, up to now, poorly characterized. Mapara et al15 showed that six of nine breast cancer patients with immunocytochemically cytokeratin-positive CD34+ specimens were also positive in an EGF receptor–specific reverse transcriptase (RT)-PCR assay. Moreover, Pantel et al35 showed that 23 of 23 of M1 and 25 of 48 M0 stage breast cancer patients expressed the Erb-B2 oncogene in bone marrow cytokeratin-positive cells, although only 10% to 30% of patients express Erb-B2 in their primary tumors. Therefore, Erb-B2 and EGF receptors are suitable targets for purging procedures in a majority of breast cancer patient samples.
Assuming that tumor cells are hierarchically ordered, the most important target for ex vivo tumor-cell purging will be tumor-cell precursors. It will also be desirable to know if micrometastatic cells, found in early stages of disease, are subject to a comparable efficiency of tumor-cell kill by ITs. Our results using a tumor-cell regrowth assay indicate that in vitro assayable tumor progenitor cells can be purged with an efficiency comparable to the overall tumor-cell population. This has not been determined with more short-term (eg, 2-hour) incubations as used previously. However, once phenotypic characterization of micrometastatic tumor cells has progressed, redesigned ITs or IT combinations may be required for depletion of more primitive tumor populations, including dormant tumor cells.
Immunotoxins as part of integrated purging strategies.
The high efficiency and specificity of ITs against breast cancer cells makes them a suitable purging tool. ITs might be used in combination with other established procedures. If several purging steps are combined, recovery rates of hematopoietic progenitor cells within each single step are crucial for the success of the manipulation procedures. Since IT-mediated tumor-cell purging results in no significant loss of hematopoietic progenitors, it is expected to be superior to immunomagnetic or immunoaffinity cell-selection procedures. These may result in an overall stem-cell loss of up to 50%. The superiority of immunotoxins to drug treatments such as 4-hydroperoxycyclophosphamide36 for ex vivo purging protocols is based not only on their high specificity, but also on the fact that toxins work by inhibiting protein synthesis and can kill nondividing cells, whereas most chemotherapeutic agents act by interfering with DNA synthesis and cell division.
Upon sequential purging procedures in tumor-cell–containing grafts, it may no longer be possible to count the number of tumor cells that remain in the graft. This is due to the detection limits in the tumor-cell measurement techniques used and the low numbers of tumor cells that remain at the end of sequential purging procedures. Instead, only a theoretic assessment of the purging efficiencies of various depletion techniques, such as surface marker isolation, ex vivo expansion in hematopoietic cytokines, and molecular depletion by ITs could serve to calculate an entire purging rate. Combinations of purging steps should then reduce the probability of retaining a single tumor cell within an entire graft. This technology may allow assessment of the value of vigorous tumor-cell purging in clinical trials. The clinical use of highly efficient purging strategies may be used to determine the role of contaminating tumor cells in solid tumors such as breast cancer.
ACKNOWLEDGMENT
We thank Prof Dr Roland Mertelsmann for the incentive to perform this cooperative study and for his continuous support. We also thank Dr Jürgen Finke for valuable suggestions.
Supported by the Deutsche Forschungsgemeinschaft through SFB 364 projects A1 (to R.H.) and C1 (to B.G. and W.W.).
Address reprint requests to R. Henschler, MD, Department of Hematology/Oncology, University Medical Center, Hugstetter Strasse 55, 79106 Freiburg, Germany.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal