Abstract
Tissue factor (TF) has been implicated in several important biologic processes, including fibrin formation, atherogenesis, angiogenesis, and tumor cell migration. In that plasminogen activators have been implicated in the same processes, the potential for interactions between TF and the plasminogen activator system was examined. Plasminogen was found to bind directly to the extracellular domain of TF apoprotein (amino acids 1-219) as determined by optical biosensor interaction analysis. A fragment of plasminogen containing kringles 1 through 3 also bound to TF apoprotein, whereas isolated kringle 4 and miniplasminogen did not. Expression of TF on the surface of a stably transfected Chinese hamster ovary (CHO) cell line stimulated plasminogen binding to the cells by 70% more than to control cells. Plasminogen bound to a site on the TF apoprotein that appears to be distinct from the binding site for factors VII and VIIa as judged by a combination of biosensor and cell assays. TF enhanced two-chain urokinase (tcuPA) activation of Glu-plasminogen, but not of miniplasminogen, in a dose-dependent, saturable manner (half maximal stimulation at 59 pmol/L). TF apoprotein induced an effect similar to that of relipidated TF, but a relatively higher concentration of the apoprotein was required (half maximal stimulation at 3.8 nmol/L). The stimulatory effect of TF on plasminogen activation was confirmed when plasmin formation was examined directly on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. In accord with this, TF inhibited fibrinolysis by approximately 74% at a concentration of 14 nmol/L and almost totally inhibited the binding of equimolar concentrations of plasminogen to human umbilical vein endothelial cells and human trophoblasts. Further, CHO cells expressing TF inhibited uPA-mediated fibrinolysis relative to a wild-type control. TF apoprotein and plasminogen were found to colocalize in atherosclerotic plaque. These data suggest that plasminogen localization and activation may be modulated at extravascular sites through a high-affinity interaction between kringles 1 through 3 of plasminogen and the extracellular domain of TF.
TISSUE FACTOR (TF) plays a critical role in the initiation of coagulation.1,2 TF apoprotein is a 263 amino acid integral membrane glycoprotein. TF is not expressed by cells that interface with the circulation under physiologic conditions,3 but its expression can be induced on monocytes and on subsets of endothelial cells4 such as those adjacent to atherosclerotic plaques5 as part of the response to inflammation or injury.6,7 TF functions in the form of a complex with membrane phospholipids to promote the activation of factors VII, X, and IX. The presence of both components are required under physiologic conditions for optimal procoagulant activity.8-11
Clot formation and lysis must be tightly coordinated to secure hemostasis while maintaining vascular patency. Fibrinolysis is dependent on the formation of plasmin through the cleavage of plasminogen by tissue-type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA) in plasma, on cell surfaces, and in some extracellular compartments. Plasmin regulates the activities of coagulation factors such as FV,12 whereas FVa and FXa have recently been reported to regulate plasminogen activation.13 These observations suggest that potentially important links may exist between the initiation of coagulation by TF and the dissolution of fibrin clots by plasmin.
Another potential link comes from recent studies in which TF has been implicated in angiogenesis,14 vasculogenesis,15tumor cell invasion,16,17 smooth muscle cell migration,18 and atherogenesis,5,19-21processes in which plasminogen activation has been implicated as well.22-25 The mechanism by which TF participates in these processes is unknown. The observation that exogenous FVIIa or FXa can bind to TF in atherosclerotic plaques,5 for example, indicates that the epitopes for these ligands are not totally occupied and suggests that binding sites for other ligands may be available as well. Furthermore, the finding that FVIIa bound to TF in these plaques expresses enzymatic activity suggests that factors other than tissue factor protease inhibitor (TFPI) may participate in its regulation at such extravascular sites. Taken together, these interactions lead us to ask whether plasminogen binds to TF and whether TF may modulate the expression of plasminogen activator activity.
MATERIALS AND METHODS
Materials.
Glu-plasminogen was purified from human plasma by affinity chromatography on lysine Sepharose as described.26Miniplasminogen, high molecular weight two-chain urokinase (tcuPA), and the plasmin chromogenic substrate Spectrazyme PL (H-D-norleucyl-hexahydrotyrosyl-lysine-p-nitroanilide diacetate salt) were provided by American Diagnostica Inc (Greenwich, CT). Murine monoclonal antibodies to human TF (no. 4509) and plasminogen (no. 3644) (antiplasminogen) were purchased from American Diagnostica Inc. Single-chain urokinase (scuPA) was the kind gift of Dr J. Henkin (Abbott Laboratories, Abbott Park, IL). TF apoproteins in 4 mmol/L CHAPS were from American Diagnostica as well as generously provided by Dr L.V.M. Rao (Tyler, TX). Relipidated TF apoproteins were from American Diagnostica, Baxter Diagnostics, Inc (Deerfield, IL), and Dr Rao. Factors VII and VIIa were from American Diagnostica and were purified from plasma (see below). Recombinant soluble extracellular domain of TF (amino acids 1-219) was the generous gift of Wolfram Ruf (Scripps Research Institute, La Jolla, CA).
Cells.
Cultures of human umbilical vein endothelial cells (HUVECs) were prepared using published methods27 in Medium 199 supplemented with 10% heat-inactivated fetal calf serum (FCS; Life Technologies, Bethesda, MD), penicillin-streptomycin, 2 mmol/L glutamine, 12 U/mL heparin sodium (SoloPak, Elk Grove Village, IL), Hydrocortisone (1 μg/mL; Clonetics Co, San Diego, CA), bovine brain extract (12 μg/mL; Clonetics Co), and human recombinant epidermal growth factor (0.01 μg/mL; Clonetics Co). The cells were passaged using trypsin-EDTA (GIBCO), frozen in liquid nitrogen, thawed, and passaged two additional times before use. Cultures of first trimester human trophoblasts were prepared and characterized as described.28 29
Development of a Chinese hamster ovary (CHO) cell line expressing TF.
TF cDNA (clone pHTF8) was obtained from American Type Culture Collection (Rockville, MD). TF cDNA, corresponding to nucleotides 21 to 1050 of the published sequence30 was cloned into theSal I site of the vector pUC18 (Life Technologies, Bethesda, MD). The vector containing this insert was then digested withSau3AI to yield an approximately 1-kb fragment beginning at nucleotides 54 of TF at its amino terminus and terminating within the multiple cloning site of the vector at its carboxyterminus. The fragment was isolated using low-melt gel electrophoresis and a Qiaex II gel purification kit (Qiagen, Chatsworth, PA). The DNA fragment was ligated into BamHI site of vector pcDNA3 (Invitrogen, Carlsbad, CA). The size and orientation of the insert was confirmed usingSma I and the nucleotide sequence was verified by dideoxynucleotide chain-termination DNA sequence analysis (University of Pennsylvania DNA Sequencing Facility, Philadelphia, PA).
The CHO cells (American Type Culture Collection) were cultured at 37°C in 5% CO2 in Iscove's modified Dulbecco's medium containing 2 mmol/L L-glutamine, 25 mmol/L HEPES, 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 0.05 mmol/L hypoxanthine, and 8 nmol/L thymidine (all from Life Technologies). Before transfection, the CHO cells were seeded onto 15-cm2 petri dishes for at least 24 hours until they reached approximately 70% confluence. The cells were incubated with lipofectamine (Life Technologies) and the pcDNA3-TF plasmid that contains a neomycin resistance gene. The remaining steps were as per the manufacturer's instruction (Life Technologies). Colonies resistant to G418 (600 μg/mL; Life Technologies) were selected for analysis. Individual colonies were trypsinized, transferred to 96-well microtiter wells (Falcon 3072; Falcon, Franklin Lakes, NJ), and grown to confluence. Colonies were chosen based on their procoagulant activity and expression of TF (see below).
The colonies were then characterized for their expression of TF antigen and FVII-dependent coagulant activity. To measure TF antigen expression, transfected and control cells were cultured in 96-well plates until they reached confluency. The cells were then washed twice with phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) and incubated with series of buffers each for 1 hour at room temperature with five washes in PBS/BSA between each incubation. The cells were incubated sequentially with PBS/2% BSA, 1 μg/mL anti-TF antibody in PBS/1% BSA, biotinylated goat antimouse IgG (H+L; Southern Biotechnology Associates, Inc, Birmingham, AL) diluted 1/10,000 in PBS/1% BSA, and streptavidin-horseradish peroxidase (Southern Biotechnology Associates, Inc) diluted 1/1,000 in PBS/1% BSA according to the manufacturer's instruction. o-phenylenediamine free base (Sigma Chemical Co, St Louis, MO) was diluted in phosphate-citrate buffer, pH 5.0, and made 0.03% H2O2 immediately before its addition to the cells, and the optical density at 490 nm was measured (Molecular Devices, Palo Alto, CA).
To measure procoagulant activity, CHO cells transfected with TF and the wild-type parental cells were cultured in 24-well dishes until they reached confluence. The cells were then washed twice in PBS (without calcium) and 0.1 mL of citrated normal plasma (0.32% final concentration) and 0.1 mL PBS (without calcium) was added. Lastly, 0.1 mL of 25 mmol/L CaCl2 was added and the time to form a visible clot was recorded.
Biosensor analysis of plasminogen binding to TF.
Kinetic analysis of the interaction of plasminogen with TF was performed using BIA2000 and IAsys optical biosensors (see Myszka31 and the references therein). Soluble and apoprotein forms of TF were separately coupled to either CM5-research grade sensor chip flow cells (BIACORE) or carboxymethyl-dextran cuvettes (IAsys), using standard amine coupling procedures described by the manufacturers.32 The coating conditions for TF immobilization were optimized by observing the electrostatic attraction to the negatively charged CM-dextran surface at different pHs (2.0 to 5.0 in 10 mmol/L NaAc buffer). TF apoprotein at a concentration of 15 μg/mL, pH 2.3, or soluble TF at a concentration of 15 μg/mL, pH 4.0, were used to coat the sensors' surfaces. The net response chosen for binding detection was 400 arc seconds and 4,000 RU for the IAsys and the BIACORE, respectively. Coupling on the BIACORE flow cell was performed with a flow of 5 μL/min. Immobilized ligand was equilibrated with PBS, pH 7.4, 0.05% Tween-20 in all experiments, except when Ca2+ was required in the reaction cell. TBS, pH 7.4, 0.05% Tween-20 was used in the presence of Ca2+cations. Regeneration of the surface after each binding interaction was achieved using 10 mmol/L EDTA, pH 9.4, or by competing with the immobilized ligand for plasminogen binding using a lysine analogue 6-amino hexanoic acid (6-AHA). All experiments were performed at 25°C.
Kinetic measurement and analysis.
Binding of the soluble analyte to the surface-immobilized ligand, in this case plasminogen and TF, respectively, was detected by a change in the refractive index as a function of time.33 The detector response is proportional to the surface concentration of bound ligand.34 The kinetics of binding were initially evaluated assuming the ligand in solution, or analyte, A (Glu-plasminogen) interacts with the immobilized ligand B, in this case TF, to form complex AB. The net rate of complex formation depends on the free concentration of A and B and on the stability of the formed complex.
Kinetics data were evaluated using relationships described previously.33 35 For a bimolecular interaction,
the association and dissociation processes are described respectively by the relationships
and
Here, kon and koff are on and offrate constants, respectively; [A] is the concentration of analyte injected onto a flow cell (or into the sensor cuvette) containing the second interactor B immobilized on a sensor surface; R is the relative response of the optical biosensor at time t and is proportional to the amount of complex formed; Rmax is the maximum response; and Ro is the response at the start of the dissociation phase. When association follows equation 2, a plot of dR/dt versus R should be linear and yield a slope of −ks = kon[A] + koff, so that a replot of ks versus [A] will yield a slope of kon. For a single exponential process (equation 3), a plot of ln(Ro/R) versus time from the dissociation part of the sensorgram should be a straight line with a slope of koff.
Immunohistochemical colocalization of TF and plasminogen in human atherosclerotic coronary arteries.
To study the colocalization of TF and plasminogen in vascular samples, a sequential double immunoenzymatic staining procedure for simultaneous localization of both antigens in the same tissue section was used. Vascular samples were obtained from human hearts removed at the time of transplantation. Replicate 2- to 3-mm sections were fixed by immersing each sample in 10% neutral buffered formalin overnight at 21°C. All samples were then embedded in paraffin and serial 5-mm sections were cut on ProbeOn Plus slides and used for immunocytochemistry. For immunostaining, a modification of the avidin-biotin peroxidase method was performed using capillary action technology and the Microprobe System (Fisher Scientific, Pittsburgh, PA). Briefly, sections were deparaffinized at 55°C for 30 minutes, placed in xylene, and hydrated in 100% and then 95% ethanol, and endogenous peroxidase activity was quenched with a 2.2% (vol/vol) H2O2/methanol solution added for 5 minutes. All subsequent incubations were performed at room temperature for the specified time. The slides were then washed with 1× automation buffer (Biomeda Corp, Foster City, CA) and 5% normal horse serum was added for 20 minutes to block nonspecific binding. Primary and secondary antibodies were diluted in 1× automation buffer containing 5% normal horse serum. Antibodies to TF and plasminogen were used at 5 μg/mL final concentration. Isotype-matched nonimmune mouse Ig was used as an irrelevant control antibody. The slides were then incubated with primary antibody for 60 minutes, washed in 1× automation buffer for 10 minutes, incubated with biotinylated antimouse antiserum (Vector Laboratories, Burlingame, CA) at a 1:300 dilution for 30 minutes, and washed again with 1× automation buffer. An alkaline phosphatase-streptavidin-biotin complex (Dako, Capiteria, CA) was used as a reporter system, and the reaction was visualized with the alkaline-phosphatase substrate (Alkaline phosphatase substrate kit III-SK 5300; Vector Laboratories) that produces a blue reaction product.
The sections were washed with 1× automation buffer, and once again blocking was performed with 5% normal horse serum for 20 minutes. Antiplasminogen antibody was then applied for 60 minutes. Sections were then incubated with the antimouse biotinylated secondary antibody for 30 minutes. The slides were then incubated for 30 minutes with the streptavidin-biotin peroxidase system (Dako) at a 1:50 dilution and developed by adding 0.05% (vol/vol) 3,3′-diaminobenzidine solution (Sigma Chemical Co) and 0.03% H2O2 (vol/vol) for 5 minutes. Stained sections were dehydrated, a coverslip was added, and the extent of staining was evaluated. Doubly stained cells showed mixtures of brown (plasminogen) and blue (TF) tones.
Expression of human FVII in vitro.
The cDNA for normal FVII (gift of Dr Darrell Stafford, University of North Carolina, Chapel Hill, NC) was inserted into the multiple cloning site of the expression vector pCMV536 as described.37 Correct insertion was confirmed by sequence analysis. Human embryonic kidney cells (293; American Type Culture Collection) were cotransfected with the pCMV5 and pSV2neo plasmids using calcium phosphate,38 and the transfected cells were selected with 500 μg/mL G418 (Life Technologies, Grand Island, NY). Colonies producing FVII as determined by enzyme-linked immunosorbent assay were grown to confluence.
Purification of recombinant wild-type FVII.
FVII-producing cell lines were expanded in Dulbecco's modified Eagle's medium (DMEM)/F12 medium contain 10% FCS, penicillin/streptomycin, L-glutamine, and 6 μg/mL vitamin K. The cells were cultured in roller bottles in serum-free medium containing 10 μg/mL of a supplement containing insulin, transferrin, and selenium (Boehringer Mannheim, Indianapolis, IN) for up to 14 days, and the culture supernatants were harvested at approximately 24-hour intervals. Clones expressed FVII at a concentration of approximately 1 mg/L/24 hr. Culture supernatants were filtered through cellulose acetate (Schleicher and Schuel, Keene, NH). Benzamidine (10 mmol/L) and protease inhibitors (Complete protease inhibitor cocktail; Boehringer Mannheim) were added (0.5 μL 25× stock/mL conditioned medium), and the samples were stored at −20°C. Thawed culture medium was diluted with 1.5 vol of 20 mmol/L Tris, pH 7.2/10 mmol/L benzamidine/5 mmol/L EDTA, mixed with 10 mL of Q-Sepharose beads (Pharmacia Biotech, Uppsala, Sweden) equilibrated in 20 mmol/L Tris, pH 7.2/60 mmol/L NaCl/10 mmol/L benzamidine/5 mmol/L EDTA and stirred at 4°C for 20 minutes. Q-Sepharose beads were washed in a scintered glass funnel with 20 mmol/L Tris, pH 7.2/10 mmol/L benazmidine before use. Bound protein was eluted with 20 mmol/L Tris (pH 7.2/750 mmol/L NaCl/10 mmol/L benzamidine). Pooled fractions containing protein were mixed with 4 vol of 20 mmol/L Tris, pH 7.4/10 mmol/L benzamidine and made 10 mmol/L in CaCl2. The pooled fractions were applied to a calcium-dependent anti-FVII monoclonal antibody-Sepharose column (gift of Dr T. Jorgensen, NovoNordisk, Copenhagen, Denmark) equilibrated with 20 mmol/L Tris, pH 7.2/100 mmol/L NaCl/10 mmol/L CaCl2/50 mmol/L benzamidine, and a buffer containing 20 mmol/L Tris (pH 7.2/1 mol/L NaCl/10 mmol/L CaCl2/50 mmol/L benzamidine) was used to elute protein nonspecifically bound to the column. The column was then re-equilibrated, and recombinant FVII was eluted with 20 mmol/L Tris, pH 7.2/100 mmol/L NaCl/20 mmol/L EDTA/50 mmol/L benzamidine. The pooled material was concentrated using Centriprep-30 concentrators (Amicon, Beverly, MA) and quantified using Coomasie blue (Pierce, Rockford, IL). Samples were assayed for purity using an 8% to 25% polyacrylamide gel gradient and visualized by silver staining (Bio-Rad, Hercules, CA). Analysis of γ-carboxyglutamic acid (Gla) content was performed as described.39 Recombinant FVII had a Gla content that was similar to plasma-derived FVII (Enzyme Research Laboratories, South Bend, IN).
Competition between FVII and plasminogen for TF binding and activity.
The effect of plasminogen on FVII binding to TF was evaluated by BIAcore biosensor assay. Increasing concentrations of plasminogen were injected into the flow cell and association allowed to reach steady state in each case. After unbound plasminogen was washed out of the flow cell by buffer alone, FVII was injected at a concentration of 149 nmol/L and its association and dissociation phases were recorded. The extents of association of FVII after varying plasminogen binding were compared.
Next, the potential for FVII and plasminogen to compete for activity was studied in two ways. First, relipidated TF (10 nmol/L) was incubated with 125I-plasminogen (100 nmol/L) in the absence or in the presence of FVII or FVIIa (100 nmol/L each) and the specific binding to HUVECs was measured as described above. Second, TF (10 nmol/L) was added to 0.1 mL of recalcified, citrated pooled normal plasma in the absence or presence of plasminogen (100 nmol/L) and the time to form a visible clot was determined.
Measurement of plasminogen activator activity.
Two methods were used. First, plasminogen activator activity was measured as previously described.40 Briefly, Glu- or mini-plasminogen (5 nmol/L), tcuPA (5 nmol/L), and Spectrazyme PL (100 to 167 μmol/L) were incubated with varying concentrations of TF in 0.1 mol/L Tris-HCl, pH 8.0, or PBS, pH 7.4 (60 μL final volume), in 96-well Falcon Multiwell tissue culture dishes (Becton Dickinson, Lincoln Park, NJ), and the optical density at 405 nmol/L was measured at various times. Second, conversion of plasminogen to plasmin was analyzed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). In these experiments, Glu-plasminogen (3 μmol/L) was incubated with tcuPA (400 nmol/L) in the absence or presence of various concentrations of TF apoprotein (0 to 450 nmol/L; American Diagnostica) in 57 mmol/L Tris-HCl, pH 8.0 (final volume, 70 μL), at 37°C for 20 minutes. To stop the reaction, 5 μL of 4× SDS-PAGE sample buffer containing 10% 2-mercaptoethanol was added to 15 μL of the reaction mix. The samples were boiled for 5 minutes, and the proteins were separated using a 10% SDS 12% polyacrylamide gel that was then stained with Coomasie blue.
Contribution of TF apoprotein and phospholipids to plasminogen activation.
Three sets of experiments were performed to explore the contribution of the phospholipid component of TF to plasminogen activation. First, the effects of the TF apoprotein and relipidated TF were compared. Second, purified phospholipids in the same ratio as incorporated into relipidated TF were studied. Third, human serum albumin (Sigma) was relipidated in the same manner as TF. To do this, a mixture containing equimolar ratios of phosphatidyl serine, phosphatidyl choline, and phosphatidyl ethanolamine (Avanti Polar Lipids Inc, Alabaster, AL) dissolved in 100% ethanol was dried under nitrogen. The phospholipids were resuspended in 10 mmol/L Tris HCl, pH 7.5, and added to an equimolar concentration of albumin overnight at 22°C in the presence of deoxycholate (0.05% final concentration). Excess detergent was removed by extensive dialysis against PBS.
Effect of TF on plasminogen binding to cells.
Two sets of experiments were performed. First, Glu-plasminogen was radiolabeled with Na 125I (Dupont-NEN, Boston, MA) using Iodo-Beads (Pierce, Rockford, IL). The cells were washed twice with 1% albumin in Dulbecco's PBS supplemented with 0.9 mmol/L CaCl2 and 0.5 mmol/L MgCl2 (GIBCO).125I-labeled plasminogen (100 nmol/L) was added to the cells alone or in the presence of various concentrations of TF apoprotein for 1 hour at 15°C in the case of trophoblasts or at 4°C when HUVECs were studied. The cells were washed four times in the same buffer to remove unbound plasminogen. The bound plasminogen was then eluted by exposing the cells to glycine buffer, pH 3.0, for 7 to 10 minutes and the radioactivity was measured. Nonspecific binding was determined by performing the experiment in the presence of 15 mmol/L 6-AHA (Sigma). Specific binding was calculated from the difference between total and specific binding. In a second set of experiments, the binding of 125I-Glu-plasminogen to CHO cells was measured. CHO cells expressing TF and parental wild-type cells (4 × 104) were incubated with 50 nmol/L125I-plasminogen in the presence or absence of 100 mmol/L 6-AHA for 2 hours at 37°C. The cells were washed three times in PBS, the cell-associated radioactivity was measured, and the specific binding was calculated.
Effect of TF on uPA-mediated fibrinolysis.
Two sets of experiments were performed to assess the effect of TF on uPA-mediated fibrinolysis. First, fibrinogen containing plasminogen (Calbiochem, San Diego, CA) was reconstituted in PBS to a concentration of 3 mg/mL. Thrombin (Sigma) at 0.025 NIH U/mL was added to 30 mL of the fibrinogen mixture and the fibrin clot was decanted onto a plastic lid. After 60 minutes at 22°C, 10 μL of PBS containing scuPA or tcuPA (1 μmol/L final concentration) was added alone or in the presence of TF (14 nmol/L). After 2 hours, the clot was washed four times with PBS, trypan blue was added, and the samples were incubated overnight at 22°C. The clot was then washed, photographed, and scanned for total reflectance using a Hoefer GS 300 Scanning Densitometer (Hoefer Scientific Instruments, San Francisco, CA). Each image was assigned a value for total reflectance in arbitrary units. The relative amount of reflectance was quantified by integrating the peak reflectance and normalizing each value to the largest area measured. Second, the effect of CHO cells expressing TF and wild-type on the lysis of fibrin clots was compared. CHO cells were used both as the source of uPA and TF in these experiments. The CHO cells (4 × 105) were detached with EDTA, washed in PBS, and added for 3 hours at 37°C to a fibrin clot formed in the presence of plasminogen (500 μmol/L) or buffer. The wells were photographed and the size of the zones of lysis was determined using a scanning densitometer as described above.
RESULTS
The purpose of this study was to determine whether plasminogen binds to TF and whether this binding modulates plasminogen activation.
Binding of plasminogen to immobilized TF.
To determine whether plasminogen interacts directly with TF, we examined the capacity of plasminogen to bind to a soluble, immobilized fragment of recombinant TF apoprotein comprising the extracellular domain of the protein (amino acids 1-219) using optical biosensor interaction analysis. As shown in Fig 1A, plasminogen was found to bind to this fragment in a dose-dependent manner. Linearization of both the association and dissociation phases according to equations 2 and 3, respectively (see the Materials and Methods), showed that these processes did not fit strictly to a single bimolecular process. In general, nonlinear dR/dt plots such as shown in Fig 1B could reflect multiple binding sites with different affinities, cooperativity or more complex models. To make approximations of binding constants in the current experiments, the dR/dt plots were divided into fast and slow components (components 1 and 2, respectively), in a manner used previously (see, for example, Casanovas and Springer41). Linear fits of components 1 and 2 to equation 2 yielded ks plots (Fig 1C) that led to konvalues of 4 × 104 and 2 × 103 mol/L−1 s−1, respectively. Fitting the early dissociation data to equation 3 (Fig1D) led to a calculated koff of 3.8 × 10−3 s−1. Hence, from these linear fits, apparent equilibrium Kd values were obtained of 100 nmol/L and 2 μmol/L for components 1 and 2, respectively.
Kinetic interaction analysis of Glu-plasminogen binding to TF as determined with a BIAcore optical biosensor. (A) Sensorgrams for interaction of denoted concentrations of Glu-plasminogen to sensor-immobilized TF extracellular domain. Association phase: 3-minute injections of Glu-plasminogen at 30 μL/min. Dissociation phase: 4-minute injections of buffer alone at 100 μL/min. (B) dR/dt plots of association data according to equation 1. Linear fits are shown to two different components of the data, the early component (cpt. 1) and the late component (cpt. 2). (C) Concentration dependence of ks values determined from linear fits of cpts. 1 and 2 to equation 1. (D) Dissociation phase data from 1 μmol/L plasminogen sensorgram plotted according to equation 2.
Kinetic interaction analysis of Glu-plasminogen binding to TF as determined with a BIAcore optical biosensor. (A) Sensorgrams for interaction of denoted concentrations of Glu-plasminogen to sensor-immobilized TF extracellular domain. Association phase: 3-minute injections of Glu-plasminogen at 30 μL/min. Dissociation phase: 4-minute injections of buffer alone at 100 μL/min. (B) dR/dt plots of association data according to equation 1. Linear fits are shown to two different components of the data, the early component (cpt. 1) and the late component (cpt. 2). (C) Concentration dependence of ks values determined from linear fits of cpts. 1 and 2 to equation 1. (D) Dissociation phase data from 1 μmol/L plasminogen sensorgram plotted according to equation 2.
We next examined whether plasminogen had an effect on FVII and FVIIa binding to TF apoprotein. FVIIa was found to bind to immobilized TF extracellular domain by biosensor analysis (data not shown), consistent with previously published results.42 43 We then tested whether plasminogen prebinding to the immobilized TF affected subsequent binding of FVII. As shown in Fig2, preinjection of plasminogen at increasing concentrations did not significantly reduce the extent of subsequent FVII binding, as judged by the maximum response signal after FVII injection, corrected for the signal due to bound plasminogen. A similar finding was observed when the order of injection was reversed, ie, FVII followed by plasminogen (data not shown). Hence, plasminogen and FVII do not appear to block each other's binding to TF completely and likely bind at independent sites.
FVII interaction with TF-plasminogen complex as determined by BIA2000 analysis. Glu-plasminogen was injected, at concentrations of 0.125 μmol/L (sensorgram 3), 0.25 μmol/L (sensorgram 2), and 1.0 μmol/L (sensorgram 1), onto sensor-immobilized soluble TF and the sensor surface then washed as in Fig 1. Then, FVII was injected in buffer containing 4 mmol/L CaCl2 for 3 minutes at 30 μL/min, followed by a wash in buffer alone containing 4 mmol/L CaCl2. Sensorgram 4 shows binding of FVII to immobilized TF after preinjection and wash with buffers containing no Glu-plasminogen. Sensorgrams labeled 1 through 4 for FVII are continuations of the same-numbered sensorgrams for Glu-plasminogen. (Inset) Bmax values for FVII bound after prebinding of Glu-plasminogen at the different concentrations. Bmax values are the difference between the maximum observed response for FVII association and the response due to bound Glu-plasminogen just before FVII was injected.
FVII interaction with TF-plasminogen complex as determined by BIA2000 analysis. Glu-plasminogen was injected, at concentrations of 0.125 μmol/L (sensorgram 3), 0.25 μmol/L (sensorgram 2), and 1.0 μmol/L (sensorgram 1), onto sensor-immobilized soluble TF and the sensor surface then washed as in Fig 1. Then, FVII was injected in buffer containing 4 mmol/L CaCl2 for 3 minutes at 30 μL/min, followed by a wash in buffer alone containing 4 mmol/L CaCl2. Sensorgram 4 shows binding of FVII to immobilized TF after preinjection and wash with buffers containing no Glu-plasminogen. Sensorgrams labeled 1 through 4 for FVII are continuations of the same-numbered sensorgrams for Glu-plasminogen. (Inset) Bmax values for FVII bound after prebinding of Glu-plasminogen at the different concentrations. Bmax values are the difference between the maximum observed response for FVII association and the response due to bound Glu-plasminogen just before FVII was injected.
Role of the kringles in the binding of plasminogen to TF.
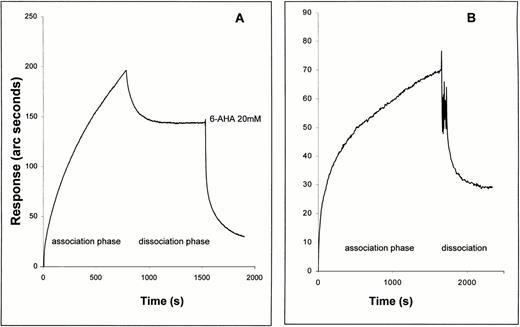
The plasminogen molecule contains 5 kringle domains, each of which contains a lysine binding site. These kringles mediate the binding of plasminogen to fibrin, to other proteins such as Lp(a), and to cells. Binding of plasminogen to these sites is prevented by the lysine analogue, 6-AHA.44-48 Therefore, we next examined the effect of 6-AHA on the binding of plasminogen to TF. As shown in Fig 3A, plasminogen bound directly to full-length TF apoprotein immobilized on a biosensor surface, in this case measured using the IAsys biosensor. Only a portion of bound plasminogen was eluted when the sensor surface was washed with buffer alone. The remaining bound plasminogen was completely washed off by injecting buffer containing 20 mmol/L 6-AHA. In accord with this finding, a plasminogen fragment containing kringles 1 through 3 bound to immobilized TF apoprotein (Fig 3B), whereas isolated kringle 4 and miniplasminogen (which lacks kringles 1 through 4) did not (data not shown).
Comparison of binding of Glu-plasminogen (A) and kringles 1 through 3 (B) to immobilized TF apoprotein as observed with an IAsys optical biosensor. Association and dissociation (wash) buffers were as for the binding of Glu-plasminogen with immobilized soluble TF (Fig 1, first part of Fig 2). Both proteins were injected into the sensor cuvette at 500 nmol/L and the association phase was observed. Dissociation was observed in dissociation buffer after 5 quick (5 seconds) rinses of the sensor cuvette with dissociation buffer. In the case of the plasminogen experiment, 20 mmol/L 6-AHA was added to the rinsed cuvette after buffer-induced dissociation.
Comparison of binding of Glu-plasminogen (A) and kringles 1 through 3 (B) to immobilized TF apoprotein as observed with an IAsys optical biosensor. Association and dissociation (wash) buffers were as for the binding of Glu-plasminogen with immobilized soluble TF (Fig 1, first part of Fig 2). Both proteins were injected into the sensor cuvette at 500 nmol/L and the association phase was observed. Dissociation was observed in dissociation buffer after 5 quick (5 seconds) rinses of the sensor cuvette with dissociation buffer. In the case of the plasminogen experiment, 20 mmol/L 6-AHA was added to the rinsed cuvette after buffer-induced dissociation.
Relationship of TF to cellular binding sites for plasminogen.
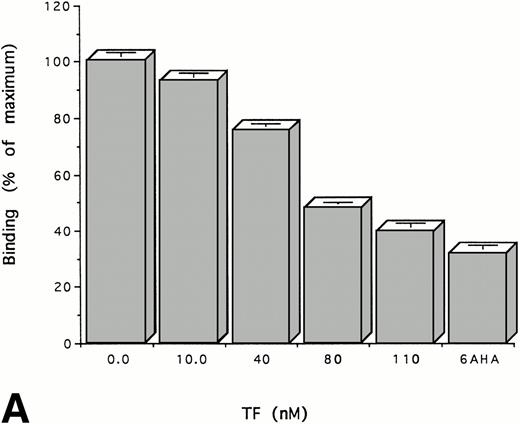
Experiments were performed to determine whether the binding of TF to plasminogen affects its binding to cells, a process that is known to be mediated through its kringles. To do this, we measured the binding of125I-plasminogen to HUVECs in the presence and absence of TF. TF inhibits the binding of plasminogen to HUVECs in a dose-dependent manner (Fig 4A). Specific binding of plasminogen was almost totally inhibited by equimolar concentrations of TF, an effect that was achieved only at much higher concentrations of 6-AHA (15 mmol/L). The same results were obtained using TF from three different sources and using another type of cell, ie, cultured human trophoblasts (Fig 4B). As shown above (Fig 2), the binding of FVII to TF apoprotein is unaffected by the binding of plasminogen. Consistent with this result, preincubation of TF (100 nmol/L) with either FVII or FVIIa (100 nmol/L) had no effect on its capacity to inhibit the binding of plasminogen (100 nmol/L) to cells or to alter FVII-dependent procoagulant activity (not shown). Thus, these data are consistent with the experiments cited above using soluble proteins that suggest that plasminogen binds to TF through its kringles at a site on the apoprotein distinct from the binding site for FVII and FVIIa.
TF inhibits the binding of Glu-plasminogen to cells.125I-Glu-plasminogen (100 nmol/L) was added to HUVECs (A) or trophoblasts (B) in the presence of the indicated concentrations of TF or 15 mmol/L 6-AHA and the cell-associated radioactivity was measured. The results are expressed relative to the binding of radiolabeled plasminogen in the absence of TF. The mean ± SD of three separate experiments performed in triplicate is shown.
TF inhibits the binding of Glu-plasminogen to cells.125I-Glu-plasminogen (100 nmol/L) was added to HUVECs (A) or trophoblasts (B) in the presence of the indicated concentrations of TF or 15 mmol/L 6-AHA and the cell-associated radioactivity was measured. The results are expressed relative to the binding of radiolabeled plasminogen in the absence of TF. The mean ± SD of three separate experiments performed in triplicate is shown.
Effect of TF on plasminogen activator activity.
Plasminogen undergoes a marked conformational change as a result of binding of certain ligands to its kringles.49 This change in conformation alters the susceptibility of the protein to activation by plasminogen activators.50-52 In view of this, we next asked whether the binding between TF and the kringles of plasminogen affected plasminogen activation by uPA. To examine this possibility, we incubated tcuPA (5 nmol/L), Glu-plasminogen (5 nmol/L), and a plasmin chromogenic substrate (100 μmol/L) in the presence and absence of 1.1 nmol/L TF. The addition of TF stimulated the formation of plasmin (Fig 5). Plasmin formation was stimulated by TF in a dose-dependent and saturable manner (Fig 6). Half-maximal stimulation was observed at a TF concentration of 59 pmol/L. Virtually identical results were obtained using each of three sources of TF (data not shown).
Effect of TF on uPA-mediated activation of Glu-plasminogen. Glu-plasminogen (5 nmol/L) was incubated with tcuPA (5 nmol/L) and the plasmin chromogenic substrate (100 μmol/L) in the absence or in the presence of TF (1.1 nmol/L). The mean ± SD of three separate experiments performed in triplicate is shown.
Effect of TF on uPA-mediated activation of Glu-plasminogen. Glu-plasminogen (5 nmol/L) was incubated with tcuPA (5 nmol/L) and the plasmin chromogenic substrate (100 μmol/L) in the absence or in the presence of TF (1.1 nmol/L). The mean ± SD of three separate experiments performed in triplicate is shown.
Concentration-dependent effect of TF on uPA-mediated activation of Glu-plasminogen. Glu-plasminogen (5 nmol/L) was incubated with tcuPA (5 nmol/L), the plasmin chromogenic substrate (100 μmol/L), and the indicated concentrations of relipidated TF for 15 minutes under the conditions described in Fig 4 and the absorbance at 405 nm was measured. The data are expressed relative to the absorbance measured in the absence of TF. The mean ± SD of three separate experiments performed in triplicate is shown.
Concentration-dependent effect of TF on uPA-mediated activation of Glu-plasminogen. Glu-plasminogen (5 nmol/L) was incubated with tcuPA (5 nmol/L), the plasmin chromogenic substrate (100 μmol/L), and the indicated concentrations of relipidated TF for 15 minutes under the conditions described in Fig 4 and the absorbance at 405 nm was measured. The data are expressed relative to the absorbance measured in the absence of TF. The mean ± SD of three separate experiments performed in triplicate is shown.
We next examined the contribution of TF apoprotein and its phospholipid component to uPA-mediated plasminogen activation. The results shown in Fig 7 indicate that TF apoprotein stimulated uPA-mediated plasmin generation. Half maximal stimulation was achieved at a concentration of 3.8 nmol/L. Thus, a higher concentration of the apoprotein was needed to stimulate plasmin formation to the same extent as relipidated protein. The results shown in Fig 7 also indicate that high concentrations of TF apoprotein attenuated the stimulatory effect on plasmin formation. The fact that higher concentrations of the apoprotein were required for plasminogen activation compared with relipidated TF suggest either that the phospholipids themselves express activity or that the phospholipids facilitate the activity of the apoprotein. To distinguish between these two possibilities, we measured the plasminogen activator activity of a mixture of purified phospholipids present in the same ratio as relipidated TF and the activity of human serum albumin reconstituted with phospholipids in the same manner as TF. In contrast to the stimulatory effect of the TF apoprotein, neither the isolated phospholipid components of TF nor relipidated human serum albumin had any effect on plasminogen activation.
Concentration-dependent effect of TF on uPA-mediated activation of Glu-plasminogen. Glu-plasminogen (5 nmol/L) was incubated with tcuPA (5 nmol/L), the plasmin chromogenic substrate (100 μmol/L), and the indicated concentrations of TF apoprotein for 15 minutes under the conditions described in Fig 4 and the absorbance at 405 nm was measured. The data are expressed relative to the absorbance measured in the absence of TF. The mean ± SD of three separate experiments performed in triplicate is shown.
Concentration-dependent effect of TF on uPA-mediated activation of Glu-plasminogen. Glu-plasminogen (5 nmol/L) was incubated with tcuPA (5 nmol/L), the plasmin chromogenic substrate (100 μmol/L), and the indicated concentrations of TF apoprotein for 15 minutes under the conditions described in Fig 4 and the absorbance at 405 nm was measured. The data are expressed relative to the absorbance measured in the absence of TF. The mean ± SD of three separate experiments performed in triplicate is shown.
Acceleration of plasmin formation by uPA in the presence of TF was also evident using gel analysis. In these experiments, Glu-plasminogen (3 μmol/L) was incubated with tcuPA (400 nmol/L) in the presence of various concentrations of TF for 20 minutes, and the reaction mixture was analyzed using SDS-PAGE under reducing conditions (Fig 8). TF apoprotein stimulated the conversion of plasminogen to plasmin in a dose-dependent manner, consistent with its effect on the generation of plasmin activity.
TF stimulates uPA-mediated conversion of plasminogen to plasmin. Glu-plasminogen (3 μmol/L) was incubated with tcuPA (400 nmol/L) and with various concentrations of TF apoprotein for 20 minutes, and the reaction mixture was analyzed using SDS-PAGE under reducing conditions. Lane 1 contains plasminogen alone. Lanes 2 through 4 contain plasminogen, tcuPA, and increasing concentrations of TF (lane 2, 40 nmol/L TF; lane 3, 100 nmol/L TF; and lane 4, 300 nmol/L TF).
TF stimulates uPA-mediated conversion of plasminogen to plasmin. Glu-plasminogen (3 μmol/L) was incubated with tcuPA (400 nmol/L) and with various concentrations of TF apoprotein for 20 minutes, and the reaction mixture was analyzed using SDS-PAGE under reducing conditions. Lane 1 contains plasminogen alone. Lanes 2 through 4 contain plasminogen, tcuPA, and increasing concentrations of TF (lane 2, 40 nmol/L TF; lane 3, 100 nmol/L TF; and lane 4, 300 nmol/L TF).
To determine whether TF stimulated uPA-mediated plasminogen activation through a mechanism similar to lysine analogues, such as 6-AHA,53-55 the activation of miniplasminogen, which lacks the kringles 1 through 4 region that contains the high-affinity Lys-binding sites,47 was analyzed. TF had no effect on the activation of miniplasminogen by tcuPA under the identical conditions in which stimulation of full-length Glu-plasminogen was observed (data not shown).
The data cited above suggest that plasminogen binds to TF through its kringles, thereby promoting its activation by uPA. Binding of other ligands, such as 6-AHA and oleic acid, to the kringles of plasminogen has been shown to inhibit plasmin(ogen)-mediated fibrinolysis, even though these same reagents stimulate its activity on small substrates.50 56 The results shown in Fig 9 indicate that TF behaves in a similar way. Addition of TF (14 nmol/L) to plasminogen inhibited fibrinolysis by 74%.
Effect of TF on uPA- and plasminogen-mediated fibrinolysis. scuPA (1 μmol/L) was added to a fibrin clot in PBS (A1 and B1) or PBS containing 14 nmol/L TF (A2 and B2) or TF without tcuPA (C1 and C2) for 2 hours. The clot was incubated with trypan blue, washed, photographed, and scanned, and its size was measured by densitinometric analysis as described in the Materials and Methods. The fibrin clot measured 0.75 and 0.84 cm2 in the absence of TF and 0.18 and 0.22 cm2 in the presence of TF. The data shown are representative of three experiments so performed. TF caused a similar increase in clot size when tcuPA (1 μmol/L) was substituted for scuPA.
Effect of TF on uPA- and plasminogen-mediated fibrinolysis. scuPA (1 μmol/L) was added to a fibrin clot in PBS (A1 and B1) or PBS containing 14 nmol/L TF (A2 and B2) or TF without tcuPA (C1 and C2) for 2 hours. The clot was incubated with trypan blue, washed, photographed, and scanned, and its size was measured by densitinometric analysis as described in the Materials and Methods. The fibrin clot measured 0.75 and 0.84 cm2 in the absence of TF and 0.18 and 0.22 cm2 in the presence of TF. The data shown are representative of three experiments so performed. TF caused a similar increase in clot size when tcuPA (1 μmol/L) was substituted for scuPA.
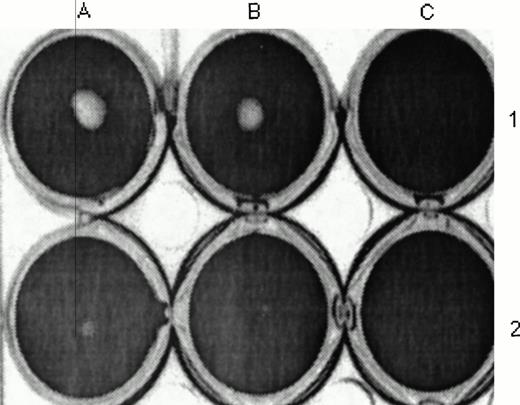
To determine whether these results using soluble TF can be generalized to the cell surface, we studied plasminogen binding and activation using a stably transfected CHO cell line that expresses full length human TF (CHO-TF). Specific binding of plasminogen to CHO-TF was stimulated 70% relative to the parental wild-type cells (Fig 10). Furthermore, the addition of CHO-TF to plasma clots inhibited fibrinolysis relative to wild-type cells (Fig 11), similar to the effect of soluble TF shown above.
Binding of TF to cell-associated TF. CHO cells expressing TF and wild-type cells (4 × 104) was incubated with 50 nmol/L 125I-plasminogen in the presence or absence of 100 mmol/L 6-AHA for 2 hours at 37°C. The cells were washed three times in PBS, the cell-associated radioactivity was measured, and the specific binding was calculated. The mean ± SEM of three experiments is shown.
Binding of TF to cell-associated TF. CHO cells expressing TF and wild-type cells (4 × 104) was incubated with 50 nmol/L 125I-plasminogen in the presence or absence of 100 mmol/L 6-AHA for 2 hours at 37°C. The cells were washed three times in PBS, the cell-associated radioactivity was measured, and the specific binding was calculated. The mean ± SEM of three experiments is shown.
Inhibition of fibrinolysis by CHO-TF. CHO cells (4 × 105) expressing TF (A) and wild-type cells (B) were detached with EDTA, washed in PBS, and added for 3 hours at 37°C to a fibrin clot formed in the presence of plasminogen (0.5 μmol/L; lanes 3 and 4) or buffer (lanes 1 and 2). The wells were photographed and the size of the zone lysis evident in the center of each well was measured by scanning densitometry. The picture shown is representative of three experiments performed under identical conditions.
Inhibition of fibrinolysis by CHO-TF. CHO cells (4 × 105) expressing TF (A) and wild-type cells (B) were detached with EDTA, washed in PBS, and added for 3 hours at 37°C to a fibrin clot formed in the presence of plasminogen (0.5 μmol/L; lanes 3 and 4) or buffer (lanes 1 and 2). The wells were photographed and the size of the zone lysis evident in the center of each well was measured by scanning densitometry. The picture shown is representative of three experiments performed under identical conditions.
Histochemical colocalization of TF and plasminogen in vivo.
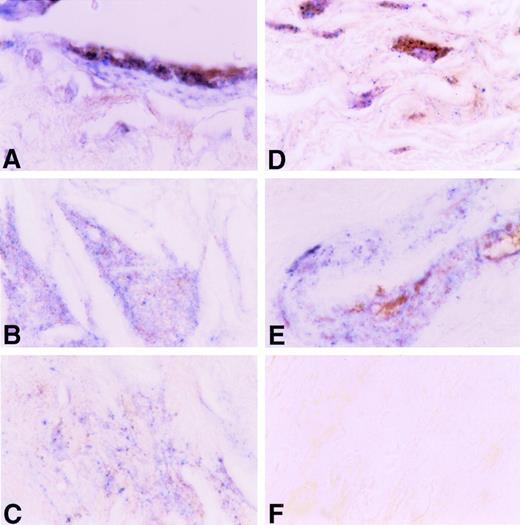
The data shown above indicate that an interaction occurs between TF apoprotein and plasminogen in vitro. To examine whether such interactions occur in vivo, we examined the relationship between the distribution of TF and plasminogen in human atherosclerotic coronary arteries using a dual-labeling approach (Fig 12). TF apoprotein was distributed in several locations within the atherosclerotic vessel wall, as described previously by others.5 19-21 The most intense staining was found in the adventitia, but TF apoprotein was also readily identified in the medial layer, in acellular plaque, and in large vessel endothelium adjacent to the plaque. Plasminogen was found in large vessel and microvascular endothelium, in medial and intimal smooth muscle cells, and in the acellular plaque. Colocalization of TF apoprotein and plasminogen was seen in luminal endothelial cells overlying the plaque (Fig 12A), within the acellular portions of the plaques (Fig 12B and C), and within medial smooth cells (Fig 12D) and smooth muscle cells surrounding small vessels in the adventitia (Fig12E). No staining was seen when an isotype control Ig was used (Fig12F).
Immunohistochemical colocalization of TF apoprotein and plasminogen in human atherosclerotic coronary artery. TF apoprotein stained blue; plasminogen stained brown (see the Materials and Methods). A large coronary artery is shown at an original magnification ×200. (A) Endothelial cells overlying plaque. (B) Acellular plaque with cholesterol cleft. (C) Acellular plaque matrix. (C) Adventitia. (E) Media of small artery within coronary artery. (F) Negative Ig control.
Immunohistochemical colocalization of TF apoprotein and plasminogen in human atherosclerotic coronary artery. TF apoprotein stained blue; plasminogen stained brown (see the Materials and Methods). A large coronary artery is shown at an original magnification ×200. (A) Endothelial cells overlying plaque. (B) Acellular plaque with cholesterol cleft. (C) Acellular plaque matrix. (C) Adventitia. (E) Media of small artery within coronary artery. (F) Negative Ig control.
DISCUSSION
The results of this study show that plasminogen binds with high affinity to an epitope(s) on the extracellular domain of TF apoprotein. Plasminogen binding to TF likely occurs through its kringles, because a fragment that contains kringles 1 through 3 also binds to TF, whereas isolated kringle 4 and miniplasminogen do not. Plasminogen and FVII/FVIIa appear to bind to different sites on TF. Preincubation of biosensor-immobilized TF extracellular domain with plasminogen did not decrease the on-rate of FVIIa binding and preincubation of TF with FVII or FVIIa had no effect on its capacity to inhibit the binding of plasminogen to cells. Furthermore, preincubation of TF with plasminogen had no effect on TF-induced procoagulant activity.
The results of the biosensor experiments, in particular the definitive response transitions in the sensorgrams showing direct mass buildup at the sensor surface upon plasminogen addition to the bulk phase and subsequent mass reduction when plasminogen is removed, demonstrate convincingly that plasminogen binds directly to the soluble domain of TF in vitro. However, the calculated rate constants obtained from the kinetics for this interaction must be considered to be estimates in that they were calculated assuming the existence of two sites of interaction with differing affinity (data fit in Fig 1B). This latter assumption is not yet proven, and the sensorgram data would also be consistent with more complicated models, such as cooperativity. Calculation of affinities using such alternative models would yield different values for kon, although it is likely that the fastest rates so calculated would not be severely different from the faster rate determined here for component 1 (Fig 1B and C). Interestingly, TF affects plasminogen activation and its binding to cells at concentrations substantially below the estimated kd (see below). This suggests that the biological (membrane-associated) and biosensor-immobilized conformations of TF may differ, that full occupancy is not required for biologic activity of TF, or that there exists a much higher affinity interaction not detectable in the time scale of the sensor method. A better understanding of the structural requirements for the interaction between plasminogen and TF will be needed to distinguish among these possibilities.
TF also stimulates the activation of plasminogen by uPA measured using a small plasmin substrate. Stimulation of urokinase-mediated plasminogen activation by TF was dose-dependent. Plasminogen activation by uPA was stimulated to the same extent by TF apoprotein and re-relipidated protein, although higher concentrations of the former were required. Neither isolated phospholipids nor human serum albumin relipidated in an identical manner as TF-accelerated plasminogen activation, suggesting that the apoprotein is the active moiety in the complex. The contribution of the lipid to plasminogen activation may be similar to its presumed role in accelerating the activation of factor X by the TF-VIIa complex and TF-VII by FXa,11 in this case by providing an additional surface to which plasminogen or uPA may bind.
TF apoprotein and Glu-plasminogen appear to form a 1:1 molar complex under the experimental conditions in that equimolar concentrations of TF almost totally inhibited the specific binding of plasminogen to HUVECs (Fig 5). Furthermore, 3.8 nmol/L TF apoprotein stimulated the activation of 5 nmol/L plasminogen by approximately 50% (Fig 6). However, the observation that the activation of 5 nmol/L plasminogen increased until the TF concentration reached 1 nmol/L suggests that a more complex interaction has occurred. This was especially evident at high concentrations of purified apoprotein (Fig 6). These data are consistent with one of the possible explanations of the biosensor binding isotherms mentioned above (Fig 1), suggesting from the dR/dt plots the possible existence of a second, lower-affinity binding site for binding of plasminogen to TF. Binding of TF to plasminogen through this second site appears to impede further stimulation. Less likely, the apoprotein may be degraded by plasmin.57
Although TF promoted the plasminogen activator activity of uPA on plasminogen when a small chromogenic substrate was used, TF inhibited uPA-mediated fibrinolysis. The capacity of TF to stimulate uPA and plasminogen-mediated cleavage of a small plasmin substrate while inhibiting fibrinolysis is similar to the effects of 6-AHA50,56 and oleic acid.58 The difference between the two situations likely results from binding of TF to the lysine binding sites of plasmin(ogen) that are involved in fibrinolysis. In accord with this interpretation, the effect of TF on plasminogen activation against the chromogenic plasmin substrate was not detected using a plasminogen fragment missing kringles 1 through 4, which are known to contain the high-affinity lysine binding sites.47 Furthermore, TF inhibited the binding of plasminogen to endothelial cells, a process mediated through its lysine binding sites,22,59 60 in accord with the results of optical biosensor studies showing that binding is mediated through kringles 1 through 3. Thus, it is likely that TF occupies the lysine binding sites because of the high affinity of this interaction, and therein prevents plasmin(ogen) from binding to fibrin.
The results of this study raise the possibility that TF may contribute a high-affinity cellular binding site for plasminogen at sites of vessel injury or inflammation. For example, TF is abundant in atherosclerotic plaques,5,19,21,61 whereas the concentration of plasminogen is very low compared with its concentration in plasma.62 We observed that plasminogen and TF colocalized in specific cellular and acellular portions of the plaque. Thus, TF may regulate the availability of plasminogen in such extracellular compartments because of its high affinity relative to other plasminogen binding sites described to date. In this manner, the capacity of TF to regulate plasminogen activation on cell surfaces may contribute to its reported effects on angiogenesis,14 tumor invasiveness,16,17 and atherosclerosis.19
Supported in part through National Institutes of Health Grants No. HL40387, HL50970, and HL49839 (D.B.C.) and Grant No. 960105000 from the American Heart Association (A.A.H.).
Address reprint requests to Douglas Cines, MD, Room 513A, Stellar-Chance Laboratories, University of Pennsylvania, 420 Curie Blvd, Philadelphia, PA 19104.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.










This feature is available to Subscribers Only
Sign In or Create an Account Close Modal