Abstract
The βIVS-2-654 C→T mutation accounts for approximately 20% of β thalassemia mutations in southern China; it causes aberrant RNA splicing and leads to β0 thalassemia. To provide an animal model for testing therapies for correcting splicing defects, we have used the “plug and socket” method of gene targeting in murine embryonic stem cells to replace the two (cis) murine adult β globin genes with a single copy of the human βIVS-2-654 gene. No homozygous mice survive postnatally. Heterozygous mice carrying this mutant gene produce reduced amounts of the mouse β globin chains and no human β globin, and have a moderate form of β thalassemia. The heterozygotes show the same aberrant splicing as their human counterparts and provide an animal model for testing therapies to correct splicing defects at either the RNA or DNA level.
β THALASSEMIA IS AN anemia of varying severity resulting from mutations that lead to a decrease in β globin subunits available to form hemoglobin (β+) or their complete absence (β0). Nearly 100 β thalassemia mutations have been described1-3: among the most frequent types are point mutations occurring in an intron, which activate aberrant splicing sites. For example, in βIVS-2-654 the C→T transition at nucleotide 654 of intron 2 creates an additional 5′ donor splice site at position 652 and activates an endogenous cryptic 3′ acceptor site at position 579.4 Spliced βIVS-2-654 mRNA retains nucleotides 580-652 of the second intron and as a result does not encode a functional β globin polypeptide.4 This particular splice mutation is frequent among patients in China and Thailand,2 5 accounting for 20% of β-thalassemia in some regions.
Splice mutations occurring in an intron of an affected gene are uniquely suited to a new type of therapeutic approach. Because the coding sequences necessary for function are intact, the blocking of the aberrant splice sites with antisense oligonucleotides may enable formation of correctly spliced and functional mRNA. Sierakowska et al6 have recently tested this idea and demonstrated that the aberrant splicing caused by the βIVS-2-654 mutation can be partially corrected in tissue culture by antisense oligonucleotides. Testing this type of oligotherapy (or any other type of therapy) in vivo requires a suitable animal model. The design and delivery of oligonucleotides to the target cells must be optimized, and the efficacies of the treatment have to be evaluated, including the therapeutic range of mRNA production, the duration of effect, and the possible occurrence of immunologic responses. The model animals should have a phenotype similar to the human condition7 and should carry the mutant globin gene in a form such that correction of the splicing mutation will yield normally functional mRNA. To achieve this goal, we have used the “plug and socket” method of gene targeting to replace the two normal murine adult β globin genes with a single copy of the human mutant βIVS-2-654 gene. Heterozygotes for this mutant gene show the same aberrant splicing as their human counterparts and have a moderate form of β thalassemia.
MATERIALS AND METHODS
Gene targeting.
The socket-containing embryonic stem (ES) cell line, B20,8 in which a neo gene and a partially deleted minigene for hypoxanthine phosphoribosyl transferase (HPRT) are inserted downstream of the murine adult β globin genes, was used to introduce the mutant form of the human gene. The construct, th-4 plug (see Fig 1), includes a 5.7-kb genomicHindIII-XbaI fragment of the human β globin gene, covering the GenBank sequence region 59611-65439, into which the human βIVS-2-654 mutation was introduced by site-directed mutagenesis9 (see Fig 1). The th-4 targeting construct also contains a 3.9-kb BamHI-HindIII fragment of BALB/c mouse DNA inserted 5′ to the murine adult β globin genes and inserted 3′ to the mouse genes, a 1.9-kbClaI-XhoI fragment, which contains the promoter, and exon 1 of the HPRT minigene, as described previously.8 The β globin and HPRT genes are in the same transcriptional orientation. Th-4 targeting DNA was introduced into B20 cells by electroporation and hypoxanthine-aminopterin-thymidine (HAT)-resistant, G418-sensitive colonies were isolated.8 The presence on Southern blots of a 10-kb NdeI fragment hybridizing to a probe specific to intron 2 of the human β globin gene was used to confirm correctly targeted colonies.8
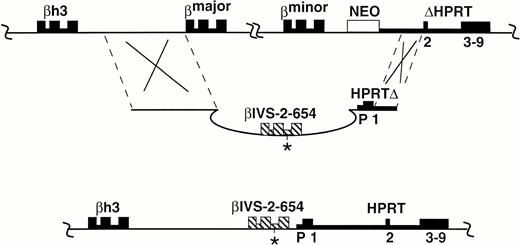
Replacement of the murine adult β globin genes by the human βIVS-2-654 gene. The socket-containing chromosome (A), the th-4 plug targeting construct (B), and the correctly targeted chromosome (C) are shown. The exons and introns of genes are represented as boxes and thick lines, respectively. The human β globin gene is cross-hatched with the position of the IVS-2-654 mutation shown with an asterisk. Promoter (P) and exons 1-9 of HPRT are marked. Upstream and downstream sequences that are identical or homologous in the targeting construct and the target chromosome are demarcated by dashed lines. βh3 is a β globin pseudogene. Recombination (indicated by Xs) occurs between the target locus (A) and the plug targeting construct (B), yielding a chromosome that contains the human β globin gene in place of the adult murine β globin genes, β major and β minor. Additionally, the neo gene is removed and a functionalHPRT gene is created by the correct targeting. TheHPRT gene and globin genes are transcribed from left to right in the figure, the neo gene is transcribed from right to left.
Replacement of the murine adult β globin genes by the human βIVS-2-654 gene. The socket-containing chromosome (A), the th-4 plug targeting construct (B), and the correctly targeted chromosome (C) are shown. The exons and introns of genes are represented as boxes and thick lines, respectively. The human β globin gene is cross-hatched with the position of the IVS-2-654 mutation shown with an asterisk. Promoter (P) and exons 1-9 of HPRT are marked. Upstream and downstream sequences that are identical or homologous in the targeting construct and the target chromosome are demarcated by dashed lines. βh3 is a β globin pseudogene. Recombination (indicated by Xs) occurs between the target locus (A) and the plug targeting construct (B), yielding a chromosome that contains the human β globin gene in place of the adult murine β globin genes, β major and β minor. Additionally, the neo gene is removed and a functionalHPRT gene is created by the correct targeting. TheHPRT gene and globin genes are transcribed from left to right in the figure, the neo gene is transcribed from right to left.
Chimera production and breeding.
Three germline transmitting chimeras were generated from one of three targeted ES cell lines that were isolated. Chimeras were bred to C57BL/6J (B6) mice to produce F1 (129×B6) offspring. The F1 animals used for the studies described here are genetically identical except for the presence (Hbbth-4/Hbb+) or absence (Hbb+/Hbb+) of the human βIVS-2-654 gene at the β globin locus.
Cellulose acetate electrophoresis.
For hemoglobin analysis by cellulose acetate electrophoresis, heparinized blood samples were collected from the retroorbital sinus and washed twice in 20× volumes of buffered saline to isolate red blood cells (RBC). The RBC were lysed in 50× volumes of cold deionized, distilled water. A total of 10 μL of lysate was then mixed with 2 μL of 75 mg/mL cystamine dihydrochloride (pH > 7) to modify the hemoglobin sulfhydryl groups.10 After incubation for 10 minutes at room temperature, approximately 3 μL of the modified lysate was analyzed by electrophoresis on Titan III-H cellulose acetate strips (76 × 60 mm) (Helena Laboratories, Beaumont, TX). Electrophoresis was performed with Supre-Heme buffer pH 8.2 (Helena Laboratories) at 40 V/cm. Bands were stained with 0.5% Ponceau S and destained with 5% acetic acid.
Peripheral blood smears.
Peripheral blood smears, made from 1 to 2 μL of blood collected in heparinized microhematocrit tubes, were air dried and stained with Wright stain.
RBC indices.
Whole blood samples from mice at least 8 weeks old were collected in 40 μL microhematocrit tubes containing 2 μL of 0.5 mol/L EDTA (pH 8). The hematocrit (Hct), RBC count, hemoglobin (Hb), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and RBC distribution width (RDW) for each sample was determined using a Roche Cobras Helios Hematology Analyzer (ABX, Montpellier, France) equipped with software to analyze murine cells.
Organ weights and preparation.
Mice at 5 months of age were given lethal doses of 2,2,2 tribromoethanol (avertin) and perfused with 4% paraformaldehyde (pH 7.4). Liver, lungs, heart, spleen, and kidneys were collected from each animal, kept overnight in Bouin's solution, blotted dry, weighed, embedded in paraffin, and sectioned. Staining was with hematoxylin and eosin or Prussian blue.
RNA isolation.
Blood was collected from mouse tail veins in microhematocrit tubes previously rinsed with sterile acid citrate dextrose. Total RNA was prepared using the Tri-Reagent BD system (Molecular Research Center, Cincinnati, OH) as described by the manufacturer.
Reverse transcriptase-polymerase chain reaction.
Reverse transcriptase-polymerase chain reaction (RT-PCR) was performed on the RNA with rTth enzyme (Perkin Elmer, Foster City, CA) with 32P-labeled nucleotide triphosphates for 18 cycles of 95°C 1 minute and 65°C for 1 minute. PCR products were separated on a 7.5% nondenaturing polyacrylamide gel.
The primers (see Fig 3A) used to determine aberrant splicing of the human β globin pre-mRNA were: i, 5′-GGACCCAGAGGTTCTTTGAGTCC-3′, and ii, 5′-GCACACAGACCAGCACGTTGCCC-3′, which correspond respectively to nucleotides 21-43 of the second exon and nucleotides 28-6 of the third exon. To identify the region of intron remaining in the processed human mRNA, the 5′ primer i was used with primer iii, 5′-GAGGCATGATACATTGTATCATTATTGCCCC-3′, corresponding to 2 bases of sequence at the end of exon 2 and 29 bases of intron 2.
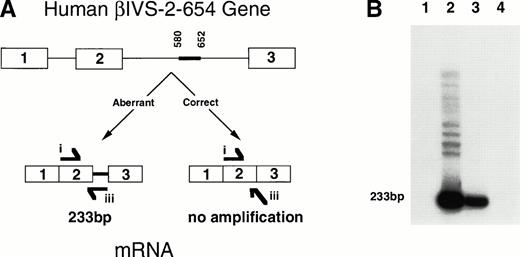
A diagram and RT-PCR results showing aberrant splicing as seen in humans of the human βIVS-2-654 transcript in theHbbth-4/Hbb+ thalassemic mice. (A) The diagram shows the human βIVS-2-654 gene with aberrantly spliced β globin mRNA produced from the mutant gene compared with the correctly spliced β globin mRNA that would have been produced from a wild-type gene. The thick line between nucleotides 580 and 652 shows the region of IVS-2 that is maintained in the βIVS-2-654 mRNA. RT-PCR primer i and iii are shown at the location and in the direction in which they anneal to the RNA. Primer i anneals to sequences within the second exon of human β globin, and primer iii anneals to two bases of the sequence at the end of the second exon and 29 bases of the region of the second intron that is maintained with this mutation in humans. (B) An autoradiograph of polyacrylamide gel electrophoresis of RT-PCR with primers i and iii on RNA from a heterozygous huβs/Hbb+ mouse (lane 1), a heterozygous Hbbth-4/Hbb+ thalassemic mouse (lane 2), a HeLa cell line transfected with a βIVS-2-654 gene (lane 3), and a normal human (lane 4) is shown. Aberrantly spliced β globin mRNA RT-PCR product is 233-bp, as labeled. Correctly spliced β globin mRNA does not amplify with primers i and iii.
A diagram and RT-PCR results showing aberrant splicing as seen in humans of the human βIVS-2-654 transcript in theHbbth-4/Hbb+ thalassemic mice. (A) The diagram shows the human βIVS-2-654 gene with aberrantly spliced β globin mRNA produced from the mutant gene compared with the correctly spliced β globin mRNA that would have been produced from a wild-type gene. The thick line between nucleotides 580 and 652 shows the region of IVS-2 that is maintained in the βIVS-2-654 mRNA. RT-PCR primer i and iii are shown at the location and in the direction in which they anneal to the RNA. Primer i anneals to sequences within the second exon of human β globin, and primer iii anneals to two bases of the sequence at the end of the second exon and 29 bases of the region of the second intron that is maintained with this mutation in humans. (B) An autoradiograph of polyacrylamide gel electrophoresis of RT-PCR with primers i and iii on RNA from a heterozygous huβs/Hbb+ mouse (lane 1), a heterozygous Hbbth-4/Hbb+ thalassemic mouse (lane 2), a HeLa cell line transfected with a βIVS-2-654 gene (lane 3), and a normal human (lane 4) is shown. Aberrantly spliced β globin mRNA RT-PCR product is 233-bp, as labeled. Correctly spliced β globin mRNA does not amplify with primers i and iii.
RESULTS
The “plug and socket” method of targeting is a two-step procedure. The first step was applied to the murine β globin locus to produce a generally useful “socket”-containing embryonic stem (ES) cell line (B20), which has been described.8 The B20 cell line, derived from an HPRT-deficient ES cell line, contains a neomycin resistance (neo) gene and a partial HPRTgene (ΔHPRT) downstream of the murine β major and β minor globin genes (Fig 1A). For the second step, which generates the desired mutation, a “plug” targeting construct (Fig1B) was used that allowed the replacement of the 21-kb of mouse genomic DNA containing the murine β major and β minor globin genes with a 5.7-kb DNA fragment containing the human βIVS-2-654 gene. The resultant mutation (Fig 1C) is designated Hbbth-4(abbreviated to th-4).
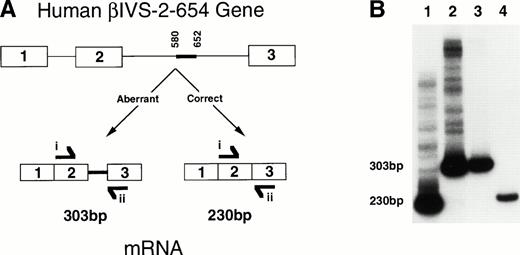
To determine if the human β globin pre-mRNA produced in theHbbth-4/Hbb+ mice was aberrantly spliced as in humans, RT-PCR was performed on RNA derived from whole blood of aHbbth-4/Hbb+ mouse, a mouse (huβs/Hbb+) heterozygous for a chromosome carrying a single copy of the human sickle globin gene in place of the murine adult β globin genes (Lewis et al, unpublished), and a normal human. Complementary primers to human (but not mouse) β globin exons 2 and 3 were used to amplify a 230-bp region in correctly spliced human β globin mRNA or a 303-bp region in aberrantly spliced βIVS-2-654 mRNA. Only correct splicing of the human β globin mRNA was detected in the huβs/Hbb+ mouse and in the normal human (Fig 2B, lanes 1 and 4). Only aberrantly spliced β globin mRNA was detected in the heterozygous β thalassemic mice (Fig 2B, lane 2). RNA from a HeLa cell line transfected with a human β globin gene containing the IVS-2-654 mutation6 was used to identify the correct size of the aberrantly spliced β globin transcript (Fig 2B, lane 3). No amplification was observed with RNA from wild-type mice (data not shown).
A diagram and RT-PCR results showing aberrant splicing of the human βIVS-2-654 transcript in theHbbth-4/Hbb+ thalassemic mice. (A) The diagram shows the human βIVS-2-654 gene with aberrantly spliced β globin mRNA produced from the mutant gene compared with the correctly spliced β globin mRNA that would have been produced from a wild-type gene. The thick line between nucleotides 580 and 652 shows the region of IVS-2 that is maintained in the βIVS-2-654 mRNA. RT-PCR primers i and ii are shown at the location and in the direction in which they anneal to the RNA. Primer i anneals to sequences within the second exon of human β globin, and primer ii anneals to sequences within the third exon of human β globin. (B) An autoradiograph of polyacrylamide gel electrophoresis of RT-PCR with primers i and ii on RNA from a heterozygous huβ s/Hbb+mouse (lane 1), a heterozygousHbbth-4/Hbb+ thalassemic mouse (lane 2), a HeLa cell line transfected with a βIVS-2-654 gene (lane 3), and a normal human (lane 4) is shown. Aberrantly spliced β globin mRNA and correctly spliced β globin mRNA RT-PCR products are 303 bp and 230 bp, as labeled.
A diagram and RT-PCR results showing aberrant splicing of the human βIVS-2-654 transcript in theHbbth-4/Hbb+ thalassemic mice. (A) The diagram shows the human βIVS-2-654 gene with aberrantly spliced β globin mRNA produced from the mutant gene compared with the correctly spliced β globin mRNA that would have been produced from a wild-type gene. The thick line between nucleotides 580 and 652 shows the region of IVS-2 that is maintained in the βIVS-2-654 mRNA. RT-PCR primers i and ii are shown at the location and in the direction in which they anneal to the RNA. Primer i anneals to sequences within the second exon of human β globin, and primer ii anneals to sequences within the third exon of human β globin. (B) An autoradiograph of polyacrylamide gel electrophoresis of RT-PCR with primers i and ii on RNA from a heterozygous huβ s/Hbb+mouse (lane 1), a heterozygousHbbth-4/Hbb+ thalassemic mouse (lane 2), a HeLa cell line transfected with a βIVS-2-654 gene (lane 3), and a normal human (lane 4) is shown. Aberrantly spliced β globin mRNA and correctly spliced β globin mRNA RT-PCR products are 303 bp and 230 bp, as labeled.
When the 5′ primer i was used in combination with a 3′ primer, iii, which includes two bases from exon 2 and continues into the intronic region that is only retained in aberrantly spliced βIVS-2-654 mRNA, theHbbth-4/Hbb+ thalassemic mouse and the transfected HeLa cell line6 showed the expected 233-bp band (Fig 3B, lanes 2 and 3). The heterozygous huβs/Hbb+ mouse and the normal human showed no amplification product (Fig 3B, lanes 1 and 4). These results show that the β globin pre-mRNA produced in theHbbth-4/Hbb+ mice was aberrantly spliced using the same IVS-2-579 and IVS-2-652 splice sites as are used in humans with the βIVS-2-654 mutation.
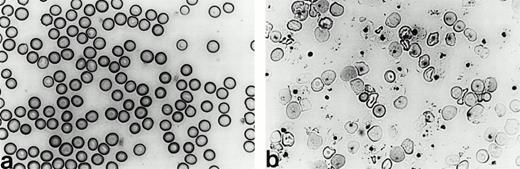
Heterozygous mice (Hbbth-4/Hbb+), where+ represents the wild-type β globin locus from strain C57BL/6J, were noticeably smaller and paler than theirHbb+/Hbb+ littermates at birth. No homozygous mutant animals survived postnatally. Cellulose acetate electrophoresis of the hemoglobin from the heterozygousHbbth-4/Hbb+ mice showed no hemoglobin from the human mutant gene (data not shown). The heterozygous Hbbth-4/Hbb+ mice showed classic signs of β thalassemia intermedia,7including anisocytosis, poikilocytosis, and target cells in the peripheral blood smear (Fig 4). Changes in RBC indices were observed inHbbth-4/Hbb+ mice when compared with their Hbb+/Hbb+littermates (Table 1), including significant decreases in RBC count, Hb, Hct, MCV, and increases in MCHC and RBC distribution width.
Peripheral blood smears from wild-type and heterozygousHbbth-4/Hbb+ mice. Blood smears stained with Wright stain from (a) a wild-type 129/Ola mouse and (b) an F1 heterozygous Hbbth-4/Hbb+thalassemic mouse (600X).
Peripheral blood smears from wild-type and heterozygousHbbth-4/Hbb+ mice. Blood smears stained with Wright stain from (a) a wild-type 129/Ola mouse and (b) an F1 heterozygous Hbbth-4/Hbb+thalassemic mouse (600X).
Hematologic Values for F1 Wild-Type and Heterozygous Thalassemic Mice
| Genotype . | No. . | RBC (106/μL) . | Hb (g/dL) . | Hct (%) . | MCV (μm3) . | MCH (pg) . | MCHC (g/dL) . | RDW . |
|---|---|---|---|---|---|---|---|---|
| Hbb+/Hbb+ | 11 | 8.7 ± 0.3 | 15.0 ± 0.6 | 43.4 ± 1.9 | 49.5 ± 0.5 | 17.2 ± 0.1 | 34.8 ± 0.4 | 18.6 ± 2.5 |
| Hbbth-4/Hbb+ | 7 | 7.4 ± 0.4 | 11.7 ± 0.3 | 28.9 ± 0.8 | 39.7 ± 1.6 | 16.3 ± 1.1 | 40.7 ± 1.1 | 43.7 ± 0.6 |
| P | .01-150 | 4 × 10−4 -150 | 2 × 10−5 -150 | 3 × 10−6 -150 | .29 | 1 × 10−5 -150 | 8 × 10−7 -150 |
| Genotype . | No. . | RBC (106/μL) . | Hb (g/dL) . | Hct (%) . | MCV (μm3) . | MCH (pg) . | MCHC (g/dL) . | RDW . |
|---|---|---|---|---|---|---|---|---|
| Hbb+/Hbb+ | 11 | 8.7 ± 0.3 | 15.0 ± 0.6 | 43.4 ± 1.9 | 49.5 ± 0.5 | 17.2 ± 0.1 | 34.8 ± 0.4 | 18.6 ± 2.5 |
| Hbbth-4/Hbb+ | 7 | 7.4 ± 0.4 | 11.7 ± 0.3 | 28.9 ± 0.8 | 39.7 ± 1.6 | 16.3 ± 1.1 | 40.7 ± 1.1 | 43.7 ± 0.6 |
| P | .01-150 | 4 × 10−4 -150 | 2 × 10−5 -150 | 3 × 10−6 -150 | .29 | 1 × 10−5 -150 | 8 × 10−7 -150 |
Hematologic values are expressed as means ± SEM. Pvalues are for two-tailed Student's t tests in comparisons between Hbb+/Hbb+ andHbbth-4/Hbb+ mice assuming equal variance.
Abbreviations: RBC, red blood cells; Hb, hemoglobin; Hct, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, RBC distribution width.
Denotes significant P values.
Body weights of F1 heterozygous mice at 2 months of age (21.8 ± 0.9 g, standard error of mean [SEM] n = 7) were 14% smaller than wild-type mice (24.8 ± 1.1 g, n = 7), although the difference did not quite reach statistical significance (P = .052). Lungs, livers, kidneys, and hearts of wild-type and thalassemic animals comprised approximately the same percentages of body weight. However, the spleens of theHbbth-4/Hbb+ mice were dramatically enlarged (P = 1.8 × 10-8) and comprised 2% of body weight compared with 0.2% of body weight in wild-type mice. This result is consistent with the splenomegaly usually seen in thalassemic patients.7
At 2 months of age, the livers from heterozygousHbbth-4/Hbb+ mice showed extramedullary hematopoiesis, similar to that observed in human thalassemia, with dilated sinusoids containing hematopoietic cells. The livers and spleens ofHbbth-4/Hbb+ mice contained regions of iron deposition that were increased by 5 months of age. Similar iron deposits are frequently seen in human thalassemia.7 The hearts, lungs, and kidneys of the 2-month old animals showed no iron deposition, but by 5 months, the convoluted tubules of the kidneys of theHbbth-4/Hbb+ mice had substantial iron deposition. TheHbbth-4/Hbb+ mice are more severely affected than comparable β°/Hbb+thalassemia intermedia humans, presumably because mice cannot compensate for a shortage of β globin subunits by maintaining production of fetal globins or by increasing δ globin synthesis.11
Overall our data establish unequivocally that the human βIVS-2-654 gene is transcribed in theHbbth-4/Hbb+ mice and that all of the corresponding processed mRNA is 73-bp larger than that expected from normal processing.
DISCUSSION
Four types of mouse models for human β thalassemia have been described, including a naturally occurring β thalassemia observed in mice. In the first model, which is a naturally occurring deletion, one of the two mouse adult β globin genes, β major, is deleted.12 About 60% of mice homozygous for this deletion (Hbbth-1/Hbbth-1) survive to adulthood. Heterozygotes (Hbb+/Hbbth-1) show very mild thalassemia. The second model for β thalassemia was created by insertional disruption by gene targeting of the mouse adult β major globin gene.13 Mice homozygous for this mutation (Hbbth-2/Hbbth-2) do not survive past a few hours after birth. The heterozygotes are anemic and have features of thalassemia similar to those found in human β thalassemia intermedia. Two models (Hbb0 14 andHbbth-3 15) were produced by complete deletions of both the murine adult β globin genes, β major, and β minor. The phenotypes of the heterozygotes for these two models are equivalent and include microcytic anemia and splenomegaly. The th-3 homozygotes die immediately after birth.
The present mouse model for β thalassemia is a heterozygote (Hbbth-4/Hbb+) carrying a human gene with βIVS-2-654 splice mutation and the normal mouse β globin locus; it shows the classic signs of a moderate form of β thalassemia. The Hbbth-4/Hbb+heterozygous mice have low RBC counts, indicating inefficient erythropoiesis and increased RBC destruction. This is seen in humans with β thalassemia and is due to inclusion body (alpha 4 tetramers) precipitation on the membrane of the RBC before they are released into circulation.7 As expected, peripheral blood smears from theHbbth-4/Hbb+ mice, showing marked anisocytosis and poikilocytosis, are similar to those of the heterozygous Hbbth-3 mice, but are more substantially pronounced than the smears from heterozygousHbbth-2 mice. The genetic defect clearly results in profound RBC morphologic abnormalities reflective of the associated erythropoietic abnormalities. These morphologic changes are very similar to those observed in human thalassemia. At 2 months, the thalassemic animals had not begun to accumulate iron in their kidneys, but by 5 months, iron deposits could be seen throughout the convoluted tubules of the thalassemic animals, presumably the consequence of ongoing hemolysis and increased iron absorption as seen in human thalassemia.7
Thus, the heterozygousHbbth-4/Hbb+ mice exhibit the β thalassemia intermedia phenotype and provide the first animal model of any disease resulting from a known human splicing mutation. In addition, unlike mouse models for thalassemia caused by complete inactivation or deletion of genes in which direct gene therapy requires the addition of a functional gene, the Hbbth-4animals can be treated in ways designed to correct the aberrant splicing at both the RNA and DNA level. Complete correction is expected to normalize their thalassemic condition because heterozygous mice carrying a human sickle globin gene in the same context as the βIVS-2-654 gene in the Hbbth-4 heterozygotes are not thalassemic (J. Lewis et al, unpublished data). But even a small increase in the production of correctly spliced mRNA should be clearly beneficial in decreasing the severity of the thalassemia and should be detectable without the need to kill the animals by testing for human β globin polypeptide or mRNA in their circulating RBC or reticulocytes. The presently generated mice will therefore provide an animal model in which the antisense and other types of therapy can be tested in vivo.
ACKNOWLEDGMENT
We thank Drs Pete Detloff, J. Barry Whitney III, Robert Reddick, and Bob Bagnell for advice concerning these experiments. We thank Drs Stuart Bentley and Suzanne Kirby for critical reading of the manuscript and expert advice concerning hematology. We thank Kim Kluckman, Jenny Lynch, and Annette Staton for excellent technical and secretarial assistance.
Supported by Grant No.GM20069 (to O.S.), HL37001 (to O.S.), HL09431 (to B.Y.) from the National Institutes of Health, Bethesda, MD and a gift from the W.M. Keck Foundation (Los Angeles, CA).
Address reprint requests to Nobuyo Maeda, PhD, Department of Pathology and Laboratory Medicine, University of North Carolina, Chapel Hill, NC 27599-7525.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal