Abstract
The Pk-1slc gene encodes a mutant red blood cell (RBC) type pyruvate kinase (PK), and adult CBA-Pk-1slc/Pk-1slc mice show a severe nonspherocytic hemolytic anemia. However, the number of RBCs and the proportion of reticulocytes were comparable between neonatal CBA-Pk-1slc/Pk-1slc mice and control -+/+ mice. Since the age-dependent increase of RBCs was much greater in CBA-+/+ mice than in CBA-Pk-1slc/Pk-1slc mice, significant anemia was observed in the latter mice on day 14 after birth. The increase of RBCs in CBA-+/+ mice was due to the prolongation of their survival time. The half life of RBCs increased in CBA-+/+ mice with ages, but it decreased in CBA-Pk-1slc/Pk-1slc mice. The relatively longer half life of RBCs in neonatal CBA-Pk-1slc/Pk-1slc mice appeared to be due to the delayed switching from M2-type PK that are expressed by undifferentiated erythroid precursor cells to RBC-type PK that are expressed by mature RBCs.
PYRUVATE KINASE (PK, EC 2.7.1.40) catalyzes the conversion of phosphoenolpyruvate to pyruvate in the glycolytic pathway. In humans, deficiency of PK activity is the most common cause of hereditary anemias due to deficiency of glycolytic enzymes.1-3 PK has four isozymes in mammals4; the red blood cell (RBC) type PK (R-PK) is expressed almost exclusively in mature RBCs.5 A unique structural profile of the R-PK is the longer amino-terminal sequence as compared with that of liver-type PK (L-PK), which is transcribed from alternative promoter of the L/R-PK gene.6 In mice, L/R-PK is encoded by the Pk-1gene.7 In contrast with mature RBCs, undifferentiated erythroid precursor cells express a different isozyme of PK (M2-PK),8-10 which is encoded by the Pk-3 gene of the mouse. The Pk-3 gene encodes both M1-PK and M2-PK; they are produced by alternative RNA splicing,11 and the former was expressed chiefly by muscle and the latter by fetus and most adult tissues.12-15 The expression of the Pk-3 gene switches to that of the Pk-1 gene during differentiation of RBCs. The persistence of M2-PK in RBCs has been described in some patients of hereditary PK deficiency.16 17
We recently found the mutant Pk-1slc gene in the CBA/N strain of mice (hereafter called CBA mice).18,19 Mice of CBA-Pk-1slc/Pk-1slc showed severe nonspherocytic hemolytic anemia. The activity of PK in RBCs of the adult mutants decreased to 16.2% of normal (+/+) adults. Because CBA-Pk-1slc/Pk-1slc mice showed a remarkable reticulocytosis (41.6%) and PK activity of reticulocytes is much higher than that of mature RBCs, the PK activity in mature RBCs of the CBA-Pk-1slc/Pk-1slc mice was calculated to be 2.8% that of mature RBCs of CBA-+/+ mice.19
The abnormality of CBA-Pk-1slc/Pk-1slcmice was characterized by comparing normal and mutant Pk-1cDNAs. A missense mutation at nucleotide 1013 GGT → GAT was identified in the cDNA sequence of the mutant, causing a single amino acid substitution of 338Gly to Asp. The missense mutant was also confirmed in genomic sequence by the analysis using polymerase chain reaction for restriction fragment length polymorphism.18
Although adult CBA-Pk-1slc/Pk-1slc mice show a severe hemolytic anemia, neonatal CBA-Pk-1slc/Pk-1slc mice are not pale. We cannot distinguish neonatal CBA-Pk-1slc/Pk-1slc mice from neonatal CBA-+/+ mice by appearance. In the present study, we investigated when the significant anemia developed in CBA-Pk-1slc/Pk-1slc mice. Moreover, we compared the survival time of RBCs between CBA-Pk-1slc/Pk-1slc and -+/+ mice of various ages.
MATERIALS AND METHODS
Mice.
CBA-Pk-1slc/Pk-1slc mice and CBA-+/+ mice were raised in the Japan SLC farm (Shuchi-gun, Shizuoka, Japan) and sent to Osaka University. As described in a previous report,19CBA-Pk-1slc/Pk-1slc mutant strain was found in the inbred CBA colony that was originally obtained from National Institutes of Health (Bethesda, MD) and kept in the Japan SLC farm. Although the CBA-Pk-1slc/Pk-1slcmice were maintained by brother-sister mating for 7 years, no difference was detectable in 16 biochemical genetic markers between the CBA-Pk-1slc/Pk-1slc and CBA-+/+ mice.19 Moreover, bone marrow transplantation from the CBA-+/+ mice to CBA-Pk-1slc/Pk-1slcmice cured the anemia.19
Female and male CBA-Pk-1slc/Pk-1slcmice were mated together to obtain mice ofPk-1slc/Pk-1slc genotypes; female and male CBA-+/+ mice were mated together to obtain mice of +/+ genotypes. On various days after birth, mice were weighed, anesthetized by ether, and killed by exsanguination from the carotid artery. Blood was collected using heparinized Pasteur pipette. The spleen was removed and weighed. To obtain fetal livers, pregnant mice were killed on day 16 postcoitus.
Hematologic parameters.
The number of RBCs was measured with a hemocytometer. Reticulocytes were counted on blood films stained with new methylene blue, and the percentage of reticulocytes was determined by counting 1,000 RBCs. In some cases, the blood films were post-stained with May-Grünwald-Giemsa to examine the contamination of nucleated erythroblasts.
Refinement of RBCs and PK activity.
Heparinized whole blood was diluted three times with ice-cold physiologic saline and passed through a column of α-cellulose (Sigma Chemical Co, St Louis, MO) and Sigmacell (Sigma) to deplete leukocytes and platelets.20 Refined RBCs were washed three times and added to the same volume of preservative composed of 28% glycerin, 2.8% sorbitol, and 0.85% NaCl, and stored frozen at −80°C. PK activity was measured spectrophotometrically by the consumption of NADH in the coupled optical assay in which LDH is used as an auxiliary enzyme as previously described.21
Survival of RBCs.
Blood was obtained from CBA-Pk-1slc/Pk-1slc and -+/+ mice of 1, 7, 42, and 90 days of age. The blood was diluted three times with Hanks' balanced salt solution (HBSS), centrifuged at 1,500gfor 5 minutes, and the supernatant and buffy coat were removed. The remaining RBCs were diluted three times with HBSS and incubated for 1 hour at 37°C with 740 kBq/mL Na251CrO4 (Amersham International, Little Chalfont, Buckinghamshire, England; specific activity, 3.7 to 18.5 MBq/μg Cr).22 The labeled RBCs were washed twice and diluted three times with HBSS, and injected into 90-day-old CBA-+/+ mice via the carotid vein in a volume of 0.5 mL. Blood samples of 0.1 mL were obtained from the tail tip on various times after the injection, and the radioactivity was measured with a Minaxi γ5550 gamma counter (Packard, Meriden, CT). Blood samples that were obtained 2 hours after the infusion were kept as controls, and the correction was made for radioactive decay. The radioactivity of each blood sample was expressed as a percentage of the control, and the percentage was plotted semi-logarithmically.
Polyacrylamide gel electrophoresis (PAGE).
Erythrocytes were lysed by sonication in the PK sample buffer containing 10 mmol/L Tris/HCl, pH 7.5, 100 mmol/L KCl, 2 mmol/L 2-mercaptoethanol, 10 mmol/L ε-aminocaproic acid, and 10 mmol/L EDTA. Protein extracts prepared from fetal liver were also homogenized in the PK sample buffer. PK activity assay, PAGE, and staining for PK activity were performed as described previously.23
Antibody neutralization of PK.
Rabbit antiserums against L-PK and M1-PK of the rat were kindly provided by Dr Tamio Noguchi of Fukui Medical School. We used them to characterize the isozyme expression in RBCs of CBA-Pk-1slc/Pk-1slc and -+/+ mice of various ages. RBC lysate was mixed with anti–L-PK or anti–M1-PK antibody, followed by overnight incubation at 4°C. The mixture was then centrifuged at 15,000g at 4°C for 15 minutes, and the PK activity in the supernatant was measured.
RESULTS
Delayed onset of anemia.
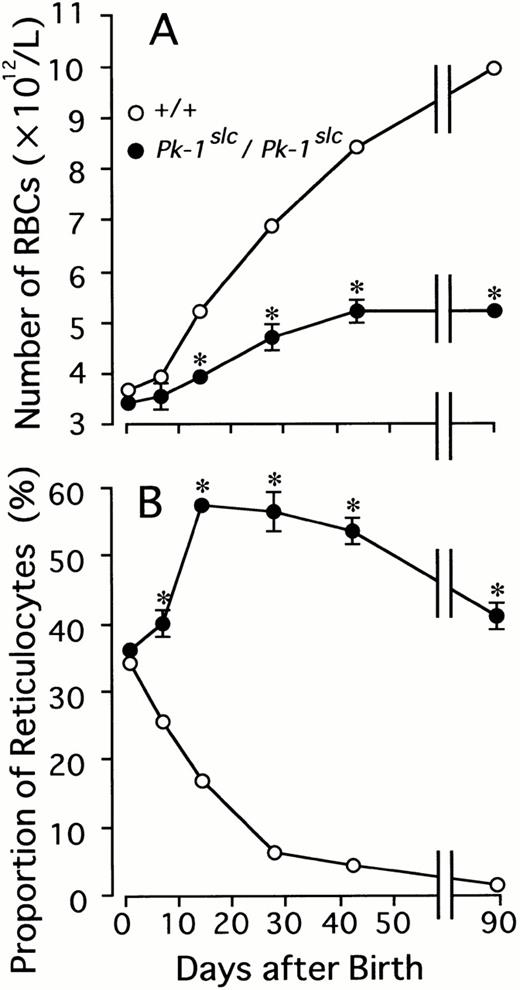
Number of RBCs and proportion of reticulocytes were measured in CBA-Pk-1slc/Pk-1slc and -+/+ mice on days 1, 7, 14, 28, and 42 after birth. No significant differences were detectable between CBA-Pk-1slc /Pk-1slcand CBA-+/+ mice on day 1 (Fig 1). Although the number of RBCs increased in both mutant and normal mice with ages, the number was significantly greater in normal mice than in mutant mice on day 14 and thereafter. As a result, significant anemia was detectable in CBA-Pk-1slc/Pk-1slc mice from day 14 after birth. The proportion of reticulocytes continuously decreased after birth in CBA-+/+ mice. On the other hand, it increased until day 14 and then retained the high value in CBA-Pk-1slc/Pk-1slc mice (Fig 1). The spleen weight was comparable between CBA-Pk-1slc/Pk-1slc and -+/+ mice on day 1 after birth, but a significant splenomegaly was observed in CBA-Pk-1slc/Pk-1slc mice on day 42 (Table 1). In spite of the severe anemia of CBA-Pk-1slc/Pk-1slc mice, body weight of CBA-Pk-1slc/Pk-1slc mice were comparable with that of CBA-+/+ mice throughout the observation period.
Number of RBCs and proportion of reticulocytes in CBA-+/+ (○) and -Pk-1slc/Pk-1slc(•) mice on various days after birth. Each point represents the mean of ten mice. Bars are the standard error. In some points the standard error was too small to be shown by bars.
Number of RBCs and proportion of reticulocytes in CBA-+/+ (○) and -Pk-1slc/Pk-1slc(•) mice on various days after birth. Each point represents the mean of ten mice. Bars are the standard error. In some points the standard error was too small to be shown by bars.
Hematologic and Anatomical Parameters in CBA-+/+ and-Pk-1slc/Pk-1slc Mice of 1 and 42 Days of Age
| Hematologic and Anatomical Parameters . | Age (d) . | Values in Mice of Each Genotype* . | |
|---|---|---|---|
| +/+ . | Pk-1slc/Pk-1slc . | ||
| No. of RBCs (×1012/L) | 1 | 3.6 ± 0.2 | 3.4 ± 0.2 |
| 42 | 8.9 ± 0.1 | 5.2 ± 0.2-151 | |
| Proportion of reticulocytes (%) | 1 | 35 ± 1 | 35 ± 1 |
| 42 | 4.4 ± 0.3 | 54 ± 2-151 | |
| Body weight (g) | 1 | 1.3 ± 0.1 | 1.3 ± 0.1 |
| 42 | 20 ± 1 | 21 ± 1 | |
| Spleen weight (mg) | 1 | 2.0 ± 0.2 | 1.8 ± 0.1 |
| 42 | 31 ± 1 | 255 ± 20-151 | |
| Hematologic and Anatomical Parameters . | Age (d) . | Values in Mice of Each Genotype* . | |
|---|---|---|---|
| +/+ . | Pk-1slc/Pk-1slc . | ||
| No. of RBCs (×1012/L) | 1 | 3.6 ± 0.2 | 3.4 ± 0.2 |
| 42 | 8.9 ± 0.1 | 5.2 ± 0.2-151 | |
| Proportion of reticulocytes (%) | 1 | 35 ± 1 | 35 ± 1 |
| 42 | 4.4 ± 0.3 | 54 ± 2-151 | |
| Body weight (g) | 1 | 1.3 ± 0.1 | 1.3 ± 0.1 |
| 42 | 20 ± 1 | 21 ± 1 | |
| Spleen weight (mg) | 1 | 2.0 ± 0.2 | 1.8 ± 0.1 |
| 42 | 31 ± 1 | 255 ± 20-151 | |
*Mean ± SE of 10 mice.
P < .01 when compared with values of CBA-+/+ mice of the same age by t-test.
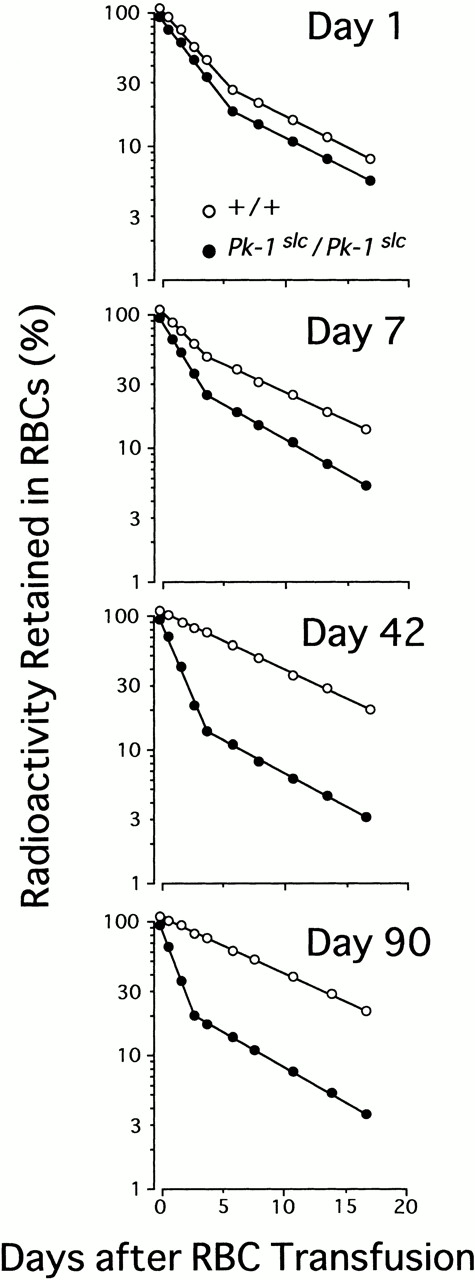
When RBCs of adult CBA-Pk-1slc/Pk-1slcand -+/+ mice were labeled with 51Cr and injected into adult CBA-+/+ mice, the disappearance of RBCs derived from CBA-Pk-1slc/Pk-1slc mice was much faster than that of RBCs from CBA-+/+ mice.19 Since the number of RBCs of 1-day-old CBA-Pk-1slc/Pk-1slc mice was comparable with that of 1-day-old CBA-+/+ mice, we compared the survival time of RBCs of CBA-Pk-1slc/Pk-1slc mice of various ages to that of RBCs of CBA-+/+ mice of the same ages. RBCs of 1-day-old, 7-day-old, 42-day-old, and 90-day-old CBA-Pk-1slc/Pk-1slc and -+/+ mice were labeled with 51Cr and injected into 90-day-old CBA-+/+ mice. Radioactivity retained in RBCs was measured at various times after the injection. In contrast with the large difference between 90-day-old CBA-Pk-1slc/Pk-1slc and -+/+ mice, the difference between 1-day-old CBA-Pk-1slc/Pk-1slc and -+/+ mice was slight (Fig 2). When the radioactivity retained in the RBCs was plotted semi-logarithmically, two-component curves were observed after the injection of labeled RBCs obtained from 1-day-old and 7-day-old CBA-Pk-1slc/Pk-1slc and -+/+ mice. Two-component curves were also observed after the injection of labeled RBCs from 42-day-old and 90-day-old CBA-Pk-1slc/Pk-1slc mice, whereas one-component curves were observed after the injection of labeled RBCs from 42-day-old and 90-day-old CBA-+/+ mice (Fig 2). Since the second-component line observed after the injection of labeled RBCs from 1-day-old and 7-day-old CBA-Pk-1slc/Pk-1slc and -+/+ mice, and 42-day-old and 90-day-old CBA-Pk-1slc/Pk-1slc mice were roughly parallel to the line observed after the injection of labeled RBCs from 90-day-old CBA-+/+ mice, these lines appeared to represent the destruction of the RBCs of 90-day-old CBA-+/+ hosts, which were secondarily labeled by the 51Cr released from the injected RBCs. The half life of RBCs decreased in CBA-Pk-1slc/Pk-1slc mice but increased in CBA-+/+ mice. The difference became greater in parallel with the increase of age (Table 2).
The effect of ages of RBC donors on the elimination of 51Cr-labeled RBCs. RBCs were collected from CBA-+/+ (○) and -Pk-1slc/Pk-1slc (•) mice of various ages, labeled, and injected into 90-day-old CBA-+/+ mice. The radioactivity retained in RBCs was measured at various times after the transfusion and the percentage was plotted semi-logarithmically. Each point represents the mean of eight mice. In all points the standard error was too small to be shown by bars.
The effect of ages of RBC donors on the elimination of 51Cr-labeled RBCs. RBCs were collected from CBA-+/+ (○) and -Pk-1slc/Pk-1slc (•) mice of various ages, labeled, and injected into 90-day-old CBA-+/+ mice. The radioactivity retained in RBCs was measured at various times after the transfusion and the percentage was plotted semi-logarithmically. Each point represents the mean of eight mice. In all points the standard error was too small to be shown by bars.
Half Life of RBCs Obtained From CBA-+/+ and-Pk-1slc/Pk-1slc Mice of Various Ages After Transfusion Into 90-Day-Old CBA-+/+
| Age of RBC Donors (d) . | Half Life (d, mean ± SE)* . | |
|---|---|---|
| +/+ . | Pk-1slc/Pk-1slc . | |
| 1 | 2.4 ± 0.1 | 1.8 ± 0.1† |
| 7 | 3.3 ± 0.2‡ | 1.8 ± 0.1† |
| 42 | 6.0 ± 0.2‡ | 1.4 ± 0.1†,1-153 |
| 90 | 6.3 ± 0.1‡ | 1.4 ± 0.1†,1-153 |
| Age of RBC Donors (d) . | Half Life (d, mean ± SE)* . | |
|---|---|---|
| +/+ . | Pk-1slc/Pk-1slc . | |
| 1 | 2.4 ± 0.1 | 1.8 ± 0.1† |
| 7 | 3.3 ± 0.2‡ | 1.8 ± 0.1† |
| 42 | 6.0 ± 0.2‡ | 1.4 ± 0.1†,1-153 |
| 90 | 6.3 ± 0.1‡ | 1.4 ± 0.1†,1-153 |
*Calculated from the same data shown in Fig 2. Half lives of RBCs obtained from 1-day-old and 7-day-old CBA-Pk-1slc/Pk-1slc and -+/+ mice and those of RBCs from 42-day-old and 90-day-old CBA-Pk-1slc/Pk-1slc mice were calculated using the first component of the two-component curves.
P < .01 when compared with values of CBA-+/+ mice of the same age by t-test.
P < .01 when compared with values of 1-day-old CBA-+/+ mice.
P < .01 when compared with values of 1-day-old CBA-Pk-1slc/Pk-1slc mice.
Age-dependent changes of PK activity.
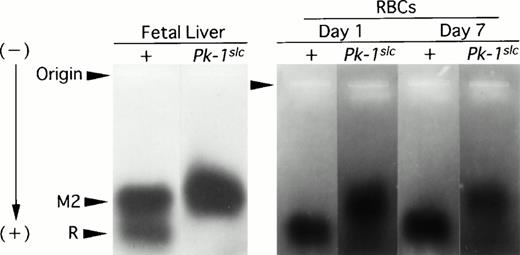
In either CBA-+/+ or CBA-Pk-1slc/Pk-1slc mice, the PK activity was much higher in neonatal mice than in 90-day-old mice (Table 3). In spite of the high activity of PK, examination of smears detected no nucleated erythroblasts. The PK activity gradually decreased with ages in both CBA-+/+ and cba-Pk-1slc/Pk-1slc mice. Although age-dependent decrease in PK activity of RBCs was observed in both CBA-+/+ and -Pk-1slc/Pk-1slc mice, the absolute value of PK activity was much greater in CBA-+/+ mice than in CBA-Pk-1slc/Pk-1slc mice. Moreover, different isozyme pattern was observed by electrophoresis between CBA-+/+ and -Pk-1slc/Pk-1slc mice of 1 and 7 days of age. Only R-PK was detectable in RBCs of 1-day-old and 7-day-old CBA-+/+ mice, whereas only M2-PK was detectable in RBCs of 1-day-old and 7-day-old CBA-Pk-1slc/Pk-1slc mice (Fig3). In the fetal liver of CBA-+/+ mice, both R-PK and M2-PK were detectable, but only M2-PK was detectable in the fetal liver of CBA-Pk-1slc/Pk-1slcmice (Fig 3). Moreover, anti–L-PK antibody neutralized the PK activity in the RBC lysate obtained from CBA-+/+ mice but did not neutralize the PK activity in the RBC lysate of CBA-Pk-1slc/Pk-1slc mice (Table 4). On the other hand, anti–M1-PK antibody neutralized the PK activity in the RBC lysate of CBA-Pk-1slc/Pk-1slc mice but did not neutralize the PK activity in the RBC lysate of CBA-+/+ mice (Table4).
Pyruvate Kinase Activity in RBCs of CBA-+/+ and-Pk-1slc/Pk-1slc Mice of Various Ages
| Age of Mice (d) . | PK Activity (U/g hemoglobin)* . | |
|---|---|---|
| +/+ . | Pk-1slc/Pk-1slc . | |
| 1 | 159 ± 16 | 17 ± 2† |
| 7 | 76 ± 10 | 16 ± 3 |
| 14 | 40 ± 4‡ | 6.9 ± 0.6†,2-153 |
| 28 | 26 ± 2‡ | 3.8 ± 0.2†,2-153 |
| 42 | 21 ± 2‡ | 3.9 ± 0.1†,2-153 |
| 90 | 25 ± 1‡ | 4.1 ± 0.1†,2-153 |
| Age of Mice (d) . | PK Activity (U/g hemoglobin)* . | |
|---|---|---|
| +/+ . | Pk-1slc/Pk-1slc . | |
| 1 | 159 ± 16 | 17 ± 2† |
| 7 | 76 ± 10 | 16 ± 3 |
| 14 | 40 ± 4‡ | 6.9 ± 0.6†,2-153 |
| 28 | 26 ± 2‡ | 3.8 ± 0.2†,2-153 |
| 42 | 21 ± 2‡ | 3.9 ± 0.1†,2-153 |
| 90 | 25 ± 1‡ | 4.1 ± 0.1†,2-153 |
*Mean ± SE of three samples; each sample was collected from various numbers of mice; for example, sample for day 1 from approximately 20 mice, sample for day 90 from 2 mice.
P < .01 when compared with values of CBA-+/+ mice of the same age by t-test.
P < .01 when compared with values of 1-day-old CBA-+/+ mice.
P < .01 when compared with values of 1-day-old CBA-Pk-1slc/Pk-1slc mice.
PAGE and staining for PK activity in extracts of fetal livers or RBCs. Fetal livers were obtained from 16-day embryos of CBA-+/+ or -Pk-1slc/Pk-1slc mice; RBCs from 1-day-old and 7-day-old CBA-+/+ or -Pk-1slc/Pk-1slc mice. M2, M2-PK; R/L, R- or L-PK.
PAGE and staining for PK activity in extracts of fetal livers or RBCs. Fetal livers were obtained from 16-day embryos of CBA-+/+ or -Pk-1slc/Pk-1slc mice; RBCs from 1-day-old and 7-day-old CBA-+/+ or -Pk-1slc/Pk-1slc mice. M2, M2-PK; R/L, R- or L-PK.
Effect of Anti-L-PK or Anti-M1-PK Antibody Treatment on PK Activity of Various RBC Lysates
| Source of RBC Lysate . | Effect of Antibody Treatment on PK Activity . | ||
|---|---|---|---|
| Genotype . | Day After Birth . | Anti–L-PK . | Anti–M1-PK . |
| +/+ | 1 | Abolished | No effect |
| 7 | Abolished | No effect | |
| 14 | Abolished | No effect | |
| 28 | Abolished | No effect | |
| 42 | Abolished | No effect | |
| 90 | Abolished | No effect | |
| Pk-1slc/Pk-1slc | 1 | No effect | Abolished |
| 7 | No effect | Abolished | |
| 14 | No effect | Abolished | |
| 28 | No effect | Abolished | |
| 42 | No effect | Abolished | |
| 90 | No effect | Abolished | |
| Source of RBC Lysate . | Effect of Antibody Treatment on PK Activity . | ||
|---|---|---|---|
| Genotype . | Day After Birth . | Anti–L-PK . | Anti–M1-PK . |
| +/+ | 1 | Abolished | No effect |
| 7 | Abolished | No effect | |
| 14 | Abolished | No effect | |
| 28 | Abolished | No effect | |
| 42 | Abolished | No effect | |
| 90 | Abolished | No effect | |
| Pk-1slc/Pk-1slc | 1 | No effect | Abolished |
| 7 | No effect | Abolished | |
| 14 | No effect | Abolished | |
| 28 | No effect | Abolished | |
| 42 | No effect | Abolished | |
| 90 | No effect | Abolished | |
RBCs lysate was mixed with anti–L-PK or anti–M1-PK antibody, followed by overnight incubation at 4°C. The mixture was then centrifuged, and the PK activity in the supernatant was measured. The antibody was diluted sequentially, and the effect of antibody treatment was evaluated at the most effective dilution. When PK activity dropped to 20% of original value after antibody treatment, the PK activity was considered to be abolished. When 70% of original PK activity was retained after antibody treatment, the treatment was considered to be of no effect. The experiments were repeated twice. Each RBC lysate for an assay was collected from the following numbers of mice: sample for day 1 or day 7 from 8 to 10 mice; sample for day 14 from 3 mice; sample for day 28, day 42, or day 90 from 2 mice.
DISCUSSION
Although PK encoded by the mutant Pk-1slc gene hardly possesses the enzymatic activity,18,19 the numbers of RBCs were comparable between 1-day-old CBA-+/+ and -Pk-1slc/Pk-1slc mice. The comparable RBC values in 1-day-old mice was chiefly attributable to the low RBC counts of 1-day-old CBA-+/+ mice. The RBC number increased continuously from day 1 to day 42 after birth in both CBA-+/+ and -Pk-1slc/Pk-1slc mice, but the magnitude of the increase was much greater in CBA-+/+ mice than in CBA-Pk-1slc/Pk-1slc mice. The greater increase in RBC number may be explained by the prolongation of the survival time observed in CBA-+/+ mice from day 1 to day 42 after birth.24 In contrast with CBA-+/+ mice, the survival time decreased with ages in CBA-Pk-1slc/Pk-1slc mice (Table 2).
The PK activity of 1-day-old CBA-+/+ mice was over six times higher than adults, and it decreased with ages (Table 3). Since the PK activity in CBA-+/+ mice paralleled with the proportion of reticulocytes, the high PK activity in neonatal CBA-+/+ mice was attributable to the high proportion of reticulocytes. This is consistent with the result reported by Chapman and Schaumburg,25 Nathan et al,26 Paglia and Valentine27 and Lakomek et al28 that PK activity was higher in reticulocytes than in mature RBCs. Although the PK activity was higher in 1-day-old and 7-day-old CBA-Pk-1slc/Pk-1slc mice than in the older mice of the same genotype, the PK activity did not parallel with the proportion of reticulocytes in mice of thePk-1slc/Pk-1slc genotype. In fact, the PK activity significantly decreased on day 14 after birth whereas the proportion of reticulocytes significantly increased on the same day (Fig 1 and Table 3). Therefore, the relatively high PK activity in neonatal CBA-Pk-1slc/Pk-1slc mice cannot be explained by the high proportion of reticulocytes. When isozyme pattern of PK was compared between 1-day-old and 7-day-old CBA-+/+ mice and CBA-Pk-1slc/Pk-1slcmice by zymograms as well as the antibody neutralization, R-PK was exclusively observed in +/+ RBCs, whereas M2-PK inPk-1slc/Pk-1slc RBCs (Fig 3, Table 4). Therefore, the relatively high PK activity in RBCs of 1-day-old and 7-day-old CBA-Pk-1slc/Pk-1slc mice may be attributable to the delayed switching from M2-PK to R-PK.
Switching of isozymes from R-PK to M2-PK during erythroid differentiation have been demonstrated by several investigators. Takegawa et al8 examined the transition of PK isozyme expression by immunologic methods; the M2-PK predominantly expressed in proerythroblasts, and R-PK expression became detectable at the stage of basophilic erythroblasts. Nijhof et al9 demonstrated that both R-PK and M2-PK activity was detected in murine erythroid precursor cells (CFU-E) and that M2-PK activity rapidly disappeared during the erythropoietin-induced differentiation. Max-Audit et al10reported that the synthetic rates of PK in human erythroid progenitors were highest in proerythroblasts. They also described that R-PK synthesis remained at the same level during differentiation and that M2-PK activity decreased due to rapid decline of synthesis as well as accelerated degradation.10
Present study showed that the half life of 51Cr-labeled RBCs of 1-day-old and 7-day-old CBA-Pk-1slc/Pk-1slc mice was longer than the half life of 42-day-old and 90-day-old CBA-Pk-1slc/Pk-1slc mice. The difference was slight but significant (Table 2). Since the PK activity was greater in the former mice than in the latter mice and since the PK activity in RBCs was attributed to M2-PK not only in suckling CBA-Pk-1slc/Pk-1slc mice but also in adult CBA-Pk-1slc/Pk-1slc mice, the relatively large amount of M2-PK expressed by RBCs of the younger CBA-Pk-1slc/Pk-1slc mice may play a significant role for the survival of the RBCs. It is most likely that the M2-PK activity beyond day 7 after birth may not be sufficient to sustain survival of RBCs in CBA-Pk-1slc/Pk-1slc mice.
There are numbers of globin gene abnormalities that influence switching of active globin gene locus during hematopoietic development in humans. Hereditary persistence of fetal hemoglobin (HPFH) is one of the genetic abnormalities that increase hemoglobin F production in RBCs. Clinical significance of HPFH has been recognized in its relationship to thalassemia and sickle cell anemia. Phenotype of β-thalassemia or the sickle cell anemia was found to be alleviated or even asymptomatic with HPFH.29-31 Beneficial effects on hemoglobinopathies would be obtained if γ globin gene expression could be reactivated in adult erythroid progenitors by pharmacologic means.32 Similarly, the persistent expression of M2-PK in mature RBCs has been reported in severe PK deficiency of humans,4,16,17,33 34 and the M2-PK activity seemed to compensate hemolysis to some extent in those cases. Further investigations are required to understand PK isozyme switching during erythroid differentiation and the significance of M2-PK expression in severe PK deficiency. Intact M2-PK gene in erythroid cells of mutants might become a novel therapeutic strategy to hemolytic anemia due to PK deficiency.
ACKNOWLEDGMENT
We thank Professor Tamio Noguchi of Fukui Medical School for supplying us with rabbit antibodies against rat L-PK and M1-PK.
Supported by grants from the Japanese Ministry of Education, Science, Sports, and Culture.
Address reprint requests to Yukihiko Kitamura, MD, Department of Pathology, Osaka University Medical School, Yamada-oka 2-2, Suita, Osaka, 565, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal