Abstract
A major obstacle in purifying either autologous or allogeneic hematopoietic stem cells from granulocyte colony-stimulating factor (G-CSF) mobilized circulating progenitor cells (CPC) is represented by the huge cellularity present in each apheretic product. To obtain a significant debulking of unwanted cells from the leukapheresis, we developed a modified protocol of immune rosetting whereby human ABO-Rh– compatible red blood cells (RBCs) are treated with chromium chloride and then coated with murine monoclonal antibodies (MoAbs) against leukocyte antigens. When experiments were performed with leukaphereses obtained from normal donors or from T-cell acute lymphoblastic leukemia (T-ALL) patients, RBCs were coated with murine MoAbs against human mature myeloid cells (CD11b) and T cells (CD6); whereas, in the case of patients with B-precursor ALL, B-cell non-Hodgkin's lymphoma (B-NHL), or multiple myeloma (MM), RBCs were coated with anti-CD11b only. After incubation with CPC, rosetting cells (myeloid precursor cells, granulocytes, monocytes, and T cells) were removed by Ficoll-Hypaque density gradient centrifugation with a blood cell processor apparatus, COBE (Lakewood, CO) 2991. After this step, a significant reduction of the initial cellularity was consistently obtained (range, 72% to 97%), whereas the median absolute recovery of the CD34+ cells was above 85% (range, 64 to 100), with a 10-fold relative enrichment ranging from 3% to 41%. In a second step, CPC can be further purged of contaminating T or B cells by incubation with lymphoid-specific magnetic microbeads (anti-CD2 and -CD7 to remove T cells; anti-CD19 to remove B cells) and elution through a type-D depletion column (composed of ferromagnetic fiber) inserted within a SuperMACS separator device (Miltenyi Biotech, Bergisch-Gladbach, Germany). By this approach, a highly effective (three to four logs) T-cell depletion was achieved in all experiments performed with normal donors or T-ALL patients (median loss of CD3+cells: 99.8% [range 99.2 to 100]) and an equally efficient B-cell depletion was obtained from B-precursor ALL, B-NHL, or MM patients. At the end of the procedure the T- or B-cell depleted fraction retained a high proportion of the initial hematopoietic CD34+ stem cells, with a median recovery above 70% (range 48% to 100%) and an unmodified clonogenic potential. In five patients (two follicular NHL and three ALL) the purified fraction of stem cells was found disease free at the molecular level as assessed by polymerase chain reaction (PCR) analysis of the t(14;18) chromosome translocation or clono-specific DNA sequences of IgH or T-cell receptor γ and δ chain genes. Purified autologous and allogeneic CPCs were transplanted in three and six patients, respectively, who showed a prompt and sustained hematologic engraftment. In conclusion, this method represents a simple and reproducible two-step procedure to obtain a highly efficient purging of T or B cells from G-CSF expanded and mobilized CPCs. This approach might lead to the eradication of the neoplastic clone in the autologous stem cell inoculum as well as for T-cell depletion during allogeneic transplantation.
IN THE NORMAL bone marrow the CD34+ cell fraction contains virtually all the hematopoietic progenitor cells (HPCs).1 After priming with hematopoietic growth factors (G-CSF or GM-CSF), either alone or in combination with high-dose chemotherapy, mobilized circulating progenitor cells (CPCs) expressing the CD34 antigen on the cell surface can be easily collected from peripheral blood in quantities approximately 10-fold higher than previously obtained from bone marrow.2 The purification of human stem cells has clinical relevance for autologous3 and allogeneic transplantation4 and for the usage of the stem cells as vehicles for gene transfer.5,6 High-dose chemotherapy and autologous transplantation are increasingly being used to treat patients with hematologic malignancies and solid tumors even though the neoplastic contamination of CPCs7-9 may contribute to subsequent relapses.10 Because many tumor cell types including multiple myeloma (MM), lymphomas, breast and ovarian cancer, and neuroblastoma do not usually express the CD34 antigen, CD34+ positive selection may be effective for the purging of the autologous grafts. Different commercial devices are now available for laboratory and clinical scale enrichment of these cells by immunoaffinity columns or immonomagnetic beads, and preliminary clinical results have already been obtained by using purified CD34+ cells for autologous transplantation.8,9,11-15 More recently, some investigators started to analyze the feasibility of using purified CD34+cells for allogeneic transplantation.4,16,17 In fact, allogeneic CPC preparations may provide the possibility to obtain T-cell depleted fractions of stem cells while preventing an unacceptable loss of hematopoietic progenitors. Therefore, CD34+ cell selection can be used as an efficient method for preventing graft versus host disease (GVHD) without an increased risk of graft failure or rejection.18 Unfortunately, at least two major concerns are opposing a wide clinical use of purified CD34+ cells. The immune reconstitution can be severely compromised if inadequate numbers of T and B cells are present in the graft, a problem that deserves particular attention, especially in the case of patients with nonhematologic malignancies. Moreover, most acute leukemia cell types express the CD34 antigen, thus preventing the use of this method of purification in the autologous setting.
The aim of this study was to develop an efficient, reproducible, and relatively inexpensive method for clinical scale preparation of CPC for transplantation procedures. Furthermore, the selective- and lineage-specific elimination of the neoplastic fraction from the autologous graft as well as the normal T cells from the allogeneic graft was the aim of the same procedure. This novel methodology consists of two distinct steps: in the first step, 85% of the initial cellularity (granulocytes, monocytes, and eventually T cells) is allowed to form rosettes with chromium chloride-treated human ABO-Rh–compatible red blood cells (RBCs) coated with murine monoclonal antibodies (MoAbs) antihuman CD11b (and CD6 when T-cell depletion is needed) and then removed by gradient sedimentation, without a significant loss of the CD34+ cells present in the input. Subsequently, in step two, this debulked apheresis is very efficiently purged of unwanted B or T cells by the use of lineage-specific monoclonal microbeads.
MATERIALS AND METHODS
Mobilization and harvesting of autologous or allogeneic CPCs.
Autologous CPCs were collected in patients with non-Hodgkin's lymphoma (NHL), acute lymphoblastic leukemia (ALL), and MM, after different consolidation protocols of high-dose chemotherapy followed by rhG-CSF (Filgrastim; Roche, Milan, Italy) administration (5 μcg/kg/day), as previously described.19 Leukapheresis was performed as soon as white blood cells (WBCs) were at least 3.0 × 109/L and peripheral blood CD34+ cells were ≥ 0.5%. Ten liters of blood were processed daily through an indwelling central venous catheter (Groshong CV Catheter; Band Inc, Salt Lake City, UT) by using a cell separator COBE spectra (COBE, Lakewood, CO). Allogeneic CPCs were collected from HLA-identical, MLC-negative normal siblings upon treatment with 2 × 5 μg/kg/day of rhG-CSF administered subcutaneously every 12 hours for 5 or 6 consecutive days. The procedure of leukapheresis was started on day 5 (after the ninth dose of G-CSF) by using a COBE spectra cell separator. Ten liters of blood were processed daily by using the cubital vein in all donors, as previously described.20 The absolute number of CD34+ cells, lymphoid cells (T, B, and NK cells), and mature myeloid cells (granulocytes and monocytes) were evaluated daily in the peripheral blood by flow cytometry with fluorescein isothiocyanate conjugated (FITC) murine MoAbs against human CD34, CD3, CD19, CD56, CD11b, CD14, CD16, or negative control and a FACScan analyzer (Becton Dickinson, Mountain View, CA). In vitro colony assay for evaluation of erythroid and myeloid colony forming units was performed as previously described.19 20 Informed consent was obtained from the patients and the donors by using forms approved by the Institutional Review Board. A fully detailed explanation of the potential risks and benefits concerning the collection of G-CSF mobilized CPCs for autologous and allogeneic transplantation was given to normal donors and the patients. Normal donors and patients were more than 18 years of age. The experimental use of apheresis products for immune rosetting and immunomagnetic depletion of T or B cells was begun when unmodified preparations of CPC (containing ≥ 6 × 106 CD34+ cells/kg of recipient body weight) were already harvested and cryopreserved.
Immune rosettes.
The method is based on a previously published procedure based on the ability to couple murine MoAbs to RBCs by chromium chloride.21 The following mouse hybridoma cell lines were obtained from American Type Culture Collection (ATCC, Rockville, MD): OKM1 (IgG2b, ATCC CRL 8026) reactive with human granulocytes, monocytes, NK cells and committed myeloid precursor cells (CD11b antigen); and T12 (IgM, clone 3Pt12B8; ATCC HB8136) reacting with human T cells and some B cells (CD6 antigen). Partially purified preparations of these MoAbs were obtained by ammonium sulphate precipitation of spent culture supernatants of each hybridoma. ABO-Rh–compatible, irradiated (25 Gy), and filtered human red blood cells (HRBCs) were obtained from the Blood Bank. For coating with MoAbs, 150 mL of packed HRBCs were washed three times in normal saline (centrifugation at 3000 RPM for 5 minutes at room temperature) with a COBE 2991 apparatus and a blood cell processor set. After the third wash, 30 mL (3 mg/mL) of partially purified MoAbs (CD11b and eventually CD6 in the case of normal donors and T-ALL patients) were added at the same blood processor set. Thereafter, and under continuous agitation, 250 mL of a 0.1% solution of chromium chloride (CrCl3.6H2O; Sigma, St.Louis, MO; prepared in normal saline from a 1% [W/V] stock solution with the pH adjusted to 5.0 with 10 N NaOH) were added dropwise over a 15-minute time period. After incubation for 5 additional minutes at room temperature the reaction was stopped by the addition of 300 mL of phosphate-buffered saline (PBS) supplemented with 2.5% Human Serum Albumin (PBS-HSA). Apparently, the isotype of the antibodies (IgM or IgG) does not affect the coupling process to RBCs. MoAb-coated HRBCs were washed twice, resuspended in 100 mL of PBS 2.5% HSA, and mixed with 11 to 58 × 109 white blood cells (200 mL final volume, hematocrit less than 5%) obtained from leukaphereses of G-CSF–treated individuals. Rosette formation was performed within the same blood cell processor set by two centrifugation steps at 3000 RPM for 30 minutes. At the end cells were resuspended in 400 mL of PBS 2.5% HSA, transferred, and layered onto the top of 200 mL Ficoll Hypaque (by using a second blood cell processor set), and centrifuged for 45 minutes at 3000 rpm. Nonrosetting cells were harvested at the Ficoll interface and washed twice with PBS-HSA. After 5 minutes of incubation with hypotonic NH4 Cl buffer (NH4 Cl 8.99 gr/L, KHCO3 1gr/L, Na4 EDTA 0.037 gr/L, pH 7.3) to lyse residual erythrocytes, cells were washed with PBS-HSA, resuspended in RPMI 1640 10% FCS, counted, and stained with MoAbs for FACS analysis. The described procedure required approximately 3 hours of work performed by one operator and the estimated cost (including production and purification of antibodies, chemical reagents, tissue culture media, two blood cell separation sets, and 1 U of filtered, irradiated, RBCs) was 400 US dollars.
Immunomagnetic purging of T or B cells.
To obtain a high degree of T- or B-cell depletion, partially purified hematopoietic progenitors (0.5 to 13 × 109 cells) obtained by immune rosetting were labelled with primary unconjugated MoAbs reacting against T cells (anti-CD2 antigen, clone 35.1; IgG2a, ATCC HB222; and anti-CD7, clone WT1, IgG1) or with a panB MoAb (clone HD37, anti CD19, IgG1; kindly provided by Dr. Moldenhauer, Heidelberg, Germany). In MM patients (two cases), cells were incubated with anti-CD19 and anti-CD5622 (clone N901, IgG1; kindly provided by Dr JD Griffin, Boston, MA). Thereafter, an indirect labelling was performed with goat antimouse magnetic microbeads, according to the manufacturer instructions (Miltenyi Biotec, Germany). After labelling, cells were washed as above, resuspended in PBS-HSA (30 mL final volume), and layered, by the use of a peristaltic pump, onto the top of a type-D depletion column (composed of ferromagnetic fibers) inserted within a SuperMACS apparatus (Miltenyi Biotec). Unstained cells were eluted with PBS-HSA from the column kept within the magnetic field of the cell separator. The T- or B-cell depleted fraction of HPCs was finally resuspended in autologous plasma, counted, phenotypically analyzed, and cryopreserved. The time required to complete this negative depletion was 3 hours and the estimated cost of two vials of goat antimouse magnetic microbeads and one type-D depletion column, necessary for each procedure, was 1,500 US dollars.
Molecular evaluation of minimal residual disease (MRD).
In patients with ALL, MRD was evaluated by molecular analysis of junctional regions of rearranged T-cell receptor (TCR) γ or δ genes. Specific patterns of recombination of TCR γ or δ genes were identified at diagnosis by Southern blot analysis, according to standard techniques.23 The TCR δ and γ gene rearrangements were subsequently amplified by polymerase chain reaction (PCR) with V δ or V γ family primers and J δ or J γ general primers, respectively. The PCR products were cloned in pMos vector (Amersham, Buckinghamshire, UK) sequenced to define the precise nucleotide sequence of the junctional regions. Based on these sequence data, leukemia-patient–specific oligonucleotide probes were synthesized to analyze subsequent marrow or leukapheretic peripheral blood samples. Reaction products were identified by hybridization studies with DNA oligonucleotide probes labeled with [γ -32 P] ATP. In one case of B-precursor ALL, MRD monitoring was performed by amplification of the CDRIII IgH with VH and JH consensus primers, as described.24 Two follicular lymphoma patients bearing the t(14;18) chromosome abnormality were evaluated by PCR analysis of BCL2-IgH rearrangement, according to published methods.25
RESULTS
Effect of immune rosetting on overall recovery and composition of G-CSF mobilized CPC.
In normal donors receiving G-CSF (10 μg/kg/d) for 5 to 6 days, the procedure of CPC mobilization and collection was safe and always well tolerated. The apheretic products were debulked of committed myeloid cells and T lymphocytes by immune rosetting with anti-CD11b and -CD6 antibodies. The experimental procedure of immune rosetting was always carried out on the second or third harvest after a sufficient amount of unmodified CPCs were used or stored in liquid nitrogen. As shown in Table 1, 18 experiments were performed with a median starting cellularity of 41.3 × 109 (range, 29.7 to 58.2) nucleated cells. After immune rosetting, the median reduction of the initial cellularity was 91% (range, 72 to 97) and the cell loss was mainly due to a marked (more than 85%) depletion of myeloid cells and T lymphocytes. A parallel enrichment of CD34+ cells was obtained (more than 10-fold) with a median recovery of HPCs of 92% (range, 67 to 100). Similar results were obtained by using autologous CPCs collected in T-ALL patients after high-dose chemotherapy and G-CSF administration. Due to the effect of high-dose chemotherapy, the starting cellularity, as well as the absolute number of CD3+ cells, was lower than that observed in the apheresis obtained from normal allogeneic donors, whereas the amount of CD34+ cells was significantly higher (Table 1). Nonetheless, the debulking effect of the procedure (88% overall cell loss, with more than 84% of mean CD3+cell loss), as well as the absolute recovery and the enrichment fold of CD34+ cells, was remarkably similar. In patients with B precursor ALL and B-NHL the apheretic products were debulked by immune rosetting with RBCs coated with CD11b only. Also, in this case, the initial cellularity and the absolute number of T and B lymphocytes were less abundant as compared to normal donors. As expected, the loss of CD3+ cells was lower, even though the overall reduction obtained by immune rosettes was similar.
Effect of Immune Rosettes on Cellularity of Leukapheresis Obtained From Normal Donors, T- or B-Precursor Acute Lymphoblastic Leukemia (ALL), B-Non-Hodgkins' Lymphoma (B-NHL) and Multiple Myeloma (MM) Patients
| Antibody Used . | Normal Donors (n = 18) . | T-ALL (n = 10) . | B-NHL, B-Precursor ALL and MM (n = 16) . | |||
|---|---|---|---|---|---|---|
| Apheresis (—) . | Immune Rosettes (CD11b + CD6) . | Apheresis (—) . | Immune Rosettes (CD11b + CD6) . | Apheresis (—) . | Immune Rosettes (CD11b) . | |
| Total cells (×109) | 41.3 (29.7-58.2) | 3.9 (1-13.7) | 28 (12.7-53.4) | 3.4 (0.4-8) | 20 (11.6-41.4) | 2.3 (1.1-4.4) |
| % overall cell loss | 91 (72-97) | 88 (77-97) | 87 (75-97) | |||
| CD34+ cells (×109) | 0.2 (0.07-1) | 0.2 (0.06-0.7) | 0.3 (0.09-0.8) | 0.2 (0.06-0.6) | 0.3 (0.1-1.3) | 0.3 (0.08-1) |
| %CD34+ cells | 0.6 (0.2-1.8) | 7.5 (2.3-22.4) | 1.2 (0.4-2.8) | 10 (4-21) | 1.9 (0.6-5.4) | 14 (2-28) |
| %CD34 recovery | 92 (67-100) | 89 (64-100) | 87 (65-100) | |||
| %CD34 enrichment fold | 15 (3-41) | 11 (4-21) | 10 (3-35) | |||
| CD11b+ cells (×109) | 25.5 (15.4-36) | 1.8 (0.01-10.8) | 21 (7.9-50.6) | 1.9 (0.1-6.3) | 16.2 (5.5-39.6) | 0.5 (0.01-1.2) |
| %CD11b+ cells | 63 (44-79) | 31 (1-83) | 70 (50-95) | 49 (16-83) | 77 (39-98) | 20 (0.6-38) |
| %CD11b+ cell loss | 93 (67-99) | 92 (73-98) | 96 (83-99) | |||
| CD3+ cells (×109) | 12.7 (7-27) | 0.9 (0.1-2.7) | 5.9 (0.6-14.3) | 0.8 (0.1-2.8) | 2.7 (0.5-7.9) | 1.3 (0.3-3) |
| %CD3+ cells | 30 (17-64) | 28 (6-62) | 29 (1-56) | 27 (3-63) | 17 (1.4-47) | 53 (15-90) |
| %CD3+ cell loss | 91 (70-99) | 84 (68-98) | 48 (11-63) | |||
| CD19+ cells (×109) | 2.2 (1-5) | 0.6 (0.08-1.2) | 0.09 (0.03-0.2) | 0.02 (0.002-0.07) | 0.06 (0-0.2) | 0.01 (0-0.05) |
| %CD19+ cells | 5 (2-12) | 22 (3-50) | 0.4 (0.1-1.4) | 0.8 (0.1-3) | 0.3 (0-1.2) | 0.5 (0-1.8) |
| %CD19+ cell loss | 71 (38-96) | 76 (38-98) | 74 (2-97) | |||
| Antibody Used . | Normal Donors (n = 18) . | T-ALL (n = 10) . | B-NHL, B-Precursor ALL and MM (n = 16) . | |||
|---|---|---|---|---|---|---|
| Apheresis (—) . | Immune Rosettes (CD11b + CD6) . | Apheresis (—) . | Immune Rosettes (CD11b + CD6) . | Apheresis (—) . | Immune Rosettes (CD11b) . | |
| Total cells (×109) | 41.3 (29.7-58.2) | 3.9 (1-13.7) | 28 (12.7-53.4) | 3.4 (0.4-8) | 20 (11.6-41.4) | 2.3 (1.1-4.4) |
| % overall cell loss | 91 (72-97) | 88 (77-97) | 87 (75-97) | |||
| CD34+ cells (×109) | 0.2 (0.07-1) | 0.2 (0.06-0.7) | 0.3 (0.09-0.8) | 0.2 (0.06-0.6) | 0.3 (0.1-1.3) | 0.3 (0.08-1) |
| %CD34+ cells | 0.6 (0.2-1.8) | 7.5 (2.3-22.4) | 1.2 (0.4-2.8) | 10 (4-21) | 1.9 (0.6-5.4) | 14 (2-28) |
| %CD34 recovery | 92 (67-100) | 89 (64-100) | 87 (65-100) | |||
| %CD34 enrichment fold | 15 (3-41) | 11 (4-21) | 10 (3-35) | |||
| CD11b+ cells (×109) | 25.5 (15.4-36) | 1.8 (0.01-10.8) | 21 (7.9-50.6) | 1.9 (0.1-6.3) | 16.2 (5.5-39.6) | 0.5 (0.01-1.2) |
| %CD11b+ cells | 63 (44-79) | 31 (1-83) | 70 (50-95) | 49 (16-83) | 77 (39-98) | 20 (0.6-38) |
| %CD11b+ cell loss | 93 (67-99) | 92 (73-98) | 96 (83-99) | |||
| CD3+ cells (×109) | 12.7 (7-27) | 0.9 (0.1-2.7) | 5.9 (0.6-14.3) | 0.8 (0.1-2.8) | 2.7 (0.5-7.9) | 1.3 (0.3-3) |
| %CD3+ cells | 30 (17-64) | 28 (6-62) | 29 (1-56) | 27 (3-63) | 17 (1.4-47) | 53 (15-90) |
| %CD3+ cell loss | 91 (70-99) | 84 (68-98) | 48 (11-63) | |||
| CD19+ cells (×109) | 2.2 (1-5) | 0.6 (0.08-1.2) | 0.09 (0.03-0.2) | 0.02 (0.002-0.07) | 0.06 (0-0.2) | 0.01 (0-0.05) |
| %CD19+ cells | 5 (2-12) | 22 (3-50) | 0.4 (0.1-1.4) | 0.8 (0.1-3) | 0.3 (0-1.2) | 0.5 (0-1.8) |
| %CD19+ cell loss | 71 (38-96) | 76 (38-98) | 74 (2-97) | |||
Highly efficient depletion of T cells by anti-CD2-CD7 or B cells by anti-CD19 magnetic microbeads.
To purge the contaminating normal or neoplastic T lymphocytes still present in the apheretic products after the debulking procedure with immune rosettes, partially purified allogeneic (9 experiments from normal donors) or autologous (7 experiments from T-ALL patients) CPCs were incubated with a mixture of anti-CD2 and -CD7 MoAbs and indirectly stained with goat antimouse magnetic microbeads. After loading onto a D-type depletion column, normal or leukemic contaminating T cells were significantly removed as judged by staining with anti-CD3 MoAbs (Table 2) and other T-cell–specific antigens like CD5, CD4, and CD8 (data not shown). The overall T-cell depletion obtained by the two combined procedures allowed a final 3 to 4 logs reduction of the T-cell content. Despite such aggressive removal of T cells, the median overall recovery of CD34+ cells was above 70% in both autologous and allogeneic CPCs (Table 2). Similar experiments were performed to remove B cells in CPCs obtained from patients with B-precursor ALL, B-NHL, and MM. As shown in Table 3, the percent and the absolute number of CD19+ cells detectable after this purification approach were very limited. Again, the absolute recovery of CD34+ cells from the initial leukapheresis was excellent, with a mean value above 80%.
T-Cell Depletion by Immune Rosettes and T-cell–specific Magnetic Microbeads (CD2 + CD7) Obtained From Normal Donors and T-ALL Patients
| Antibody Used . | Normal Donors (n = 9) . | T-ALL (n = 7) . | ||||
|---|---|---|---|---|---|---|
| Apheresis (−) . | Immune Rosettes (CD11b + CD6) . | D-column (CD2 + CD7) . | Apheresis (−) . | Immune Rosettes (CD11b + CD6) . | D-column (CD2 + CD7) . | |
| Total cells (×109) | 43.7 (17.2-66) | 5.7 (1-13.8) | 3.5 (0.1-10.8) | 33.9 (18.2-53.4) | 4.4 (1.7-8) | 2.5 (0.9-6) |
| % overall cell loss | 88 (76-96) | 93 (83-99) | 86 (77-96) | 92 (83-97) | ||
| CD34+cells (×109) | 0.3 (0.07-1) | 0.2 (0.08-0.7) | 0.2 (0.06-0.5) | 0.4 (0.2-0.8) | 0.3 (0.2-0.6) | 0.3 (0.1-0.6) |
| %CD34+ cells | 0.7 (0.2-1.8) | 5.3 (2.7-7.9) | 14 (3-37) | 1.3 (0.4-2.8) | 9 (4-21) | 14 (4-25) |
| %CD34 recovery | 87 (59-100) | 76 (53-100) | 92 (69-100) | 73 (57-100) | ||
| %CD34 enrichment fold | 12 (3-41) | 28 (3-74) | 9 (4-21) | 14 (5-28) | ||
| CD11b+cells (×109) | 29.3 (11.4-50.2) | 3 (0.1-11.6) | 1.9 (0.02-10.2) | 25.9 (9.1-50.6) | 2.7 (6.3-9.7) | 1.2 (0.02-3) |
| %CD11b+ cells | 67 (54-79) | 38 (7-84) | 38 (2-95) | 71 (50-95) | 59 (18-83) | 39 (0.2-78) |
| %CD11b+cell loss | 91 (72-99) | 95 (80-100) | 89 (73-96) | 94 (87-100) | ||
| CD3+cells (×109) | 11.6 (3.8-20.9) | 1.4 (0.3-4.1) | 0.02 (0.0002-0.1) | 6 (0.6-14.3) | 1 (0.1-2.8) | 0.008 (0.0007-0.03) |
| %CD3+ cells | 26 (9-49) | 36 (6-81) | 0.3 (0.1-1) | 23 (1-49) | 23 (3-63) | 0.2 (0.1-0.5) |
| %CD3+ cell loss | 84 (57-98) | 99.7 (99.4-100) | 82 (68-96) | 99.9 (99.2-100) | ||
| CD19+ cells (×109) | 1.8 (0.6-2.8) | 0.6 (0.08-1) | 0.3 (0.02-0.7) | 0.1 (0.06-0.2) | 0.03 (0.003-0.07) | 0.01 (0.0006-0.05) |
| %CD19+cells | 4 (2-7) | 16 (3-50) | 29 (0.2-69) | 0.4 (0.1-1.4) | 0.5 (0.1-1.5) | 0.3 (0.1-0.8) |
| %CD19+ cell loss | 71 (61-87) | 82 (66-98) | 74 (38-98) | 84 (20-99) | ||
| Antibody Used . | Normal Donors (n = 9) . | T-ALL (n = 7) . | ||||
|---|---|---|---|---|---|---|
| Apheresis (−) . | Immune Rosettes (CD11b + CD6) . | D-column (CD2 + CD7) . | Apheresis (−) . | Immune Rosettes (CD11b + CD6) . | D-column (CD2 + CD7) . | |
| Total cells (×109) | 43.7 (17.2-66) | 5.7 (1-13.8) | 3.5 (0.1-10.8) | 33.9 (18.2-53.4) | 4.4 (1.7-8) | 2.5 (0.9-6) |
| % overall cell loss | 88 (76-96) | 93 (83-99) | 86 (77-96) | 92 (83-97) | ||
| CD34+cells (×109) | 0.3 (0.07-1) | 0.2 (0.08-0.7) | 0.2 (0.06-0.5) | 0.4 (0.2-0.8) | 0.3 (0.2-0.6) | 0.3 (0.1-0.6) |
| %CD34+ cells | 0.7 (0.2-1.8) | 5.3 (2.7-7.9) | 14 (3-37) | 1.3 (0.4-2.8) | 9 (4-21) | 14 (4-25) |
| %CD34 recovery | 87 (59-100) | 76 (53-100) | 92 (69-100) | 73 (57-100) | ||
| %CD34 enrichment fold | 12 (3-41) | 28 (3-74) | 9 (4-21) | 14 (5-28) | ||
| CD11b+cells (×109) | 29.3 (11.4-50.2) | 3 (0.1-11.6) | 1.9 (0.02-10.2) | 25.9 (9.1-50.6) | 2.7 (6.3-9.7) | 1.2 (0.02-3) |
| %CD11b+ cells | 67 (54-79) | 38 (7-84) | 38 (2-95) | 71 (50-95) | 59 (18-83) | 39 (0.2-78) |
| %CD11b+cell loss | 91 (72-99) | 95 (80-100) | 89 (73-96) | 94 (87-100) | ||
| CD3+cells (×109) | 11.6 (3.8-20.9) | 1.4 (0.3-4.1) | 0.02 (0.0002-0.1) | 6 (0.6-14.3) | 1 (0.1-2.8) | 0.008 (0.0007-0.03) |
| %CD3+ cells | 26 (9-49) | 36 (6-81) | 0.3 (0.1-1) | 23 (1-49) | 23 (3-63) | 0.2 (0.1-0.5) |
| %CD3+ cell loss | 84 (57-98) | 99.7 (99.4-100) | 82 (68-96) | 99.9 (99.2-100) | ||
| CD19+ cells (×109) | 1.8 (0.6-2.8) | 0.6 (0.08-1) | 0.3 (0.02-0.7) | 0.1 (0.06-0.2) | 0.03 (0.003-0.07) | 0.01 (0.0006-0.05) |
| %CD19+cells | 4 (2-7) | 16 (3-50) | 29 (0.2-69) | 0.4 (0.1-1.4) | 0.5 (0.1-1.5) | 0.3 (0.1-0.8) |
| %CD19+ cell loss | 71 (61-87) | 82 (66-98) | 74 (38-98) | 84 (20-99) | ||
B-Cell Depletion by Immune Rosettes and B-Cell–Specific Magnetic Microbeads (CD19) of Leukaphereses Obtained From B-NHL, B-precursor ALL, or MM Patients
| Antibody Used . | B-NHL, B-precursor ALL and MM (n = 17) . | ||
|---|---|---|---|
| Apheresis (—) . | Immune Rosettes (CD11b) . | D-column (CD19*) . | |
| Total cell (×109) | 19.4 (8.6-41.4) | 2.4 (1.1-4.4) | 2 (0.9-3.6) |
| % overall cell loss | 85 (52-97) | 87 (59-98) | |
| CD34+cells (×109) | 0.3 (0.07-1.3) | 0.3 (0.06-1) | 0.2 (0.05-1) |
| %CD34+ cells | 1.8 (0.6-5.4) | 12 (1.5-28) | 14 (1.5-37) |
| %CD34 recovery | 85 (53-100) | 80 (48-100) | |
| %CD34 enrichment fold | 9 (2-35) | 10 (2-42) | |
| CD11b+cells (×109) | 15 (3.1-39.6) | 0.4 (0.01-1.2) | 0.2 (0.005-0.9) |
| %CD11b+ cells | 72 (37-98) | 19 (0.6-38) | 11 (0.4-28) |
| %CD11b+ cell loss | 96 (83-100) | 97 (87-100) | |
| CD3+ cells (×109) | 3.3 (0.5-8.4) | 1.4 (0.4-3) | 1.3 (0.4-2.6) |
| %CD3+ cells | 22 (1.4-55) | 58 (34-90) | 62 (42-92) |
| %CD3+ cell loss | 48 (11-78) | 52 (16-79) | |
| CD19+cells (×109) | 0.06 (0-0.2) | 0.02 (0-0.09) | 0.0004 (0-0.004) |
| %CD19+ cells | 0.4 (0-2) | 0.6 (0-2.3) | 0.02 (0-0.1) |
| %CD19+ cell loss | 72 (2.2-97) | 99 (94-100) | |
| Antibody Used . | B-NHL, B-precursor ALL and MM (n = 17) . | ||
|---|---|---|---|
| Apheresis (—) . | Immune Rosettes (CD11b) . | D-column (CD19*) . | |
| Total cell (×109) | 19.4 (8.6-41.4) | 2.4 (1.1-4.4) | 2 (0.9-3.6) |
| % overall cell loss | 85 (52-97) | 87 (59-98) | |
| CD34+cells (×109) | 0.3 (0.07-1.3) | 0.3 (0.06-1) | 0.2 (0.05-1) |
| %CD34+ cells | 1.8 (0.6-5.4) | 12 (1.5-28) | 14 (1.5-37) |
| %CD34 recovery | 85 (53-100) | 80 (48-100) | |
| %CD34 enrichment fold | 9 (2-35) | 10 (2-42) | |
| CD11b+cells (×109) | 15 (3.1-39.6) | 0.4 (0.01-1.2) | 0.2 (0.005-0.9) |
| %CD11b+ cells | 72 (37-98) | 19 (0.6-38) | 11 (0.4-28) |
| %CD11b+ cell loss | 96 (83-100) | 97 (87-100) | |
| CD3+ cells (×109) | 3.3 (0.5-8.4) | 1.4 (0.4-3) | 1.3 (0.4-2.6) |
| %CD3+ cells | 22 (1.4-55) | 58 (34-90) | 62 (42-92) |
| %CD3+ cell loss | 48 (11-78) | 52 (16-79) | |
| CD19+cells (×109) | 0.06 (0-0.2) | 0.02 (0-0.09) | 0.0004 (0-0.004) |
| %CD19+ cells | 0.4 (0-2) | 0.6 (0-2.3) | 0.02 (0-0.1) |
| %CD19+ cell loss | 72 (2.2-97) | 99 (94-100) | |
*In two MM patients purging experiments were performed by using anti-CD19 and anti-CD56 magnetic microbeads.
Evaluation of MRD.
A PCR-based quantitation of MRD was performed on CPC samples obtained from five patients before and after depletion of contaminating tumor B or T lymphocytes by purging with lineage-specific immunobeads. Molecular analysis was performed by demonstration of chimeric genomic products generated by the t(14;18) chromosomal translocation or by analysis of leukemia-specific DNA sequence, amplified from the rearranged TCR γ, δ, or IgH chain genes. As shown in Table 4, after the purification procedure, CD19-purged HPCs obtained from two follicular NHLs and two B-precursor ALLs were judged as PCR negative within the sensitivity limits of our assays (between 10-4 to 10-5). Similarly, after purging with T-cell–specific microbeads the PCR evaluation of DNA samples from a T-ALL patient showed the absence of the leukemic-specific clone (Table 4 and Fig1). Interestingly, in this case the amount of MRD was very limited and not detectable if searched within the whole unmodified apheretic product (Fig 1). However, the leukemic contamination was clearly shown in the unwanted, wasted, T-cell fraction retained within the magnetic column. Similar results were obtained in all the analyzed cases in which the neoplastic B-lymphoid cells were similarly trapped within the depletion column (Table 4).
Molecular Evaluation of Minimal Residual Disease After Lineage-Specific Purging
| Patients . | Disease . | . | Apheresis . | Wasted Cells* . | Purified Stem Cells . | Sensitivity3-151 . |
|---|---|---|---|---|---|---|
| 1 | NHL | t(14;18) BCL2/IgH | + | + | − | 105 |
| 2 | NHL | t(14;18) BCL2/IgH | + | + | − | 105 |
| 3 | preB-ALL | TcRγ (Vγ4-Jγ1.3) | + | + | − | 104 |
| 4 | T-ALL | TcRδ (Vδ1-Jδ1) | − | + | − | 104 |
| 5 | preB-ALL | IgH | + | + | − | 104 |
| Patients . | Disease . | . | Apheresis . | Wasted Cells* . | Purified Stem Cells . | Sensitivity3-151 . |
|---|---|---|---|---|---|---|
| 1 | NHL | t(14;18) BCL2/IgH | + | + | − | 105 |
| 2 | NHL | t(14;18) BCL2/IgH | + | + | − | 105 |
| 3 | preB-ALL | TcRγ (Vγ4-Jγ1.3) | + | + | − | 104 |
| 4 | T-ALL | TcRδ (Vδ1-Jδ1) | − | + | − | 104 |
| 5 | preB-ALL | IgH | + | + | − | 104 |
*Wasted cells were eluted after removal of the D column from the magnetic field of the SuperMACS apparatus.
The sensitivity of the PCR reaction was determined by log dilution of the patient's DNA obtained at diagnosis with DNA from a normal donor.
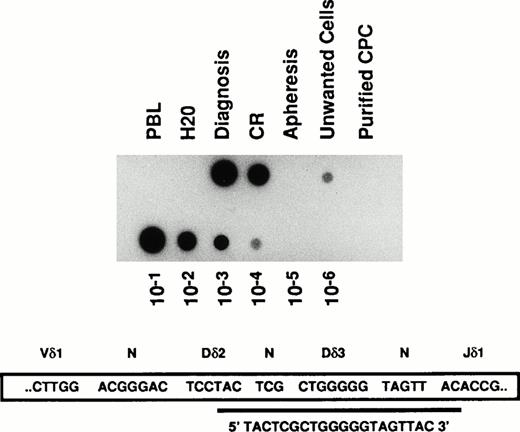
Detection of MRD by PCR analysis of V δ 1-J δ 1 junction of the TCR δ gene in a T-ALL patient. DNA samples were extracted from bone marrow aspirates performed at the onset of the disease (Diagnosis), at the end of induction chemotherapy when it was considered in complete hematologic remission (CR), from unmanipulated G-CSF mobilized CPC obtained after a consolidation course with high dose chemotherapy (Apheresis), from cells coated by anti-CD2 and -CD7 magnetic microbeads and retained within the depletion column (Unwanted cells), from the purified T-cell depleted fraction of circulating progenitor cells (Purified CPC), and from the peripheral blood lymphocytes of a normal donor (PBL). The sensitivity of the PCR reaction was checked by serial log dilution of the patient's DNA obtained at diagnosis with DNA from a normal donor (lower lane of the figure). PCR products were blotted onto nylon membranes and hybridized with the indicated clonospecific probe labeled with (γ -32 P) ATP.
Detection of MRD by PCR analysis of V δ 1-J δ 1 junction of the TCR δ gene in a T-ALL patient. DNA samples were extracted from bone marrow aspirates performed at the onset of the disease (Diagnosis), at the end of induction chemotherapy when it was considered in complete hematologic remission (CR), from unmanipulated G-CSF mobilized CPC obtained after a consolidation course with high dose chemotherapy (Apheresis), from cells coated by anti-CD2 and -CD7 magnetic microbeads and retained within the depletion column (Unwanted cells), from the purified T-cell depleted fraction of circulating progenitor cells (Purified CPC), and from the peripheral blood lymphocytes of a normal donor (PBL). The sensitivity of the PCR reaction was checked by serial log dilution of the patient's DNA obtained at diagnosis with DNA from a normal donor (lower lane of the figure). PCR products were blotted onto nylon membranes and hybridized with the indicated clonospecific probe labeled with (γ -32 P) ATP.
Transplantation of T- or B-cell purged autologous and allogeneic CPC.
We performed autologous transplantation in three patients by using CPCs highly purified by immune rosetting and immunomagnetic purging. In the case of the T-ALL patient presented in Fig 1, after a myeloablative therapy with high-dose Ara-C (2 g/m2 × 2/d for 6 days) and fractionated total body irradiation (TBI, 12cGy), the purified, leukemia-free fraction of HPCs obtained at the end of the purification procedure (6.8 × 106 /kg CD34+ cells) was autografted. The neutrophil engraftment (more than 1.5 × 109/L) was observed after 12 days and more than 20 and 100 × 109 /L platelets were counted at days +15 and + 30, respectively. Interestingly, the molecular evaluation of the bone marrow 100 days after transplantation did not show persistence of leukemic cells within the patient (data not shown). Similar results were obtained in two MM patients resistant to conventional chemotherapy and who underwent transplantation after a high-dose Melphalan (200 mg/m2) conditioning regimen, with CD19 and CD56 purged stem cells.26 27 The numbers of infused CD34+ cells were 8.3 and 10 × 106 /kg CD34+ cells, respectively. A rapid hematologic engraftment was observed in both patients, because more than 1.5 × 109/L neutrophils were counted at days +10 and +11 and more than 20 × 109/L platelets were counted at day +14 (Table 5).
Hematologic Reconstitution After Autologous Transplantation On Lineage-Specific Purging With Magnetic Microbeads
| Patients . | Disease . | Age . | Conditioning Regimen . | Composition of CPCs . | Days From BMT Graft Infusion Until: . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TNC ×106/kg . | CD34+ ×106/kg . | CD3+ ×106/kg . | CD19+ ×106/kg . | ANC > 500/mcl . | ANC > 1500/mcl . | Plt > 20000/mcl . | Plt > 50000/mcl . | ||||
| 1 | T-ALL | 47 | Ara-C/TBI | 74 | 6.8 | 0.1 | 0.1 | 9 | 11 | 14 | 16 |
| 2 | MM | 61 | Melphalan | 18 | 8.3 | 2.3 | 0.01 | 10 | 11 | 14 | 15 |
| 3 | MM | 59 | Melphalan | 29 | 10 | 13 | 0.09 | 10 | 11 | 10 | 12 |
| Patients . | Disease . | Age . | Conditioning Regimen . | Composition of CPCs . | Days From BMT Graft Infusion Until: . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TNC ×106/kg . | CD34+ ×106/kg . | CD3+ ×106/kg . | CD19+ ×106/kg . | ANC > 500/mcl . | ANC > 1500/mcl . | Plt > 20000/mcl . | Plt > 50000/mcl . | ||||
| 1 | T-ALL | 47 | Ara-C/TBI | 74 | 6.8 | 0.1 | 0.1 | 9 | 11 | 14 | 16 |
| 2 | MM | 61 | Melphalan | 18 | 8.3 | 2.3 | 0.01 | 10 | 11 | 14 | 15 |
| 3 | MM | 59 | Melphalan | 29 | 10 | 13 | 0.09 | 10 | 11 | 10 | 12 |
Abbreviations: ANC, absolute neutrophil count; Plt, platelets; TNC, total nucleated cells.
Because the use of unmodified allogeneic CPC has been possibly associated with increased chronic GVHD28 in six acute leukemia patients undergoing transplantation from HLA-identical siblings, allogeneic CPCs were extensively T-cell depleted by immune rosetting and immunomagnetic purging. However, to prevent an increased rate of graft failure and leukemia relapse,29 escalating moderate30 amounts of donor T lymphocytes (from a minimum of 2.5 to a maximum of 50 × 106 /kg) were rescued from the rosetted cells (by hypotonic lysis with NH4 Cl buffer, as described in Materials and Methods), enumerated by immunophenotyping with anti-CD3 antibody, and added back to the stem cell fraction just before cryopreservation. Only two apheretic procedures were necessary to infuse more than 5 × 106/kg CD34+ cells and despite the in vitro manipulation, a prompt hematologic reconstitution was observed in each patient (Table6). According to previously published experience,16,17 a conventional GVHD prophylaxis with a combination of Methotrexate and Cyclosporine A was performed20 31 and neither acute (more than grade I) or chronic GVHD was observed in these patients.
Effect of T-Cell Dose on the Quality and Timing of Short-Term Hematological Engraftment after CPC Allogeneic Transplantation
| Patients . | Disease . | Age . | Conditioning Regimen . | Composition . | Days from BMT Graft Infusion Until: . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| TNC ×106/kg . | CD34+ ×106/kg . | CD3+ ×106/kg . | ANC > 500/mcl . | ANC > 1500/mcl . | Plt > 20000/mcl . | Plt > 50000/mcl . | ||||
| 1 | T-ALL | 24 | TBI/Melphalan | 120 | 7.5 | 2 | 12 | 13 | 14 | 50 |
| 2 | AML | 59 | TBI/Melphalan | 90 | 9 | 10 | 10 | 11 | 12 | 14 |
| 3 | B-precursor ALL | 43 | TBI/Melphalan | 120 | 10.5 | 15 | 20 | 21 | 26 | 40 |
| 4 | B-precursor/ALL | 18 | TBI/Melphalan | 290 | 19.5 | 15 | 9 | 11 | 10 | 16 |
| 5 | B-precursor ALL | 45 | TBI/Melphalan | 80 | 5.2 | 40 | 11 | 12 | 11 | 14 |
| 6 | T-ALL | 31 | TBI/Melphalan | 470 | 11.4 | 50 | 14 | 15 | 14 | 16 |
| Media | 36.7 (18 to 59) | 195 (80 to 470) | 10.5 (5.2 to 19.5) | 22 (2 to 50) | 12.6 (9 to 20) | 13.8 (11 to 21) | 14.5 (10 to 26) | 25 (14 to 50) | ||
| Patients . | Disease . | Age . | Conditioning Regimen . | Composition . | Days from BMT Graft Infusion Until: . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| TNC ×106/kg . | CD34+ ×106/kg . | CD3+ ×106/kg . | ANC > 500/mcl . | ANC > 1500/mcl . | Plt > 20000/mcl . | Plt > 50000/mcl . | ||||
| 1 | T-ALL | 24 | TBI/Melphalan | 120 | 7.5 | 2 | 12 | 13 | 14 | 50 |
| 2 | AML | 59 | TBI/Melphalan | 90 | 9 | 10 | 10 | 11 | 12 | 14 |
| 3 | B-precursor ALL | 43 | TBI/Melphalan | 120 | 10.5 | 15 | 20 | 21 | 26 | 40 |
| 4 | B-precursor/ALL | 18 | TBI/Melphalan | 290 | 19.5 | 15 | 9 | 11 | 10 | 16 |
| 5 | B-precursor ALL | 45 | TBI/Melphalan | 80 | 5.2 | 40 | 11 | 12 | 11 | 14 |
| 6 | T-ALL | 31 | TBI/Melphalan | 470 | 11.4 | 50 | 14 | 15 | 14 | 16 |
| Media | 36.7 (18 to 59) | 195 (80 to 470) | 10.5 (5.2 to 19.5) | 22 (2 to 50) | 12.6 (9 to 20) | 13.8 (11 to 21) | 14.5 (10 to 26) | 25 (14 to 50) | ||
DISCUSSION
The manipulation of specific cell subpopulations of marrow or peripheral blood origin has become an interesting way to increase the applicability and reduce the toxicity of hematopoietic transplantation. However, the manipulation of CPCs obtained from G-CSF treated normal donors or patients is hampered by the huge cellularity present in the apheretic products, which often exceeds the absolute number of 50 × 109 cells, and by the fact that under the stimulatory effect of G-CSF, mature myeloid cells (mostly granulocytes) acquire different characteristics of cell density preventing their sedimentation on normal Ficoll gradients. Therefore, two main approaches to the CD34+ purification have been taken either by positive or by negative selection. Indeed, in the perspective of genetic manipulation, positive selection of CD34+ cells is likely to be the ideal option to ensure that the selected gene is transduced only in the small target population of pluripotent progenitor cells.32 Although transplantation of positively selected CD34+ cells purified by immunoaffinity columns or immunomagnetic bead adsorption has been shown as a feasible procedure,4,9,11-15 the specific depletion of unwanted cells seems preferable for several reasons including the preservation of the manipulation of CD34+ cells from binding with murine MoAbs and the need of their subsequent detachment by using chemical or physical methods. Moreover, most acute leukemias of both myeloid and lymphoid origin are positive for CD34 antigen expression thus reducing in this setting the clinical applicability of methods that rely only on the positive selection of the stem cell fraction. On the contrary, the selective elimination of residual neoplastic cells detectable in the stem cell fraction obtained from some patients with MM,33follicular lymphoma,34 and breast cancer35could be achieved by the use of MoAbs either in association with complement,36 conjugated to toxic compounds,37or by the use of magnetic microbeads proven to eradicate the neoplastic clone at the molecular level.25,38 However, it has to be mentioned that positive selection of CD34+ cells, combined with negative depletion steps, could also achieve high levels of purity,39 even though purging strategies based only on depletion techniques could avoid an extensive removal of T cells from the autograft of patients with B-lymphoproliferative disorders and solid tumors, thus reducing the risk of long-lasting immunodeficiency.
In this manuscript, we described a simple and reproducible method based on a two-step negative selection of cytokine mobilized circulating progenitor cells. With the first step of immune rosetting and subsequent Ficoll gradients we were able to obtain a drastic reduction of the massive starting cellularity of the leukapheretic products. The use of human RBCs suitable for transfusion and the fact that the whole procedure was performed by using clinical grade devices without the use of tubes or pipettes reduces the risk of microbiological contaminations and represents a crucial advantage of this approach. Most importantly, the consistent reduction of the mature myeloid cells without a significant loss of CD34+ cells allows a subsequent further effective purification of the human hematopoietic stem cells either by positive selection of the CD34+ cells or by further purging of the contaminating T- or B-cell fraction depending on the specific medical need. The results we obtained showed that we can specifically and reproducibly remove from the graft several logs of normal T cells as well as of contaminating tumor cells as assessed by PCR analysis.
The infusion of sibling-matched allogeneic hematopoietic stem cells purified according to this method, along with the add back of escalating amounts of T cells, was followed by a rapid hematologic engraftment, thus confirming the lack of any toxicity associated with the purification procedure.
In the setting of autologous transplantation, our data showed that an efficient purification of neoplastic B- or T-lymphoid cells could be reached and in the five cases analyzed at the molecular level, a disappearance of the neoplastic clone could be shown. Several obvious advantages are potentially associated with the use of purified stem cells for autologous hematopoietic transplantation in acute leukemia40 and lymphoma.41 We know that many patients can eventually relapse and die from their disease because of the infusion of tumor cells along with the autologous graft.42,43 Indeed, MRD persistence after autologous transplantation in NHL patients is associated with poor outcome, thus suggesting that neoplastic contamination of the graft should be eradicated for cure.41 The possibility of eradicating the residual leukemic cells in the apheretic product represents an important result because very little data about effective immunologic purging of ALL are available so far. Obviously, whereas these data represent a clear demonstration of the technical feasibility of a rapid and reproducible two-step purging procedure of cytokine-mobilized HPCs, the clinical outcome of autologous and allogeneic transplants in the diverse proposed clinical settings waits for future validation.
Supported in part by grants from the Associazione Italiana per la ricerca contro il cancro (AIRC); the Consiglio Nazionale per le Ricerche (CNR) Target Project on Biotechnology; the Associazione Paolo Belli, Lotta alla Leucemia; and Fondazione Tettamanti.
Address correspondence to Alessandro Rambaldi, MD, Division of Hematology, Ospedali Riuniti di Bergamo, Largo Barozzi, 1, 24100 Bergamo, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal