Abstract
Overexpression of P-glycoprotein (P-gp), the protein product of the multidrug resistance gene (MDR1), confers a drug resistant phenotype on cells. This phenotype is reminiscent of human T-cell leukemia virus (HTLV)-transformed leukemic cells, for which no consistently effective chemotherapeutic regime has been found. The presence of an active multiple drug resistance (MDR) phenotype in freshly isolated peripheral blood mononuclear cells (PBMC) from HTLV-I–infected subjects was investigated. Significant P-gp–mediated efflux activity and enhanced MDR1 mRNA expression was observed in nine of 10 HTLV-infected subjects. The development of MDR phenotypes was found to be independent of disease type or status with significant MDR activities being observed in adult T-cell leukemia (ATL), HTLV-associated myelopathy (HAM)/tropical spastic paraparesis (TSP), and asymptomatic HTLV-infected individuals. P-gp–mediated drug efflux was also found to be restricted to CD3+ T-cell populations. Furthermore, we show the novel finding that theMDR1 gene promoter is transcriptionally activated by the HTLV-I tax protein, suggesting a molecular basis for the development of drug resistance in HTLV-I infections. These observations open up the possibility of new chemotherapeutic approaches to HTLV-associated diseases through the use of P-gp inhibitors.
ATOTAL OF 10 to 20 million people worldwide are estimated to be infected with human T-cell leukemia virus (HTLV).1 Infection is associated with at least two distinct disease syndromes.2 Adult T-cell leukemia (ATL) is an aggressive T-cell malignancy, which occurs worldwide in populations where HTLV-I infection is endemic. HTLV-I infection has also been linked with a syndrome known as HTLV-associated myelopathy (HAM) or tropical spastic paraparesis (TSP), and more recently, a variety of other pathologies. The pathogenesis of these conditions remains unclear.
Treatment of ATL patients has traditionally consisted of combination chemotherapy, but this approach has limited clinical benefit. Four generations of combination chemotherapy have shown an increase in remission rates from 11% to 42%, but a corresponding improvement in overall or disease-free survival time has not occurred.3,4Similarly other novel treatments, including bone marrow transplantation, total body irradiation, and treatment with interferons β and γ have failed to show clinical improvement. Limited clinical improvement in some patients has been reported using deoxycoformycin (DCF), an adenosine deaminase inhibitor,5 MST-16, a topoisomerase II inhibitor,6 and anti-Tac antibodies.7 Improved remission rates have recently been reported in small studies combining α-interferon and azidothymidine (AZT).8 9 Generally, ATL is highly resistant to traditional chemotherapeutic strategies, of which none confer significant clinical improvement. This observation suggests that ATL cells have innate drug resistance, a phenotype that could be one result of HTLV-I–mediated gene deregulation.
The multiple drug resistance phenotype (MDR) results in a broad spectrum of resistance to chemotherapy. There are several known mechanisms by which an MDR phenotype may arise in cells, but one of the best characterized is that caused by the overexpression of P-glycoprotein (P-gp).10,11 P-gp, the protein product of the MDR gene family, is able to confer a MDR phenotype by pumping a wide spectrum of chemotherapeutic agents (including anthracyclines, vinca alkaloids, epipodophylline, and dactinomycins) from the cell. The result is lower intracellular drug concentrations and reduced drug efficacy. There are two MDR genes in humans, 1 and 2. Only MDR1is able to confer a MDR phenotype in transfection analysis.12-14 Increased MDR1 gene expression has been associated with poor prognosis and drug resistance in acute myeloid leukemia (AML), adult lymphoblastoid leukemia (ALL),15-17 and various other leukemias, lymphomas, myelomas, and sarcomas.18-22 The expression of membrane bound P-gp and MDR1 RNA has been shown in cells from ATL patients.23-25 The incidence of P-gp expression was also suggested to be higher in ATL than in other non-HTLV–induced leukemias.25 However, these preliminary experiments did not establish an association between P-gp expression and drug resistance activity, only that membrane P-gp and MDR1 RNA could be detected in ATL cells. Similarly, no evidence for a mechanism or the extent of enhancement of P-gp expression in HTLV infections was given.
In the present study, we show significant P-gp–mediated drug efflux associated with enhanced MDR1 gene transcription in T cells of individuals infected with HTLV regardless of disease status. Using specific assays to measure MDR1 gene expression and P-gp–mediated drug efflux activity, we have confirmed and extended preliminary studies to include asymptomatics, HAM/TSP, and ATL subjects. The molecular mechanism of P-gp upregulation in HTLV infections was also investigated, and we show here for the first time activation of the MDR1 gene promoter by the HTLV tax protein. These observations raise the possibility of new and alternative chemotherapeutic approaches to HTLV-associated diseases.
MATERIALS AND METHODS
Cells.
Preservative-free heparinized fresh whole blood was obtained by venipuncture of 10 HTLV-I–seropositive subjects and six healthy uninfected controls. Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation over Ficoll-Paque (Amersham Pharmacia Biotech, UK). Isolated PBMCs were assayed immediately for MDR activity or RNA extracted. COS cells26were maintained in Dulbecco's modified Eagle's medium (DMEM) medium supplemented with 10% calf serum at 37°C, 5% C02 in a humidified atmosphere.
Rhodamine 123 (R123) dye efflux MDR assay.
R123 efflux assays were performed as described by Davies et al,27 except that the assays presented here used 2 × 106 PBMC in 2 mL RPMI 1640 medium supplemented with 2.5% fetal calf serum (FCS) and 0.4 μmol/L R123. After incubation and propidium iodide staining as described, cells were fixed using 1% paraformaldehyde before FACscan analysis (Becton Dickinson, Franklin Lakes, NJ). The effects of P-gp inhibitors were assessed by performing R123 assays on cells in the presence 5 μmol/L reserpine or verapamil (Sigma, St Louis, MO). This assay is highly specific for MDR1 because R123 accumulation correlates with monoclonal antibody (MoAb) MRK16 binding (specific for MDR1) in cells that express MDR1 and MDR2.27
Determination of cell surface phenotypes of MDR+cells.
PBMC subset analysis of R123 efflux cells was performed using phycoerythrin (PE)-conjugated lymphocyte cell typing MoAbs (VERItype) from Harlan Sera-lab (Sussex, UK). R123 efflux assays were performed on cells as described above except that the cells were not labeled with propidium iodide. The cells after R123 dye efflux were washed in once in phosphate-buffered saline (PBS) and resuspended at 1 × 106 cells/mL in PBSH (PBS + 2% human AB serum). Cells (2 × 105) were incubated with 2 μL (1:100 dilution) of PE-conjugated mouse anti-CD3, CD14, or CD22 VERItype antibodies. Cells were directly stained at 4°C for 30 minutes, then washed in PBS and fixed in 1% paraformaldehyde before flow cytometric analysis. High and low R123 dye efflux cell populations were analyzed for PE fluorescence with either anti-CD3, anti-CD14, and anti-CD22 antibodies on a Becton Dickinson FACscan.
Immunofluorescent detection of P-gp.
Indirect immunofluorescent detection of cell surface-associated P-gp was performed using the anti–P-gp MoAb MRK-16 (Kamiya Biomedical Co, Thousand Oaks, CA). Freshly isolated PBMCs were washed in PBS and resuspended at 1 × 106 cells/mL in PBSG (PBS + 2% goat serum). Cells (2 × 105) were incubated with 2 μg (10 μg/mL) of either anti–P-gp MRK-16 or IgG2a control primary antibody at 4°C for 1 hour. Cells were washed once with PBST (PBS + 10% Tween-20), then resuspended in 200 μL cold PBSG containing 10 μL (1:10 dilution) of fluorescein isothiocyanate (FITC)-conjugated goat antimouse IgG secondary detection antibody (Dako, Glostrup, Denmark). Cells were incubated at 4°C for 1 hour, then washed twice with PBST and fixed in 1% paraformaldehyde before FACscan analysis.
Quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) of MDR1 mRNA.
RNA for RT-PCR analysis was isolated by guanidium isothiocyanate/phenol extraction (RNAzol B, Biogenesis, Poole, UK) according to the supplier's recommend protocol. A total of 100 to 500 ng of total cellular RNA were reverse transcribed by incubation with 2 U AMV-RT (Promega, Madison, WI) at 42°C for 1 hour. After inactivation at 90°C for 5 minutes, the entire mix was subjected to PCR amplification using 1.25 U Taq polymerase (Promega) using theMDR1-specific primers and internal standard RNA,27 28 giving rise to an amplified product of 161 bp and not 425 bp, as originally published. Negative controls consisted of mock reactions lacking RT, positive controls provided by internal standard RNA. PCR products were visualized under ultraviolet (UV) light on 3% GTG Nusieve agarose gels (Flowgen, Staffordshire, UK). Relative band intensities were calculated by densitometry.
Plasmids.
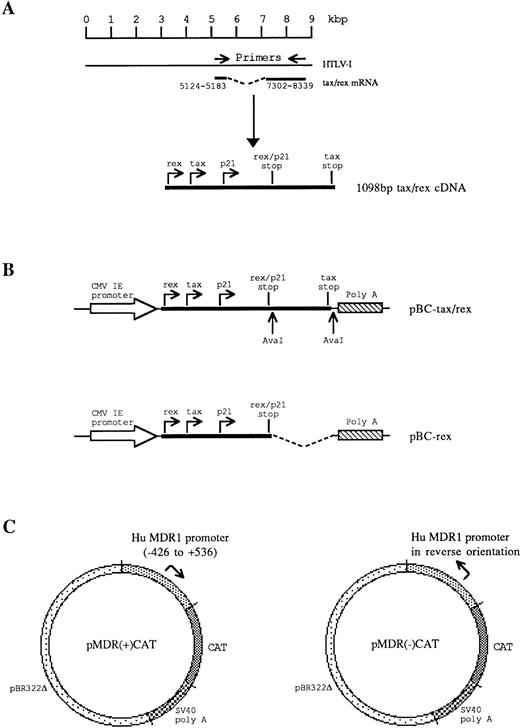
Plasmids pBC-tax/rex, pBC-rex, and pMDR-CAT were constructed as shown in Fig 1. pBC-tax/rex contains a 1,098-bp fragment of cDNA corresponding to the tax/rex mRNA of HTLV-I, which encodes both the tax and rex open reading frames (orf). Total cellular RNA was extracted from virus-infected C91/PL cells29 by guanidium isothiocyanate/phenol extraction (RNAzol B, Biogenesis). A total of 400 ng of total cellular RNA was reverse transcribed with 200 U M-MLV RT (GIBCO-BRL, Gaithersburg, MD) and 200 ng oligo (dT)12-18primer at 37°C for 1 hour. One quarter of the cDNA products were then PCR amplified for 30 cycles using 1 U pfu DNA polymerase (Stratagene, La Jolla, CA) and 100 ng each of sense and antisense tax/rex primer (sense, 5′-TTAAGCTTGCGAGCTGCATGCCCAAGAC-3′; antisense, 5′-AACTCGAGTCAGACTTCTGTTTCTCGGAAATG-3′). The amplified tax/rex fragment was inserted into pBC12/CMV/IL-230 under the control of the huCMV immediate early promoter, replacing the interleukin-2 (IL-2) sequences. pBC-rex only contains the rex orf and was constructed by deletion of a 534-bp Ava I-Ava I fragment from pBC-tax/rex (Fig 1). This deletion removes the 3′-end 470 bp of the tax orf, which is not present in rex. pRK7-tax expresses tax, but not rex from the huCMV immediate early promoter.31
Construction and structure of recombinant plasmids pBC-tax/rex, pBC-rex, and pMDR-CAT. (A) RT-PCR amplification from HTLV tax/rex mRNA was used to generate a 1,098-bp fragment, which encodes the complete tax, rex, and p21 proteins. (B) The tax/rex protein expression construct, pBC-tax/rex, was derived by insertion of the 1,098-bp tax/rex cDNA into pBC12/CMV/IL-2 under the control of the CMV immediate early (CMV IE) promoter. The rex protein expression construct, pBC-rex, was derived by deletion of an AvaI-Ava I fragment in pBC-tax/rex, which removes the C-terminal half of tax. (C) MDR1 promoter-CAT reporter gene constructs. pMDR(+)CAT contains the MDR1 promoter in tandem with the CAT gene. pMDR(-)CAT contains the MDR1 promoter in a reverse orientation to the CAT gene.
Construction and structure of recombinant plasmids pBC-tax/rex, pBC-rex, and pMDR-CAT. (A) RT-PCR amplification from HTLV tax/rex mRNA was used to generate a 1,098-bp fragment, which encodes the complete tax, rex, and p21 proteins. (B) The tax/rex protein expression construct, pBC-tax/rex, was derived by insertion of the 1,098-bp tax/rex cDNA into pBC12/CMV/IL-2 under the control of the CMV immediate early (CMV IE) promoter. The rex protein expression construct, pBC-rex, was derived by deletion of an AvaI-Ava I fragment in pBC-tax/rex, which removes the C-terminal half of tax. (C) MDR1 promoter-CAT reporter gene constructs. pMDR(+)CAT contains the MDR1 promoter in tandem with the CAT gene. pMDR(-)CAT contains the MDR1 promoter in a reverse orientation to the CAT gene.
pMDR-CAT contains the 1kb Pst I-Pst I promoter region (−426 to +536) surrounding the transcription start site of the human MDR1 gene derived from plasmid pMDR-P3,32kindly provided by M. Cornwell (Fred Hutchinson Cancer Research Centre, WA). The SV40 promoter and origin of replication were excised from pSV2CAT (ATCC #37155, positions 1-328) and replaced with the MDR1 fragment. pMDR(+)CAT contains the MDR1promoter in tandem with the CAT gene; pMDR(-)CAT contains the promoter opposed to the CAT gene and was used as a negative control. pMDR-CAT (M.M. Gottesman, Laboratory of Cell Biology, NCI, NIH) contains a longer 1.8 kb promoter region of the human MDR1 gene inserted upstream of the CAT reporter gene33 and was kind gift of M.M. Gottesman. pLTR-I-CAT contains the HTLV-I LTR promoter inserted upstream of the CAT reporter gene and is described elsewhere.34
Trans-activation of the MDR1 promoter by HTLV-I tax.
Electroporation of COS cells and chloramphenicol acetyl transferase (CAT) assays were performed as previously described35except that 3 × 106 COS cells were electroporated at 250 V, 960 μF with 25 μg of total plasmid DNA (12.5 μg of each plasmid to be coelectroporated). Forty-eight hours after electroporation, cells were harvested and cells lysed by freeze-thawing. Total protein concentrations were determined using the Bio-Rad (Hertfordshire, UK) protein assay kit. A total of 50 μg of cell lysates were incubated with 4 mmol/L Acetyl-CoA (Sigma) and 0.05 μCi [14C]-chloramphenicol (ICN, Costa Mesa, CA) at 37°C for 3 hours. Reactions were extracted with ethyl acetate, then acetylated products resolved by thin layer chromatography and quantified by scintillation counting.
RESULTS
R123 dye efflux analysis of PBMC from HTLV and non–HTLV-infected subjects.
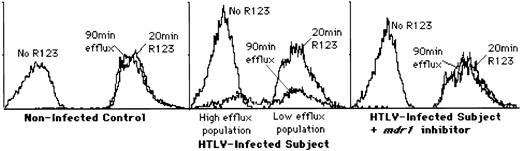
PBMC from 10 HTLV-I–seropositive subjects (6 asymptomatic, 2 HAM/TSP, 1 lymphoma, 1 ATL) and 6 healthy uninfected controls were examined using a rhodamine 123 (R123) dye efflux assay (Fig 2, Table 1). None of the non–HTLV-infected control subjects tested showed any significant level of P-gp efflux activity in PBMC, whereas all but one (no. 1) of the HTLV-infected subjects showed at least some activity, indicated by the presence of a high efflux cell population. The role of P-gp in the observed R123 efflux was confirmed by abolition of the high efflux population of cells by P-gp inhibitors, reserpine and verapamil (Fig 2, Table 1).
Analysis of P-gp–mediated drug efflux activity in freshly isolated PBMCs from HTLV and non-HTLV–infected subjects. Rhodamine 123 (R123) dye efflux assays were used to measure the extent of drug resistance. This figure shows the results of a single representative sample; data from other subjects examined is summarized in Table 1. (Left panel) FACscan analysis of cells from a typical HTLV-negative control subject. No decrease in fluorescence is evident after a 90-minute incubation, indicating no P-gp activity. (Center panel) FACscan analysis of cells from a typical HTLV-I–infected subject. After a 90-minute incubation, two populations of cells are present, a high efflux, low fluorescence population and a low efflux, high fluorescence population. (Right panel) Inhibition of R123 efflux by P-gp inhibitors. In the presence of 5 μmol/L reserpine or 5 μmol/L verapamil (shown here), the dye efflux is blocked, indicating the specificity of this assay for P-gp.
Analysis of P-gp–mediated drug efflux activity in freshly isolated PBMCs from HTLV and non-HTLV–infected subjects. Rhodamine 123 (R123) dye efflux assays were used to measure the extent of drug resistance. This figure shows the results of a single representative sample; data from other subjects examined is summarized in Table 1. (Left panel) FACscan analysis of cells from a typical HTLV-negative control subject. No decrease in fluorescence is evident after a 90-minute incubation, indicating no P-gp activity. (Center panel) FACscan analysis of cells from a typical HTLV-I–infected subject. After a 90-minute incubation, two populations of cells are present, a high efflux, low fluorescence population and a low efflux, high fluorescence population. (Right panel) Inhibition of R123 efflux by P-gp inhibitors. In the presence of 5 μmol/L reserpine or 5 μmol/L verapamil (shown here), the dye efflux is blocked, indicating the specificity of this assay for P-gp.
R123 Dye Efflux Assay of P-gp Activity in PBMC
| Category . | Patient No. . | High Efflux Population %R123 Efflux . | Low Efflux Population %R123 Efflux . |
|---|---|---|---|
| Asymptomatic | 1 | 0 | 11 |
| 2 | 88 | 6 | |
| 3 | 90 | 3 | |
| 4 | 85 | 1 | |
| 5 | 83 | 1 | |
| 6 | 92 | 10 | |
| Average | 73 | 5 | |
| TSP/HAM | 7 | 92 | 7 |
| 8 | 86 | 15 | |
| Average | 89 | 11 | |
| ATLL | 9 | 90 | 11 |
| Lymphoma | 10 | 87 | 2 |
| Overall HTLV+ Average: | 79 | 7 | |
| Asymptomatic No. 6 + 5 μmol/L reserpine | 0 | 27 | |
| Asymptomatic No. 6 + 5 μmol/L verapamil | 0 | 25 | |
| Non–HTLV-infected | 1 | 0 | 22 |
| 2 | 0 | 12 | |
| 3 | 0 | 2 | |
| 4 | 0 | 3 | |
| 5 | 0 | 15 | |
| 6 | 0 | 17 | |
| Overall HTLV− Average | 0 | 12 |
| Category . | Patient No. . | High Efflux Population %R123 Efflux . | Low Efflux Population %R123 Efflux . |
|---|---|---|---|
| Asymptomatic | 1 | 0 | 11 |
| 2 | 88 | 6 | |
| 3 | 90 | 3 | |
| 4 | 85 | 1 | |
| 5 | 83 | 1 | |
| 6 | 92 | 10 | |
| Average | 73 | 5 | |
| TSP/HAM | 7 | 92 | 7 |
| 8 | 86 | 15 | |
| Average | 89 | 11 | |
| ATLL | 9 | 90 | 11 |
| Lymphoma | 10 | 87 | 2 |
| Overall HTLV+ Average: | 79 | 7 | |
| Asymptomatic No. 6 + 5 μmol/L reserpine | 0 | 27 | |
| Asymptomatic No. 6 + 5 μmol/L verapamil | 0 | 25 | |
| Non–HTLV-infected | 1 | 0 | 22 |
| 2 | 0 | 12 | |
| 3 | 0 | 2 | |
| 4 | 0 | 3 | |
| 5 | 0 | 15 | |
| 6 | 0 | 17 | |
| Overall HTLV− Average | 0 | 12 |
Summary of R123 efflux assay data. With one exception, all HTLV-infected subjects showed a high efflux population of cells, irrespective of clinical status, indicating elevated P-gp activity (Fig2). The one exception (asymptomatic subject no. 1) was confirmed HTLV-I seropositive and genome-positive by PCR; it is presently unclear what distinguishes this subject from all of the others examined. In contrast, all of the non–HTLV-infected subjects examined are P-gp negative and show only a single low efflux population of cells (Fig2).
Cell surface phenotypes of MDR+ PBMC subpopulations from HTLV-infected subjects.
The identity of cells in the high and low R123 efflux populations was tested by labeling with cell surface MoAb markers and measuring the relative distribution of the markers in each of the populations (Table 2). The majority of stained cells in the high efflux population were CD3 positive (pan T-cell marker), while the low efflux population contained a mixture of T cells (CD3+), B cells (CD22+), and monocyte-macrophages (CD14+). This result indicates that the high efflux population contains mostly T cells with very few B cells or monocyte-macrophages.
Surface Phenotype of PBMC From Subjects Examined
| . | % Total Cells Staining . | % High Efflux . | % Low Efflux . |
|---|---|---|---|
| CD3 | 59, 61, 58 (59) | 29, 29, 27 (28) | 24, 31, 24 (26) |
| CD14 | 12, 13, 15 (13) | 0, 0, 0 (0) | 9, 10, 14 (11) |
| CD22 | 15, 11, 12 (13) | 1, 1, 1 (1) | 10, 12, 10 (11) |
| . | % Total Cells Staining . | % High Efflux . | % Low Efflux . |
|---|---|---|---|
| CD3 | 59, 61, 58 (59) | 29, 29, 27 (28) | 24, 31, 24 (26) |
| CD14 | 12, 13, 15 (13) | 0, 0, 0 (0) | 9, 10, 14 (11) |
| CD22 | 15, 11, 12 (13) | 1, 1, 1 (1) | 10, 12, 10 (11) |
The surface phenotype of PBMC in HTLV-I–infected subjects (subjects no. 4 and 5 [asymptomatic] and no. 7 [HAM/TSP]) were examined. The percentage of cells staining with MoAbs against CD3 (T-cell marker), CD14 (monocyte-macrophage cell marker), and CD22 (B-cell marker) are shown (mean frequency in parentheses). In each case, almost all of the cells in the high efflux population are T cells, whereas the low efflux P-gp negative population of cells contains a mixture of T cells, B cells, and monocyte-macrophages.
Immunofluorescent detection of P-gp.
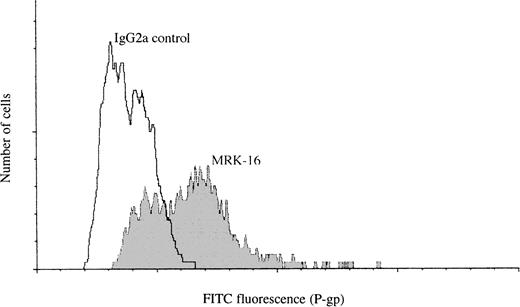
The presence of surface expressed P-gp on PBMC from HTLV-infected subjects was confirmed by indirect immunofluorescence using anti–P-gp MoAb MRK-16 (Fig 3). Staining of cells with MRK-16 antibody showed the presence of a high P-gp cell surface expressing subpopulation in PBMC. The proportion of high P-gp expressing cells was found to be around 50% of the total number of PBMCs. This is consistent with the proportion of MDR+ cells found by the R123 dye efflux assay method, suggesting that the high P-gp expressing and high R123 dye efflux populations are the same cells.
Immunofluorescent detection of P-gp on the surface of freshly isolated PBMCs from an HTLV-infected subject. Staining of cells with MRK-16 shows the presence of both high and low P-gp–expressing cell populations. A matched isotype IgG2a antibody was used instead of MRK-16 as a control.
Immunofluorescent detection of P-gp on the surface of freshly isolated PBMCs from an HTLV-infected subject. Staining of cells with MRK-16 shows the presence of both high and low P-gp–expressing cell populations. A matched isotype IgG2a antibody was used instead of MRK-16 as a control.
Quantitative RT-PCR analysis of MDR1 mRNA.
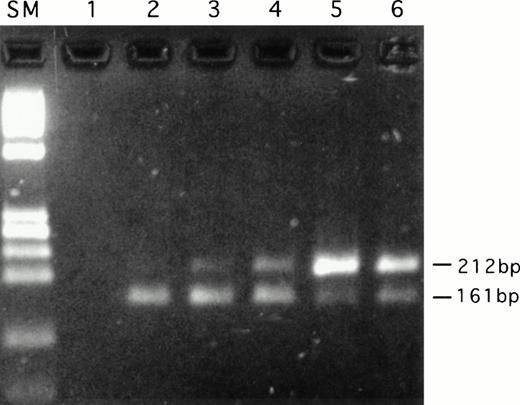
To confirm the R123 efflux results, quantitative RT-PCR analysis ofMDR1 mRNA was performed (Fig 4, Table 3). The results obtained are entirely consistent with those obtained from the R123 efflux assay. Only very low levels ofMDR1 mRNA were observed in all of the non–HTLV-infected subjects examined, whereas all but one (no. 1) of the HTLV-infected subjects showed enhanced MDR1 mRNA levels. These results suggest that the MDR activity seen in HTLV-infected subjects is the result of transcriptional activation of the MDR1 gene or possibly stabilization of MDR1 mRNA.
Quantitative RT-PCR analysis of MDR1 mRNA. Reactions were performed as described in Materials and Methods. A representative experiment is shown. All reactions contained 100 ng total RNA. Internal standard (upper band) 212 bp, MDR1 mRNA (lower band) 161 bp. SM: Size marker (HaeIII digest ◊X174 DNA); 1: negative control (no RT); 2: 0.05 pg ISC RNA; 3: 0.25 pg ISC RNA; 4: 0.5 pg ISC RNA; 5: 1.0 pg ISC RNA; 6: 2.5 pg ISC RNA.
Quantitative RT-PCR analysis of MDR1 mRNA. Reactions were performed as described in Materials and Methods. A representative experiment is shown. All reactions contained 100 ng total RNA. Internal standard (upper band) 212 bp, MDR1 mRNA (lower band) 161 bp. SM: Size marker (HaeIII digest ◊X174 DNA); 1: negative control (no RT); 2: 0.05 pg ISC RNA; 3: 0.25 pg ISC RNA; 4: 0.5 pg ISC RNA; 5: 1.0 pg ISC RNA; 6: 2.5 pg ISC RNA.
Quantitative RT-PCR Analysis of MDR1 mRNA
| Category . | Patient No. . | Molecules MDR1 mRNA/ pg total RNA . |
|---|---|---|
| Asymptomatic | 1 | 2 |
| 2 | 22 | |
| 3 | 27 | |
| 4 | 21 | |
| 5 | 22 | |
| 6 | 24 | |
| Average | 20 | |
| TSP/HAM | 7 | 42 |
| 8 | 27 | |
| Average | 35 | |
| ATLL | 9 | 49 |
| Overall HTLV+ Average | 26 | |
| Non–HTLV-infected | 1 | 6 |
| 2 | 7 | |
| 3 | 3 | |
| 4 | 5 | |
| 5 | 8 | |
| 6 | 13 | |
| Overall HTLV− Average | 7 |
| Category . | Patient No. . | Molecules MDR1 mRNA/ pg total RNA . |
|---|---|---|
| Asymptomatic | 1 | 2 |
| 2 | 22 | |
| 3 | 27 | |
| 4 | 21 | |
| 5 | 22 | |
| 6 | 24 | |
| Average | 20 | |
| TSP/HAM | 7 | 42 |
| 8 | 27 | |
| Average | 35 | |
| ATLL | 9 | 49 |
| Overall HTLV+ Average | 26 | |
| Non–HTLV-infected | 1 | 6 |
| 2 | 7 | |
| 3 | 3 | |
| 4 | 5 | |
| 5 | 8 | |
| 6 | 13 | |
| Overall HTLV− Average | 7 |
MDR1 mRNA levels in the subjects tested were measured by a quantitative RT-PCR assay as described in the text. The values obtained reflect closely the results of the functional R123 efflux assay (Table1), indicating that the upregulation of P-gp activity in HTLV-I–infected subjects is due to transcriptional upregulation of theMDR1 gene.
Trans-activation of the MDR1 promoter by HTLV-I tax.
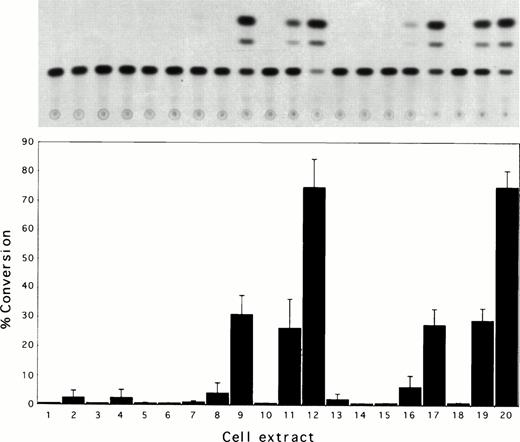
We examined the transcriptional activation of the human MDR1gene by constructing tax and rex protein expression vectors and MDR-CAT reporter constructs (Fig 1). Figure 5 shows typical CAT assay results when MDR-CAT reporter plasmids were introduced into COS cells together with HTLV tax or rex protein expression constructs. When pMDR(+)CAT and pMDR-CAT (M.M. Gottesman) were coelectroporated with the HTLV-I tax protein expression construct, pBC-tax/rex, 19-fold and 10-fold activation of the human MDR1promoter were observed, respectively. The pBC-tax/rex construct potentially encodes rex2 and p21rex 36 proteins in addition to tax, and their involvement in MDR1 gene activation was assessed using the rex/p21rex protein expression construct pBC-rex. Extracts of cells coelectroporated with pBC-rex and pMDR(+)CAT or pMDR-CAT (M.M. Gottesman) showed negligible CAT activity and suggests the trans-activation activity is due to transcriptional activation by the tax protein alone. This was confirmed using plasmid pRK7-tax, which expresses tax, but not rex31and still transactivates the pMDR-CAT plasmid. The specificity of tax trans-activation was further confirmed by coelectroporation with pMDR(-)CAT in which the MDR1 gene promoter is linked upstream of the CAT reporter gene in a reverse orientation. This construct was found to be unable to direct expression of CAT and cell extracts showed negligible CAT activity. Together these results suggest that the increase in MDR1 mRNA levels seen may be due to trans-activation of the MDR1 gene in HTLV-I–infected individuals.
Trans-activation of the MDR1 gene promoter by the HTLV-I tax protein. (A) Representative experiment showing chloramphenicol acetyl transferase (CAT) assays of COS cell extracts. (B) Quantification of CAT activity in COS cell extracts from three independent experiments. The percentage of chloramphenicol converted into acetylated forms is shown. COS cells were electroporated, as described in the text, with the following plasmids: 1: No DNA; 2: pBC-tax/rex; 3: pBC-rex; 4: pRK7-tax; 5: pMDR(+)CAT; 6: pMDR(-)CAT; 7: pMDR-CAT (M.M. Gottesman); 8: pLTR-I-CAT; 9: pMDR(+)CAT + pBC-tax/rex; 10: pMDR(-)CAT + pBC-tax/rex; 11: pMDR-CAT (M.M. Gottesman) + pBC-tax/rex; 12: pLTR-I-CAT + pBC-tax/rex; 13: pMDR(+)CAT + pBC-rex; 14: pMDR(-)CAT + pBC-rex; 15: pMDR-CAT (M.M. Gottesman) + pBC-rex; 16: pLTR-CAT + pBC-rex; 17: pMDR(+)CAT + pRK7-tax; 18 pMDR(-)CAT + pRK7-tax; 19: pMDR-CAT (M.M. Gottesman) + pRK7-tax; 20: pLTR-I-CAT + pRK7-tax. Therefore, lanes 1 to 8, 10, 14, and 18 are negative controls; lanes 12 and 20 are positive controls. Trans-activation of the MDR1 promoter-CAT constructs by tax can be seen in lanes 9, 11, 17, and 19. Lanes 13 to 16 show negligible activation of the MDR1 promoter-CAT constructs by rex.
Trans-activation of the MDR1 gene promoter by the HTLV-I tax protein. (A) Representative experiment showing chloramphenicol acetyl transferase (CAT) assays of COS cell extracts. (B) Quantification of CAT activity in COS cell extracts from three independent experiments. The percentage of chloramphenicol converted into acetylated forms is shown. COS cells were electroporated, as described in the text, with the following plasmids: 1: No DNA; 2: pBC-tax/rex; 3: pBC-rex; 4: pRK7-tax; 5: pMDR(+)CAT; 6: pMDR(-)CAT; 7: pMDR-CAT (M.M. Gottesman); 8: pLTR-I-CAT; 9: pMDR(+)CAT + pBC-tax/rex; 10: pMDR(-)CAT + pBC-tax/rex; 11: pMDR-CAT (M.M. Gottesman) + pBC-tax/rex; 12: pLTR-I-CAT + pBC-tax/rex; 13: pMDR(+)CAT + pBC-rex; 14: pMDR(-)CAT + pBC-rex; 15: pMDR-CAT (M.M. Gottesman) + pBC-rex; 16: pLTR-CAT + pBC-rex; 17: pMDR(+)CAT + pRK7-tax; 18 pMDR(-)CAT + pRK7-tax; 19: pMDR-CAT (M.M. Gottesman) + pRK7-tax; 20: pLTR-I-CAT + pRK7-tax. Therefore, lanes 1 to 8, 10, 14, and 18 are negative controls; lanes 12 and 20 are positive controls. Trans-activation of the MDR1 promoter-CAT constructs by tax can be seen in lanes 9, 11, 17, and 19. Lanes 13 to 16 show negligible activation of the MDR1 promoter-CAT constructs by rex.
DISCUSSION
The expression of P-gp has been reported previously on ATL cells.23-25 These earlier studies detected the presence of P-gp on membrane fractions derived from ATL cells by immunoblotting using a MoAb. The report by Kuwazuru et al24 also showed P-gp–like photoaffinity drug labeling and MDR1 RNA in cells from a single ATL patient after clinical relapse. However, these reports did not correlate P-gp expression with cellular drug resistance activity or provide any evidence for a mechanism of P-gp enhancement in HTLV-induced disease. Moreover, these studies did not include appropriate controls from non–HTLV-infected subjects, or did they investigate MDR1 gene expression in HTLV-infected subjects other than those with full-blown ATL. P-gp has subsequently been found to be expressed at low levels on normal lymphocytes (but not enough to mediate significant drug resistance) and by the use of the same MoAb (C219) used in the above studies.37-39 Hence, the actual enhancement of P-gp expression in ATL cells over normal lymphocyte cell populations was unclear. The data presented here addresses all of these points and clarifies the involvement of HTLV in the induction of multiple drug resistance phenotypes.
Our results show that the MDR1 gene is expressed at relatively high levels in all categories of HTLV-I–infected individuals (asymptomatic, HAM/TSP, ATL, and lymphoma). The molecular mechanisms involved in the normal cellular control of MDR1 gene expression remain unclear. Upstream promoter sequences from the MDR1 gene have been isolated and sequenced.40 The MDR1promoter does not contain a TATA box, but does contain several distinct regulatory motifs including a CAAT box (−113 to −118), two GC-rich regions (-51 to −61, −103 to −110), which are putative Sp-1 binding sites, a heat shock consensus element, and an AP-1–like binding site. Sequences immediately downstream of the transcription start site are also involved in MDR1expression.41 42 The region of DNA present in pMDR-CAT and pMDR-CAT (M.M. Gottesman) contains all of these putative regulatory elements.
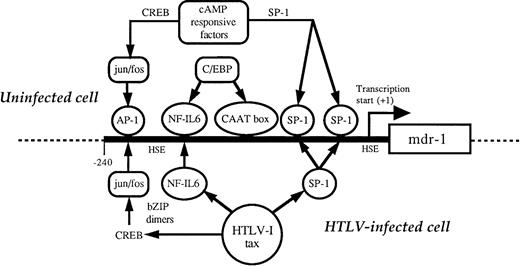
The HTLV-I trans-activator protein, tax, is a 40-kD nonstructural nuclear protein encoded by a doubly spiced subgenomic viral mRNA. The tax protein is a positive trans-activator of both viral and cellular transcription and is thought to be the main cause of cellular transformation, due to aberrant expression of a large range of heterologous cellular genes.2 The mechanism of tax trans-activation is indirect, mediating enhanced transcription through interactions with cellular transcription factors rather than by direct binding to DNA. Such transcription factors include the cAMP-responsive element binding protein/activating transcription factor (CREB/ATF), NF-κB, and serum response factor (SRF) families.43
Evidence for trans-acting transcriptional activation of MDR1comes from observations of differential expression of MDR1 in normal tissues and tumors in the absence of gene amplification, which suggests transcription factors are involved.11 Enhanced transcription has also been shown in cells that additionally containMDR1 gene amplifications.44MDR1 gene expression can be activated when cells are subjected to chemical or physical stress45,46 and increased MDR1 promoter activity is seen in transfected cells treated with anticancer agents.47 NF-IL–6 (or C/EBP-β), a bZIP motif-containing member of the C/EBP (CCAAT enhancer binding protein) family of transcription factors involved in regulating cytokine IL-6 transcription, has been associated with trans-activation ofMDR1 promoter.48 This suggests a possible mechanism for trans-activation of MDR1 by tax (Fig 6), as the tax protein is capable of modulating the transcription factor CREB through interactions with the basic domain of bZIP proteins, increasing dimerization, and DNA binding affinities to increase transcription from CRE-containing promoters.2 A similar mechanism could be postulated with NF-IL–6, whereby associations with tax via the bZIP domain may lead to increased active NF-IL–6 dimers and upregulation of MDR1transcription, overexpression of P-gp, and development of the MDR phenotype. Although no direct protein associations between tax and the CEBP family of proteins have been reported, tax has been shown to be able to modulate granulocyte colony-stimulating factor (G-CSF) expression via a NF-IL–6 consensus sequence.49
Possible mechanisms for trans-activation of MDR1by tax. See text for detailed discussion.
Possible mechanisms for trans-activation of MDR1by tax. See text for detailed discussion.
The presence of GC-rich elements (putative SP1 binding sites) and an AP-1 like element in the MDR1 promoter are also other potential targets for tax trans-activation (Fig 6). Both of these elements have been implicated in mediating trans-activation by tax the protein. HTLV-I tax upregulates c-fos50 and HTLV-infected cells show increased AP-1 binding activity.51 AP-1 induction may be critically involved in the trans-activation of other genes, such as transforming growth factor-β1 (TGF-β1).52 Moreover, development of the MDR phenotype in mouse carcinoma cells has been associated with drug inducible increased c-fos expression,53 suggesting a possible mechanism whereby the induction of AP-1 complexes stimulate MDR1 gene expression. Hence, possible trans-activation mechanisms known to be active for tax can also be implied for the MDR1 gene, suggesting a molecular basis for the involvement of HTLV-I infection and MDR development in the resulting tumor. Studies of tax-mediated activation of MDR1 may aid in the elucidation of the complex molecular mechanisms involved in normal MDR1 gene regulation.
The possibility of drug resistance phenotypes manifesting themselves in HTLV-induced tumors seems likely based on the evidence outlined above. The obvious clinical benefits could include alternative treatments based on reversion of the MDR phenotype, such as using chemosensitizing drugs such as cyclosporin A, reserpine, tamoxifen, or verapamil11 54 or newer specific MDR inhibitors currently in clinical trials. Such compounds act as competitive inhibitors for drug binding or transport by P-gp and, if used in conjunction with cytotoxic drugs, may allow full chemotherapeutic potentials to be achieved. Ultimately, this could lead to a greatly improved prognosis for patients with HTLV-induced tumors. Moreover, an improved understanding of the mechanisms underlying control of expression of theMDR1 gene would be a very important contribution to the management and therapy of many different types of leukemia and other tumors in which this gene is believed to play a role.
ACKNOWLEDGMENT
We are grateful to the HTLV-infected patients and families who generously donated materials for this study and to K. Khazaie for plasmid pRK7-tax.
Supported by Grant No. 92/13 from the Leukaemia Research Fund (to A.J.C.) and MRC Grant No. G9622810. A.L. was the recipient of an MRC PhD studentship.
Address reprint requests to Alan J. Cann, PhD, Department of Microbiology and Immunology, University of Leicester, Leicester LE1 9HN, United Kingdom.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal