Abstract
Flavopiridol is a novel semisynthetic flavone derivative of the alkaloid rohitukine. Flavopiridol is known to inhibit potently the activity of multiple cyclin-dependent kinases. We have assessed its effects on normal and malignant cells in preclinical animal models of localized and disseminated human hematopoietic neoplasms. Flavopiridol, when administered as daily bolus intravenous (IV) injections, produced selective apoptosis of cells in the thymus, spleen, and lymph nodes, resulting in atrophy of these organs. With the exception of the intestinal crypts, apoptosis or tissue damage was absent in all other organs investigated (kidneys, liver, lungs, bone/bone marrow, muscle, and heart). Flavopiridol had a marked apoptotic effect documented by DNA nick-end labeling, or DNA agarose gels in xenografts of human hematopoietic tumors HL-60, SUDHL-4, and Nalm/6. After treatment with 7.5 mg/kg flavopiridol bolus IV or intraperitoneal on each of 5 consecutive days, 11 out of 12 advanced stage subcutaneous (s.c.) human HL-60 xenografts underwent complete regressions, and animals remained disease-free several months after one course of flavopiridol treatment. SUDHL-4 s.c. lymphomas treated with flavopiridol at 7.5 mg/kg bolus IV for 5 days underwent either major (two out of eight mice) or complete (four out of eight mice) regression, with two animals remaining disease-free for more than 60 days. The overall growth delay was 73.2%. The acquired immunodeficiency syndrome–associated lymphoma AS283 showed no significant response when flavopiridol was used in advanced s.c. tumors, but when treatment was initiated in early stages, there was a complete regression of the early tumors, and a significant overall growth delay (>84%). When flavopiridol was used in severe combined immunodeficient mice bearing disseminated human acute lymphoblastic leukemia Nalm/6 cells, there was 15-day prolongation in survival (P = .0089). We conclude that flavopiridol greatly influences apoptosis in both normal and malignant hematopoietic tissues. This activity was manifested in our study as a potent antileukemia or antilymphoma effect in human tumor xenografts, which was dose and schedule dependent. These findings provide compelling evidence for the use of flavopiridol in human hematologic malignancies.
FLAVOPIRIDOL, previously designated Behringwerke L86-8275,1,2 is a flavone synthetically derived from the plant alkaloid rohitukine isolated from the leaves and stems of Amoora rohituka,3 and later fromDysoxylum binectariferum.4 Both plants are indigenous to India, where they are widely used in traditional medicine.5-7 Initial studies documented that flavopiridol reversibly inhibited the in vitro growth of MDA468 human breast carcinoma cells, a phenomenon that was associated with arrest of cells in G1 or G2 phases of the cell cycle.2 This drug was subsequently shown to inhibit potently cyclin-dependent kinases (CDKs), a family of kinases which govern progression of cells through the cell cycle.8,9 Induction of G2 arrest by flavopiridol appears to be caused by both direct inhibition of CDK1 and interference with the regulatory phosphorylations of CDK1.10,11 The arrest of cells in G1 by flavopiridol can be related to inhibition of both CDK2 and CDK4.12 Cocrystallization studies using a des–chloro-flavopiridol and CDK213 have shown that the aromatic portion of the compound binds to the hydrophobic adenine-binding pocket of the adenosine triphosphate (ATP) site of CDK2. Studies summarized by Sedlacek et al14 have documented that higher concentrations of flavopiridol than those associated with inhibition of CDKs can also inhibit a variety of other kinases.
The antitumor activity of flavopiridol has been evaluated in vivo by Czech et al1 using a variety of human solid-tumor cell lines xenografted in the subrenal capsule and/or subcutaneous (s.c.) space of athymic nude mice. Their studies have included seven human carcinoma cell lines of the lung, five of the colon, four of the ovary, two of the breast, one of kidney, and one of the stomach, as well as one glioma and one melanoma tumor cell lines. None of the evaluated tumors underwent complete regressions, but 14 out of 21 tumor cell lines responded to flavopiridol treatment with an average growth delay of about 35% to 45%.1 More recently, Drees et al15 have shown sensitivity of both soft agar colonies and tumors in vivo from prostate cancer cells. Initial clinical studies have commenced with a 72-hour continuous infusion because of the need for frequent dosing in animal models to show cytostatic effect.16
While surveying the effect of flavopiridol in vitro on different cell types, Parker et al17 noted that certain hematopoietic cells were notably sensitive to apoptosis. To extend our experience with flavopiridol's antitumor activity and in an effort to improve the bioactivity of flavopiridol against malignant tumors in vivo, we examined the dose-limiting toxicity and antitumor effect of flavopiridol in murine hosts bearing hematopoietic human tumor xenografts. In this report, we document that flavopiridol, when administered as a daily intravenous (IV) bolus administration, produces selective apoptosis of cells of the thymus, spleen, and lymph nodes, and has marked antitumor activity in several localized or disseminated human hematologic tumor xenografts in severe combined immunodeficient (SCID) or nude mice.
MATERIALS AND METHODS
Drugs and treatment into animals.
Flavopiridol (NSC: 649890) was synthesized and supplied to us by Behringwerke AG (Marburg, Germany). For use in laboratory animals, flavopiridol was dissolved in a pyrogen-free, sterile 0.9% NaCl solution (McGaw, Ontario, Canada) containing 1% or 5% dimethyl sulfoxide (DMSO). The flavopiridol solution was sterilized by filtration using a pyrogen-free 0.22μ sterile filter. For daily injections, a freshly prepared solution was stored at 4°C. At 4°C, flavopiridol tended to precipitate, thus before injection the flavopiridol solution was warmed up at 37°C and vigorously agitated in a vortex. Flavopiridol was administered as a bolus IV or intraperitoneal (IP), or as a continuous infusion s.c. using osmotic mini-pumps (ALZA, Palo Alto, CA), as previously described in detail.18 The antibiotic cephalexin (Dista Products & Eli Lilly, Indianapolis, IN) was purchased as a pediatric oral suspension (125 mg/5 mL). Except for the animals treated by continuous infusion, all flavopiridol and control animals received 5 mL of cephalexin in 250 mL of drinking water, left at libitum, starting 24 hours before, during, and up to 48 hours after flavopiridol treatment.
Animals.
Female athymic nude NCr-nu/nu (Taconic Farm, Germantown, NY), SCID/NCr, immunocompetent C57BL/6 NCr, and CD2F1 mice (National Cancer Institute [NCI]-Animal Production Program, Frederick, MD), ages 6 to 14 weeks, were used in our studies. Animals were maintained according to the guidelines established by the National Institutes of Health.
Tumor cell lines and injection of tumor cells into animals.
The human promyelocytic leukemia HL-60, human B-cell follicular lymphoma SUDHL-4, and acquired immunodeficiency syndrome (AIDS)-related human B-cell lymphoma AS283 were obtained from the Tumor Cell Repository of the Division of Cancer Treatment, Diagnosis and Centers, NCI–Frederick Cancer Research and Development Center (Frederick, MD). The human acute lymphoblastic leukemia Nalm/619 was the generous gift of Dr Daniel H. Ryan (University of Rochester Medical Center, Rochester, NY). All cell lines were grown in RPMI-1640 medium with 10% fetal calf serum and L-glutamine, and the cells were maintained using standard tissue culture conditions. For the production of s.c. tumors, 1 × 107 cells in 0.3 mL of medium without serum were inoculated in the right flank of mice. To produce disseminated disease in SCID mice, Nalm/6 and AS283 cells were injected IV in a dose of 5 × 106 or 1 × 107 cells in 0.1 mL of medium without serum, as previously described in detail.19
Toxicologic studies.
To evaluate the effect of flavopiridol on the normal function of diverse tissues or organs, blood chemistry analyses were performed with the Abbot Vision System (Abbott Laboratories, Abbott Park, IL) and T-Stat portable analyzer (Sensor Devises, Inc, Waukesha, WI). For white cell counts, the red cells were lysed first by mixing 10 μL of blood with 440 μL of a 2% solution of acetic acid. Leukocytes were counted manually using a hemocytometer. Differential leukocyte counts were made by using standard staining with Wright and Giemsa. For histological examination of the bone marrow, the bones of the legs were decalcified, as described previously.20
Assays of immunosuppression.
Human peripheral blood lymphocytes (PBL) were obtained from different healthy donors by using standard density gradient centrifugation techniques. PBL were pretreated with different concentrations of flavopiridol for 24 hours at 37°C, washed, and assayed in their ability to incorporate 3H-methyl thymidine21after stimulation with a variety of mitogens, including phytohemagglutinin-P (PHA-P), concanavalin A (CON-A), and pokeweed mitogen (PWM), all acquired from Sigma Chemical Co (St Louis, MO). The mouse anti-human CD3 monoclonal antibody (MoAb) (clone HIT3a; Pharmingen, San Diego, CA) was used as a selective mitogen of T cells. Flavopiridol-treated PBL were stimulated with serially diluted mitogens in microtiter plate format for 72 hours, and triplicate samples for each flavopiridol and mitogen concentration were pulsed with3H-methyl thymidine (ICN Biomedicals, Inc, Costa Mesa, CA), at 1 μCi/well for the final 20 hours of the 72-hour incubation period. The degree of stimulation by mitogens in flavopiridol-treated and control cells was determined using standard scintillation counting. Appropriate controls were included in all assays. These consisted of untreated PBL (with and without mitogens) and the use of actinomycin D which inhibits replicative responses of PBL.21 22
Pharmacokinetic studies.
Pharmacokinetic studies of flavopiridol were conducted after five daily bolus IV injections of 5 mg/kg flavopiridol in male, CD2F1 mice. The dose volume was 1 μL/g body weight. After the first, third and fifth dose, groups of three mice were exsanguinated via the suborbital plexus at close intervals from 2 minutes through 8 hours after injection of flavopiridol. For 3-day continuous s.c. infusions, we used osmotic minipumps to release flavopiridol at a rate of 0.9 mg/kg/h during three consecutive days. Blood was collected and processed as above from groups of three mice, 24, 48, 72, and 96 hours after pump implantation. Aliquots (50 μL) of each plasma sample were prepared for assay by the addition of 50 μL of 0.0125 mol/L sodium borate buffer (pH 8.0), followed by extraction with 7.5 mL of t-butylmethyl ether. The organic phase was removed, evaporated to dryness using a centrifugal vacuum concentrator (Jouan, Inc, Winchester, VA). The residue was dissolved in 250 μL of mobile phase (see below), and 200 μL were analyzed by high performance liquid chromatography. The analytical system consisted of a Hewlett-Packard (Palo Alto, CA) model 1050 pump, autosampler, and ultraviolet detector with associated software for system control and spectrum analysis. The system was equipped with a stainless steel 4.6 × 150 mm column containing J'Sphere H-80 packing (YMC, Inc, Wilmington, NC). Chromatography was effected with an isocratic eluent at 1.0 mL/min using a mobile phase consisting of acetonitrile–0.05 mol/L, pH 3.0 ammonium formate buffer (25:75, vol/vol). Ultraviolet detection at a wavelength of 267 nm was used. Plasma standard curves consisting of 5 standards were prepared and processed identically to samples. For pharmacokinetic analysis, plots of flavopiridol concentration as a function of time were constructed using the geometric mean of the plasma concentrations and the mean of the time intervals for each time point.
Evaluation of antitumor response in animals with localized s.c. tumors.
Subcutaneous tumors were measured with caliper, and the weight of the animals recorded at least three times a week. Tumor weight was estimated from caliper measurements of two perpendicular dimensions of the tumor in millimeters using the formula:
To calculate the percent growth delay, the median tumor weight (MTW) for the treated group was divided by the MTW of the control group on each individual day of the experiment in which tumor weights were measured during the course of the experiment. The results were then averaged and multiplied by 100 to transform a proportion into a percent. The percent growth delay was then computed with this formula:
Complete tumor regression, represented by the disappearance of an existing measurable tumor in the animal, is reported as a ratio of the number of complete regressions observed to the total number of animals in the group. In animals injected with leukemia and lymphoma cells IV, in which tumor growth is not amenable to direct observation, blood samples were obtained at timed intervals to quantify total levels of lactic dehydrogenase (LDH) and LDH isoenzymes, as described by Arguello et al.19
Documentation of drug-induced apoptosis.
To document drug-induced apoptosis in situ, we used ApopTag In Situ Apoptosis Kit (Oncor, Gaithersburg, MD) immunostaining on paraffin sections according to the manufacturer's instructions, and DNA was also extracted from frozen tissue by homogenization for 5 minutes at 37°C, followed by DNA isolation as described by Distelhorst et al.23 Thirty micrograms of DNA was then analyzed on a 0.8% agarose gel.
Statistical analysis.
The statistical test for growth delay was accomplished as follows. Each treatment condition in a group of experiments was compared with its appropriate control group by taking the ratio of the MTW of the treated group to the MTW of the control group on a particular day, and comparing over all days how the ratios of the treated to control median tumor weights differ from 100%. Under the null hypothesis of no growth delay, this random variable has the expectation of unity across the days of the experiment. A one sample t-test was performed to determine whether the percent growth delay was significant.
RESULTS
Effect of flavopiridol on normal cells and tissues.
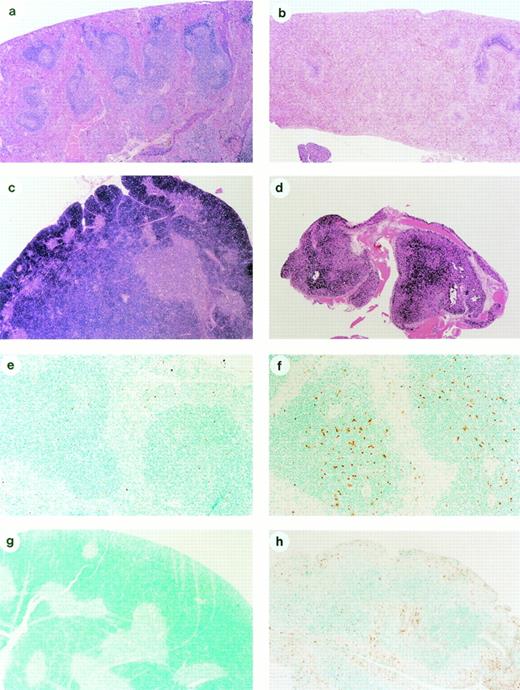
Histological evaluation of different organs (heart, lung, kidney, spleen, thymus, bone/bone marrow, and small intestines) of immunocompetent C57BL/6 mice treated with the maximal tolerable dose (MTD) in this mouse species (10 mg/kg bolus IV for 4 consecutive days) showed a series of abnormalities when compared with control nontreated animals. The spleen of flavopiridol-treated mice were smaller, with markedly diminished numbers of lymphocytes in white pulp areas, and follicular centers were completely absent (Fig 1a and b). The thymus in the treated animals also showed a marked depletion of lymphoid cells, and the organ was about 10% to 20% the size of that of control animals by the fourth day of flavopiridol treatment (Fig 1c and d). ApopTag immunohistological analysis of the thymus and spleen from mice treated with flavopiridol showed increased apoptosis of lymphoid cells in these organs (Fig 1e through h). Peripheral lymph nodes in the intestines (Peyer's patches) were significantly depleted of lymphoid cells and the follicular centers were also absent, and some apoptotic epithelial cells were seen in the intestinal crypts (data not shown). These intestinal changes were identical to that described in patients with severe immunodeficiencies, grade I acute graft versus host disease, or T-cell defects.24 25 None of the vehicle-treated control animals had these lesions.
Effect of flavopiridol on normal cells and tissues. (a) Histological section (10×) of the spleen of an untreated immunocompetent C57BL/6 mouse, showing a rich population of lymphocytes forming the germinal centers and marginal zone of the folliculi of the white pulp, surrounded by the blood-filled sinusoids of the red pulp. (b) Histological section (10×) of the spleen of an immunocompetent mouse 96 hours after initiation of treatment with daily IV bolus injection of flavopiridol, showing a marked depletion of lymphocytes, and only remnants of the white pulp. (c) Histological section of the thymus (10×) of a nontreated immunocompetent mouse showing the densely populated cortex by lymphocytes, surrounding the medullary areas of the lobules. (d) Histological section of a thymus (10×) of a flavopiridol-treated mouse showing an atrophic thymus, in which most lymphoid areas have disappeared. (e) ApopTag immunohistochemistry of the spleen (50×) of a nontreated mouse showing the rare presence of apoptotic brown-stained cells. (f) ApopTag immunohistochemistry of the spleen (50×) of a mouse 48 hours after initiation of treatment with flavopiridol, showing multiple brown-stained apoptotic lymphocytes in the follicular centers of the white pulp. (g) ApopTag immunostaining of the thymus (10×) of a nontreated immunocompetent mouse showing the lack of apoptosis in the lymphocyte-formed cortex. (h) ApopTag immunostaining of the thymus (10×) of a flavopiridol-treated mouse, showing a brown-stained, atrophic cortex caused by the death of thymocytes through apoptosis.
Effect of flavopiridol on normal cells and tissues. (a) Histological section (10×) of the spleen of an untreated immunocompetent C57BL/6 mouse, showing a rich population of lymphocytes forming the germinal centers and marginal zone of the folliculi of the white pulp, surrounded by the blood-filled sinusoids of the red pulp. (b) Histological section (10×) of the spleen of an immunocompetent mouse 96 hours after initiation of treatment with daily IV bolus injection of flavopiridol, showing a marked depletion of lymphocytes, and only remnants of the white pulp. (c) Histological section of the thymus (10×) of a nontreated immunocompetent mouse showing the densely populated cortex by lymphocytes, surrounding the medullary areas of the lobules. (d) Histological section of a thymus (10×) of a flavopiridol-treated mouse showing an atrophic thymus, in which most lymphoid areas have disappeared. (e) ApopTag immunohistochemistry of the spleen (50×) of a nontreated mouse showing the rare presence of apoptotic brown-stained cells. (f) ApopTag immunohistochemistry of the spleen (50×) of a mouse 48 hours after initiation of treatment with flavopiridol, showing multiple brown-stained apoptotic lymphocytes in the follicular centers of the white pulp. (g) ApopTag immunostaining of the thymus (10×) of a nontreated immunocompetent mouse showing the lack of apoptosis in the lymphocyte-formed cortex. (h) ApopTag immunostaining of the thymus (10×) of a flavopiridol-treated mouse, showing a brown-stained, atrophic cortex caused by the death of thymocytes through apoptosis.
A dose-related leukopenia was also observed. Peripheral leukocyte counts in three immunocompetent C57BL/6 mice treated with 5 mg/kg IV during 5 consecutive days developed a moderate leukopenia (average, 5,512/μL; n = 3; day 4), when compared with their leukocyte counts before initiation of treatment (average, 12,599/μL). When other C57BL/6 mice were treated with 10 mg/kg for three consecutive days, the leukopenia was more pronounced (average, 2,054/μL; n = 3; day 3), as compared with leukocyte counts before treatment (average, 9,506/μL). However, 24 hours after cessation of treatment, there was a rebound in the number of leukocyte counts to 19,000/μL average (∼50% neutrophils). Thus, persistent leukopenia is not a feature of flavopiridol action in mice. The bone marrow sections of femurs and tibias examined from two out of three flavopiridol-treated mice were not significantly different from controls, whereas one mouse showed depletion of both red and white cell elements.
Despite extensive blood chemistry analyses to evaluate a variety of physiological functions, no abnormalities were observed, even when immunodeficient nude mice were treated with lethal doses of flavopiridol at 10 mg/kg bolus IV daily for 3 or more consecutive days. However, histological examination of some organs showed bacterial colonies. This prompted us to examine immunocompetent C57BL/6 mice treated with a high dose of flavopiridol bolus IV (10 mg/kg/d during 7 days) for evidence of sepsis, while we tested in another group of mice whether the prophylactic use of a broad-spectrum antibiotic, cephalexin, would prevent the suspected septicemia. We found that four out of five mice treated with flavopiridol alone developed mixed positive hemocultures with Escherichia coli, Staphylococcus aureus, and S saprophyticus, whereas only one out of five mice treated with flavopiridol plus cephalexin developed bacteremia by E coli. After that study, all animals treated with flavopiridol and their respective control mice received cephalexin in drinking water starting 24 hours before initiation of treatment with flavopiridol, and ending 48 hours after the last day of IV flavopiridol treatment. The prophylactic use of cephalexin allowed us to increase our previous MTD in nude mice of 5 mg/kg bolus IV during 5 consecutive days to 7.5 mg/kg IV for 5 days.
Immunosuppressive effects of flavopiridol.
The prominent effect of flavopiridol on lymphoid cell elements in normal, nontumored animals (Fig 1), raised the possibility that the drug could cause defects in immune cell function in response to antigenic stimulation. Thus, we assessed the potential for flavopiridol to interdict mitogen-stimulated lymphocyte proliferation. Substantial decreases in thymidine incorporation were observed in flavopiridol-pretreated PBL incubated with T-specific mitogens, PHA and CON-A, and CD3 MoAbs, as well as B-specific mitogen, PWM (Table1). Table 1 also shows that this suppressive effect is dose dependent. The threshold inhibitory flavopiridol concentration was shown within the range of 100 to 250 nmol/L in PBL, in which cell viability remained above 80%. At these concentrations, previous studies2 have shown little effect on 3[H] thymidine incorporation into exponentially growing cells until cell cycle arrest is established.
Thymidine Incorporation of Flavopiridol-Treated Human Lymphocytes After Mitogen Stimulation in Vitro
| Pretreatment . | Stimulation Indices From 3H-Methyl Thymidine Incorporation According to the Mitogen Used . | |||
|---|---|---|---|---|
| CON-A 1.56 μg/mL . | PHA-P 1.56 μg/mL . | PWM 1.56 μg/mL . | Anti-CD3 10 ng/mL . | |
| Flavopiridol (nmol/L) | ||||
| 0 | 252.2 | 155.8 | 86.4 | 35.3 |
| 100 | 68.1 | 89.9 | 23.2 | −0.7 |
| 250 | 1.0 | 4.7 | 2.8 | −0.9 |
| 500 | −0.3 | −0.4 | 0.1 | −0.8 |
| Actinomycin D (μg/mL) | ||||
| 10 | −0.5 | −0.1 | −0.1 | −0.9 |
| Pretreatment . | Stimulation Indices From 3H-Methyl Thymidine Incorporation According to the Mitogen Used . | |||
|---|---|---|---|---|
| CON-A 1.56 μg/mL . | PHA-P 1.56 μg/mL . | PWM 1.56 μg/mL . | Anti-CD3 10 ng/mL . | |
| Flavopiridol (nmol/L) | ||||
| 0 | 252.2 | 155.8 | 86.4 | 35.3 |
| 100 | 68.1 | 89.9 | 23.2 | −0.7 |
| 250 | 1.0 | 4.7 | 2.8 | −0.9 |
| 500 | −0.3 | −0.4 | 0.1 | −0.8 |
| Actinomycin D (μg/mL) | ||||
| 10 | −0.5 | −0.1 | −0.1 | −0.9 |
Antitumor effect of flavopiridol in immunodeficient nude and SCID mice bearing localized (s.c.) human lymphohematopoietic tumors.
Preliminary studies in our lab had shown that 5 mg/kg bolus IV during 5 days, without antibiotics, was the MTD of flavopiridol in nude mice. This MTD produced complete regressions of large s.c. HL-60 xenografts (five out of five) which lasted for about 15 days in three animals, and two remained disease-free for months (data not shown). Table2 summarizes the treatment of several xenograft models of mice bearing large s.c. HL-60 tumors with 7.5 mg/kg/d × 5 by IV injection plus the prophylactic use of cephalexin. This treatment resulted, except for one animal, in complete regressions. When the same dose was given IP, all six tumors also underwent complete regressions. All animals with complete regressions have remained tumor-free for more than 90 days. On the other hand, s.c. SUDHL-4 lymphoma treated with flavopiridol at 7.5 mg/kg bolus IV for 5 days plus antibiotic underwent either 50% (two out of eight mice) or 100% (four out of eight mice) regression, but only two animals remained disease-free for more than 60 days, with an overall growth delay of greater than 73.2%. The AIDS-related AS283 lymphoma did not experience tumor regressions when flavopiridol was used in large s.c. tumors, other than an evident growth delay of 45.8%. However, when the treatment was implemented in early stages (tumors ∼2 to 4 mm in diameter), there was a complete regression of the early tumors and a significant growth delay of greater than 84%. In experiments not shown, we treated athymic mice bearing human HCT15 colon and U251 glioma tumors with the same regimen which caused prominent responses in hematopoietic tumors. No tumor regressions were observed; however, there was a modest (37.8% to 44.7%) growth delay, concordant with prior experience with flavopiridol in solid tumor xenografts.
Effect of Flavopiridol on s.c. Human Leukemia and Lymphoma Xenografts
| Human Cell Line . | Mouse Strain . | Flavopiridol Treatment (days of treatment) . | 100% Tumor Regressions (50% regression) . | Tumor- Free >3 months . | Growth Delay (prob. value) . | Day Euthanasia (no. of mice) . |
|---|---|---|---|---|---|---|
| HL-60 Promyelocytic Leukemia | nu/nu | Sham controls 1% DMSO | 0/6 (0/6) | 0/6 | — | 20 (6/6) |
| 20.6 mg/kg/d 72-h continuous infusion (days 10-13) | 0/6 (0/6) | 0/6 | 20.5% (P = .0248) | 20 (6/6) | ||
| 10.8 mg/kg/d 72-h continuous infusion (days 10-13) | 0/6 (0/6) | 0/6 | 17.2% (P = .0014) | 20 (6/6) | ||
| Sham controls 1% DMSO | 0/5 (0/5) | 0/5 | 21 (4/5) | |||
| 7.5 mg/kg/d bolus IV × 5 (days 13-17) | 4/5 (1/5) | 4/5 | N/A | >90 (5/6) | ||
| 7.5 mg/kg/d IP × 5 (days 13-17) | 6/6 (N/A) | 6/6 | N/A | >90 (6/6) | ||
| SUDHL-4 Follicular Lymphoma | SCID | Sham controls 1% DMSO | 0/5 (0/5) | 0/5 | 38 (5/5) | |
| 7.5 mg/kg/d bolus IV × 5 (2 cycles) (days 25-30), and 38-42) | 4/8 (2/8) | 2/8 | >73% (P = <.0001) | 52 (8/8) | ||
| AS283 AIDS-Associated Lymphoma | SCID | Sham control 1% DMSO | N/A Early tumor | 0/5 | 32 (5/5) | |
| 7.5 mg/kg/d bolus IV × 5 (2 cycles) (days 3-8, and 18-22) | N/A Early tumor | 0/7 | >84% (P = <.0001) | 32 (5/7) | ||
| Sham control 1% DMSO | 0/5 | 0/5 | 20 (5/5) | |||
| 7.5 mg/kg/d bolus IP × 5 (days 13-17) | 1/6 (2/6) | 0/6 | 45.8% (P = .013) | 20 (6/6) |
| Human Cell Line . | Mouse Strain . | Flavopiridol Treatment (days of treatment) . | 100% Tumor Regressions (50% regression) . | Tumor- Free >3 months . | Growth Delay (prob. value) . | Day Euthanasia (no. of mice) . |
|---|---|---|---|---|---|---|
| HL-60 Promyelocytic Leukemia | nu/nu | Sham controls 1% DMSO | 0/6 (0/6) | 0/6 | — | 20 (6/6) |
| 20.6 mg/kg/d 72-h continuous infusion (days 10-13) | 0/6 (0/6) | 0/6 | 20.5% (P = .0248) | 20 (6/6) | ||
| 10.8 mg/kg/d 72-h continuous infusion (days 10-13) | 0/6 (0/6) | 0/6 | 17.2% (P = .0014) | 20 (6/6) | ||
| Sham controls 1% DMSO | 0/5 (0/5) | 0/5 | 21 (4/5) | |||
| 7.5 mg/kg/d bolus IV × 5 (days 13-17) | 4/5 (1/5) | 4/5 | N/A | >90 (5/6) | ||
| 7.5 mg/kg/d IP × 5 (days 13-17) | 6/6 (N/A) | 6/6 | N/A | >90 (6/6) | ||
| SUDHL-4 Follicular Lymphoma | SCID | Sham controls 1% DMSO | 0/5 (0/5) | 0/5 | 38 (5/5) | |
| 7.5 mg/kg/d bolus IV × 5 (2 cycles) (days 25-30), and 38-42) | 4/8 (2/8) | 2/8 | >73% (P = <.0001) | 52 (8/8) | ||
| AS283 AIDS-Associated Lymphoma | SCID | Sham control 1% DMSO | N/A Early tumor | 0/5 | 32 (5/5) | |
| 7.5 mg/kg/d bolus IV × 5 (2 cycles) (days 3-8, and 18-22) | N/A Early tumor | 0/7 | >84% (P = <.0001) | 32 (5/7) | ||
| Sham control 1% DMSO | 0/5 | 0/5 | 20 (5/5) | |||
| 7.5 mg/kg/d bolus IP × 5 (days 13-17) | 1/6 (2/6) | 0/6 | 45.8% (P = .013) | 20 (6/6) |
Abbreviation: N/A, not applicable.
To mimic schedules used in initial clinical trials with flavopiridol, in which the drug is administered by continuous infusion during 72 hours,16 we implanted s.c. 3-day continuous infusion osmotic pumps in mice bearing the flavopiridol-sensitive cell line HL-60. We found that a 72-hour continuous infusion of flavopiridol at doses as high as 0.9 mg/kg/h (61.4 mg/kg/72 h) had at best very modest effects on the HL-60 tumors.
Flavopiridol-mediated apoptosis of tumor cells.
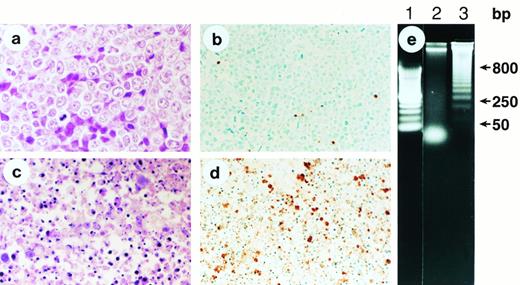
Recent in vitro studies have shown that flavopiridol has the ability to induce apoptosis of human lymphoma cells.17 To examine whether apoptosis could be shown in tumors after treatment in vivo with flavopiridol, we removed s.c. HL-60 tumors after treatment with flavopiridol at 7.5 mg/kg IV every other day × 4. Immunohistochemistry staining for apoptotic cells in situ showed by 96 hours evidence of extensive apoptosis (Fig 2a through d), which was first evident by 24 hours (data not shown). A DNA “ladder” was observed in SUDHL-4 tumors 72 hours after initiation of treatment (Fig2e). Of interest, AS283 tumors, which did not show evidence of persistent regressions (only growth delays), did not show evidence of extensive apoptosis by immunohistochemistry nor agarose gels, despite the obvious reduction in tumor mass (data not shown).
Apoptotic death of human promyelocytic leukemia HL-60 cells in vivo after flavopiridol therapy. (a) Histological section of a s.c. HL-60 tumor (100×) removed from a nontreated control nude mouse, showing a large number of predominantly viable and dividing malignant blasts. (b) ApopTag immunostaining of the same HL-60 tumor as (a), showing the sporadic presence of brown-stained cells indicative of rare spontaneous apoptosis. (c) Histological section of an HL-60 tumor (100×) 96 hours after initiation of treatment with daily IV bolus injection of flavopiridol, showing multiple fragments of condensed chromatin (“apoptotic bodies”) indicative of cell death through apoptosis. (d) ApopTag immunostaining of an HL-60 tumor 96 hours after initiation of treatment with bolus IV flavopiridol. Multiple cells densely stained brown indicate DNA fragmentation. (e) Agarose gel analysis of DNA isolated from SUDHL-4 nontreated lymphoma (lane 2), and DNA isolated from a tumor 72 hours after initiation of treatment with flavopiridol (lane 3), which shows the typical “ladder” pattern of internucleosomal cleavage of DNA into multiples of 180 to 250 bp. Lane 1 includes DNA molecular mass markers.
Apoptotic death of human promyelocytic leukemia HL-60 cells in vivo after flavopiridol therapy. (a) Histological section of a s.c. HL-60 tumor (100×) removed from a nontreated control nude mouse, showing a large number of predominantly viable and dividing malignant blasts. (b) ApopTag immunostaining of the same HL-60 tumor as (a), showing the sporadic presence of brown-stained cells indicative of rare spontaneous apoptosis. (c) Histological section of an HL-60 tumor (100×) 96 hours after initiation of treatment with daily IV bolus injection of flavopiridol, showing multiple fragments of condensed chromatin (“apoptotic bodies”) indicative of cell death through apoptosis. (d) ApopTag immunostaining of an HL-60 tumor 96 hours after initiation of treatment with bolus IV flavopiridol. Multiple cells densely stained brown indicate DNA fragmentation. (e) Agarose gel analysis of DNA isolated from SUDHL-4 nontreated lymphoma (lane 2), and DNA isolated from a tumor 72 hours after initiation of treatment with flavopiridol (lane 3), which shows the typical “ladder” pattern of internucleosomal cleavage of DNA into multiples of 180 to 250 bp. Lane 1 includes DNA molecular mass markers.
Antitumor effect of flavopiridol in SCID mice bearing disseminated human lymphohematopoietic tumors.
Because localized s.c. tumors do not really reflect the systemic nature of human leukemia, or advanced stages of lymphomas in humans, SCID mice received human acute lymphoblastic leukemia (ALL) Nalm/6 cells, or AS283 human lymphoma cells IV to produce systemic disease.19 When flavopiridol was used in SCID mice bearing disseminated human ALL Nalm/6 cells, at a dose of 7.5 mg/kg bolus IV during five consecutive days from days 3 to 7 after tumor cell injection, and repeated again at days 17 to 21, there was 15-day prolongation in survival (P = .0089) (Fig3a). Also, serum levels of total LDH in nontreated mice increased progressively over time, reaching total LDH levels as high as 43,000 U/L, whereas LDH in mice treated with flavopiridol remained within the basal levels (∼2,000 U/L) for almost the same 30-day period. We have recently shown that serum levels of total LDH, human-specific LDH isoenzymes, and NMP 41/7 are highly reliable serum markers to monitor the progression of human leukemias in SCID mice.19 An enhanced survival was also seen in SCID mice bearing systemic AIDS-related lymphoma AS283. Whereas control nontreated animals developed paralysis approximately 25 days after injection of AS283 cells because of the involvement of the central nervous system (meningeal infiltration), animals treated with flavopiridol from days 3 to 7 after tumor cell injection had an improvement in survival, as compared with controls (P = .0027) (Fig 3b). The SUDHL-4 did not produce systemic disease in SCID mice after IV injection of 107 cells/mouse, and HL-60 produced diseases in a very unpredictable fashion; thus, these cell lines could not be used as models of human systemic disease.
Effect of flavopiridol therapy in systemic leukemia and lymphoma xenografts. (a) Kaplan and Meier survival curve of SCID mice bearing systemic human acute lymphoblastic leukemia (Nalm/6) treated with flavopiridol 7.5 mg/kg IV every other day × 5 at days 3 to 7, and repeated again at days 17 to 21 posttransplantation of cells (•). Control mice (▪) received the vehicle 1% DMSO in NaCl. (b) Kaplan and Meier survival curve of SCID mice bearing systemic human AS283 human lymphoma after one 5-day cycle of flavopiridol bolus IV therapy from days 3 to 7 posttransplantation of cells (•). Control mice received the vehicle (▪).
Effect of flavopiridol therapy in systemic leukemia and lymphoma xenografts. (a) Kaplan and Meier survival curve of SCID mice bearing systemic human acute lymphoblastic leukemia (Nalm/6) treated with flavopiridol 7.5 mg/kg IV every other day × 5 at days 3 to 7, and repeated again at days 17 to 21 posttransplantation of cells (•). Control mice (▪) received the vehicle 1% DMSO in NaCl. (b) Kaplan and Meier survival curve of SCID mice bearing systemic human AS283 human lymphoma after one 5-day cycle of flavopiridol bolus IV therapy from days 3 to 7 posttransplantation of cells (•). Control mice received the vehicle (▪).
Pharmacokinetic studies.
To correlate the pharmacological behavior of flavopiridol with the occurrence of drug-induced apoptosis, we studied nontumored mice treated in an identical fashion to animals with s.c. or disseminated tumors. There was no significant difference in the plasma concentration-time profiles obtained after 1, 3, or 5 daily injections of 5 mg/kg flavopiridol, so the groups were combined for the purpose of pharmacokinetic analysis. After IV injection of flavopiridol, plasma concentrations declined in a biexponential manner from maximum levels after 2 minutes of approximately 7 μmol/L to approximately 0.1 μmol/L after 8 hours (Fig 4). The half-life for the initial phase was 18 minutes, and for the terminal phase (biological half-life) was 158 minutes. A comparison of the apparent volumes of distribution for the central compartment (1.4 L/kg) and for the whole body (7.1 L/kg) suggest that flavopiridol is highly distributed into the tissues. The plasma clearance was 31 mL/min/kg. In our s.c. infusion studies, of the 12 plasma samples collected between 24 and 96 hours from mice receiving 0.9 mg/kg/h flavopiridol, the drug was not detectable in four (2, 24-hour; 1, 48-hour; 1, 72-hour; and 1, 96-hour samples), suggesting possible pump failure. The remaining seven samples had a mean flavopiridol concentration of 427 nmol/L (range, 175 to 907 nmol/L).
Plasma concentrations of flavopiridol observed in combined groups receiving 1, 3, and 5 daily IV injections of 5 mg/kg in mice. The experimental data points (•), representing the geometric mean of the assayed plasma concentrations for each time point, and the best-fit curve generated by nonlinear regression analysis, are shown.
Plasma concentrations of flavopiridol observed in combined groups receiving 1, 3, and 5 daily IV injections of 5 mg/kg in mice. The experimental data points (•), representing the geometric mean of the assayed plasma concentrations for each time point, and the best-fit curve generated by nonlinear regression analysis, are shown.
DISCUSSION
We have found that flavopiridol has a marked proapoptotic effect on normal lymphoid organs, such as spleen, thymus, and intestinal lymphoid tissues, when administered into animals using a daily bolus IV or IP schedule. With the exception of the intestinal crypt mucosa, there was no evidence of apoptosis or tissue damage in several other nonlymphoid organs studied. Similarly, good antitumor activity characterized as tumor regression with cell apoptosis was observed in human tumors of lymphohematopoietic origin. These include HL-60 and SUDHL-4 s.c. xenografts, and Nalm/6 and AS283 disseminated disease models. This effect correlated with exposure of the animals to transient plasma peak concentrations of the drug of 7 to 9 μmol/L over each of five days. Evidence of impaired immunologic response to several mitogens on the part of flavopiridol-treated human lymphocytes has also been documented.
The ability of a drug to selectively affect cells of a certain lineage is determined by many factors, including selective binding/uptake of the drug, increased or specific presence of the drug's molecular target, and/or intrinsic metabolic and detoxifying systems present in the cells. Flavonoid compounds have been previously shown to interact with normal and malignant hematopoietic cells,26-29 through apparent specific binding to nuclear “receptors” referenced as type II estrogen binding sites (type II EBS).30,31 Larocca et al27,28 found that all blast cells from seven patients with ALL and 16 with acute myeloblastic leukemia expressed variable amounts of type II EBS, ranging between 3,109 and 239,450 sites/cell. They also discovered that the growth of these cells could be inhibited by compounds that interact with type II EBS, mainly estrogens and plant flavonoids.26-29 The relation of type II EBS as potential flavonoid receptors to protein kinases may be of interest to consider. Although flavopiridol most potently affects CDKs,10-12 at the concentrations achieved here many other protein kinases, eg, protein kinase C,14 may be affected. Other targets, especially those with nucleotide binding sites, may also exist, but these have yet to be defined.
The organs affected by flavopiridol, eg, spleen, lymph nodes, and thymus, are formed predominantly by lymphoid cells in resting, G0 phase, unless they are stimulated by mitogens.32-34 Thus, induction of apoptosis in these populations indicate a capacity of the drug to affect noncyclin cells of certain lineage. Similar observations were recently made by Bible and Kaufmann,35 who found that confluent noncycling A549 human lung carcinoma, as well as cells A549 arrested in G1 after aphidicolin treatment, could be killed with high doses of flavopiridol (eg, >500 nmol/L). The ability of flavopiridol to kill nondividing cells raises the following possibilities: (1) Flavopiridol at high concentrations may have an additional molecular target(s), other than CDKs. This possibility is reinforced by the capacity of the drug to inhibit other kinases when used at concentrations greater than 1 μmol/L.14 (2) CDKs may have important unsuspected cell functions in resting cells. For example, Perkins et al36have recently shown that CDK is a component of the p300-associated regulatory apparatus that regulates the transcriptional activation of nuclear factor kappaB, a factor that is responsive to specific cytokines and stress and is often activated in association with cell damage and growth cell arrest in eukaryotes. Nevertheless, future experiments must address the basis for flavopiridol-induced apoptosis of normal and malignant hematopoietic cells.
We have also observed that apoptosis was readily apparent in some tumors, eg, HL-60, SUDHL-4, or Nalm/6, whereas the partial tumor regression of AS283 lymphoma tumors occurred without evidence of apoptosis (DNA fragmentation). Similar observations were made in vitro by Bible and Kaufmann.35 They found that cultured A549 human lung carcinoma cells, in contrast to HL-60 cells, at 72 hours after exposure to greater than 300 nmol/L flavopiridol died without the classic changes of apoptosis. However, they pointed out that other well-established apoptotic agents, eg, topotecan and etoposide, also failed to induce apoptosis of A549 cells. These studies highlight the fact that after a cytocidal stimulus, the occurrence of apoptosis seems to be determined not by universally applicable actions of the drug, but by response properties of the cells, particularly their ability to engage in “programmed cell death.” Others have pointed out that it may not be coincidence that chemotherapy in humans has been successful in tumors which have risen from the types of cells that can readily die by apoptosis, eg, hematopoietic and germ cells.37 Flavopiridol may be uniquely suited to trigger this process in certain cell types.
We found that continuous infusion of flavopiridol for 3 days resulted in plasma levels (average, 427 nmol/L) that exceed the in vitro IC50 reported for most human tumor cells tested (20 nmol/L to 200 nmol/L), including those in the NCI's 60-cell line in vitro screen panel.14 However, this concentration resulted in very modest antitumor effect in animals bearing HL-60. The best antitumor effect in xenografted animals was observed after daily bolus IV or IP administration of flavopiridol that resulted in peak plasma levels of about 7 μmol/L, followed by a progressive decline to approximately 100 nmol/L in 8 hours. Thus, relatively short-lived, but repetitive high plasma levels of flavopiridol in the μmol/L range seem to be an effective way to produce the maximum antitumor effect with flavopiridol. Thus, efforts to achieve this concentration versus time relationship in clinical trials should be pursued. Current clinical trials with flavopiridol have consistently achieved concentrations in the range of 500 to 1,000 nmol/L with a continuous infusion scheme.16 The apparent need for high levels of flavopiridol in vivo, as compared with those required in vitro, may be dependent on a variety of factors, including high concentrations of competing ATP in vivo, binding proteins in blood and tissues, rapid metabolism into inactive compounds, presence of pharmacological barriers, among other factors.38
Our preliminary studies indicate that flavopiridol has the potential for immunosuppressive activity. Human lymphocytes pretreated in vitro with flavopiridol were unable to respond to well-established mitogens (Con-A, PHA-P, PWM, and anti-CD3 antibodies), as evidenced in this study by their inability to incorporate radioactive thymidine. Indeed, the results of our preliminary immunologic studies suggest a broad immunosuppressive effect on both T and B lymphocytes by flavopiridol. The recently concluded Phase I trial of flavopiridol16 did not suggest neutropenia as a noted side effect at the maximal tolerated dose. However, that trial used continuous infusion and did not achieve the transient high drug concentrations seen here. Our results raise the possibility that flavopiridol may have immunosuppressive activity in humans when administered in an IV bolus schedule.
The potential use of flavopiridol in the treatment of human hematologic tumors is clear. Flavopiridol has the ability to produce cure of animals bearing large HL-60 tumor masses. Also, SUDHL-4 or AIDS-related lymphoma AS283 tumors underwent transient, but complete regressions, and/or had a growth delay of greater than 70% after flavopiridol treatment. Flavopiridol also produced a substantial and statistically significant prolongation in survival of SCID mice bearing disseminated AIDS-related lymphoma AS283 and human ALL Nalm/6 cells. We believe that the results obtained in these preclinical studies using athymic nude and SCID mice bearing human leukemia and lymphoma xenografts may predict the therapeutic potential of flavopiridol in humans with hematologic malignancies.
NOTE ADDED IN PROOF
Quantitation of lymphocytes in results from the recently concluded Phase I trial in humans with a 72-hour infusion does suggest dose-related lymphopenia (A. Senderowicz, unpublished results), but not neutropenia.
ACKNOWLEDGMENT
The authors express their gratitude to Drs Adrian M. Senderowicz, Alan D. Harmon, Alan J. Bitonti, Jennifer A. Dumont, and Barry M. Markaverich for their helpful discussions and suggestions during the performance of this study. Our special thanks to Salvatore Gangliano for his assistance in the measurement of total LDH.
Address reprint requests to Edward A. Sausville, MD, PhD, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, EPN/843 MSC 7458, 6130 Executive Blvd, Rockville, MD 20852.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal