Current data support the notion that the thymus is seeded by a yet uncommitted progenitor cell able to generate T cells, B cells, natural killer (NK) cells, and dendritic cells (DCs). We assess in this report the developmental relationship of DCs and NK cells derived from a small subset of CD34+ human postnatal thymocytes that, like the earliest precursors in the fetal thymus, display low CD33 surface expression. Culture of these isolated CD34+CD33lo thymic progenitors with a mixture of cytokines, including interleukin-7 (IL-7), IL-1α, IL-6, granulocyte-macrophage colony-stimulating factor, and stem cell factor, results in predominant generation of DCs. However, the addition of IL-2 to the cytokine mixture leads to the simultaneous development of DCs and NK cells. Both developmental pathways progress through a transient population of CD34+CD44brightCD5lo/−CD33+ large-sized cells, distinct from small-sized T-lineage precursors, that contain bipotential NK/DC progenitors. These data provide evidence of linked pathways of NK cell and DC development from intrathymic precursors and suggest that NK cells and DCs branch off the T lineage through a common intermediate progenitor.
ALL LYMPHOID CELL types derive, ultimately, from pluripotent hematopoietic stem cells (HSCs) that reside in the liver during embryonic development and then in the bone marrow during the postnatal life.1 A major issue is whether commitment of HSCs into a given lymphoid lineage is an abrupt process or whether there exist oligopotent intermediate progenitors common to all lymphoid lineages, but no longer able to generate myeloid, erythroid, or megakaryocytic cells.2,3 Although the existence of a common lymphoid progenitor has been postulated for years,4 only very recently has formal proof been provided by Galy et al5 that such a cell type does exist in the human bone marrow. This cell population represents a phenotypically defined subset of CD34+ bone marrow cells (CD34+CD10+CD45RA+CD38+HLA-DR+) that is distinct from CD34+ HSCs and has a unique differentiation potential restricted to the production of all lymphoid cells (T, B, and natural killer [NK] cells) and also of dendritic cells (DCs). The identification of such a common lymphoid/DC progenitor challenges current views on the myeloid lineage affiliation of most DCs6-8 and supports the notion that the DC lineage, or at least a particular lineage of DCs, is developmentally more closely related to lymphoid cells than to myeloid cells.
This appears to be the case for the so-called thymic DCs. In fact, the earliest mouse intrathymic precursors that are capable of forming T, B, and NK cells also serve as precursors of DCs on transfer to irradiated recipients, without concomitant formation of erythroid or myeloid cells.9-13 This situation can be extended to humans, in whom equivalent lymphoid/DC progenitors have been identified both in the fetal and in the postnatal thymus and have also been found in the fetal liver.14-17 As a whole, the hematopoietic developmental potential of the earliest intrathymic progenitor pool matches that of the lymphoid/DC-restricted progenitor identified in the bone marrow, suggesting that such a oligopotent precursor subset might include the initial cells that migrate out of bone marrow to seed the thymus.3 18
Little is known about the critical points of lineage decision leading such intrathymic lymphoid/DC precursors to commit to each alternative (T, B, NK, and dendritic) lymphoid cell lineage. Intrathymic precursors in mice had shown a sequential rather than simultaneous loss of other developmental potentials en route to T cells, suggesting that non–T-cell lineages stem from distinct branch points in the differentiation pathway of a common progenitor. Particularly, it has been shown that the capacity to form B cells is lost in the murine thymus in a T-cell precursor subset that still retains a full potential to generate DCs19 and also forms NK cells, albeit with lower efficiency than its immediate progenitor,13indicating that this precursor population must be tripotential.18 In addition, a formal clonal proof has been provided that human T cells and NK cells share a common intrathymic precursor,15 leading to the current view that the branching of NK and T cells occurs downstream of the divergence of DCs.20 However, it is not known whether such a T/NK bipotential progenitor can also derive DCs; therefore, no formal evidence has been produced that DCs branch off the main T-cell developmental pathway before NK cells. We now assess the developmental relationship of thymic DCs and NK cells derived from the most immature CD34+CD33lo human thymocyte precursors. Our data provide evidence of linked pathways of NK cell and DC development from intrathymic precursors in vitro and suggest that NK cells and DCs branch off the T lineage through a common intermediate bipotential progenitor.
MATERIALS AND METHODS
Isolation of subpopulations of human postnatal thymocytes.
Normal human postnatal thymocytes were isolated from thymus fragments removed during corrective cardiac surgery of patients aged 1 month to 4 years.14 Thymocyte suspensions were enriched in immature thymocytes by using the sheep red blood cell (SRBC) rosetting technique described by Poggi et al.21 Recovered cells (1% to 2% of cellular input, 30% to 60% CD34+) were depleted of B, myeloid, and NK cells by treatment with anti-CD19–coated magnetic beads (Dynabeads; Dynal, Oslo, Norway) and anti-CD14 and anti-CD56 (Becton Dickinson & Co, San José, CA) monoclonal antibodies (MoAbs) bound to sheep antimouse Ig-coated magnetic beads (Dynal). To isolate CD34+CD1− thymocytes, CD1+ cells were first removed by treatment with anti-CD1a (OKT6, CRL8020; American Type Culture Collection [ATCC], Gaithersburg, MD) MoAbs bound to magnetic beads (Dynal) and then cells expressing intermediate to bright levels of CD34 (CD34int-bright) were positively isolated with the Dynal CD34 Progenitor Cell Selection System following the manufacturer's instructions. The isolated cell preparation (0.1% to 0.25% of total thymocytes) was highly enriched in CD34+CD1− thymocytes (>98%). Twenty percent to 30% of these cells expressed low levels of CD33. These CD34+CD1−CD33lo cells were then isolated by cell sorting (see below). In some experiments, thymocyte preparations depleted of B, myeloid, and NK cells (as described above) were also depleted of mature T cells by treatment with anti-CD3– and anti-CD8–coated magnetic beads (Dynal). Such lin− (CD19−, CD14−, CD56−, CD3−) thymocytes were finally treated with the CD34 Progenitor Cell Selection System (Dynal), without previous treatment with anti-CD1a. The remaining cellular population was shown to include two distict subsets of thymocytes expressing low CD34 levels, one CD44brightCD5lo/−CD33+ and the other CD44loCD5+CD33−. Sheep defibrinated erythrocytes in Alsever solution were purchased from Unipath Ltd (Hampshire, UK).
Immunofluorescence, flow cytometry, and cell sorting.
MoAbs against the following antigens were used: CD2 (T11-RD1), CD11b (MO1-fluorescein isothiocyanate [FITC]), CD14 (MO2-FITC), and CD25 (IL2-R1-FITC) from Coulter Clone (Hialeah, FL); CD1a (T6-RD1 [from Coulter Clone] and OKT6 [from the ATCC]), CD34 (CD34-phycoerythrin [PE]-Cy5 [from Immunotech, Marseille, France] and HPCA-2-PE [from Becton Dickinson & Co]); CD3 (Leu-4-PE), CD5 (Leu-1-PE), CD8 (Leu-2a-FITC), CD13 (Leu-M7-PE), CD19 (Leu-12-FITC), CD22 (Leu-14-PE), CD38 (Leu-17-PE), CD80 (B7-BB1), and HLA-DR (anti-HLA-DR-PE) from Becton Dickinson & Co; CD44 (CD44-FITC), CD4 (CD4-Tri-Color), and CD11c (CD11c-FITC), from Caltag Laboratories (South San Francisco, CA); CD33 (Leu-M9-PE [from Becton Dickinson & Co] and CD33 biotin-conjugated [from Caltag]); CD7 (Leu 9-FITC [from Becton Dickinson & Co] and T3-3A1 and HB 2 [from the ATCC]); CD40 (MoAb89 [kindly provided by Dr J. Banchereau, Schering-Plough, Dardilly, France] and CD40-FITC [from Caltag]); CD86 (CD86-FITC [from Serotec, Oxford, UK]); CD56 (Leu-19-PE [from Becton Dickinson & Co] and CD56-Tri-Color [from Caltag]); and CD122 (DU-2) from Olimpus Corp Immunochemicals (New York, NY). The anti-CD161 specificity (anti-NKR-P1A) of HP-3G10 MoAb was assigned at the Sixth International Workshop on Human Leukocyte Differentiation Antigens. The anti-CD45RA MoAb was generously provided by Dr J.C. Gutiérrez-Ramos (Millennium Pharmaceuticals Inc, and Department of Medicine, Boston University Medical Center, Boston, MA) and the anti-HLA-DP and -DQ MoAbs were the kind gift of Dr J. Bodmer (Imperial Cancer Research Fund, London, UK).
Single-, two-, or three-color immunofluorescence stainings were performed on cells previously incubated with PBS/EDTA buffer for 15 minutes, as described elsewhere.14 Second-step reagents including FITC-, PE-, or PE-Cy5-conjugated goat antimouse F(ab2)′ IgGs or IgG1 and PE- or PE-Cy5-conjugated streptavidine were purchased from Caltag. Stained cells were analyzed in a flow cytometer (EPICS Profile; Coulter Electronics Inc, Hialeah, FL). Data were collected on 1 to 3 × 104 viable cells as determined by electronic gating on forward and side scatter light parameters. Isotype-matched irrelevant antibodies from Caltag were used as negative controls to define background fluorescence. Cell sorting of CD34+CD44+CD33− and CD34+CD44+CD33lo thymocytes was performed with a FACStar plus (Becton Dickinson & Co) on isolated CD34+CD1− thymocytes after labeling with anti-CD44-FITC, anti-CD33-PE, and anti-CD34-PE-Cy5. Sorted cells were 95% to 98% pure as determined by post-sort analysis.
Cell cultures.
Either CD34+CD1−CD33−or CD34+CD1−CD33lo thymocytes (5 × 105 cells/mL) were cultured in 96-well flat-bottomed microtiter plates (Costar, Cambridge, MA) in RPMI 1640 medium (GIBCO, Paisley, UK) supplemented with 10% fetal calf serum (FCS; GIBCO), in the presence of 100 International Units (IU)/mL recombinant human interleukin-7 (rhIL-7), 60 IU/mL rhIL-1α, 100 IU/mL recombinant human stem cell factor (rhSCF), 50 IU/mL rhIL-6, and 75 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; from the National Institute of Biological Standards and Controls, Potters Bar, Hertfordshire, UK). When indicated, rhIL-2 (from Hoffman La Roche, Basel, Switzerland) was added to the multicytokine-supported cultures at doses favoring high-affinity IL-2 receptor binding (10 IU/mL, 40 pmol/L). When used singly, IL-2 and IL-7 were used at 200 IU/mL and 1000 IU/mL, respectively (Table 1).
Absolute Frequencies of NK Cells and DCs Generated From CD34+CD33lo Postnatal Thymocytes in Different Culture Conditions
| Culture Conditions . | Total Cell Numbers (×10−3) . | NK Cell Numbers (×10−3) . | DC Numbers (×10−3) . |
|---|---|---|---|
| Medium | 5 | ND | ND |
| IL-7 | 420 | 20 | 42 |
| IL-2 | 6 | ND | ND |
| Cytokine mix | 1,500 | 30 | 1,480 |
| Cytokine mix + IL-2 | 1,480 | 780 | 650 |
| Culture Conditions . | Total Cell Numbers (×10−3) . | NK Cell Numbers (×10−3) . | DC Numbers (×10−3) . |
|---|---|---|---|
| Medium | 5 | ND | ND |
| IL-7 | 420 | 20 | 42 |
| IL-2 | 6 | ND | ND |
| Cytokine mix | 1,500 | 30 | 1,480 |
| Cytokine mix + IL-2 | 1,480 | 780 | 650 |
CD34+CD33lo thymocytes (105/well) were cultured for 9 days in the conditions listed, as described in the Materials and Methods. Absolute numbers of NK cells and DCs were deduced from relative numbers of CD7+CD56+ and CD13+CD1+cells, respectively, as determined by flow cytometry. Results are representative of one of three experiments.
Abbreviation: ND, not determined due to low cell recovery.
Limiting dilution analysis.
CD34+CD1−CD33lo thymocytes were diluted in RPMI 1640 medium supplemented with 10% FCS in the presence of the cytokine mixture described above either with or without IL-2 (200 IU/mL). Cells were plated at 300, 100, 33, 10, 3, and 1 cell per well onto Terasaki microplates (Costar; 1 plate per cellular dilution) and cultured for 16 days. Cytokine-supplemented medium was changed every 6 to 7 days by demidepletion. In some experiments, cells were first maintained during 3 or 4 days under high-density culture conditions (5 × 105 cells/mL), as described above, in the presence of the cytokine mixture without IL-2. Afterwards, cells were plated by limiting dilution and cultured in the presence of the cytokine mixture either with or without IL-2 for 16 days. At the end of the culture, all wells were microscopically inspected, and the number of wells containing cells with a typical dendritic morphology (veiled cells and cells with dendritic processes) was scored. The same wells were analyzed for the presence of CD56+ cells after incubation for 30 minutes at 4°C with anti-CD56 (Leu-19; Becton Dickinson & Co) MoAbs bound to goat antimouse IgG-coupled magnetic beads (Dynal). Wells containing cells coated with magnetic particles were scored as positive. The maximum likelihood estimate of clonogenic precursors was calculated with the single hit Poisson model.22
RESULTS
CD34+CD33lo postnatal thymocytes differentiate efficiently to DCs in response to multiple cytokines.
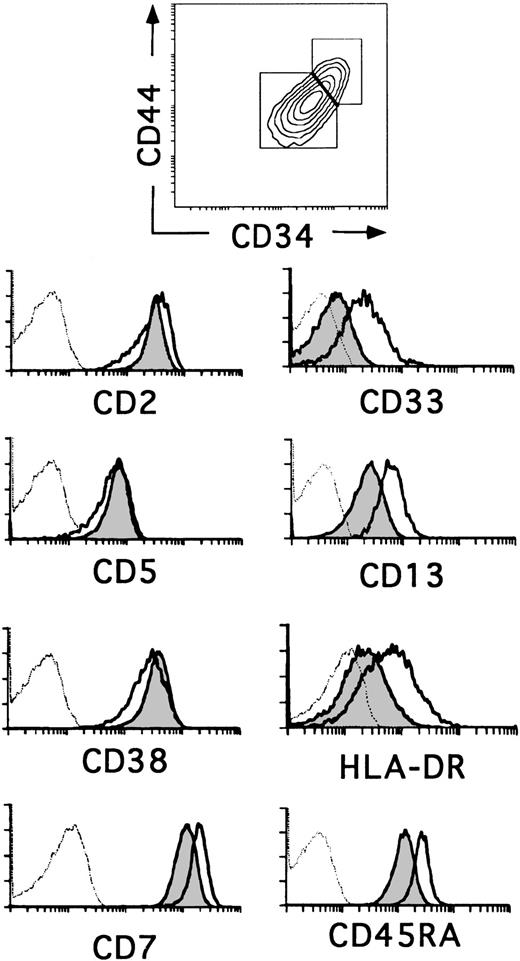
The relative expression of CD34 on thymocyte subsets is useful as a marker for defining different stages of human thymic development.23,24 The most primitive progenitors in the human fetal thymus have been identified as CD34brightCD1− thymocytes that express low surface density CD38, but lack the T-lineage markers CD2 and CD5, whereas the equivalent population in the postnatal thymus displays a more mature CD2+CD5+CD38+phenotype. In addition, fetal progenitors express low surface density CD33 and intermediate levels of CD13 and HLA-DR, whereas cells with this phenotype represent only 20% to 30% of CD34+CD1− postnatal thymocytes (0.037% ± 0.011% of total thymocytes).14 20 Flow cytometry analysis in Fig 1 shows that such cells reside within the population of CD34+CD1−postnatal thymocytes that display the highest levels of CD34 and also of CD44, suggesting that they represent the most immature progenitors in the postnatal thymus. Conversely, thymocytes with a lower expression of CD34 and CD44 (Fig 1) lack CD33 and display lower levels of CD13 and HLA-DR. Despite these phenotypic differences, both cell subsets (hereafter referred to as CD34+CD33lo and CD34+CD33−, respectively) were similar in terms of CD2, CD5, and CD38 expression, although a slightly lower expression of these markers was consistently found in the former. In addition, CD34+CD33lo thymocytes displayed higher levels of CD7 and CD45RA (Fig 1).
Three-color flow cytometry analysis of postnatal CD34+ CD1− thymocytes. CD34+CD1− thymocytes isolated as described in the Materials and Methods were analyzed by flow cytometry for the correlated expression of CD44, CD34, and one of the indicated MoAbs. Electronic gates were set as shown in the upper biparametric plot to analyze the expression of CD2, CD5, CD38, CD7, CD33, CD13, HLA-DR, and CD45RA antigens on CD44brightCD34bright(unshaded areas) and CD44intCD34int (shaded areas) thymocytes. Background values (dashed histograms) were determined with isotype-matched irrelevant antibodies.
Three-color flow cytometry analysis of postnatal CD34+ CD1− thymocytes. CD34+CD1− thymocytes isolated as described in the Materials and Methods were analyzed by flow cytometry for the correlated expression of CD44, CD34, and one of the indicated MoAbs. Electronic gates were set as shown in the upper biparametric plot to analyze the expression of CD2, CD5, CD38, CD7, CD33, CD13, HLA-DR, and CD45RA antigens on CD44brightCD34bright(unshaded areas) and CD44intCD34int (shaded areas) thymocytes. Background values (dashed histograms) were determined with isotype-matched irrelevant antibodies.
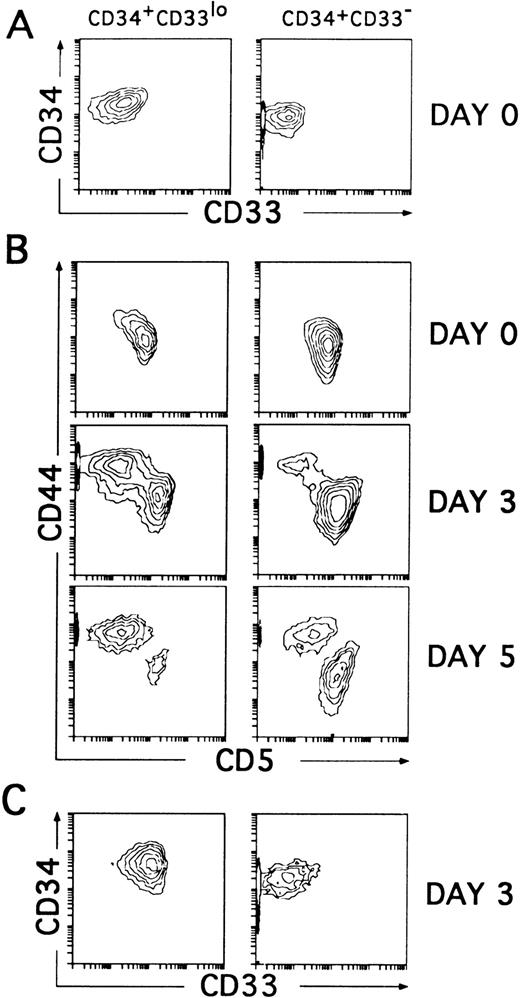
To assess whether the CD34+CD33lo and the CD34+CD33− thymocytes represent sequential developmental stages within the postnatal CD34+CD1− thymic compartment, we sought to determine their intrinsic differentiative capacity. In particular, both isolated cell subsets were compared for their T/DC precursor potential in culture, because unfractionated CD34+CD1− thymocytes cultured with IL-7 have been shown to produce simultaneously T-lineage cells (80% to 90%) and DCs (10% to 20%).14 However, in our initial studies (Table 1), we observed that cell proliferation was greatly improved and DC production was highly increased, mostly at the expense of T-lineage cells (not shown), when IL-7 was used together with various cytokines, namely IL-1α, IL-6, SCF, and GM-CSF, used to generate DCs in culture by others.5,25,26 Thus, CD34+CD33lo and CD34+CD33− thymocytes sorted as shown in Fig 2A were cultured with the optimized mixture of cytokines, and their respective progenies were analyzed by flow cytometry. As previously shown with unfractionated CD34+CD1− thymocytes,14 cells expressing upregulated levels of CD44 arised within 2 to 3 days from both the CD34+CD33lo and CD34+CD33− cultures, although in different proportions. These were large cells (mean forward scatter, 554 ± 30) that had gradually lost surface CD5 to become CD44brightCD5lo/−. Interestingly, cells with these morphologic and phenotypic features represented the major cell progeny (80% to 90% by day 5) in all cultures initiated with CD34+CD33lo thymocytes. The remaining population (10% to 20%) was composed of small-sized cells (mean forward scatter, 285 ± 15) that, as expected from T-lineage precursors,14 displayed downregulated levels of CD44 but retained CD5 expression. The opposite situation was found in the CD34+CD33− cultures, in which a minor fraction of cells (<25%) displayed the CD44brightCD5lo/− phenotype, whereas most of them (>75%) were CD44loCD5+. As shown in Fig 2C, three-color analysis showed that the CD44bright and the CD44lo cell progenies were significantly different in terms of CD33 expression. CD44bright cells expressed upregulated CD33 levels, whereas CD44lo cells were CD33−. Both populations were shown to keep relatively high CD34 levels during the initial 3 to 4 days of culture (Fig 2C), but thereafter they downregulated gradually CD34 expression (see below), suggesting that they represent progenitor cells downstream from the earliest CD34+CD33lo thymocytes.
CD34+CD33lo and CD34+CD33− postnatal thymocytes cultured with multiple cytokines give rise to phenotypically distinct cell progenies. (A) Sorted CD34+CD33lo and CD34+CD33− postnatal thymocytes were reanalyzed for the expression of CD34 and CD33. (B) The correlated expression of CD44 and CD5 was independently analyzed on sorted CD34+CD33lo (left panels) and CD34+CD33− (right panels) cells either before (day 0) or after 3 and 5 days of culture with a mixture of IL-7, IL-1α, IL-6, SCF, and GM-CSF. (C) The correlated expression of CD34 and CD33 was analyzed on electronically gated CD44bright(left panel) and CD44lo (right panel) cell progenies recovered at day 3 from the multicytokine-supported cultures of CD34+CD33lo thymocytes.
CD34+CD33lo and CD34+CD33− postnatal thymocytes cultured with multiple cytokines give rise to phenotypically distinct cell progenies. (A) Sorted CD34+CD33lo and CD34+CD33− postnatal thymocytes were reanalyzed for the expression of CD34 and CD33. (B) The correlated expression of CD44 and CD5 was independently analyzed on sorted CD34+CD33lo (left panels) and CD34+CD33− (right panels) cells either before (day 0) or after 3 and 5 days of culture with a mixture of IL-7, IL-1α, IL-6, SCF, and GM-CSF. (C) The correlated expression of CD34 and CD33 was analyzed on electronically gated CD44bright(left panel) and CD44lo (right panel) cell progenies recovered at day 3 from the multicytokine-supported cultures of CD34+CD33lo thymocytes.
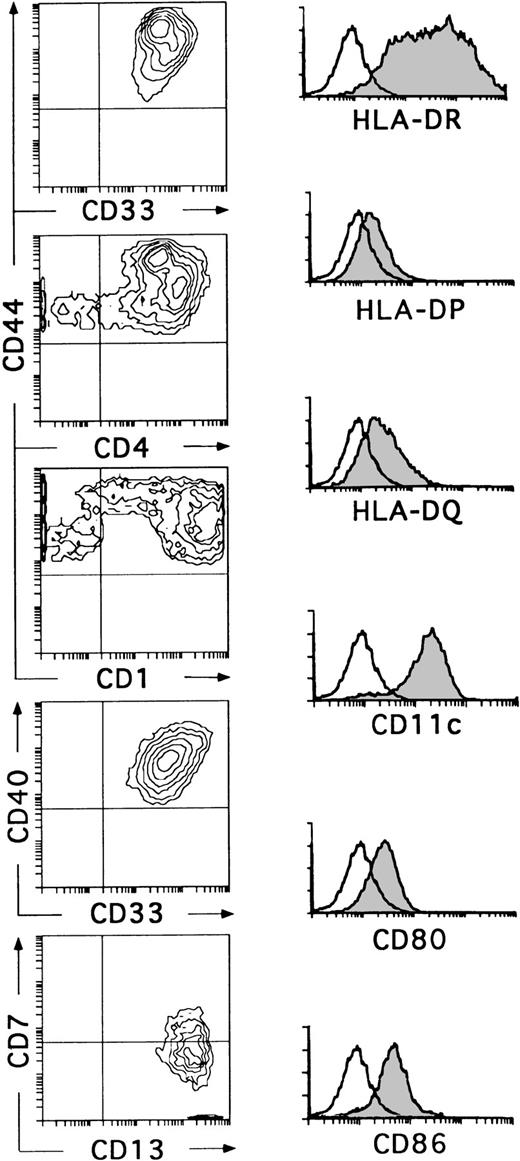
Kinetic studies showed that the small-sized CD34+CD44loCD5+CD33−progeny differentiated within 6 to 8 days into CD34−CD1+CD4+CD8+double-positive immature thymocytes, indicating that they represent T-lineage precursors (data not shown). These cells died in culture by day 10. In contrast, essentially all CD34+CD44brightCD5lo/−CD33+cells had differentiated by day 10 into large non–T-lineage cells lacking CD34 (data not shown) and expressing high levels of CD33, CD4, CD40, and HLA-DR (Fig 3). These cells showed a variable expression of CD1, although about 60% of them were CD1bright. In all experiments, cells with this phenotype had downregulated the CD7 molecule, but had acquired very high levels of CD13, thus becoming homogeneously CD13brightCD7lo/−. In addition, a variable proportion coexpressed CD11c, CD80, CD86, and HLA-DR, -DP, and -DQ (Fig 3). Although no markers of mature T (CD3), B (CD19, CD22), and NK (CD56) cells were found on these cells, the myeloid-related markers CD14 and CD11b were detected at low and high expression levels, respectively, on a variable proportion of them (data not shown). Cells with this antigenic profile were irregularly shaped, displayed long membrane processes, and behaved as professional antigen presenting cells in a mixed lymphocyte reaction (Márquez et al14and data not shown). Interestingly, these cells represented the only cell progeny recovered from the CD34+CD33locultures by day 10 (Fig 3). Therefore, the optimized mixture of cytokines used in this study induces CD34+CD33lo intrathymic precursors to generate CD34+CD44bright CD5 lo/- CD33+intermediate progenitors that differentiate exclusively into cells with the phenotypic, morphological, and functional features associated with DCs.27-29 Together, our data suggest that the CD34+ CD33lo and CD34+CD33− thymocyte precursors differ markedly in their ability to generate DCs. In terms of absolute cell numbers, CD34+ CD33lo thymocytes generate up to 10 times more DCs than CD34+ CD33− thymocytes. Therefore, CD34+ CD33lo postnatal thymocytes are capable of serving as efficient DC precursors, while CD34+ CD33− thymocytes seem to display a reduced DC precursor potential but an increased fitness to generate T-lineage cells.
CD34+CD33lo thymocytes develop efficiently into DCs in multicytokine-supported cultures. CD34+CD33lo thymocytes were cultured for 10 days with the cytokine mixture described in Fig 2. Biparametric histograms on the left show the correlated expression of CD44 and CD33, CD44 and CD4, CD44 and CD1, CD40 and CD33, and CD7 and CD13 on the cultured cells. Background fluorescence values were set by use of FITC- and PE-conjugated isotype-matched irrelevant antibodies. Monoparametric histograms on the right show the expression of HLA-DR, -DP, and -DQ, CD11c, CD80, and CD86 (shaded histograms) on the same cultured cells. Background fluorescence (unshaded histograms) was determined by staining with isotype-matched irrelevant MoAbs plus PE-conjugated goat antimouse Igs.
CD34+CD33lo thymocytes develop efficiently into DCs in multicytokine-supported cultures. CD34+CD33lo thymocytes were cultured for 10 days with the cytokine mixture described in Fig 2. Biparametric histograms on the left show the correlated expression of CD44 and CD33, CD44 and CD4, CD44 and CD1, CD40 and CD33, and CD7 and CD13 on the cultured cells. Background fluorescence values were set by use of FITC- and PE-conjugated isotype-matched irrelevant antibodies. Monoparametric histograms on the right show the expression of HLA-DR, -DP, and -DQ, CD11c, CD80, and CD86 (shaded histograms) on the same cultured cells. Background fluorescence (unshaded histograms) was determined by staining with isotype-matched irrelevant MoAbs plus PE-conjugated goat antimouse Igs.
CD34+CD33lo postnatal thymocytes display NK cell precursor potential.
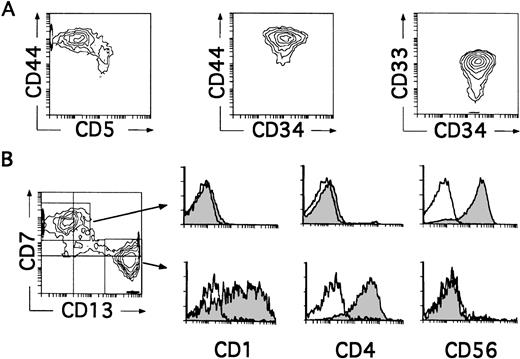
We next investigated whether CD34+CD33lothymocytes represent oligopotent progenitors able to generate not only DCs, but other non–T-lymphoid cells such as NK cells. Differentiation of CD34+CD33lo thymocytes into cells with the characteristic CD34−CD56+CD3−phenotype of mature NK cells was observed in all experiments in which IL-2 was added to the optimized cytokine mixture used for the generation of DCs. As shown in Table 1, such culture conditions also supported the production of DCs, so that both NK cells and DCs were produced at equivalent numbers. Indeed, cells recovered by day 8 comprised a mixed population with two reciprocal phenotypic patterns (Fig 4B). About 50% of these cells displayed the CD13brightCD7lo/− phenotype associated with DCs, whereas the other 50% showed high CD7 expression but low to undectetable levels of CD13. Electronic gates set independently on each cell subset showed that, as expected for DCs, CD13brightCD7lo/− cells expressed both CD1 and CD4 and lacked CD56. In contrast, CD13lo/−CD7+ cells were homogeneously positive for the NK cell marker CD56, but lacked CD1 and CD4 (Fig 4B). Thus, CD34+CD33lo thymocytes cultured with the cytokine mixture plus IL-2 are able to generate simultaneously both DCs and NK cells. Because no NK cells were formed in the absence of IL-2, these data suggest a strong dependence on IL-2 by these precursors for the development of NK cells. However, IL-2 by itself was unable to induce the generation of NK cells, a common feature of all cytokines included in the mixture when used singly (Table 1 and data not shown). It did not induce the proliferation or support the viability of CD34+CD33lo cells (Table 1), indicating that NK cell development requires a broad spectrum of cytokines in addition to IL-2. A striking finding was that the majority (∼90%) of cells recovered by day 4 from these cultures were large-sized cells that displayed the CD34+CD44brightCD5lo/−CD33+non–T-lineage phenotype of large-sized DC precursors identified in cultures lacking IL-2 (Fig 4A).
CD34+CD33lo thymocytes develop simultaneously into NK Cells and DCs in multicytokine-supported cultures containing IL-2. CD34+ CD33lothymocytes were cultured with the mixture of IL-7, IL-1α, IL-6, SCF, and GM-CSF described in Fig 2, plus IL-2. (A) Depicts the correlated expression of CD44 versus CD5, CD44 versus CD34, and CD34 versus CD33 at day 4. CD34+CD44brightCD5lo/− cells represent 90% of total cells. High surface CD33 expression was observed on 75% of total cells. (B) Shows the phenotype of the cellular progeny at day 8. Cells were analyzed for the correlated expression of CD7, CD13, and either CD1, CD4, or CD56. Monoparametric histograms show the expression of CD1, CD4, and CD56 (shaded areas) on the CD7+CD13lo/− and CD7lo/−CD13+ cell subsets electronically gated as shown in the biparametric plot. Background fluorescence (unshaded histograms) was determined by staining with isotype-matched irrelevant MoAbs plus PE-Cy5-conjugated goat antimouse Igs.
CD34+CD33lo thymocytes develop simultaneously into NK Cells and DCs in multicytokine-supported cultures containing IL-2. CD34+ CD33lothymocytes were cultured with the mixture of IL-7, IL-1α, IL-6, SCF, and GM-CSF described in Fig 2, plus IL-2. (A) Depicts the correlated expression of CD44 versus CD5, CD44 versus CD34, and CD34 versus CD33 at day 4. CD34+CD44brightCD5lo/− cells represent 90% of total cells. High surface CD33 expression was observed on 75% of total cells. (B) Shows the phenotype of the cellular progeny at day 8. Cells were analyzed for the correlated expression of CD7, CD13, and either CD1, CD4, or CD56. Monoparametric histograms show the expression of CD1, CD4, and CD56 (shaded areas) on the CD7+CD13lo/− and CD7lo/−CD13+ cell subsets electronically gated as shown in the biparametric plot. Background fluorescence (unshaded histograms) was determined by staining with isotype-matched irrelevant MoAbs plus PE-Cy5-conjugated goat antimouse Igs.
Differentiation kinetics of thymic NK cells and DCs derived from CD34+CD33lo intrathymic precursors.
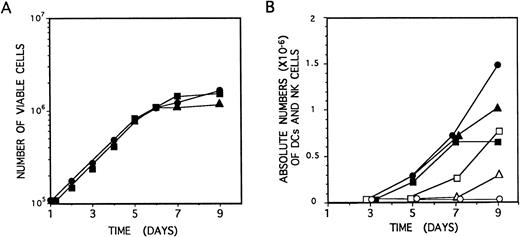
Identical proliferation kinetics were observed in multicytokine-supported cultures of CD34+CD33lothymocytes either containing or lacking IL-2. As shown in Fig 5A, CD34+CD33loprecursors grew exponentially, with a 30-hour doubling time, during the initial (5 to 6 days) period of culture, resulting in a 15-fold increase of cell numbers by day 6. Thereafter, cell expansion decreased, but cell viability was maintained (>99%), and cell numbers remained essentially unchanged from day 6 to day 10 through 15. Cellular recovery declined steadily afterwards, although viable cells could be observed for up to 2 to 3 weeks. Thus, IL-2 had no effect on the proliferation induced by the combination of IL-7, IL-1α, IL-6, SCF, and GM-CSF on CD34+CD33lo precursors (Fig5A).
Growth and differentiation kinetics of NK cells and DCs derived from CD34+CD33lo thymocytes. (A) CD34+CD33lo thymocytes (105/well) were cultured during 9 days in 0.2 mL of medium supplemented with the mixture of IL-7, IL-1α, IL-6, SCF, and GM-CSF either without IL-2 (•) or with IL-2 (▪). An additional culture was set up without IL-2, and IL-2 was added at day 4 (▴). The number of total viable cells recovered at the indicated days was determined by trypan blue dye exclusion. (B) CD34+CD33lo thymocyte cultures set up as described in (A) were analyzed by flow cytometry for the correlated expression of either CD7, CD13, and CD56, or CD7, CD13, and CD1, at the indicated days. The data show the absolute numbers of CD7+CD13lo/−CD56+ NK cells (open symbols) and CD7lo/−CD13+CD56− DCs (solid symbols) recovered from cultures lacking IL-2 (•, ○) or supplemented with IL-2 either from day 0 (▪, □) or from day 4 (▴, ▵).
Growth and differentiation kinetics of NK cells and DCs derived from CD34+CD33lo thymocytes. (A) CD34+CD33lo thymocytes (105/well) were cultured during 9 days in 0.2 mL of medium supplemented with the mixture of IL-7, IL-1α, IL-6, SCF, and GM-CSF either without IL-2 (•) or with IL-2 (▪). An additional culture was set up without IL-2, and IL-2 was added at day 4 (▴). The number of total viable cells recovered at the indicated days was determined by trypan blue dye exclusion. (B) CD34+CD33lo thymocyte cultures set up as described in (A) were analyzed by flow cytometry for the correlated expression of either CD7, CD13, and CD56, or CD7, CD13, and CD1, at the indicated days. The data show the absolute numbers of CD7+CD13lo/−CD56+ NK cells (open symbols) and CD7lo/−CD13+CD56− DCs (solid symbols) recovered from cultures lacking IL-2 (•, ○) or supplemented with IL-2 either from day 0 (▪, □) or from day 4 (▴, ▵).
Microscopic inspection of cultures showed that, as reported for precursors cultured only with IL-7,14CD34+CD33lo cells growing in multicytokine-supported cultures either with or without IL-2 became aggregated after 24 hours to form large colonies containing greater than 50 cells/colony. Such a colony formation was highly dependent on cell density. Cells arising under both culture conditions were morphologically indistinguishable during the initial 4 to 5 days. From day 6 on, cells displaying a typical DC morphology were evident in both cultures. However, these were the only cells arising in cultures lacking IL-2, whereas DCs together with regularly shaped blastic cells represented the end-product cells in cultures supplemented with IL-2. No specific NK cell or DC maturation patterns were evident during the initial (4 to 5 days) period of cellular expansion that was characterized by the gradual loss of surface CD34. As shown in Fig 5B, cells with the CD13brightCD7lo/−phenotype associated with DCs were first detectable in both culture conditions by day 5, whereas CD56+ NK cells arose 2 days later, but exclusively in IL-2–supplemented cultures. Thereafter, the absolute numbers of these two cell types increased gradually in their respective cultures and peaked at day 9. Because total cell numbers remained essentially unchanged from day 5 through 9, final differentiation into cells with the immunophenotypic features of mature DCs and NK cells may occur independently of cell proliferation.
Thymic DCs and NK cells develop simultaneously through a common CD34+CD44brightCD5lo/−CD33+intermediate stage.
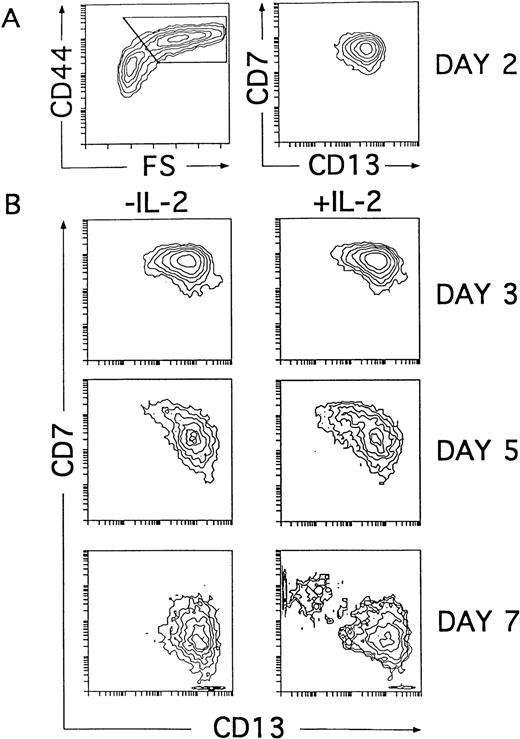
Our kinetic studies provided evidence that the generation of CD56+ NK cells in IL-2–supported cultures was accompanied by a proportional decrease in the absolute numbers of DCs as compared with numbers obtained from cultures lacking IL-2 (Fig 5B). This indicates that NK cell production occurred at the expense of DCs. Further support for this notion comes from additional studies in which generation of CD56+ NK cells was also observed when addition of IL-2 to the cytokine-supported cultures was delayed up to day 4. In this last condition, NK cells appeared with delayed kinetics, and the net NK cell production was reduced, whereas the absolute numbers of DCs increased proportionally (Fig 5B). Thus, our data indicate that IL-2 is dispensable for the initial generation of NK cell precursors. Moreover, they suggest that the cytokine mixture without IL-2 supports the generation of intermediate progenitors able to produce both DCs and NK cells. Based on our previous data, we reasoned that such progenitors might reside within the population of CD34+CD44brightCD5lo/−CD33+ large cells representing the immediate progeny arising from CD34+CD33lothymocytes cultured both with and without IL-2 (Figs 2 and 4). To address this point, cells recovered at early time points (day 2) from cultures lacking IL-2 were analyzed for their developmental potential upon reculture for 5 additional days with the cytokine mixture, either containing or lacking IL-2. In particular, we followed the kinetics of CD7 and CD13 expression on ellectronically gated CD44brightlarge cells arising under both culture conditions, because those markers were shown to define reciprocal phenotypic patterns on mature NK cells and DCs (Fig 4B). At day 2, the CD44brightlarge-sized progeny, gated as shown in Fig6A, displayed a homogeneous CD7+CD13+ phenotype that was maintained during the initial 4 days of culture, regardless of the presence of IL-2. Thus, as shown in Fig 6B, the CD44bright large cells recovered from both cultures at day 3 displayed an overlapping CD7+CD13+ phenotype. Afterwards, CD44bright cells with upregulated CD13 and downregulated CD7 surface levels increased gradually in cultures lacking IL-2, giving rise to a homogeneous CD13brightCD7lo/− DC progeny by day 7. In contrast, a mixed population of CD44bright cells showing opposite CD7 and CD13 expression patterns was evident by day 5 in cultures containing IL-2. These cells segregated into two different cell populations: one showing the characteristic CD13brightCD7lo/− DC phenotype and the other expressing upregulated levels of CD7 but low to undetectable surface CD13 by day 7. As expected from our previous data, day 7 CD13lo/−CD7+ cells had acquired the CD56 NK cell marker (Fig 7). Taken together, our data provide evidence that the CD34+CD44brightCD5lo/−CD33+cells represent intermediate progenitors common to NK cells and DCs.
NK cells and DCs develop simultaneously through a common CD44bright intermediate stage. Cells derived from CD34+CD33lo thymoctes cultured with IL-7, IL-1α, IL-6, SCF, and GM-CSF were recovered at day 2 and cultured for 5 additional days with the same cytokine mixture either with or without IL-2. (A) Shows CD44 expression versus cell size (FS, forward scatter; left plot) and the correlated expression of CD7 and CD13 on electronically gated CD44bright large (forward scatter >350) cells (right plot) at day 2. (B) Shows the kinetics of CD7 and CD13 expression on electronically gated CD44bright large cells recovered at the time points indicated after reculture with the cytokine mixture either with or without IL-2.
NK cells and DCs develop simultaneously through a common CD44bright intermediate stage. Cells derived from CD34+CD33lo thymoctes cultured with IL-7, IL-1α, IL-6, SCF, and GM-CSF were recovered at day 2 and cultured for 5 additional days with the same cytokine mixture either with or without IL-2. (A) Shows CD44 expression versus cell size (FS, forward scatter; left plot) and the correlated expression of CD7 and CD13 on electronically gated CD44bright large (forward scatter >350) cells (right plot) at day 2. (B) Shows the kinetics of CD7 and CD13 expression on electronically gated CD44bright large cells recovered at the time points indicated after reculture with the cytokine mixture either with or without IL-2.
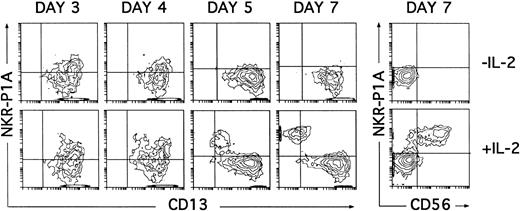
Induction of NKR-P1A expression on cultured CD34+ CD33lo thymocytes. Cultures of CD34+ CD33lo thymocyes were set up as described in Fig 6. The correlated expression of NKR-P1A and CD13 was analyzed by flow cytometry on electronically gated CD44bright large cells at the indicated time points. Percentages of NKR-P1A+ CD13+ cells recovered at days 3, 4, 5, and 7 were 27%, 22%, 7%, and 5%, respectively, in cultures lacking IL-2; and 29%, 40%, 30%, and 40%, respectively, in cultures containing IL-2. The correlated expression of NKR-P1A and CD56 was analyzed on electronically gated CD44bright large cells recovered under both culture conditions at day 7. Background fluorescence was determined by sequential staining with isotype-matched (IgG1) irrelevant MoAbs, FITC-conjugated goat antimouse IgG1, and PE-conjugated isotype-matched irrelevant MoAbs.
Induction of NKR-P1A expression on cultured CD34+ CD33lo thymocytes. Cultures of CD34+ CD33lo thymocyes were set up as described in Fig 6. The correlated expression of NKR-P1A and CD13 was analyzed by flow cytometry on electronically gated CD44bright large cells at the indicated time points. Percentages of NKR-P1A+ CD13+ cells recovered at days 3, 4, 5, and 7 were 27%, 22%, 7%, and 5%, respectively, in cultures lacking IL-2; and 29%, 40%, 30%, and 40%, respectively, in cultures containing IL-2. The correlated expression of NKR-P1A and CD56 was analyzed on electronically gated CD44bright large cells recovered under both culture conditions at day 7. Background fluorescence was determined by sequential staining with isotype-matched (IgG1) irrelevant MoAbs, FITC-conjugated goat antimouse IgG1, and PE-conjugated isotype-matched irrelevant MoAbs.
NK cell progenitors derived from CD34+CD33lo thymocytes can be identified by the expression of the NKR-P1A receptor.
It has recently been shown that expression of the NKR-P1A surface receptor in the absence of the CD56 NK cell marker defines an immature pre-NK cell stage.21 30 Thus, kinetic studies on the expression of the NKR-P1A molecule were then performed to assess whether NKR-P1A+ intermediate NK cell precursors were produced in our cultures. As shown in Fig 7, NKR-P1A was expressed on 25% to 30% of CD13+ cells recovered after 3 days from cultures both with and without IL-2. These NKR-P1A+ cells decreased gradually from day 4 in cultures lacking IL-2, so that by day 7 essentially all cells were NKR-P1A− and displayed the CD13bright (CD7lo/−) DC phenotype. However, in the presence of IL-2, increasing NKR-P1A expression levels were detected throughout culture on a fraction of CD13+cells that gradually downregulated surface CD13 to become NKR-P1A+CD13lo/−. All these cells displayed a mature CD56+ phenotype by day 7 (Fig 7).
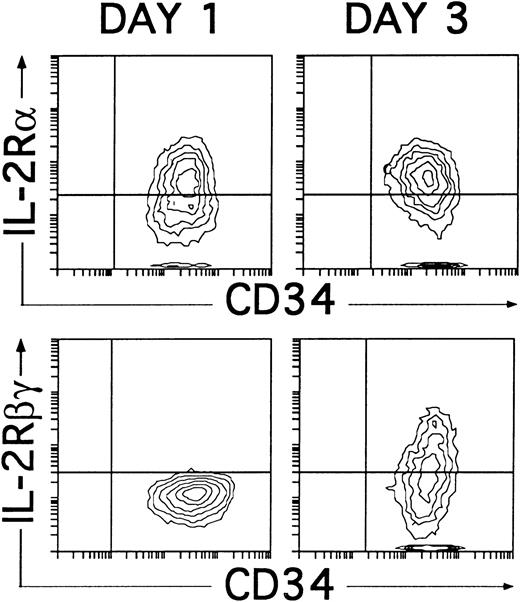
These data confirm that IL-2 is dispensable for the initial generation of NKR-P1A+CD56− NK cell progenitors, although it is required for their progression into NKR-P1A+CD56+ mature NK cells. Related to this point, it is worth noting that, regardless of the presence of IL-2, the cytokine combination used in our studies induced the expression of CD25 (IL-2Rα) within the first 24 hours of culture. Such culture conditions also supported the induction of CD122 (IL-2Rβγ), which was first detectable 2 days later (day 3), coincident with the appearance of NKR-P1A (Fig 8), and reached a maximal expression by day 4. Thus, NK cell progenitors may become sensitive to IL-2 only after they reach the NKR-P1A+ stage of differentiation, at which they express both CD25 and CD122.
Induction of CD25 (IL-2Rα) and CD122 (IL-2Rβγ) expression on cultured CD34+CD33lothymocytes. CD34+CD33lo thymocytes cultured for 1 or 3 days with IL-7, IL-1α, IL-6, SCF, and GM-CSF were analyzed for the correlated expression of CD34 and CD25 or CD34 and CD122. Background fluorescence was determined by sequential staining with isotype-matched irrelevant MoAbs, FITC-conjugated goat antimouse Igs, and PE-Cy5-conjugated isotype-matched irrelevant MoAbs.
Induction of CD25 (IL-2Rα) and CD122 (IL-2Rβγ) expression on cultured CD34+CD33lothymocytes. CD34+CD33lo thymocytes cultured for 1 or 3 days with IL-7, IL-1α, IL-6, SCF, and GM-CSF were analyzed for the correlated expression of CD34 and CD25 or CD34 and CD122. Background fluorescence was determined by sequential staining with isotype-matched irrelevant MoAbs, FITC-conjugated goat antimouse Igs, and PE-Cy5-conjugated isotype-matched irrelevant MoAbs.
Frequency of DC- and NK cell-producing progenitors.
The overall differentiative capacity of CD34+CD33lo thymocytes suggested that we had identified culture conditions able to support the simultaneous generation of NK cells and DCs with similar efficiencies. To directly analyze this possibility, we sought to determine the frequency of DC and NK cell progenitors in CD34+ CD33lo thymocytes by limiting dilution assays. Thus, cells were plated at 300, 100, 33, 10, 3, and 1 cell per well and cultured with the cytokine mix plus IL-2 for 16 days. At this time, all wells were microscopically examined for the presence of live cells expressing CD56 (NK cells) or lacking CD56 but showing a typical dendritic morphology (DCs). The nature of such NK cells and DCs was confirmed by carefully analyzing the composition of wells plated at high cell concentrations (300 and 100 cells per well). As shown in Table 2 (experiment no. 1), the maximum likelihood estimate of clonogenic precursors was 0.033 (1/30), calculated with the single hit Poisson model.22 The frequencies of DC and NK cell clonogenic precursors in this assay were quite similar (0.031 [1/32] and 0.028 [1/35], respectively). Interestingly, a substantial fraction of the positive wells seeded under clonal conditions contained both type of cells, indicating that they were seeded with bipotential DC/NK progenitors. However, the individual DC and NK cell precursor frequencies obtained were lower than expected considering the rapid increase in cell counts recorded in high-density cultures. This suggested that proliferation and DC/NK cell production would be favored by cell-cell contacts occurring in dense cultures. As shown in Table 2, we found that the frequency of clonogeneic precursors was about 20- to 30-fold higher when cells were plated by limiting dilution after short-term (3 to 4 days) high-density cultures. In addition, these experiments allowed us to compare the DC and NK cell precursor frequencies of the cell progeny generated in the absence of IL-2 upon reculture with or without IL-2. Thus, cells arising at day 3 (experiment no. 2) or day 4 (experiment no. 3) from CD34+CD33lo multicytokine-supported cultures lacking IL-2 (88% and 90% CD34+CD44bright, respectively, <1% CD56+) were plated by limiting dilution and cultured for 16 additional days with the cytokine mixture either with or without IL-2. As shown in Table 2, the maximum likelihood estimate of clonogenic precursors in the absence of IL-2 was 0.909 (1/1.10) and 0.952 (1/1.05) in experiments no. 2 and 3, respectively. All positive wells in these assays contained exclusively DCs, indicating that close to 100% of cells recovered from short-term cytokine-supplemented cultures lacking IL-2 can develop into DCs. Interestingly, when the same cell samples were supplemented with IL-2, 25% (1/4) and 14% (1/7) of cells (in experiments no. 2 and 3, respectively) developed into NK cells, indicating that a significant proportion of them were bipotential DC/NK cell precursors. The estimated frequencies of such bipotential clones in experiments 2 and 3 was 19% (1/5.3) and 14% (1/7), respectively. Analysis of wells seeded under clonal conditions in experiment no. 2 showed that, from a total of 12 wells containing NK cells (of 60 plated), 10 wells contained also DCs.
Limiting Dilution Analysis for the Production of DCs and NK Cells
| Experiment No. . | Cytokines . | Frequencies of . | ||
|---|---|---|---|---|
| Clonogenic Precursors . | DC Precursors . | NK Cell Precursors . | ||
| 1 | Mix + IL-2 | 3.3% | 3.1% | 2.8% |
| 2 | Mix | 91% | 91% | 0% |
| Mix + IL-2 (day 3) | 77% | 67% | 25% | |
| 3 | Mix | 95% | 95% | 0 |
| Mix + IL-2 (day 4) | 87% | 78% | 14% | |
| Experiment No. . | Cytokines . | Frequencies of . | ||
|---|---|---|---|---|
| Clonogenic Precursors . | DC Precursors . | NK Cell Precursors . | ||
| 1 | Mix + IL-2 | 3.3% | 3.1% | 2.8% |
| 2 | Mix | 91% | 91% | 0% |
| Mix + IL-2 (day 3) | 77% | 67% | 25% | |
| 3 | Mix | 95% | 95% | 0 |
| Mix + IL-2 (day 4) | 87% | 78% | 14% | |
Freshly isolated CD34+CD33lo postnatal thymocytes (experiment no. 1) were plated by limiting dilution and cultured with a mixture of IL-7, IL-1α, IL-6, SCF, GM-CSF, and IL-2, as described in the Materials and Methods. After a total culture period of 16 days, all wells were microscopically inspected for the presence of live hematopoietic cells reactive with anti-CD56-coated magnetic beads (NK cells) or unreactive with anti-CD56-coated beads but displaying a typical dendritic morphology (DCs). In experiments no. 2 and 3, CD34+CD33lo thymocytes were first cultured at high density for 3 and 4 days, respectively, with the cytokine mixture described for experiment no. 1 lacking IL-2. Afterwards, recovered cells were phenotyped (88% and 90% CD34+CD44brightCD56−, in experiments no. 2 and 3, respectively), plated by limiting dilution, and cultured for 16 additional days with the cytokine mixture either with or without IL-2. Positive wells were analyzed for the presence of DCs and/or NK cells as described for experiment no. 1.
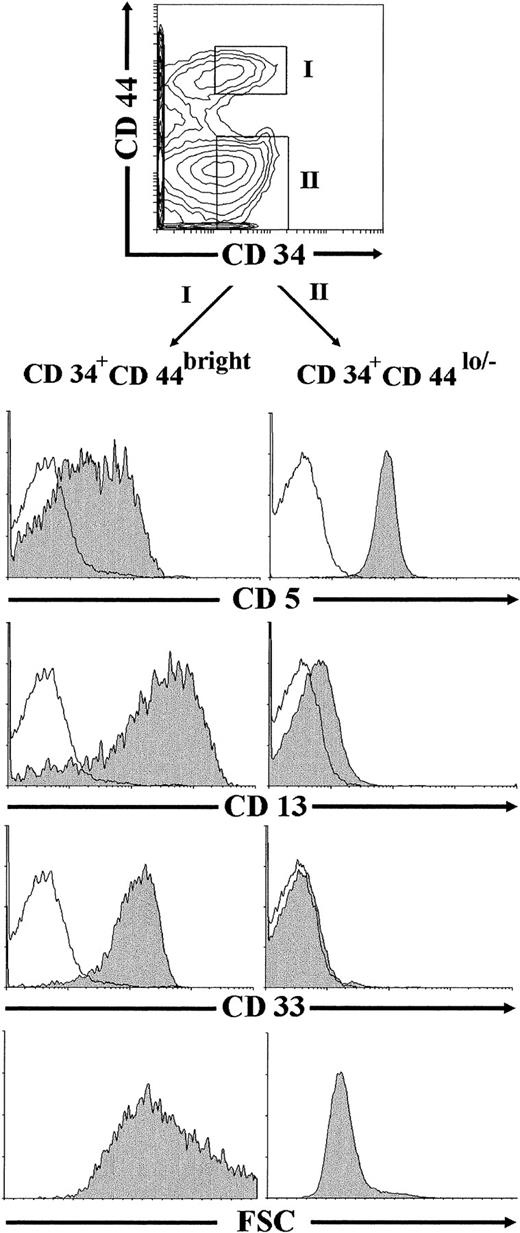
Identification of CD34+CD44brightCD5lo/−CD33+intermediate thymocytes in vivo.
Flow cytometric studies were finally performed on ex vivo-isolated thymocytes to investigate whether CD34+CD44brightCD5lo/−CD33+progenitors, equivalent to the NK/DC intermediate precursors generated in vitro under our culture conditions, could be identified in the human postnatal thymus. To this end, thymocyte preparations depleted of mature T, B, NK, and myeloid cells (lin−) were analyzed by flow cytometry after removal of early CD34int-bright thymic progenitors, as described in the Materials and Methods. As shown in Fig 9, most thymocytes (∼75%) within the remaining population were CD44lo/− cells, whereas only up to 25% of them expressed high levels of CD44. Both the CD44lo/− and the CD44bright cell subsets were mostly composed of CD34− cells, but also included a variable proportion of cells expressing low but readily detectable surface CD34 (30% and 40% of CD44lo/− and CD44brightthymocytes, respectively, in this experiment). Electronic gates set independently on the CD34+ CD44lo/− and the CD34+CD44bright populations showed that thymocytes within the former subset displayed a CD5+CD33−CD13−T-lineage phenotype. In contrast, CD34+CD44bright cells were homogeneously CD33+CD13+, and most of them (>70%) displayed low to negative CD5 surface expression. No expression of the NKR-P1A receptor could be detected on cells within any cell subset (data not shown). According to our results in vitro, CD34+CD44brightCD5lo/−CD33+CD13+thymocytes identified in vivo were large-sized cells (mean forward scatter, 656), whereas CD34+CD44lo/−CD5+CD33−CD13−thymocytes were small-sized cells (mean forward scatter, 319; Fig 9).
Identification of CD34+CD44brightCD5lo/−CD33−intermediate thymocytes in vivo. Lin−thymocytes depleted of the most immature CD34int-bright precursors, as described in the Materials and Methods, were analyzed by flow cytometry for the correlated expression of CD44, CD34, and one of the indicated MoAbs. Electronic gates were set as shown in the upper biparametric plot to analyze either the cell size (mean forward scatter, FSC) or the expression of CD5, CD13, and CD33 antigens (shaded histograms) on CD34+CD44bright (gate I) and CD34+CD44lo/− (gate II) thymocytes. Background staining values (unshaded histograms) were determined with isotype-matched irrelevant antibodies.
Identification of CD34+CD44brightCD5lo/−CD33−intermediate thymocytes in vivo. Lin−thymocytes depleted of the most immature CD34int-bright precursors, as described in the Materials and Methods, were analyzed by flow cytometry for the correlated expression of CD44, CD34, and one of the indicated MoAbs. Electronic gates were set as shown in the upper biparametric plot to analyze either the cell size (mean forward scatter, FSC) or the expression of CD5, CD13, and CD33 antigens (shaded histograms) on CD34+CD44bright (gate I) and CD34+CD44lo/− (gate II) thymocytes. Background staining values (unshaded histograms) were determined with isotype-matched irrelevant antibodies.
DISCUSSION
We have shown previously that CD34+CD1−human postnatal thymocytes cultured with IL-7 differentiate efficiently to T cells and also produce DC, albeit with a fivefold to 10-fold lower efficiency. Both developmental pathways progress through separate intermediate progenitors of different cell size that lose surface CD34 and simultaneously acquire opposite CD44 expression patterns.14 Human pro-T cells, like their mouse counterparts, downregulate CD44 to give rise to small-sized CD44lo pre-T cells,31 whereas upregulation of CD44 identifies large-sized thymic progenitors developing into DCs. These observations suggested that attainment of the CD44bright stage may be intimately linked to commitment of intrathymic precursors into non-T cells. A relevant question is whether these CD44bright cells represent intermediate precursors already committed to the DC lineage or, alternatively, they include cells able to form other thymic non-T cells such as NK cells. This has important implications regarding the currently accepted premise that the branching of human NK cells and T cells occurs downstream of the divergence of DCs.20 We have addressed this point in the present study by analyzing the developmental relationship between DCs and NK cells derived in culture from a minor subset of CD34+CD1− thymocytes that display the highest levels of CD34 and may thus represent the most primitive progenitors so far defined and isolated from the human postnatal thymus. Such CD34bright postnatal thymocytes, like their fetal counterparts,15,16 display a CD33loCD13intHLA-DRint phenotype equivalent to that of bone marrow CD34bright cells recently identified as oligopotent progenitors common to all (T, B, NK, and dendritic) lymphoid cell lineages.5 Therefore, it is likely that the postnatal precursors analyzed in this study (referred to as CD34+CD33lo) correspond to the initial bone marrow-derived lymphoid progenitors that seed the postnatal thymus and give rise to more differentiated CD34+CD33− thymocytes. This possibility is supported by the fact that isolated CD34+CD33lo precursors differentiate preferentially to T-lineage cells in IL-7–supported cultures (Table 1and data not shown), but can also derive DCs and NK cells when more complex cytokine combinations are used, whereas CD34+CD33− thymocytes have a diminished DC (present study) and NK cell (not shown) precursor potential but display more immediate T-cell precursor activity. Although the B-cell precursor potential of the CD34+CD33lo precursors has not been analyzed in this study, it is likely that these early progenitors, like their mouse counterparts,19 are also able to form B cells.
An important aspect of our study was the development of a multicytokine-supported cell culture system (namely IL-7, IL-1α, IL-6, SCF, and GM-CSF) that induced CD34+CD33loprecursors to differentiate efficiently to DCs, but not to T-lineage cells. Similar cytokine combinations without GM-CSF have recently been reported by Saunders et al26 to induce CD4lomouse intrathymic progenitors to develop almost exclusively to DCs, although the T/B/NK precursor potential of such CD4lointrathymic precursors has been extensively reported.9-13Strikingly, addition of IL-2 to our cultures resulted in the simultaneous production of NK cells and DCs in similar proportions, but had no effect on the cellular yield, indicating that NK cell generation occurs at the expense of DCs. A more relevant finding was that greater than 90% of cells recovered at day 3 to 4 under both culture conditions were large-sized cells that displayed the non-T CD44bright phenotype. Therefore, upregulation of CD44 may be a necessary step common to the development of both DCs and NK cells. These CD44bright cells still expressed the CD34 molecule, albeit at reduced levels, indicating that they were relatively primitive progenitors, but they had acquired high CD33 and CD13 surface density. In contrast, the low proportion (<10%) of small-sized CD44lo T-lineage progenitors arising at early time points in our cultures displayed a CD34+CD33−CD13lo/−phenotype. It is worth noting that both the CD44bright and the CD44lo cell subsets were identified in vivo, thus providing additional support for their physiological relevance as intermediate progenitors in the human postnatal thymus. Although we cannot at present determine whether the CD44bright cells still retain the capacity to form T cells, this possibility seems very unlikely considering that progression to the CD44bright stage results in the downregulation of the T-cell–associated antigen CD5, whereas T-cell progenitors go through a reciprocal CD44loCD5+ stage. Such a differential expression of CD5 has proved particularly useful to trace T-cell and NK cell lineage development from a subset of CD34+CD5+CD33+CD13+fetal thymocytes shown to display bipotential T/NK precursor activity.15 These data had been taken as evidence that, once CD5 is lost from the cell surface, intrathymic precursors are no longer able to form T cells, but rather they display NK cell precursor potential. However, it remains to be determined whether the CD5− NK cell progenitors previously described15 are able to produce DCs as well.
We provide evidence that the CD34+CD44brightprogeny arising by day 3 to 4 under the cytokine-supported culture conditions used in this study is a homogeneous DC progenitor population (close to 100% clonogenic), able to form both DCs and NK cells upon addition of IL-2, and propose that such CD44bright cells include bipotential DC/NK cell progenitors. Interestingly, we found that progression of such progenitors to the NK cell pathway could be followed by the expression of the NKR-P1A receptor before acquisition of the CD56+ mature NK cell phenotype, indicating that these cells represent the thymic counterparts of a pre-NK cell developmental stage recently identified in vivo in human thymus21 as well as in adult and neonatal circulating lymphocytes.30 Similarly, pre-NK cell precursors have recently been identified in the mouse fetal thymus by the expression of a different member of the NKR-P1 gene family, NKR-P1C.32
Although the minimal cytokine requirements for the generation of NK cells have not been addressed in this study, our results support the notion that IL-2 is dispensable for the production of CD44brightNKR-P1A+CD56−intermediates with NK cell precursor potential. However, it is required for the progression of NKR-P1A+CD56−precursors to NKR-P1A+CD56+ mature NK cells. Thus, the effects of IL-2 in our NK cell differentiation system appear to be stage-specific. This supports the current view that NK cell progenitors at distinct developmental stages display differential cytokine requirements: the most mature NK precursors respond readily to IL-2, whereas more immature progenitors need complex mixtures of cytokines and/or stromal cells.33-36 It is worth noting that IL-15, a cytokine known to play a role in NK cell differentiation, has recently been reported to display similar stage-specific effects,36-38 this probably reflecting the fact that both IL-2 and IL-15 use the γ and β chains of the IL-2R. This concurs with our observation that, as shown for bone marrow CD34+ NK cell progenitors,34 the thymus-derived NK cell progenitors identified in this study become sensitive to IL-2 only after they reach the stage of differentiation at which they express the IL-2Rβγ (CD122) complex. Given the coincident expression kinetics of IL-2Rβγ and NKR-P1A, it can be speculated that signaling through the IL-2Rβγ complex is the key molecular event that determines the final developmental fate of NKR-P1A+ precursors. In addition, the finding that expression of the IL-2Rα (CD25) precedes that of the IL-2Rβγ, together with the fact that low doses of IL-2 promote efficient NK cell production, may suggest that IL-2 responsiveness in our cultures involves the high-affinity IL-2Rβγα. However, it is likely that, during the process of intrathymic NK cell development in vivo, IL-15 that is efficiently produced by the thymic epithelium,37rather than IL-2, is the physiological ligand that binds the IL-2Rβγ complex and promotes final differentiation of pre-NK cells to mature NK cells.
Taken together, our results provide evidence that the intrathymic DC and NK cell developmental pathways share a common transitional stage that is morphologically and phenotypically distinct from the most immature T-lineage precursors. However, recent results in mice by Wu et al19 indicate that there is a coincidence between the thymic DC precursors and the earliest T-cell precursors. Because it was shown in a previous study13 that the cellular precursors analyzed by Wu et al19 can also give rise to NK cells, such cells may thus represent tripotential T/DC/NK precursors. Although the T-cell precursor potential of the human DC/NK precursor subset described in this study remains to be examined, we favor the concept that, once the B-cell potential is lost, intrathymic NK cells and DCs branch off the T lineage through a common intermediate progenitor. It has to be stressed that, in support of this view, it has recently been shown that postnatal T-cell precursors, but neither NK cells nor DCs, are produced in mice mutant for the transcription factor Ikaros, indicating that Ikaros is essential for establishing early branch points into these two pathways from the main T-cell pathway in the postnatal thymus.39 The simultaneous development of NK cells and DCs may provide new insights into the physiological mechanisms that control the MHC-dependent NK cell education process in the thymus.40
ACKNOWLEDGMENT
The authors thank Drs J. Banchereau, J.C. Gutiérrez-Ramos, I. Mérida, J. Bodmer, and E. Leonardo for the generous gift of antibodies; Dr A. Alvarez for assistance with cell sorting; and the Pediatric Cardiosurgery Units from the Centro Especial Ramón y Cajal and Ciudad Sanitaria La Paz (Madrid) for the thymus samples.
C.M. and C.T. are joint first authors.
Supported in part by grants from Glaxo Wellcome S.A., Grants No. SAF95-0006 and SAF97-0161 from Comisión Interministerial de Ciencia y Tecnologı́a (CICYT), and Grant No. FIS-94/0266 from the Fondo de Investigaciones Sanitarias. The Centro de Biologı́a Molecular “Severo Ochoa” is partially supported by the Fundación Ramón Areces. C.M. has a postdoctoral contract from the Consejo Superior de Investigaciones Cientı́ficas (CSIC)-Fundación Ramón Areces, and C.T., J.M.F., and A.R.R. are fellows from FIS, Glaxo Wellcome S.A., and Ministerio de Educación y Ciencia, respectively.
Address reprint requests to Marı́a L. Toribio, PhD, Centro de Biologı́a Molecular “Severo Ochoa,” Universidad Autónoma de Madrid, Cantoblanco, 28049 Madrid, Spain.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal