Replication-deficient adenoviral vectors (AdVec), which infect cycling and noncycling cells with high efficiency, low toxicity, and ease of delivery, provide ideal vehicles to study the expression of regulatory genes controlling different stages of hematopoiesis. To examine the infection efficiency of AdVec in hematopoietic precursor and progenitor cells, we used a replication-deficient adenovector expressing the humanized form of the cDNA for green fluorescent protein (AdGFP), permitting assessment of infection efficiency and kinetics of transgene expression in viable hematopoietic cells using flow cytometry and fluorescence microscopy. Flow-cytometric analysis of ex vivo expanded hematopoietic precursor cells infected with a multiplicity of infection (MOI) of 100 of AdGFP show that 78% of megakaryocytic (CD41a+ and CD42b+) cells, 82% of dendritic (CD1a+) cells, 41% of RBC precursors (glycophorin A+), and 32% of monocytic (CD14+) cells expressed GFP. Nineteen percent ± 1% of freshly isolated CD34+ cells from peripheral blood leukapheresis products infected under the same conditions expressed GFP. Morphologic evaluation of ex vivo expanded, AdGFP-infected CD34+ cells showed normal maturation. The functional capacity of AdGFP-infected CD34+ cells was analyzed by quantifying clonogeneic efficiency and proliferative capacity. Infection of CD34+ progenitor cells with MOIs of 1 to 100 did not impair clonogeneic efficiency of CD34+ cells. However, MOI greater than 100 resulted in a significant inhibition of colony-forming unit–granulocyte/granulocyte-macrophage (CFU-G/GM) formation. In sequential dilution expansion over 3 weeks (Delta assay), the cytokine-driven proliferative potential of CD34+cells was not impaired following exposure to AdGFP at MOIs of 1 to 1,000. The GFP+ population expanded 10- to 15-fold at high MOIs (500 to 1,000), indicating multiple copies of the transgene in the initially infected CD34+ cells, which were expressed in subsequent progenies. These data show that AdVec deliver transgenes with high efficiency and low toxicity to hematopoietic progenitor and precursor cells. Introduction of marker genes such as GFP into hematopoietic cells by AdVec will provide a valuable system for study of development, homing, and trafficking of hematopoietic precursor and progenitor cells in vitro and in vivo. Furthermore, these results provide insights into the design of gene therapy strategies for treatment of hematologic disorders by AdVec.
INDUCED EXPRESSION OF regulatory or marker genes in hematopoietic cells provides a powerful tool for the study of regulation of hematopoietic progenitor- and precursor-cell proliferation, differentiation, maturation, and trafficking. Furthermore, overexpression of cytokines, lymphokines, surface receptors, and chemoresistance proteins in hematopoietic precursor cells may lead to new approaches for the treatment of neoplastic, inflammatory, or inherited hematologic disorders.
Transfer of genes by retroviral vectors requires cycling of the target cells to achieve integration and stable transgene expression. Such an approach is thus not suitable for targeting of differentiated, postmitotic hematopoietic precursor cells. Introduction of genes into such cells by traditional transfection techniques such as calcium phosphate, electroporation, or lipofection results in low transduction efficiency, significant toxicity, and cell loss.1-3Adenoviral vectors (AdVec) have several advantages over other strategies for gene delivery to hematopoietic precursor cells. AdVec do not require cycling of the target cell for gene transfer and their integrin-dependent mechanism for cell entry,4,5 as well as their efficient mechanism for gene delivery from the cell surface to the nucleus,6 renders them ideal candidate vectors for gene targeting into hematopoietic progenitor and precursor cells.
We evaluated transduction efficiency and duration of transgene expression mediated by AdVec expressing the jellyfish Aequorea victoria green fluorescent protein (AdGFP) in ex vivo expanded and terminally differentiated, postmitotic hematopoietic cells, as well as freshly isolated CD34+ progenitor cells. Recent reports have shown that adenoviral mediated gene transfer into hematopoietic progenitors using Escherichia coli β-galactosidase as a marker gene is feasible, although a high multiplicity of infection (MOI) was necessary to achieve adequate expression.7 8 Our data show that AdVec deliver and express transgenes efficiently in CD34+ progenitor cells, as well as in more mature hematopoietic precursor cells in a number of lineages. Maturation of precursor cells was not impaired and the proliferative capacity of CD34+ cells, measured by cloning efficiency and expansion potential, was not negatively influenced by the presence of the transgene. Our experimental design provides a new model to study the physiology of hematopoietic progenitor and precursor cells in vitro and in vivo. In addition, this model provides new insights into the design of gene therapy protocols for the treatment of hematologic disorders using transient gene expression mediated by AdVec.
MATERIALS AND METHODS
Purification of Human CD34+ Cells
Human CD34+ cells were purified from frozen leukapheresis products collected from patients with solid tumors after stem-cell mobilization with granulocyte colony-stimulating factor (G-CSF)/cyclophosphamide. Permission to obtain leukapheresed samples was obtained from the Sloan-Kettering Institute and Cornell University Medical College institutional review boards. Briefly, the units were thawed at 37°C and washed several times with phosphate-buffered saline (PBS)/0.1% fetal calf serum (FCS) at room temperature (23°C). The cells were then concentrated to 5 to 10 × 108cells/mL in PBS/0.1% FCS and incubated at 4°C for 30 minutes with monoclonal mouse antihuman CD34 antibodies, IgG1, clone 11.1.6 (developed by M.A.S. Moore, licensed to Organon Teknika, Cambridge, MA) at a concentration of 50 μg antibodies/108 cells. Thereafter, the cells were washed twice with PBS/0.1% FCS and incubated with magnetic immunobeads (30 μL/108 cells; Dynal beads, Dynal Inc, Oslo, Norway) at 4°C for 30 minutes. Then, the CD34+ cells, now rosetted by immunobeads, were collected using a magnetic particle concentrator (MPC; Dynal) and placed in Iscove's modified Dulbecco's medium (IMDM)/20% FCS at 37°C overnight. After the CD34+ cells were detached from the beads, the cells were collected in fresh IMDM/20% FCS. The purity of the CD34+ cell fraction ranged routinely between 85% to 95% as assessed by flow cytometry.
Ex vivo Expansion of CD34+ Cells
CD34+ cells (4 × 104 to 1 × 105/mL) were expanded in 12-well polystyrene plates (Corning, New York, NY) using conditions and cytokine cocktails designed to generate precursors of choice. Megakaryocytic (CD41a+) and erythrocytic precursors (glycophorin A+) were generated in serum-free medium X-Vivo 15 (Bio Whittaker, Walkersville, MD). For expansion of CD41a+cells, thrombopoietin (TPO) and c-kit ligand (KL) with either interleukin-3 (IL-3) or IL-6 were added to the medium. To generate erythrocytic precursor cells, erythropoietin (EPO) and KL were used. Myelomonocytic cells (CD14+/CD15+) were expanded in IMDM/20% FCS in the presence of G-CSF, KL, and IL-3 and dendritic cells were expanded in IMDM/20% FCS in the presence of KL, tumor necrosis factor-α (TNF-α), and granulocyte-macrophage colony-stimulating factor (GM-CSF).9-11 All expansion cultures were incubated at 37°C in 100% humidity, 5.2% CO2, and replenished with fresh medium containing the appropriate cytokines every 7 days.
Antibodies and Cytokines
Monoclonal antibodies.
All of the monoclonal antibodies used for flow cytometry experiments were directly conjugated with phycoerythrin (PE). Dendritic cells were detected by CD1a-PE (SK9, IgG2b; Becton Dickinson, San Jose, CA), monocytic cells by CD14-PE (UCHM-1, IgG2a; Sigma, St Louis, MO), myeloid cells by CD15-FITC (DU-HL60-3, IgM; Sigma), stem cells by CD34-PE (8G12, IgG1; Becton Dickinson), megakaryocytic precursor and progenitor cells by CD41a-PE (GPllbIllla, HIP8, IgG1; Pharmingen, San Diego, CA), mature megakaryocytes by CD42b-PE (GPIb SZ2, IgG1) and CD62-PE (GMP140, AC 1.2, IgG1; Becton Dickinson), and erythroid precursor cells by rhodamine-conjugated antibody to glycophorin (AD2, 10 Immunotech, Westbrook, ME).
Cytokines.
The following cytokines were used Kit ligand (20 ng/mL; Amgen, Thousand Oaks, CA), G-CSF (100 ng/mL; Amgen), GM-CSF (100 ng/mL; Sandoz, Basel, Switzerland), TPO (50 ng/mL; R&D Systems, Minneapolis, MN), IL-3 (50 ng/mL; Sandoz), IL-6 (20 ng/mL; Amgen); EPO (6 U/mL; Amgen), and TNF-α (100 ng/mL; Genentech, San Francisco CA).
AdVec Construction and Preparation
The humanized GFP cDNA12 was subcloned as a NotI fragment into the plasmid pCMVSV2+,13 which creates an expression cassette with the CMV immediate-early promoter and a synthetic splice site upstream of the gene and the SV40 early polyadenylation site downstream of the gene. In pCMVSV2+, the expression cassette lies between the left end of the adenovirus genome (nucleotides 1 to 355) and the truncated E1B, pIX region (nucleotides 3333 through 5790). Coinfection of human embryonic kidney (HEK) 293 cells with the pCMVSV2+ GFP plasmid and the adenoviral backbone prepared from delE3 adenovirus backbone plasmid14 produced a full-length, replication-incompetent, E1- and E3-deficient adenovector expressing GFP. AdGFP was prepared by expansion of a single plaque generated in HEK 293 cells, which gave fluorescence. Large-scale preparations were routinely tested for titer (plaque-forming units [PFU]) by plaquing on HEK 293 cells and replication-competent adenovirus (RCA) by plaquing on A549 cells.15 16 A PFU to RCA ratio of greater than 106 was considered acceptable. The absolute amount of RCA present in the preparations is less than 1 RCA in the total dose administered. This is the same criteria the Food and Drug Administration has established for clinical studies, and the method used to detect RCA is the same as we used for clinical preparations. There is no detectable RCA+ virus or E1+ GFP virus in the preparation, and assessment of the cultures demonstrated no RCA+ virus.
Adenovector Infection of Ex Vivo Expanded Hematopoietic Precursor Cells
On day 10 of expansion, the cells were incubated with AdGFP (12 hours at 37°C) at various MOIs in 200 to 300 μL of serum-free medium (X-Vivo 15; BioWhittaker, Walkersville, MD) in flat-bottom 12-well plates. Following incubation, the cells were resuspended in the original expansion medium, including cytokines and serum as appropriate, and expanded for 7 to 14 more days. Subsequently, aliquots of cells were stained with lineage-specific, fluorescein-conjugated antibodies and analyzed by two-color flow cytometry for GFP expression.
Flow Cytometry
Before flow cytometry, viability of the cells was routinely determined by trypan blue exclusion. On average, 80% to 95% of cells were viable. AdGFP-infected hematopoietic precursor cells were washed and stained (30 minutes at 4°C) with PE- or rhodamine 1 (RD1)-conjugated monoclonal antibodies. Noninfected ex vivo expanded hematopoietic cells were stained with PE- or fluorescein isothiocyanate (FITC) conjugated IgG control isotype. Immediately after staining, the cells were washed with PBS/0.1% bovine serum albumin (BSA) and analyzed by two-color flow cytometry using an Elite Profile II flow cytometer (Coulter, Hialeah, FL). For determination of cells coexpressing GFP and the lineage-specific marker, a fluorescence intensity 1/fluorescence intensity 2 (FL1/FL2) dot-plot display was used. Log FL1 (x-axis) indicates the fluorescence intensity of GFP+ cells. Log FL2 (y-axis) represents the fluorescence intensity of ex vivo expanded hematopoietic precursor cells labeled with the PE- or RD1-conjugated monoclonal antibody. GFP+ cells labeled with lineage-specific antibody are designated as FL1(GFP+) · FL2+ and GFP−cells labeled with lineage-specific antibody are designated as FL1(GFP−) · FL2+. The quantification of cells in the different subgroups was performed by analyzing at least 10,000 cells and the proportion of transgene-expressing lineage-differentiated cells was calculated using the following formula:
Cell Sorting of GFP-Expressing CD34+ Cells
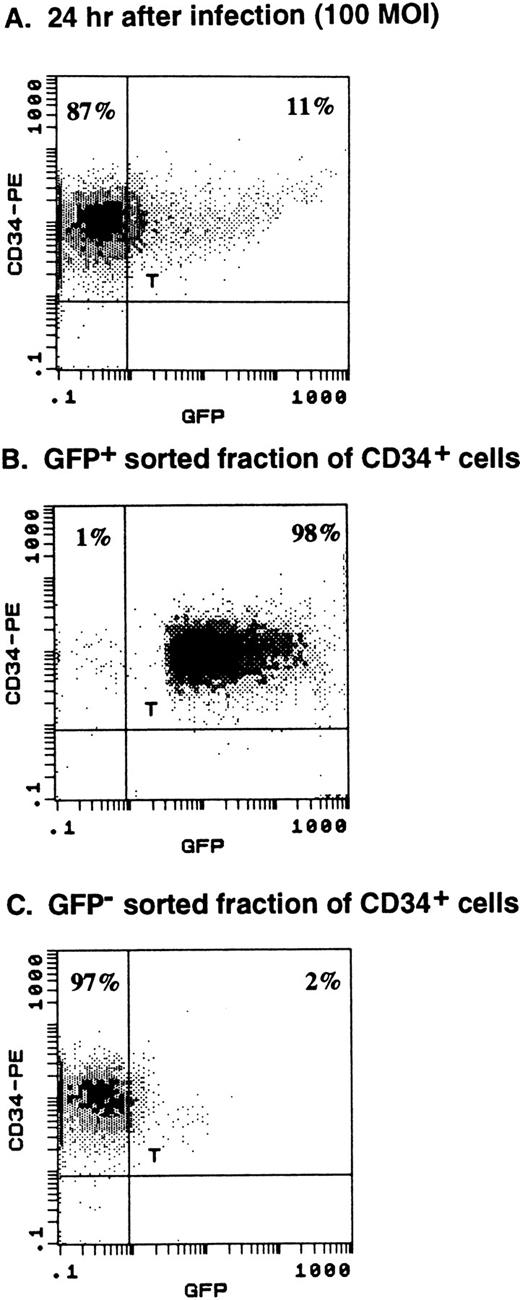
Purified human CD34+ cells (purity, 99.6%) were infected with AdGFP at MOI of 100 as described earlier. Twenty-four hours after infection, 11% of the CD34+ cells expressed GFP. Subsequently, the cells were stained with propidium iodine and the viable cells (>90%) were sorted into GFP+ and GFP− fractions using fluorescence-activated cell sorting (FACS Star Plus cell sorter and LYSIS Program; Becton Dickinson). Analysis after sorting showed a recovery of 70% of input CD34+ cells with viability of greater than 90% for both fractions.
Blockage of Vitronectin Receptor Before AdGFP Infection
CD41a+ cells expanded with TPO/KL/IL-6 were incubated with 0.1 μg/mL of monoclonal antibody to vitronectin receptor (VnR) (αvβ3 integrin, L203, a gift from T.J. Wickham, GenVec, Rockville, MD) for 2 hours before AdGFP infection. After washing, the cells were infected with AdGFP (100 MOI, 12 hours, 37°C). Twelve hours and 36 hours after infection, the expression of GFP by CD41a+ cells was analyzed by flow cytometry and compared with AdGFP-infected CD41a+ cells that were not preincubated with anti-VnR antibodies.
Morphology and Cell Counts
Liquid cultures and cytospin preparations were assayed by UV microscopy for intracellular GFP expression. Phase-contrast microscopy of liquid culture and light microscopy (Nikon, UFX-IIA) of Wright-Giemsa–stained cytospin preparations were used to document cell morphology and maturation features (camera, Nikon FX-35A; color print film, Kodak ASA 200 to 400). Cell numbers and viability of expanded cells were routinely determined by trypan blue exclusion using Neubauer hematocytometer chambers.
Southern Blot Analysis for Adenoviral DNA
Total DNA was extracted using protease K digestion and phenol-chloroform extraction. The DNA was digested over night withHindIII at 37°C. Ten micrograms of digested DNA were electrophoresed on a 1.2% agarose gel and subsequently transferred to nitrocellulose membrane. Hybridization (30 minutes at 65°C) was performed with a 32P-labeled adenovirus-specific probe detecting the 2.9-kb digestion product of the E4 gene.
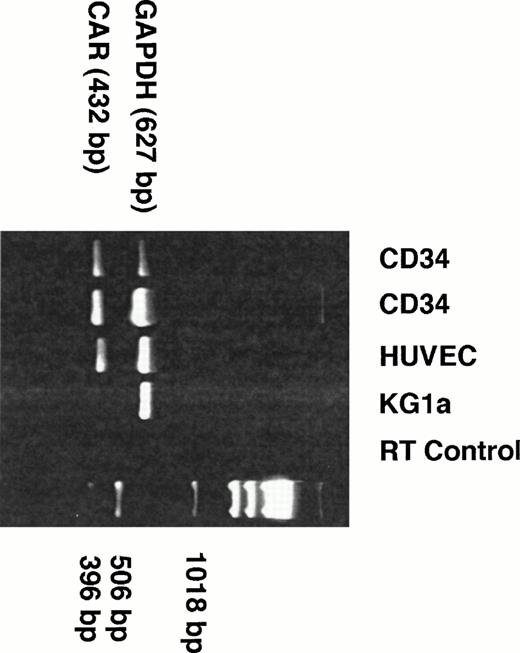
Semiquantitative Reverse-Transcriptase Polymerase Chain Reaction for the Common Coxsackie and Adenovirus Receptor (CAR)
Total RNA extracts were prepared from CD34+ cells and expanded CD41a+ cells, which were enriched for megakaryocytic cells to 98% purity using immunoaffinity columns.17 RNA was extracted using the Ultraspec RNA isolation system (Biotecx Laboratories, Houston, TX). Reverse-transcriptase polymerase chain reaction (RT-PCR) was performed for simultaneous amplification of the transcription products of the recently described common receptor for coxsackie B and adenovirus (CAR)18 and the housekeeping gene for human glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primers for CAR were designed using the cDNA sequence from the National Center for Biotechnology Information gene bank (accession no. Y07593) to provide an amplification product of 432 bp (5′-primer: TCTCATCTGTGCTCTCCGTG; 3′-primer: TAATTTGGGGGAGACTGGTG). The primers for GAPDH were designed using the gene sequence published at NCBI gene bank (accession no.M33197) to provide an amplification product of 627 bp (5′-primer: GGAAGGTGAAGGTCGGAGTC; 3′-primer: AACATCATCCCTGCCTCTAG). Briefly, primers were synthesized using 392 DNA/RNA Synthesizer (Applied Biosystem, Perkin-Elmer Co, Foster City, CA) oligo synthesizer and purified with NAP-25 columns (Pharmacia LKB Biotechnology, Arlington, IL) according to the manufacturer's instructions. Then, 1 μg of RNA was mixed with 5 U AMV RT (Promega, Madison, WI) and 5 U Tfl DNA polymerase (Promega) in the presence of dNTP mix and of 5× reaction buffer (Tris base 242 mg/mL, glacial acetic acid 57.1 μL/mL, 50 mmol/L EDTA, pH 8.0; Promega). For the RT reaction, the mixture was incubated at 48°C for 45 minutes followed by a denaturation step (94°C, 2 minutes) using a Perkin Elmer Thermocycler. Subsequently, 35 cycles of PCR reaction (94°C for 30 seconds, 56°C for 1 minute, and 68°C for 2 minutes) plus one cycle of extension (68°C for 7 minutes) were performed. The RT-PCR product was then electrophoresed on a 2% TBE-agarose gel and stained with ethidium bromide. RNA from CD34+ and CD41a+ cells was compared with RNA isolated from human umbilical cord vein endothelial cells (HUVEC; positive control) and the KG1a leukemia cell line (negative control). For control of the RT-reaction, RNA from the first sample of CD34+ cells was treated with RNAse H (Boehringer Mannheim, Indianapolis, IN) 1 U/μL at 37°C for 15 minutes before RT-PCR.
Clonogeneic Assay
CD34+ cells (103 cells/mL) were plated in IMDM/20% FCS and 0.36% agarose in the presence of KL, EPO, IL-3, IL-6, and G-CSF (Table 1) and incubated at 37°C in 100% humidity and 5.2% CO2 for 14 days. Colonies that consisted of more than 50 cells were quantified using an inverted microscope (×40).
Sequential Dilution Expansion (Delta Assay)
AdGFP-infected CD34+ cells (4 × 104cells/mL) were expanded for 7 days in IMDM/20% FCS in the presence of KL, IL-3, IL-6, G-CSF, and EPO. After 7 days, the cells were washed with IMDM/20% FCS and the viable cells were quantified using trypan blue exclusion. An aliquot of 4 × 104 cells was expanded again in the same conditions for another 7 days. This was repeated for three rounds and the cumulative number of cells produced over 21 days of expansion was calculated.19 Additionally, every week, the number of GFP+ cells was determined by flow cytometry and the cumulative number of GFP+ progeny generated during the expansion period was quantified.
Statistics
Results are shown as the mean ± SD of at least three experiments. For statistical comparison Student's t test for nonparametric data was used. For multivariable analysis, two-way analysis of variance (ANOVA) was performed.
RESULTS
Adenovirus Infection Efficiency of Hematopoietic Precursor Cells
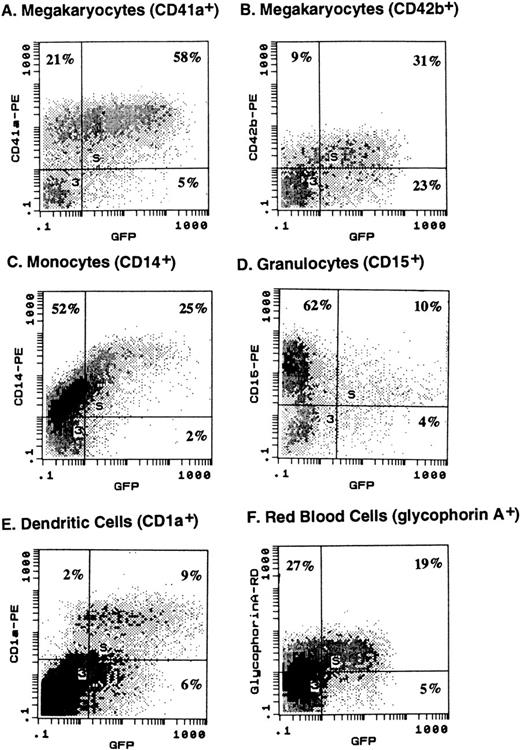
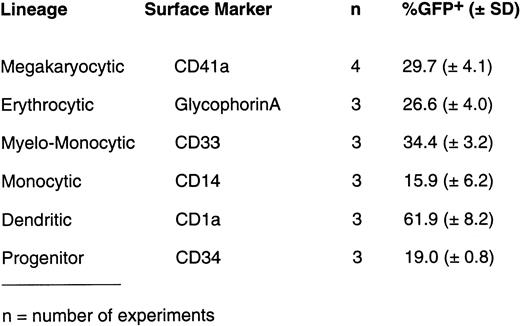
Human CD34+ cells purified from leukapheresed peripheral blood were expanded ex vivo for 10 days with various cytokine cocktails to generate cell populations enriched for lineage-committed precursor cells. Ex vivo expanded cells were then infected with different MOIs of AdGFP and analyzed for GFP expression by lineage-committed precursor cells using two-color flow cytometry 3 days after infection. A typical profile of GFP expression in different ex vivo expanded precursor cells with a MOI of 100 is shown in Fig 1A. Using PE-conjugated monoclonal antibody to different lineages, GFP expression (FITC expression) could be detected in 78% of megakaryocytic (CD41a+ and CD42b+) cells, 82% of dendritic (CD1a+) cells, 41% of RBC precursors (glycophorin A+), and 32% of monocytic (CD14+) cells. As summarized in Fig 1B, on average (n = 3 to 4), infection with 50 MOI of AdGFP resulted in GFP expression of 61.9% ± 8.2% of dendritic cells (CD1a+) generated with KL, GM-CSF, and TNF-α; 29.7% ± 4.1% of megakaryocytic cells (CD41a+) generated with TPO, KL, and IL-6; 15.9% ± 6.2% of monocytic cells (CD14+) obtained with GM-CSF, KL, and IL-3; and 26.6% ± 4.0% of glycophorin A+ RBC precursors obtained with KL and EPO. Higher MOIs (100 to 500) of AdGFP resulted in a higher proportion of cells expressing GFP.
(A) Expression of GFP by various hematopoietic precursor cells following infection with AdGFP. Human CD34+ cells were expanded in liquid culture in the presence of various cytokine cocktails for 10 days to generate lineage-committed precursor cells. Infection with AdGFP (100 MOI for 12 hours) was performed in serum-free medium. Seventy-two hours after infection, cells were stained with lineage-specific monoclonal antibody and evaluated for GFP expression by the respective differentiation lineage using two-color flow cytometry. Based on two-color dot-plot analysis, 73% of megakaryocytic (CD41a+) precursor (A), 78% of more mature megakaryocyte (CD42b+) (B), 32% of monocytic precursor (CD14+) (C), 14% of myeloid precursor cells (CD15+) (D), 85% of dendritic (CD1a+) (E) cells, and 41% of RBC precursors (glycophorin A+) (F) coexpressed GFP. (B) On average (n = 3 to 4), infection with 50 MOI of AdGFP resulted in GFP expression of 61.9% ± 8.2% of dendritic cells (CD1a+) generated with KL, GM-CSF, and TNF-α; 29.7% ± 4.1% of megakaryocytic cells (CD41a+) generated with TPO, KL, and IL-6; 15.9% ± 6.2% of monocytic cells (CD14+) obtained with GM-CSF, KL, and IL-3; and 26.6% ± 4.0% of glycophorin A+ RBC precursors obtained with KL and EPO.
(A) Expression of GFP by various hematopoietic precursor cells following infection with AdGFP. Human CD34+ cells were expanded in liquid culture in the presence of various cytokine cocktails for 10 days to generate lineage-committed precursor cells. Infection with AdGFP (100 MOI for 12 hours) was performed in serum-free medium. Seventy-two hours after infection, cells were stained with lineage-specific monoclonal antibody and evaluated for GFP expression by the respective differentiation lineage using two-color flow cytometry. Based on two-color dot-plot analysis, 73% of megakaryocytic (CD41a+) precursor (A), 78% of more mature megakaryocyte (CD42b+) (B), 32% of monocytic precursor (CD14+) (C), 14% of myeloid precursor cells (CD15+) (D), 85% of dendritic (CD1a+) (E) cells, and 41% of RBC precursors (glycophorin A+) (F) coexpressed GFP. (B) On average (n = 3 to 4), infection with 50 MOI of AdGFP resulted in GFP expression of 61.9% ± 8.2% of dendritic cells (CD1a+) generated with KL, GM-CSF, and TNF-α; 29.7% ± 4.1% of megakaryocytic cells (CD41a+) generated with TPO, KL, and IL-6; 15.9% ± 6.2% of monocytic cells (CD14+) obtained with GM-CSF, KL, and IL-3; and 26.6% ± 4.0% of glycophorin A+ RBC precursors obtained with KL and EPO.
Phenotypic and functional maturation of hematopoietic precursor cells is controlled in part by the cytokine milieu.29 The influence of cytokines on infectibility of hematopoietic precursor cells was studied on megakaryocytes (CD41a+). For all vector MOIs used (10 to 500), CD41a+ cells generated in the presence of IL-6/TPO/KL showed the most intense fluorescence as compared with CD41a+ cells grown in the presence of IL-3/TPO/KL or TPO/KL (data not shown). This suggests that either the vector load per cell or transgene expression or both were enhanced at a given MOI in the presence of IL-6 as compared with other cytokine combinations.
Seven days after infection of CD41a+ cells with AdGFP, the viability as assessed by trypan blue exclusion and propidium iodide staining was between 80% and 90% over a range of MOIs of 10 to 500. There was no difference in cell viability in three different cytokine combinations used for expansion of the megakaryocytes. Southern blot analysis was performed on total DNA extracts from megakaryocytes 7 days after infection; the amount of adenoviral DNA recovered was proportional to the initial MOIs.
Kinetics of AdGFP Expression in Megakaryocytic and Erythroid Progenitor Cells
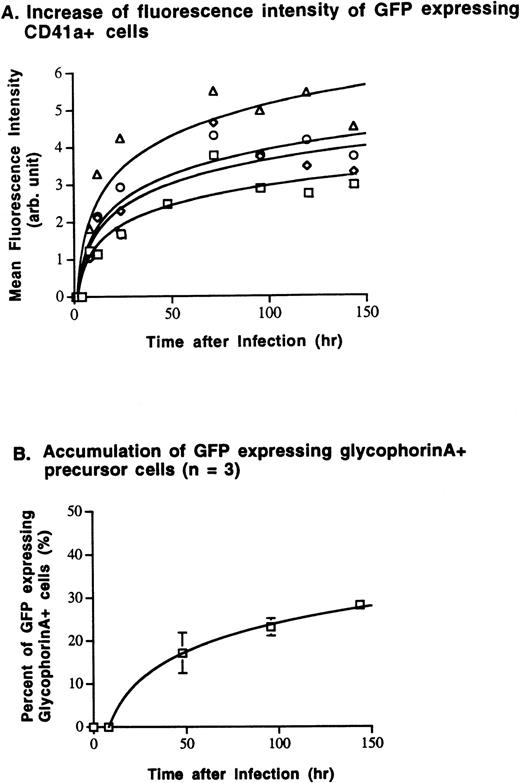
The expression of the transgene over time was investigated in megakaryocytic (CD41a+) and erythrocytic (glycophorin A+) cells. After a lag phase of 2 to 4 hours following infection, the number of GFP+ CD41a+ cells, as well as the intensity of GFP expression (mean fluorescence intensity), increased as a function of time after infection and of the MOI used for infection (Fig 2). Seventy-two to 96 hours after infection, the number of GFP-expressing cells and their fluorescence intensity plateaued. Subsequently, the number of GFP-expressing megakaryocytes decreased, particularly if the cells were exposed to high MOI levels (100 to 500) (Fig 2A). The number of GFP-expressing glycophorin A+ cells increased with similar kinetics, reaching a maximum 120 to 144 hours after infection and remaining unchanged thereafter (Fig 2B).
Kinetics of GFP expression studied in megakaryocytic and erythrocytic precursor cells. Megakaryocytic and RBC precursor cells were infected with various MOIs of AdGFP on day 10 of expansion. Subsequently, expression of GFP by lineage-committed cells was analyzed using two-color flow cytometry. (A) After a lag phase of 2 to 4 hours following infection, mean GFP fluorescence intensity (mean Log FL1) and the number of GFP-expressing megakaryocytes (CD41a+) increased depending on the MOI used for infection and time after infection. Steady-state expression of GFP in CD41a+ was reached 72 to 96 hours after infection. (B) Glycophorin A+ RBC precursor cells expressed GFP with similar kinetics. Following infection with an MOI of 100, the number of GFP+/glycophorin A+ cells increased continuously after a lag phase of 8 hours. Maximal transgene expression is reached 96 to 144 hours after infection. (□, MOI 10; ◊, MOI 50; ○, MOI 100; ▵, MOI 500).
Kinetics of GFP expression studied in megakaryocytic and erythrocytic precursor cells. Megakaryocytic and RBC precursor cells were infected with various MOIs of AdGFP on day 10 of expansion. Subsequently, expression of GFP by lineage-committed cells was analyzed using two-color flow cytometry. (A) After a lag phase of 2 to 4 hours following infection, mean GFP fluorescence intensity (mean Log FL1) and the number of GFP-expressing megakaryocytes (CD41a+) increased depending on the MOI used for infection and time after infection. Steady-state expression of GFP in CD41a+ was reached 72 to 96 hours after infection. (B) Glycophorin A+ RBC precursor cells expressed GFP with similar kinetics. Following infection with an MOI of 100, the number of GFP+/glycophorin A+ cells increased continuously after a lag phase of 8 hours. Maximal transgene expression is reached 96 to 144 hours after infection. (□, MOI 10; ◊, MOI 50; ○, MOI 100; ▵, MOI 500).
Blockage of Adenovector Infection by Anti-VnR Antibodies
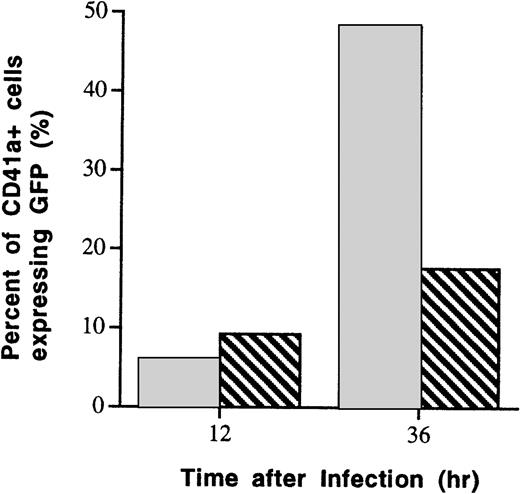
AdVec use a variety of integrins expressed on hematopoietic cells for attachment and cell entry. Megakaryocytes express αvβ3 integrins (VnR), which function as adenoviral vector coreceptors.20 21 CD41a+cells were infected with AdGFP (MOI, 100) after preincubation with blocking monoclonal antibody (L203) to VnR. Subsequently, infection efficiency was assessed by flow cytometry. Compared with CD41a+ cells that had not been preincubated with VnR monoclonal antibody, blockage of αvβ3integrins partially inhibited the infection of CD41a+ cells. As shown in Fig3, 36 hours after AdGFP infection, the frequency of GFP-expressing CD41a+ cells was reduced from 50% (no preincubation with antibodies) to 15% (preincubation with 0.1 μg/mL of VnR monoclonal antibody).
Suppression of AdGFP infection by blocking of VnR (αvβ3 integrin). VnR expressed on megakaryocytic precursor cells was blocked with a saturable dose of monoclonal antibody to VnR (L203) before incubation of the cells with AdGFP (500 MOI). As assessed by flow cytometry 36 hours after infection, the number of GFP-expressing megakaryocytic cells (CD41a+) was reduced from 50% in control culture to 18% in culture that was preincubated with VnR antibodies. (□) Preincubation with anti-VnR antibody (0.1 μg/mL). (▧) No anti-VnR antibody.
Suppression of AdGFP infection by blocking of VnR (αvβ3 integrin). VnR expressed on megakaryocytic precursor cells was blocked with a saturable dose of monoclonal antibody to VnR (L203) before incubation of the cells with AdGFP (500 MOI). As assessed by flow cytometry 36 hours after infection, the number of GFP-expressing megakaryocytic cells (CD41a+) was reduced from 50% in control culture to 18% in culture that was preincubated with VnR antibodies. (□) Preincubation with anti-VnR antibody (0.1 μg/mL). (▧) No anti-VnR antibody.
Infection Efficiency of Purified CD34+ Cells
A maximal expression of GFP in 19% ± 1% of CD34+ cells was achieved with an MOI of 50, 72 hours after infection (Fig 1B). Twenty-four hours after infection (MOI, 100), CD34+ cells expressing GFP were sorted by FACS. As shown in Fig4A, 11% of the CD34+ cells expressed GFP. Analysis after sorting showed 98% of positively sorted cells expressing GFP (Fig 4B). The assessment of viability of GFP+ and GFP− fraction by trypan blue exclusion showed greater than 90% viable cells in both populations.
Cell sorting of GFP-expressing CD34+ cells. Purified CD34+ cells were infected with 100 MOI of AdGFP and analyzed 24 hours after infection for GFP expression. (A) Eleven percent of CD34+ cells expressed GFP, allowing sorting of the cells into GFP+ and GFP− fractions. (B) Reanalysis after cell sorting showed 98% purity of the GFP-expressing population of CD34+ cells. (C) The GFP−fraction was 97% pure.
Cell sorting of GFP-expressing CD34+ cells. Purified CD34+ cells were infected with 100 MOI of AdGFP and analyzed 24 hours after infection for GFP expression. (A) Eleven percent of CD34+ cells expressed GFP, allowing sorting of the cells into GFP+ and GFP− fractions. (B) Reanalysis after cell sorting showed 98% purity of the GFP-expressing population of CD34+ cells. (C) The GFP−fraction was 97% pure.
Proliferative Potential and Plating Efficiency of AdGFP-Infected CD34+ Cells
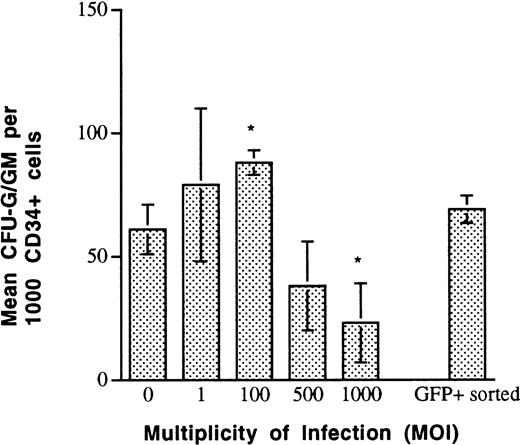
The progenitor content of AdGFP-infected CD34+ cells was determined by clonogeneic assay in 0.36% agarose. The plating efficiency of unsorted, adenovector-exposed CD34+ cells remained unchanged over a range of 0 to 100 MOI (Fig5). At an MOI of 1,000, there was a significant decrease in clonogeneic capacity to 20% to 30% of noninfected CD34+ cells. FACS-sorted 98% pure CD34+/GFP+ cells that were previously exposed to AdGFP with an MOI of 100 maintained normal plating efficiency (Fig5).
Plating efficiency of AdGFP-infected CD34+cells measured by colony-forming assays performed in 0.36% agarose, in the presence of IL-3, IL-6, G-CSF, KL, and EPO. CD34+cells that were exposed to AdGFP at MOIs <500 had normal plating efficiency. Cells exposed to MOIs >500 demonstrated significant reduction of colony formation. Cell-sorted CD34+GFP+ cells that were exposed to AdGFP (MOI, 100) before cell sorting, demonstrated normal plating efficiency, suggesting that an adenovector load corresponding to an MOI of 100 does not negatively influence primary plating efficiency of CD34+ cells (*P < 0.05 v MOI of 0).
Plating efficiency of AdGFP-infected CD34+cells measured by colony-forming assays performed in 0.36% agarose, in the presence of IL-3, IL-6, G-CSF, KL, and EPO. CD34+cells that were exposed to AdGFP at MOIs <500 had normal plating efficiency. Cells exposed to MOIs >500 demonstrated significant reduction of colony formation. Cell-sorted CD34+GFP+ cells that were exposed to AdGFP (MOI, 100) before cell sorting, demonstrated normal plating efficiency, suggesting that an adenovector load corresponding to an MOI of 100 does not negatively influence primary plating efficiency of CD34+ cells (*P < 0.05 v MOI of 0).
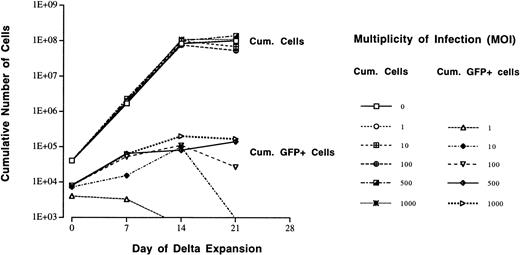
The proliferative capacity of AdGFP-infected CD34+ cells was analyzed in a cytokine-driven sequential dilution expansion assay over 3 weeks (Delta assay19). Before expansion, CD34+ cells were exposed to AdGFP using MOIs of 0 to 1,000. The proliferative capacity of CD34+ cells remained unchanged independent of the dose of AdGFP used for infection and showed a cumulative expansion of 2,000- to 3,000-fold over 21 days (Fig6). Only CD34+ cells that were exposed to a high MOI (>100) cumulatively generated increasing numbers of GFP+ progeny (expansion, 10- to 15-fold). If CD34+ cells were exposed to lower MOIs (<100), there was no detectable expansion of GFP+ cells or they disappeared completely during cell expansion.
Sequential dilution expansion (Delta assay) of AdGFP-infected CD34+ cells in the presence of IL-3, IL-6, G-CSF, KL, and EPO. Increasing concentrations of AdGFP (MOI of 0 to 1,000) used for infection of CD34+ cells before expansion did not impair the proliferative capacity of the cells. In all conditions, the cells cumulatively expanded by 3 log. The cumulative number of GFP-expressing progeny derived from AdGFP-infected CD34+ cells was measured throughout the expansion culture. If MOIs >100 were used for infection of CD34+cells, moderate expansion (1 log) of GFP+ progeny was observed. However, with lower MOIs (<100) used for infection, the number of GFP+ progeny did not proportionally increase during expansion.
Sequential dilution expansion (Delta assay) of AdGFP-infected CD34+ cells in the presence of IL-3, IL-6, G-CSF, KL, and EPO. Increasing concentrations of AdGFP (MOI of 0 to 1,000) used for infection of CD34+ cells before expansion did not impair the proliferative capacity of the cells. In all conditions, the cells cumulatively expanded by 3 log. The cumulative number of GFP-expressing progeny derived from AdGFP-infected CD34+ cells was measured throughout the expansion culture. If MOIs >100 were used for infection of CD34+cells, moderate expansion (1 log) of GFP+ progeny was observed. However, with lower MOIs (<100) used for infection, the number of GFP+ progeny did not proportionally increase during expansion.
Morphologic Assessment of AdGFP-Infected Precursor Cells
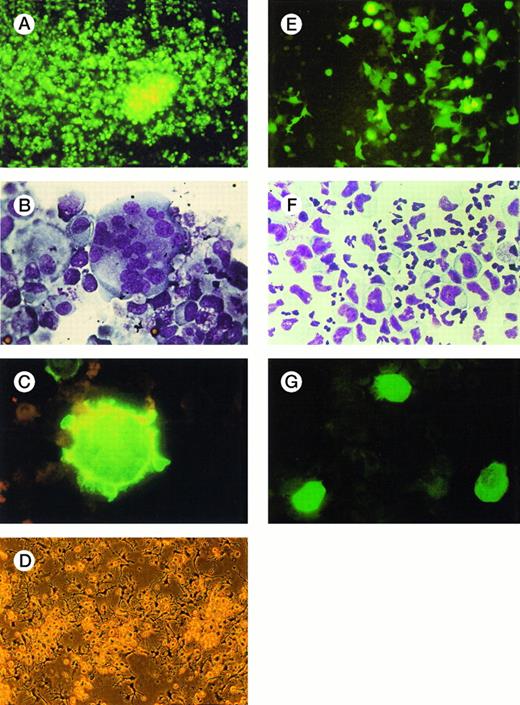
Labeling of hematopoietic cells with GFP facilitates morphologic evaluation of stability of transgene expression and the fate of infected precursor cells over time during ex vivo expansion. Ninety-six hours after infection of CD41a+ megakaryocytes, the majority of the cells showed intensive GFP expression (Fig7A). Cytospin preparations of the same expansion culture confirmed the presence of normal, polyploid megakaryocytes (Fig 7B) that elicited bright green fluorescence upon excitation with UV light (Fig 7C). Morphologic details, such as cytoplasm pseudopods, blebs, and platelet-like particles, were recognizable. Dendritic cells in liquid culture generated from CD34+ cells with GM-CSF, KL, and TNF-α showed typical maturation features, such as long cytoplasm veils (Fig 7D), and displayed striking cytoplasmic GFP expression in UV light (Fig 7E). In expansion cultures of myelomonocytic cells (Fig 7F), mainly monocytic cells showed bright GFP expression (Fig 7G).
Morphologic analysis of hematopoietic precursor cells several days after infection with AdGFP. Forty-eight to 96 hours after infection of expanded precursor cells with AdGFP, fluorescence and light microscopic analysis of liquid cultures and cytospin preparations were performed. (A) Megakaryocytic cells in liquid culture demonstrated bright fluorescence 48 hours after infection (100 MOI). (B) Cytospin preparations of the same culture confirmed the presence of large multilobulated and monolobulated megakaryocytes (Wright-Giemsa staining; original magnification ×1,000). (C) Examined under UV light, these cells were brightly fluorescing (×1,000). Cytoplasm pseudopods and blebs, typical features of polyploid mature megakaryocytes, are recognizable. (D) Liquid culture of dendritic cells shown in phase-contrast microscopy before infection. (E) The same cell culture 48 hours after infection with 100 MOI of AdGFP shows numerous dendritic cells expressing GFP (×100). (F) Cytospin preparations of myelomonocytic expansion cultures 72 hours after infection (100 MOI) show morphologically normal granulocytic and monocytic precursor cells (Wright/Giemsa staining, ×600). (G) Examined with UV microscopy, mainly large monoblastic cells expressed GFP.
Morphologic analysis of hematopoietic precursor cells several days after infection with AdGFP. Forty-eight to 96 hours after infection of expanded precursor cells with AdGFP, fluorescence and light microscopic analysis of liquid cultures and cytospin preparations were performed. (A) Megakaryocytic cells in liquid culture demonstrated bright fluorescence 48 hours after infection (100 MOI). (B) Cytospin preparations of the same culture confirmed the presence of large multilobulated and monolobulated megakaryocytes (Wright-Giemsa staining; original magnification ×1,000). (C) Examined under UV light, these cells were brightly fluorescing (×1,000). Cytoplasm pseudopods and blebs, typical features of polyploid mature megakaryocytes, are recognizable. (D) Liquid culture of dendritic cells shown in phase-contrast microscopy before infection. (E) The same cell culture 48 hours after infection with 100 MOI of AdGFP shows numerous dendritic cells expressing GFP (×100). (F) Cytospin preparations of myelomonocytic expansion cultures 72 hours after infection (100 MOI) show morphologically normal granulocytic and monocytic precursor cells (Wright/Giemsa staining, ×600). (G) Examined with UV microscopy, mainly large monoblastic cells expressed GFP.
CD34+ Cells Express CAR
CD34+ cells do not express αvβ3or αvβ5 integrins.22,23Therefore, we speculated that the CD34+ population may express the recently described common receptor for coxsackie B virus and adenovirus (CAR).18 In this regard, we performed RT-PCR on RNA derived from purified populations of CD34+ cells. As controls, RNA derived from HUVEC (positive control) and RNA from the leukemic cell line KG1a, which is not infectable with AdVec vectors even at MOIs greater than 500 (negative control), were used. RT-PCR performed with RNA extracts from two different samples of CD34+ cells was positive for the expected amplification product for CAR of 432 bp (Fig8).
CD34+ cells express CAR. RNA extracts from isolated human CD34+ cells were subjected to 35 cycles of RT-PCR reaction coamplifying CAR18 and GAPDH (see Materials and Methods). For comparison, RNA from HUVEC (positive control) and from the human leukemic cell line KG1a (negative control) were included in this experiment. RT control was performed with RNA from CD34+ cells that were treated with RNAse H before RT-PCR. Two different samples of peripheral blood CD34+ cells (lanes 1 and 2), as well as HUVEC (lane 4), demonstrated a strong signal of expected size (432 bp) for CAR. The presence of GAPDH (627 bp) in all samples demonstrates equal representation of mRNA from each sample.
CD34+ cells express CAR. RNA extracts from isolated human CD34+ cells were subjected to 35 cycles of RT-PCR reaction coamplifying CAR18 and GAPDH (see Materials and Methods). For comparison, RNA from HUVEC (positive control) and from the human leukemic cell line KG1a (negative control) were included in this experiment. RT control was performed with RNA from CD34+ cells that were treated with RNAse H before RT-PCR. Two different samples of peripheral blood CD34+ cells (lanes 1 and 2), as well as HUVEC (lane 4), demonstrated a strong signal of expected size (432 bp) for CAR. The presence of GAPDH (627 bp) in all samples demonstrates equal representation of mRNA from each sample.
DISCUSSION
Noncycling hematopoietic cells are resistant to stable transgene expression using currently available gene-transfer techniques. Introduction of transgenes with standard techniques, including calcium phosphate transfection, lipofection, or electro-poration, are inefficient, induce significant cell loss, and result in transient gene expression.1-3 Unlike retrovirally mediated gene transfer, AdVec infect noncycling cells without the need for significant physical manipulation and pathogenicity to the target cell. In this regard, AdVec provide suitable vehicles for transferring genes into hematopoietic progenitor cells and their precursors.
There are several lines of evidence indicating the susceptibility of hematopoietic cells to AdVec infection. First, adenovirus binds to integrins expressed on certain hematopoietic precursor cells and uses these adhesion molecules for cell entry.24 Second, cells of hematopoietic origin play an important role as a reservoir for adenoviruses in immunocompromised individuals.25 26 Third, in this report, we show that various molecules, known to function as AdVec receptors, are expressed on CD34+ progenitors, as well as on differentiated progeny.
To assess infection efficiency and subsequent transgene expression kinetics in human hematopoietic progenitor and precursor cells, we used a replication-deficient adenovector expressing the humanized GFP gene derived from the jellyfish Aequorea victoria that is driven by CMV promoter. The intracellular expression of GFP facilitates real-time analysis and quantification of infected cells by fluorescence microscopy, flow cytometry, and functional analysis of the infected cells following FACS sorting.12 27
To evaluate infection and expression efficiency of adenovirally delivered transgenes in progenitor and lineage-committed hematopoietic cells, we infected ex vivo expanded hematopoietic cells of various lineages with AdGFP. Purified human peripheral blood CD34+cells were expanded in the presence of cytokine combinations designed to promote lineage-specific differentiation. On day 10 of expansion, the cells were infected with AdGFP at MOIs of 10 to 500. Relative to erythroid and myeloid lineages, AdGFP showed higher affinity for megakaryocytic and dendritic cells. At MOIs greater than 100, more than 80% of dendritic cells and 75% of megakaryocytes expressed GFP. Myeloid and monocytic precursors showed the lowest infection efficiency, while glycophorin A+ RBC precursor cells showed intermediate susceptibility of infection to AdVec.
Time-course studies performed with megakaryocytic and glycophorin A+ RBC precursors showed maximal transgene expression occurring 72 to 96 hours after infection, resulting in 10% to 60% transduced cells depending on the MOI of AdGFP used. Due to maturation and disintegration of megakaryocytes into proplatelets,52the relative number of GFP expressing CD41a+ cells decreased later on. In contrast, the proportion of transduced RBC precursors remained unchanged upon reaching maximal expression over at least 6 days.
Dendritic cells represent one of the most versatile type of antigen-presenting cells (APC) that reside in bone marrow, skin, parenchymatous organs, and lymphoid tissue. By circulation in the blood stream dendritic cells and their precursor cells orchestrate cellular immunity through interaction with T cells. Dendritic cells are characterized by a unique morphology and repertoire of cell-surface molecules, including major histocompatibility antigens (MHC) class I and II, adhesion, and costimulatory molecules.30 Ex vivo expansion of large numbers of functionally active dendritic cells10,11,31-33 has sparked interest in the potential of genetically modified dendritic cells for the treatment of cancer, autoimmune diseases, and human immunodeficiency virus.34AdVec deliver and express transgenes with high efficiency into dendritic cells.54 55 Forced expression of target antigens in dendritic cells using AdVec provides a novel strategy to engineer dendritic cells for these therapies.
Infection of hematopoietic precursor cells with AdVec does not impair the final differentiation of the targeted cells. Despite the presence and expression of the GFP gene, precursor cells underwent normal differentiation into megakaryocytes, neutrophils, monocytes, nucleated RBCs, and dendritic cells. The amount of transgene recovered from megakaryocytes 7 days after infection correlated with the vector dose used for infection, suggesting stable coexistence of the transgene in maturing precursor cells. Based on these findings, adenovirally mediated transgene expression in hematopoietic cells can lead to measurable amount of transgene product in postmitotic, mature hematopoietic cells and their progeny. This may have therapeutic implications for the treatment of inherited hematologic disorders, such as sickle cell disease and hemophilia, where transient and high level expression of normal hemoglobin in RBCs or clotting factors in platelets may alleviate complications of the underlying genetic disorders.
Our results show that purified CD34+ cells can be reproducibly infected with AdGFP. Overall, we found a saturable infection efficiency of CD34+ cells of 20% to 25%, which was accomplished by using adenovirus doses of 50 to 100 MOI. Plating efficiency of AdGFP-infected CD34+ cells is not diminished by virus load corresponding to an MOI of 1 to 100. However, a higher MOI (>500) leads to significantly reduced colony formation. These results are in agreement with Watanabe et al7 and Neering et al,8 who have shown, that 6% to 45% of CD34+ cells obtained from bone marrow or peripheral blood can be infected with AdVec expressing Escherichia coliβ-galactosidase using high MOIs (>500) of AdVec.
However, the fate of adenoviral transgene in transduced CD34+ progenitor cells during the process of proliferation and differentiation has not well studied. Adenoviral transgenes delivered to CD34+ progenitor cells remain epichromosomal and are not duplicated during cell division. Since it is difficult to study the fate of adenoviral transferred gene to the CD34 progeny by β-galactosidase, we took advantage of GFP expression in viable replicating progenitor cells to evaluate the adenoviral gene expression in proliferating CD34+ progenitor cells. We show in Delta expansion cultures of infected CD34+ cells that progeny derived from GFP+ CD34+ cells express GFP if high MOIs (>100) are used for infection of the original CD34+ cells, suggesting that the introduced transgenes were transferred from CD34+ cells to the daughter cells. The expansion capacity of the whole population of CD34+ cells that were exposed to AdGFP was 2,000- to 3,000-fold and independent of the MOI used for infection of the CD34+ cells (MOI, 1 to 1,000). In summary, this implies that adenovirally infected CD34+ cells disappear during cell expansion, either by loss of the transgene during cell division or by dilution and overgrowth of the GFP+ cells with noninfected cells. Therefore, adenoviral gene transfer for therapeutic purposes may achieve optimal results if mature postmitotic precursor cells are targeted. On the other hand, AdVec used for tumor-purging purposes should be approached with caution, since uncommitted CD34+ cells can demonstrate significant susceptibility to AdVec infection and toxicity upon exposure to higher MOIs (>100).
Infection efficiency of hematopoietic cells varies considerably in the different lineages. This may be explained by the differential expression of integrins on the cell surface of various hematopoietic cell populations.35-48 Expression of αv and β3/β5 integrins by hematopoietic cells may play a critical role in adenovirus entry into precursor cells.4,20,24 During differentiation of uncommitted hematopoietic progenitors into lineage-restricted precursors and mature hematopoietic cells, the expression of integrins is controlled in part by cytokines released by the stromal cells in the bone marrow microenvironment.49,51 Megakaryocytic and dendritic precursor cells express integrins35,36,39,43 46 and were found to be most susceptible to AdVec infection. Our finding that uptake of AdGFP by megakaryocytes is only partially inhibited by αvβ3 antibodies imply that adenovirus internalization by megakaryocytes may partially be mediated through integrins.
We found that mRNA of the recently described CAR18 is expressed by peripheral blood CD34+ cells. Transfection with CAR has been shown to enhance the susceptibility of rodent cells to transduction by AdVec. Expression of CAR protein may thus be important for gene delivery to CD34+cells.18 53 Differential expression of CAR and integrins may account for the broad range of susceptibility for adenovector infection observed in the different stages of differentiation and maturation of hematopoietic precursor cells. Identification of factors that modulate CAR and integrin expression may allow augmentation of infectibility of hematopoietic precursor and progenitor cells with AdVec.
GFP cDNA delivered by AdVec provides an excellent tool for quantifying infection and expression efficiency of these vectors over time. Labeling of hematopoietic cells, particularly megakaryocytic progenitor and precursor cells, with GFP in vitro and in vivo will allow studies that will lead to new insights into proliferation, maturation, trafficking, and intercellular interactions of various hematopoietic subpopulations. Such studies will be facilitated by the fact that GFP-expressing hematopoietic cells maintain their functional capacity, can be FACS sorted, and can ultimately be used for proliferation and differentiation assays. Furthermore, based on the presented data, we speculate that transient expression of therapeutic transgenes in terminally differentiated hematopoietic cells using an adenoviral strategy may be used for the treatment of various inherited and acquired disorders. High-level expression of therapeutic proteins such as cytokines, growth factors, hemoglobin, clotting factors, receptor molecules, and tumor antigens in terminally differentiated hematopoietic cells may become applicable for the treatment of acute and chronic hematologic and infectious disorders, as well as lymphoproliferative and myeloproliferative diseases.
B.M.F. was the recipient of a stipend of the Dr Arnold U. und Susanne Huggenberger-Bischoff Stiftung zur Krebsforschung (Krebsstiftung), Zürich, and is supported by the Fondazione San Salvatore, Lugano (Switzerland). S.R. is supported by National Institutes of Health Grants No. K08HL 02926 and R01-HL-58707-01, the Dorothy Rodbell Foundation for Sarcoma Research, and the Rich Foundation. M.A.S.M. was supported by R01-DK-42693, R01-CA-59461, R01-HL-46546, and Gar Reichman Fund of the Cancer Research Institute. R.G.C. is supported by the National Heart, Lung and Blood Institute Cystic Fibrosis Gene Therapy Program Project Grant No. P01 HL 51746-01, the Cystic Fibrosis Foundation, the Will Rogers Memorial Fund, White Plains, NY, and GenVec, Inc, Rockville, MD.
Address reprint requests to Shahin Rafii, MD, Cornell University Medical College, Hematology-Oncology Division, 1300 York Ave, Room C-616, New York, NY 10021; e-mail: srafii@Mail.Med.Cornell.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal