Understanding anti-Rh(D) antibodies on a molecular level would facilitate the genetic analysis of the human immune response to Rh(D), lead to the design of therapeutically useful reagents that modulate antibody binding, and provide relevant information regarding the structural organization of Rh(D) epitopes. Previously, we described a Fab/phage display-based method for producing a large array of anti-Rh(D) antibodies from the peripheral blood lymphocytes of a single alloimmunized donor. In the current study, we present a detailed analysis of 83 randomly selected clones. Sequence analysis showed the presence of 28 unique γ1 heavy chain and 41 unique light chain gene segments. These paired to produce 53 unique Fabs that had specificity for at least half of the major Rh(D) epitopes. Surprisingly, despite this diversity, only 4 closely related heavy chain germline genes were used (VH3-30, VH3-30.3, VH3-33, and VH3-21). Similarly, nearly all Vκ light chains (15/18) were derived from one germline gene (DPK9). λ light chains showed a more diverse VL gene usage, but all (23/23) used the identical Jλ2 gene. Several Fabs that differed in epitope specificity used identical heavy chains but different light chains. In particular, 2 such clones differed by only 3 residues, which resulted in a change from epD2 to epD3 specificity. These results suggest a model in which footprints of anti-Rh(D) antibodies are essentially identical to one another, and Rh(D) epitopes, as classically defined by panels of Rh(D) variant cells, are not discrete entities. Furthermore, these data imply that the epitope specificity of an anti-Rh(D) antibody can change during the course of somatic mutation. From a clinical perspective, this process, which we term epitope migration, has significance for the design of agents that modulate antibody production and for the creation of mimetics that block antibody binding in the settings of transfusion reactions and hemolytic disease of the newborn.
CLINICALLY, THE HUMAN Rh(D) antigen is the most important red blood cell (RBC) membrane protein in transfusion medicine. The alloimmune response against Rh(D) produces high-affinity IgG antibodies that cause hemolytic transfusion reactions and hemolytic disease of the newborn (HDN). The prophylactic use of Rh(D)-immune globulin in pregnant Rh(D)-negative women has been a major advance in the prevention of HDN,1 yet the mechanism by which the drug exerts its immune modulatory effect is not well understood.2 Monoclonal antibodies (MoAbs) derived from the B cells of Rh(D)-immune globulin donors have defined several dozen Rh(D) epitopes3; paradoxically, the Rh(D) antigen, an approximately 30-kD transmembrane protein, has minimal extracellular mass and presents a very limited surface area for epitope expression.4-9 The molecular cloning of large repertoires of anti-Rh(D) antibodies would help reconcile these observations. In addition, it would facilitate the rational development of recombinant formulations of Rh(D)-immune globulin and aid in the design of therapeutic agents that block antibody binding. Furthermore, the comprehensive genetic analysis of anti-Rh(D) antibodies within a given alloimmunized individual would serve as a paradigm for human immune repertoire development, an area in which limited information is currently available.
Previously, no more than 8 IgG anti-Rh(D) human MoAbs have been derived from a single individual.10 The primary challenge in studying the Rh(D) immune response has been technical difficulties inherent in human B-cell immortalization. Epstein-Barr virus (EBV) transformation results in relatively low transformation efficiencies11 that can undergo a decline in antibody production,12-15 whereas cell fusion methods have been hampered by the lack of good fusion partners.16,17 More recently, molecular approaches have been developed that bypass the need for cell transformation.18-20 Conceptually, these techniques, referred to as repertoire cloning or Fab/phage display, seek to immortalize Ig mRNA rather than the B cells from which they were derived. In an earlier report, our laboratory adapted these techniques for isolating Fab/phage antibodies directed against conformation-dependent antigens expressed on cell surfaces.21 Using intact human RBCs, we isolated highly diverse γ1κ and γ1λ Fab/phage libraries against the Rh(D) antigen from the B cells of a single Rh(D)-immune globulin donor.22
In the following report, we present a detailed genetic and serological analysis of 53 unique anti-Rh(D) antibodies derived from 83 randomly chosen clones. The results complement previous reports on the genetic and biochemical makeup of monoclonal anti-Rh(D) antibodies derived from multiple donors.10 23-25 Significantly, our data also demonstrate extensive genetic homology between antibodies directed against different Rh(D) epitopes. We provide evidence that antibodies directed against different epitopes can be clonally related. Finally, we suggest a model that reconciles the serological diversity of anti-Rh(D) antibodies with the topological constraints imposed by the Rh(D) antigen.
MATERIALS AND METHODS
Production of Monoclonal Anti-Rh(D) Phage-Displayed and Soluble Fab Molecules
Methods for the isolation of human anti-Rh(D)–specific antibodies from γ1κ and γ1λ Fab/phage display libraries using the pComb3H phagemid vector and a cell-surface panning protocol have been previously published.22 Soluble anti-Rh(D) Fab preparations for inhibition studies were produced from bacterial cultures transfected with plasmid DNA from which the M13 gene III coat protein sequence had been excised as described.21 26Cultures were grown by shaking at 300 RPM at 37°C in superbroth (30 g/L tryptone, 20 g/L yeast, 10 g/L MOPS, pH 7.00) containing 20 mmol/L MgCl2 and 50 μg/mL carbenicillin to an OD600of 0.5. Isopropyl-β-D-thiogalactopyranoside (IPTG) was added to 1 mmol/L and cultures were shaken overnight at 30°C. Bacterial pellets were harvested and resuspended in 1/50th of the initial culture volume with osmotic shock buffer (500 mmol/L sucrose, 1 mmol/L EDTA, 100 mmol/L Tris, pH 8.00), incubated for 30 minutes at 4°C, and centrifuged at 16,000g for 15 minutes at 4°C. Fab-containing supernatants were dialyzed against phosphate-buffered saline (PBS) and used in agglutination experiments without further purification.
Anti-Rh(D) Antibody Binding Assays
The binding of anti-Rh(D) Fab/phage or soluble Fab molecules to normal or partial Rh(D) antigens was assessed by indirect agglutination assays as described.21,22 Briefly, 100 μL aliquots of phage-displayed Fabs or soluble Fabs were incubated with 50 μL of a 3% suspension of RBCs. After 1 hour of incubation at 37°C, the RBCs were washed three times with 2 mL of cold PBS to remove unbound antibody. The resulting RBC pellets were resuspended in 100 μL of a 10 μg/mL solution of sheep anti-M13 antibody (5 Prime → 3 Prime, Boulder, CO) for Fab/phage experiments or goat antihuman κ or λ light chain antibody (Tago, Burlingame, CA) for γ1κ or γ1λ soluble Fab experiments, respectively. The RBC suspensions were transferred to the round-bottomed wells of a 96-well microplate and left undisturbed for 2 hours. Negative reactions show sharp approximately 2-mm diameter RBC spots, whereas the RBCs in agglutinated wells form a thin carpet coating the entire floor of the well.22 Agglutination titers for recombinant antibodies were determined by performing serial twofold dilutions in 1% bovine serum albumin (BSA)/PBS. Typically, Fab/phage had agglutination titers of 1/1,024 to 1/2,048 (where neat is defined as 5 × 1012 tfu/mL),22 and soluble Fabs had agglutination titers of 1/64 to 1/128 when prepared as described above.
For determining Rh(D) epitope specificity for anti-Rh(D) Fab/phage antibodies, the following reference Rh(D) variant cells were obtained from the MRC Blood Group Unit (London, UK), The New York Blood Center (New York, NY), or Gamma Biologicals, Inc (Houston, TX): O/DIIIaCce, G positive; B/DIIIcCce; A/DIVace; A/DIVace; O/DIVace; O/DIVbCce; B/DIVbCce, Goa negative, Rh32 negative; O/DVaCce; O/DVacEe, Dw positive; O/DVICce; B/DVICce; AB/DVICce; A/DVIcEe; O/DVIICce; and O/DVIICce. Each Fab/phage antibody was tested on at least three separate occasions against at least two different examples of each variant cell type, and identical epitope assignments were obtained each time. For antibodies that demonstrated previously undescribed patterns of reactivity or repeatedly weak reactivity against one type of cell (see the Results), monoclonal Fab/phage were prepared on a least four separate occasions to verify the patterns of reactivity.
For inhibition studies, the ability of antibodies with different Rh(D) epitope specificities to compete with each other for binding was assessed by preparing stocks of each clone in both a soluble Fab form and a phage-displayed form. Pairwise combinations of soluble Fabs and Fab/phage were prepared and added to Rh(D)-positive RBCs. The resulting incubation mixes comprised 50 μL of a 3% suspension of RBCs, 100 μL of undiluted soluble Fab, and 100 μL of Fab/phage diluted to its highest agglutinating titer. After 1 hour of incubation at 37°C, RBCs were washed, resuspended in anti-M13 antibody, and placed in microplate wells as described above. That the amount of soluble Fab present in an incubation mixture was sufficient to compete away a Fab/phage that shared the same binding site was determined by verifying that each soluble Fab preparation could block its own Fab/phage (see the Results).
Inhibition experiments were also performed using pairwise combinations of soluble Fabs instead of soluble Fab and Fab/phage combinations. In this type of experiment, pairs of soluble Fabs specific for different epitopes were chosen such that one Fab contained a λ light chain and the other a κ light chain. Incubations with RBCs were performed with one Fab in excess and the other in limiting amounts. Blocking of the latter antibody was assessed using a secondary antibody (anti-λ or anti-κ) specific for its light chain isotype.
Nucleotide Sequencing and Analysis
Plasmid DNA for sequencing was prepared using the Qiawell system (Qiagen, Chatsworth, CA). Double-stranded DNA was sequenced using light chain or heavy chain Ig constant region reverse primers or a set of unique pComb3H vector primers that anneal 5′ to the respective Ig chain26,27 and automated fluorescence sequencing (Applied Biosystems, Foster City, CA; DNA Sequencing Facility, University of Pennsylvania Department of Genetics and Cancer Center, Philadelphia, PA). Sequence analysis and variable region germline assignments were performed using DNAplot28 and the V Base Directory of Human V Gene Sequences (March 1997 update).29 Germline assignments were corroborated with the MacVector (v. 6.0) software package (Oxford Molecular Group, Oxford, UK) against the same database. Multiple sequence alignments and predictions of isoelectric point were calculated using the Pileup and Isoelectric programs of the GCG software package (v. 8.0.1; GCG, Madison, WI). Statistical analysis was performed with Statview (Abacus Concepts, Berkeley, CA).
Because of the large number of heavy and light chain sequences (N = 69), only alignments of the predicted amino acid sequences are presented. Nucleotide sequences of all clones are available in Genbank.
RESULTS
Sequence Analysis of Anti-Rh(D) Heavy and Light Chains
We previously reported on the use of Fab/phage display and cell-surface panning to isolate a large array of anti-Rh(D) antibodies from the peripheral blood lymphocytes of a single hyperimmunized donor.22,30 Separate γ1κ and γ1λ Fab/phage display libraries had been constructed and contained 7 × 107 and 3 × 108independent transformants, respectively, based on electroporation efficiency. Each library was panned independently using a simultaneous positive/negative selection strategy with magnetically labeled Rh(D)-positive RBCs and unmodified Rh(D)-negative RBCs as described. After two rounds of panning, 32 of 36 γ1λ and 15 of 15 γ1κ randomly chosen clones were positive for anti-Rh(D) activity. After the third round of panning, 24 of 24 γ1λ and 12 of 12 γ1κ clones were positive. Nucleotide sequencing of the 83 positive clones showed a total of 28 unique heavy and 41 unique light chains. Because of combinatorial effects during phage display library construction, heavy and light chain gene segments paired to produce 53 unique Fab antibodies.22
Anti-Rh(D) heavy chains.
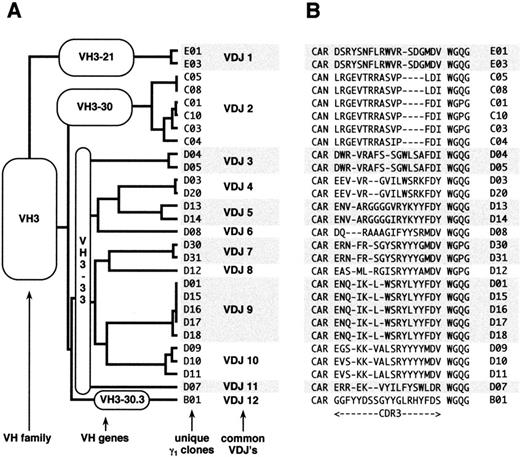
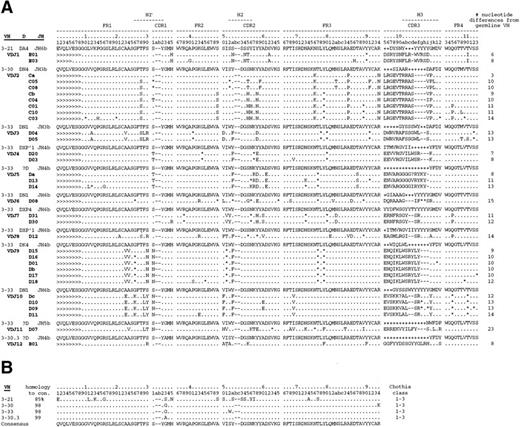
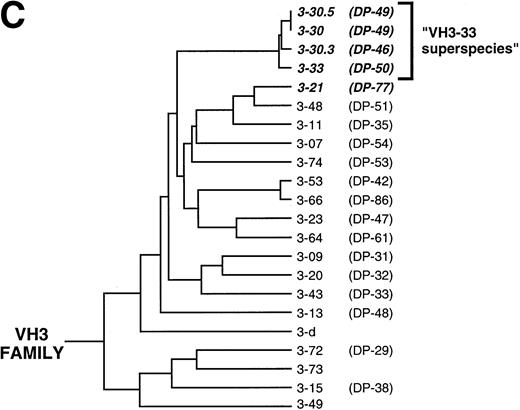
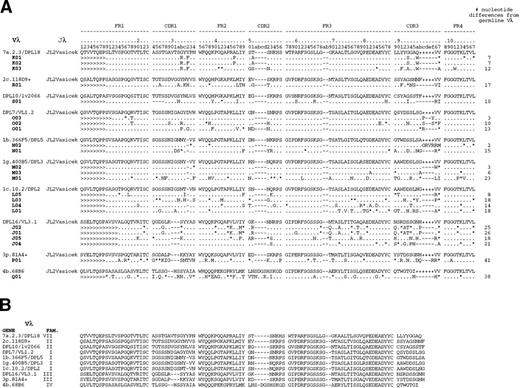
All of the heavy chain sequences used VHIII family-encoded gene products (Figs 1 and2). Several sequences shared identical VDJ joining regions, and 12 unique VDJ rearrangements were identified and designated VDJ1 through VDJ12. Alignment of these sequences against the V Base Directory of Human V Gene Sequences29 showed that only four VHIII genes were used by these antibodies: VH3-21, VH 3-30, VH 3-33, and VH 3-30.3. VH3-21 was used by 1 of the 12 VDJs and 2 of the 28 clones; VH3-30 by 1 VDJ and 6 clones; VH3-33 by 9 VDJs and 19 clones; and VH3-30.3 by 1 VDJ and 1 clone. Interestingly, VH3-30, VH3-33, and VH3-30.3 comprise a set of closely related genes (>98% homology; Fig 2B) and their next nearest neighbor, VH3-07, is only 90% homologous (Fig 2C). Hereafter, these three genes are referred to as the VH3-33 superspecies. Heavy chain E1 differed from VH3-21 by 6 mutations and from VH3-48 by 10 mutations; hence, it was assigned to the former germline gene. Because there were no common mutations among the VH3-33 clones, it is highly probable that the donor possessed the VH3-33 germline gene. However, we could not formally rule out gene duplication with allelic variants of VH3-33 or the existence of variant alleles of the other germline genes in the donor. The isolation of clones sharing multiple VDJ joining regions argues that cloning artifacts cannot account for the VH gene restrictions observed.
(A) Dendrogram and (B) CDR3 alignment of anti-Rh(D) heavy chains. The 28 unique heavy chain clones are organized by VH family, VH germline gene, and VDJ rearrangement. Each heavy chain clone is identified by a numeral preceded by a letter (B through E) that denotes its germline gene. The 28 heavy chains comprised 12 distinct VDJ regions, designated VDJ1 through VDJ12. Clones with identical VDJ joins putatively result from intraclonal diversity of 12 original B lymphocytes.
(A) Dendrogram and (B) CDR3 alignment of anti-Rh(D) heavy chains. The 28 unique heavy chain clones are organized by VH family, VH germline gene, and VDJ rearrangement. Each heavy chain clone is identified by a numeral preceded by a letter (B through E) that denotes its germline gene. The 28 heavy chains comprised 12 distinct VDJ regions, designated VDJ1 through VDJ12. Clones with identical VDJ joins putatively result from intraclonal diversity of 12 original B lymphocytes.
(A) Alignment of anti-Rh(D) heavy chains to their nearest germline V, D, and J genes. Also shown are the putative intermediate heavy chain sequences (Ca, Cb, Da, Db, and Dc; see text and Fig 3). The number of nucleotide differences from a germline VH is tabulated to the right of each sequence. In general, D segments showed poor homology with known D genes, so mutations were not scored in these regions. Key: Replacement mutations indicated with letters, silent mutations as “*”, identities as “.”, and insertions as “-”. Sequences derived from the 5′ VH primers used in library construction22 are marked as “>”. CDR region designations are determined as per Kabat59; numbering and H region designations per Chothia et al.31(B) Alignment of the four VH3 genes used by anti-Rh(D) heavy chains and (C) dendrogram of all human VH3 family germline genes shows relatedness of VH3-21, VH3-30, VH3-33, and VH3-30.3 and the surprising restriction in VH gene usage. Note that the VH3-30.5 gene is present in only certain haplotypes and is identical to VH3-30.60Genbank accession numbers for anti-Rh(D) heavy chains are listed in the Appendix.
(A) Alignment of anti-Rh(D) heavy chains to their nearest germline V, D, and J genes. Also shown are the putative intermediate heavy chain sequences (Ca, Cb, Da, Db, and Dc; see text and Fig 3). The number of nucleotide differences from a germline VH is tabulated to the right of each sequence. In general, D segments showed poor homology with known D genes, so mutations were not scored in these regions. Key: Replacement mutations indicated with letters, silent mutations as “*”, identities as “.”, and insertions as “-”. Sequences derived from the 5′ VH primers used in library construction22 are marked as “>”. CDR region designations are determined as per Kabat59; numbering and H region designations per Chothia et al.31(B) Alignment of the four VH3 genes used by anti-Rh(D) heavy chains and (C) dendrogram of all human VH3 family germline genes shows relatedness of VH3-21, VH3-30, VH3-33, and VH3-30.3 and the surprising restriction in VH gene usage. Note that the VH3-30.5 gene is present in only certain haplotypes and is identical to VH3-30.60Genbank accession numbers for anti-Rh(D) heavy chains are listed in the Appendix.
Neither JH nor D segments showed restriction. At least 9 different D segments were used and JH gene use comprised JH6 (5 VDJs and 9 clones), JH4 (4 VDJs and 10 clones), JH3 (2 VDJs and 8 clones), and JH5 (1 VDJ and 1 clone). All four VH genes were Chothia class 1-3,31 and the CDR3s showed a narrow range of length from 15 to 19 residues.
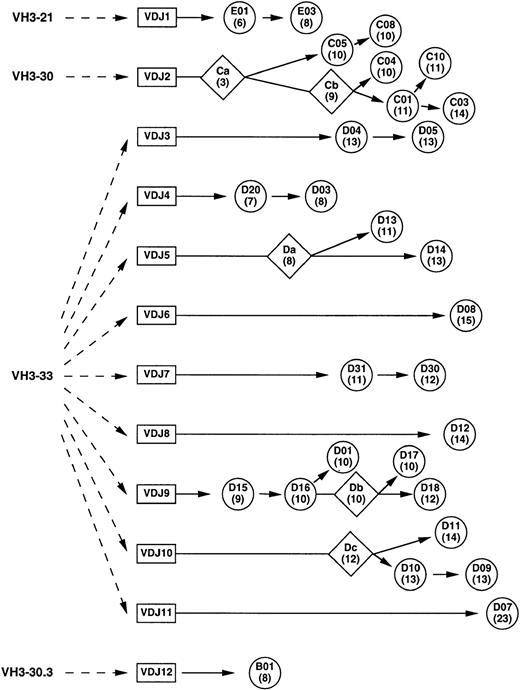
Because rearranged heavy chain genes demonstrate extensive diversity, clones sharing identical VDJ rearrangements are generally considered to have arisen from the same clone. Based on nucleotide alignment with the germline genes (data not shown), an ontogeny tree was constructed for the 12 VDJs and 28 clones (Fig 3). By using the most parsimonious mutation scheme (ie, postulating the minimum number of mutations), putative intermediate antibodies were derived for several of the VDJs and were designated Ca, Cb, Da, Db, and Dc (Figs 2A and 3). Compared with the isolated heavy chain clones, which displayed between 6 and 23 nucleotide differences from their germline counterparts, these putative intermediates had between 3 and 12 mutations from germline. Based on the ontogeny tree, the number of independent mutations could be tabulated among the clones. The most commonly mutated residues were 52a and 58 (7 independent mutations), followed by residues 30, 31, and 50 (6 mutations) and residue 55 (5 mutations). In the VH3-33 superspecies, residues 52a and 58 in CDR2 are tyrosines and residue 52a was mutated to phenylalanine in 6 of the 11 VDJs derived from VH3-33 superspecies VH genes. Mutations at residue 58 comprised glutamate (3), aspartate (2), histidine (1), and asparagine (1). The AGY serines at residues 30, 31, and 55 were mutated to a number of different amino acids, although the AGY serine at 82b was conserved in all clones. The valine at residue 50 in the VH3-33 superspecies also had a diverse set of mutations. This distribution of hot spots is similar to that seen with nonproductive rearrangements as previously reported by Dörner et al.32
Ontogenic tree of anti-Rh(D) heavy chains constructed using nucleotide alignment data. Circles represent isolated and sequenced clones and diamonds represent putative intermediates (see Fig2A). The number of nucleotide mutations from its germline VH gene is shown in parentheses below the clone name. The distance along the horizontal axis represents the degree of mutation (including J segments) within the constraints of the diagram.
Ontogenic tree of anti-Rh(D) heavy chains constructed using nucleotide alignment data. Circles represent isolated and sequenced clones and diamonds represent putative intermediates (see Fig2A). The number of nucleotide mutations from its germline VH gene is shown in parentheses below the clone name. The distance along the horizontal axis represents the degree of mutation (including J segments) within the constraints of the diagram.
Anti-Rh(D) light chains.
Seventeen of the 18 κ light chains were from the VκI family and the remaining light chain originated from a VκII family member germline gene (Fig 4). Only 4 Vκ germline genes were used (15 clones were derived from DPK9 alone), and the κ light chain clones had between 1 and 49 mutations from their corresponding Vκ germline genes. All 5 of the known Jκ genes were used and were each joined to the DPK9 gene in one or more clones. Because the light chains showed considerably less diversity in their joining regions than the heavy chains, it was difficult to assign common clonal origins. However, an ontogeny tree was constructed by grouping common V and J gene segments along with common mutations (data not shown). Based on this analysis, the 18 κ chains comprised at least 10 different recombination events.
(A) Alignment of anti-Rh(D) κ light chains to their nearest germline V and J genes shows predominance of DPK-9 usage from the VκI family. Nomenclature for clones is similar to that for heavy chains but uses the letters F through I. (B) Alignment of the four Vκ genes used by anti-Rh(D) light chains. The key is the same as that used in Fig 2A. Genbank accession numbers for anti-Rh(D) κ light chains are listed in the Appendix.
(A) Alignment of anti-Rh(D) κ light chains to their nearest germline V and J genes shows predominance of DPK-9 usage from the VκI family. Nomenclature for clones is similar to that for heavy chains but uses the letters F through I. (B) Alignment of the four Vκ genes used by anti-Rh(D) light chains. The key is the same as that used in Fig 2A. Genbank accession numbers for anti-Rh(D) κ light chains are listed in the Appendix.
λ light chains were restricted by their Jλ gene usage but showed no restriction in their use of Vλ genes (Fig 5). The 23 λ light chains all used the Jλ2Vasicek gene but were derived from VλI (12 clones), VλIII (5), VλVII (3), VλII (2), and VλIV (1) family genes. The number of mutations ranged from 2 to 41 from the nearest germline Vλ gene. Based on common joining regions and mutations, these 23 λ light chains were derived from at least 13 different B cells.
(A) Alignment of anti-Rh(D) λ light chains to their nearest germline V and J genes and (B) alignment of the 10 Vλ germline genes used shows the use of a diverse set of variable region genes derived from multiple families. However, all of the clones use the identical Jλ gene segment. Nomenclature for the clones is similar to that for heavy chains but uses the letters J through S. The key is the same as that used in Fig2A. Genbank accession numbers for anti-Rh(D) λ light chains are listed in the Appendix.
(A) Alignment of anti-Rh(D) λ light chains to their nearest germline V and J genes and (B) alignment of the 10 Vλ germline genes used shows the use of a diverse set of variable region genes derived from multiple families. However, all of the clones use the identical Jλ gene segment. Nomenclature for the clones is similar to that for heavy chains but uses the letters J through S. The key is the same as that used in Fig2A. Genbank accession numbers for anti-Rh(D) λ light chains are listed in the Appendix.
Assessment of the Diversity of the Unpanned Libraries
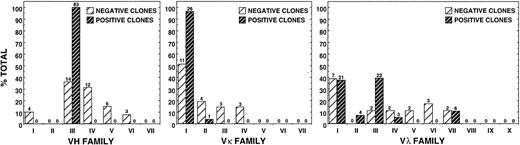
To determine whether the apparent restriction in gene usage of the anti-Rh(D) antibodies could have been due to preselection factors (ie, cloning artifacts), we assessed the diversity of the unpanned γ1κ and γ1λ Fab/phage libraries. By sequencing 39 randomly picked clones, we determined that there were no duplicate heavy or light chain sequences and that there was significant heterogeneity in V gene family representation before selection (Fig 6). In fact, the variable region gene family distribution was not unlike that found by other investigators for IgG-secreting lymphocytes in adult peripheral blood.33Furthermore, of the 14 VHIII-encoded negative clones, only one used a VH3-33 superspecies germline gene (VH3-30.3); the other 13 were encoded by VH3-07 (3), 3-09 (2), 3-15 (2), 3-48 (2), 3-72 (2), 3-23 (1), and DP-58 (1). Therefore, the restriction of the 83 anti-Rh(D) clones to the VH3-33, 3-30, 3-30.3, and 3-21 genes is significant and not a result of skewed representation of certain germline genes within the originally constructed γ1κ and γ1λ Fab/phage libraries.
Comparison of variable region gene family usage for anti-Rh(D)–specific clones with those used by randomly picked, non-Rh(D)-binding clones from original γ1κ and γ1λ unselected libraries. Lightly hatched bars reveal heterogeneity in VH (left panel), Vκ (middle panel), and Vλ (right panel) family representation before selection for anti-Rh(D) specificity. Numbers above bars represent absolute number of clones in that group.
Comparison of variable region gene family usage for anti-Rh(D)–specific clones with those used by randomly picked, non-Rh(D)-binding clones from original γ1κ and γ1λ unselected libraries. Lightly hatched bars reveal heterogeneity in VH (left panel), Vκ (middle panel), and Vλ (right panel) family representation before selection for anti-Rh(D) specificity. Numbers above bars represent absolute number of clones in that group.
Heavy and Light Chain Contribution to Rh(D) Epitope Specificity
Because of the conformational dependency of Rh(D) antigenicity, Rh(D) epitopes have been classically defined through the use of RBCs obtained from rare individuals whose cells appear to produce Rh(D) antigens lacking certain epitopes.34 Examining the pattern of agglutination of a particular anti-Rh(D) MoAb with such sets of partial Rh(D) RBCs enables one to categorize that antibody's fine specificity.
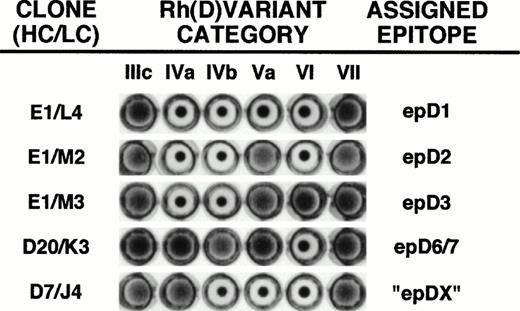
Monoclonal Fab/phage preparations were prepared in triplicate for each of the 53 anti-Rh(D) clones and tested against a panel of Rh(D) category cells IIIa/c, IVa, IVb, Va, VI, and VII. This panel of cells can differentiate between the Rh(D) epitope specificities as described by Lomas et al6 (designated epitopes epD1, epD2, epD3, epD4, epD5, and epD6/7). Agglutination experiments with the Fab/phage clones showed five different patterns of reactivity, including a new pattern that had not been described in the original study by Lomas et al6 or in the more recently described 9, 30, or 37 epitope systems (Figs 7 and8).3 35 Although nearly all Fab/phage gave unequivocal agglutination reactions, a few antibodies gave repeatedly weak patterns of reactivity against one of the panel cells. For these reactions, monoclonal Fab/phage were prepared on at least four separate occasions to verify the patterns of reactivity.
Determination of the Rh(D) binding epitope of anti-Rh(D) Fab/phage clones. The fine specificities of monoclonal Fab/phage clones were determined by their ability to agglutinate members of a panel of six Rh(D) variant RBCs. Shown are the five different agglutination patterns obtained from screening all of the 53 Fab/phage clones. The particular clones shown are identified by their unique heavy chain/light chain pairings using the nomenclature defined in Figs 1, 4, and 5. For E1/M3, reactivity with additional Rh(D) variant cells would be required to distinguish its specificity for epD3 versus epD9.3 Rh(D) epitope assignments are as per Lomas et al.6 Note that inclusion of the category IVb cell (not available in our previous study)22 permits the identification of a new epitope designated epDX (see text).
Determination of the Rh(D) binding epitope of anti-Rh(D) Fab/phage clones. The fine specificities of monoclonal Fab/phage clones were determined by their ability to agglutinate members of a panel of six Rh(D) variant RBCs. Shown are the five different agglutination patterns obtained from screening all of the 53 Fab/phage clones. The particular clones shown are identified by their unique heavy chain/light chain pairings using the nomenclature defined in Figs 1, 4, and 5. For E1/M3, reactivity with additional Rh(D) variant cells would be required to distinguish its specificity for epD3 versus epD9.3 Rh(D) epitope assignments are as per Lomas et al.6 Note that inclusion of the category IVb cell (not available in our previous study)22 permits the identification of a new epitope designated epDX (see text).
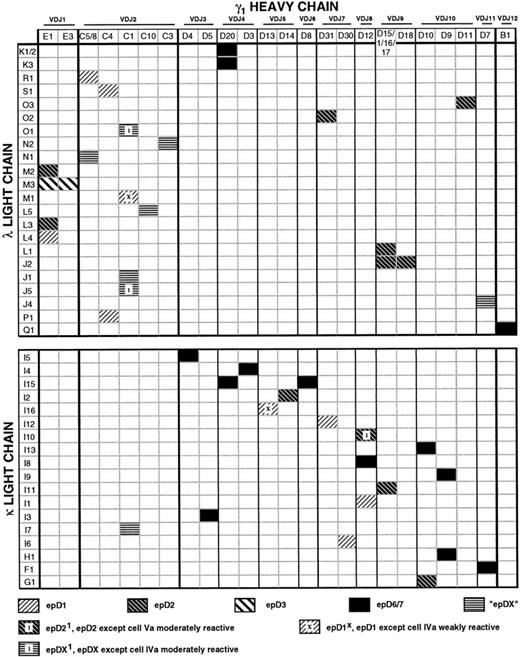
Matrix illustrating the genetic composition and epitope specificity of anti-Rh(D) antibodies. The horizontal axis represents the unique γ1 heavy chains and the vertical axis represents the unique λ and κ light chains (based on amino acid sequence). A shaded pattern at the intersection of a heavy chain/light chain pair indicates the Rh(D) epitope specificity observed for that Fab/phage antibody. A few clones gave mixed patterns of reactivity, as shown (see text). The order of heavy chains (left to right) and light chains (top to bottom) was determined by the multiple alignment of amino acid sequences as in Figs 2, 4, and 5. Note that heavy chains D1, D15, D16, and D17, although differing in nucleotide sequence, have the identical amino acid sequences and thus comprise a single column. Similarly, heavy chains C5 and C8 and λ light chains K1 and K2 encode the same proteins. The pairings of these 28 heavy and 41 light chain nucleotide gene segments, which produced 53 unique Fab transcripts, encoded 43 different Fab proteins, as indicated in the matrix.
Matrix illustrating the genetic composition and epitope specificity of anti-Rh(D) antibodies. The horizontal axis represents the unique γ1 heavy chains and the vertical axis represents the unique λ and κ light chains (based on amino acid sequence). A shaded pattern at the intersection of a heavy chain/light chain pair indicates the Rh(D) epitope specificity observed for that Fab/phage antibody. A few clones gave mixed patterns of reactivity, as shown (see text). The order of heavy chains (left to right) and light chains (top to bottom) was determined by the multiple alignment of amino acid sequences as in Figs 2, 4, and 5. Note that heavy chains D1, D15, D16, and D17, although differing in nucleotide sequence, have the identical amino acid sequences and thus comprise a single column. Similarly, heavy chains C5 and C8 and λ light chains K1 and K2 encode the same proteins. The pairings of these 28 heavy and 41 light chain nucleotide gene segments, which produced 53 unique Fab transcripts, encoded 43 different Fab proteins, as indicated in the matrix.
The most commonly recognized epitope was epD6/7, against which 13 clones were directed. Interestingly, monoclonal anti-Rh(D) clones isolated using conventional tissue culture methods are most often specific for epD6/7.34 epD2, epD1, and epD3 were recognized by 10, 7, and 2 clones, respectively. Six clones agglutinated cells of categories IIIa/c, IVa, and VII, but not of categories IVb, Va, and VI, and were designated anti-epDX. This pattern is identical to epD1, except that the IVa cell is agglutinated. Three clones gave intermediate reactions with cell IVa, but otherwise showed patterns consistent with epDX or epD1. These clones were designated epDX1 or epD1X, depending on whether this reactivity against cell IVa was stronger or weaker, respectively (Fig8). Similarly, reaction patterns for epD1 and epD2 differ by a positive reaction with the category Va cell; therefore, one clone was given epD21 specificity because it gave only moderate reactivity against that cell. Such variable reactions against one or more partial Rh(D) cells have been observed for anti-Rh(D) MoAbs produced through conventional tissue culture methods.36
Because of the reassortment of heavy and light chain gene segments that occurs during the construction of a phage display library, a number of clones were isolated that shared either a heavy (eg, E1) or light (eg, M3) chain sequence (Fig 8). Some heavy chains were found to have paired with both κ and λ light chains (eg, C1, D20), and each demonstrated anti-Rh(D) specificity. Interestingly, some heavy chains (eg, E1, D12) mapped to different epitopes depending on the light chains with which they were paired. In particular, the light chains of two such clones, E1/M2 and E1/M3, differed by only 3 amino acid residues (Fig 5) and these differences appear to confer specificity for epD2 versus epD3.
Inhibition Studies
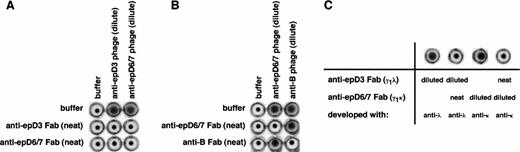
To investigate the topological relationships among the Rh(D) epitopes, inhibition studies were performed. Previous work by Gorick et al37 using pairs of unlabeled and 125I-labeled anti-Rh(D) MoAbs demonstrated that antibodies to at least 3 different Rh(D) epitopes (subsequently identified as epD1, D6, and D7)6 could inhibit one another. We have confirmed and extended these findings using recombinant antibodies to 5 Rh(D) epitopes (Fig 9). In one series of experiments, we exploited the ability to express each antibody in both a soluble Fab as well as phage-displayed form to ask whether a soluble Fab against one epitope would inhibit the agglutination induced by an Fab/phage directed against a different epitope. Reciprocal pairs of soluble Fab and Fab/phage specific for epD1, epD2, epD3, epD6/7, and epDX were tested. All 10 combinations showed mutual inhibition patterns (shown in Fig 9A for an anti-epD3/anti-epD6/7 combination). To show that this inhibition was not due to nonspecific factors, a control with an irrelevant RBC-binding recombinant antibody (an anti-blood group B antibody) was performed (Fig 9B). That sufficient inhibitory amounts of soluble Fab was present were first verified by demonstrating that each soluble Fab could inhibit its own Fab/phage (Fig 9A and B; samples on diagonal). Similar results were obtained using pairs of soluble Fabs which differed in their light chain isotype composition (Fig 9C).
Inhibition studies with recombinant anti-Rh(D) antibodies. Panels show results of representative experiments demonstrating the mutual inhibition of antibodies directed at 2 different Rh(D) epitopes (in this example, epD3 and epD6/7; A and C), but not between an Rh(D) antibody and an unrelated recombinant anti-RBC antibody (an anti-blood group B antibody; B). In (A), Rh(D)-positive RBCs were incubated with soluble Fabs only, phage-displayed Fabs only, or combinations of the two, as indicated. In (B), Rh(D)-positive RBCs that were blood group B were used. After washing, RBCs were resuspended in anti-M13 antibody and assessed for agglutination induced by phage-displayed Fabs. Soluble Fabs were used full-strength, whereas Fab/phage preparations were present in limiting amounts to increase the sensitivity of the inhibition assay (see the Materials and Methods). In (C), mutual inhibition of epD3 and epD6/7 anti-Rh(D) antibodies was demonstrated with Rh(D)-positive RBCs, γ1κ and γ1λ soluble Fabs, and light chain isotype-specific antisera (see text for details). In these examples, the anti-epD3 and anti-epD6/7 antibodies were clones E1/M3 and D5/I3, respectively. The anti-blood group B antibody was isolated from an IgG phage display library made from the splenic B cells of a blood group O donor.61
Inhibition studies with recombinant anti-Rh(D) antibodies. Panels show results of representative experiments demonstrating the mutual inhibition of antibodies directed at 2 different Rh(D) epitopes (in this example, epD3 and epD6/7; A and C), but not between an Rh(D) antibody and an unrelated recombinant anti-RBC antibody (an anti-blood group B antibody; B). In (A), Rh(D)-positive RBCs were incubated with soluble Fabs only, phage-displayed Fabs only, or combinations of the two, as indicated. In (B), Rh(D)-positive RBCs that were blood group B were used. After washing, RBCs were resuspended in anti-M13 antibody and assessed for agglutination induced by phage-displayed Fabs. Soluble Fabs were used full-strength, whereas Fab/phage preparations were present in limiting amounts to increase the sensitivity of the inhibition assay (see the Materials and Methods). In (C), mutual inhibition of epD3 and epD6/7 anti-Rh(D) antibodies was demonstrated with Rh(D)-positive RBCs, γ1κ and γ1λ soluble Fabs, and light chain isotype-specific antisera (see text for details). In these examples, the anti-epD3 and anti-epD6/7 antibodies were clones E1/M3 and D5/I3, respectively. The anti-blood group B antibody was isolated from an IgG phage display library made from the splenic B cells of a blood group O donor.61
Isoelectric Point (pI) Analysis of Anti-Rh(D) Antibodies
The restriction in VH germline gene usage to only four VHIII family members was intriguing in light of their ability to confer specificity to a number of Rh(D) epitopes. As suggested by Boucher et al,10 VH germline gene segments used to encode anti-Rh(D) antibodies are among the most cationic segments available in the human VH repertoire that may be used to account for the relatively high pI of polyclonal anti-Rh(D)–containing antisera.38,39 Although the cationic nature of the antibodies may be important for binding to Rh(D), it has also been suggested that a constitutive net positive charge may be necessary to permeate the highly negative RBC ζ potential, thus permitting antibody to contact antigen.34In either case, analysis of the predicted pI for the 28 heavy chains and 41 light chains isolated here showed an interesting phenomenon for the heavy versus light chains. Using the pI interval scale of Boucher et al,10 the average pI of the 4 germline VHsegments used to encode the 28 heavy chains is high (9.87 ± 0.15) and significantly higher than that of 39 randomly picked, non-Rh(D) binding clones from the original unpanned libraries (9.24 ± 0.80,P < 10−5). Similar to the results of Boucher et al,10 the addition of D and JHsegments and the introduction of somatic mutation did not significantly change the pI of the average anti-Rh(D) heavy chain (9.81 ± 0.33,P < .37). However, for the light chains, the average pI of their germline counterparts was not cationic, but the light chains became so through the addition of JL segments and somatic mutation. Overall, for all 18 κ and 23 λ light chains, pairedt-test analyses before and after somatic mutation showed a significant increase in net positive charge when comparing germline VL (6.63 ± 1.47) with expressed VL (7.28 ± 1.51, P < 10−3) or germline VLJL (7.43 ± 1.47) with expressed VLJL (8.55 ± 1.35, P < 10−7). There was no significant increase in a similar analysis of 16 non-Rh(D) binding clones (P < .59 andP < .19, respectively). Examination of the light chain sequences (Figs 4 and 5) showed that this increase in pI resulted from mutations that not only introduced positively charged residues, but also eliminated some negatively charged residues. There were 31 such events, 29 (91%) of which occurred in the light chain CDR regions.
DISCUSSION
Conventional and Phage-Displayed Anti-Rh(D) MoAbs
Because of differences in methodology, we were interested in comparing our phage-display–derived anti-Rh(D) clones with those produced by conventional tissue culture techniques (EBV transformation and cell fusion). Despite the relatively small number of previously published sequences for IgG anti-Rh(D) antibodies (N = 21) and the fact that they were derived from over 10 different donors,10 23-25 there was surprisingly good correlation between the two groups (Table 1). Both cohorts demonstrated a predominance of VHIII-family encoded germline genes, particularly those of the VH3-33 superspecies. CDR3 regions showed similar lengths, ranging from 15 to 19 residues for Fab/phage antibodies and 16 to 20 for conventional monoclonals, although one heterohybridoma was an outlier with a CDR3 length of 28 residues. κ light chains were biased towards Vκ1 family members and λ light chains demonstrated the preferential use of the Jλ2Vasicek gene. The only qualitative discrepancy was in Vλ family usage, where Fab/phage clones demonstrated a slight preference for VλI versus VλIII family members for conventional monoclonals. However, in both cohorts, DPL16 was used more often than any other λ light chain gene.
Comparison of Current IgG Fab/Phage Library-Derived Anti-Rh(D) MoAbs With Those Previously Produced by Conventional Tissue Culture Methods
| Attribute . | Previously Published* . | Current Study . | |
|---|---|---|---|
| Heavy Chains | (by clone)-151 | (by VDJ) | |
| VH3 family derived | 12/21 (57%) | 28/28 (100%) | 12/12 (100%) |
| VH3-33 superspecies-152/VH3 | 10/12 (83%) | 26/28 (93%) | 11/12 (92%) |
| VH3-33/VH3 | 9/12 (75%) | 19/28 (68%) | 9/12 (75%) |
| VH3-21/VH3 | 1/12 (8%) | 2/28 (7%) | 1/12 (8%) |
| VH4-34 derived | 2/21 (10%) | 0/28 (0%) | 0/12 (0%) |
| JH6 usage | 15/21 (71%) | 9/28 (32%) | 5/12 (42%) |
| CDR3 length | 16-20 (28-153) | 15-19 | |
| κ Light Chains | |||
| Vκ1 family derived/total κ | 8/12 (67%) | 17/18 (94%) | |
| Jκ1 usage/total κ | 4/12 (33%) | 6/18 (33%) | |
| Jκ2 usage/total κ | 4/12 (33%) | 6/18 (33%) | |
| λ Light Chains | |||
| Vλ1 family derived/total λ | 2/8 (25%) | 12/23 (52%) | |
| Vλ3 family derived/total λ | 5/8 (63%) | 5/23 (22%) | |
| DPL16 derived/Vλ3 family | 3/5 (60%) | 4/5 (80%) | |
| Jλ2Vasicek usage/total λ | 6/8 (75%) | 23/23 (100%) | |
| Attribute . | Previously Published* . | Current Study . | |
|---|---|---|---|
| Heavy Chains | (by clone)-151 | (by VDJ) | |
| VH3 family derived | 12/21 (57%) | 28/28 (100%) | 12/12 (100%) |
| VH3-33 superspecies-152/VH3 | 10/12 (83%) | 26/28 (93%) | 11/12 (92%) |
| VH3-33/VH3 | 9/12 (75%) | 19/28 (68%) | 9/12 (75%) |
| VH3-21/VH3 | 1/12 (8%) | 2/28 (7%) | 1/12 (8%) |
| VH4-34 derived | 2/21 (10%) | 0/28 (0%) | 0/12 (0%) |
| JH6 usage | 15/21 (71%) | 9/28 (32%) | 5/12 (42%) |
| CDR3 length | 16-20 (28-153) | 15-19 | |
| κ Light Chains | |||
| Vκ1 family derived/total κ | 8/12 (67%) | 17/18 (94%) | |
| Jκ1 usage/total κ | 4/12 (33%) | 6/18 (33%) | |
| Jκ2 usage/total κ | 4/12 (33%) | 6/18 (33%) | |
| λ Light Chains | |||
| Vλ1 family derived/total λ | 2/8 (25%) | 12/23 (52%) | |
| Vλ3 family derived/total λ | 5/8 (63%) | 5/23 (22%) | |
| DPL16 derived/Vλ3 family | 3/5 (60%) | 4/5 (80%) | |
| Jλ2Vasicek usage/total λ | 6/8 (75%) | 23/23 (100%) | |
*Compiled from a total of 21 sequences of IgG anti-Rh(D) antibodies isolated from multiple subjects originally published by Bye et al,25 Hughes-Jones et al,23 Chouchane et al,24 and Boucher et al10 and available from Genbank. One light chain (Oak-3)25 was not available in Genbank and was not included in the assessment.
For heavy chains, the left column tabulates each clone separately and the right column tabulates clones on the basis of shared V-D-J joining regions.
VH3-33 superspecies defined as the group of VH3 family germline genes comprising VH3-33, VH3-30, and VH30.3.
CDR3 length outlier.
It has been suggested in the literature that the VH4-34 (VH4.21) germline gene, a gene used by many autoantibodies and cold agglutinins,40-42 may play an important role in the immune response to Rh(D).43 However, these conclusions arose from the analysis of IgM monoclonals and only 2 of the 21 published anti-Rh(D) IgG sequences used VH4-34.25 In a related series of experiments, we pooled aliquots of the γ1κ and γ1λ libraries obtained after the second and third rounds of selection and then panned them against the VH4-34 specific rat anti-idiotypic MoAb (9G444). Although we successfully enriched for VH4-34 encoded antibodies, the Fab/phage were not specific for Rh(D) and displayed serological characteristics similar to those of cold agglutinins (data not shown). We are currently examining a μ phage display library from the same donor to compare gene usage.
Rh(D) Epitopes and Significance of Antibody Sequences
Since the initial report by Argall et al45 in 1953, it has been recognized that rare individuals who type as Rh(D)-positive can produce allo-anti-Rh(D) antibodies in response to Rh(D) immunization by transfusion or pregnancy. This phenomenon was explained by hypothesizing that the Rh(D) antigen is a mosaic structure and that these individuals were producing alloantibodies to parts of the mosaic they lack. By systematically examining patterns of reactivity between their cells and sera, RBCs expressing partial Rh(D) antigens were divided into categories, each presumed to have a different abnormality in their Rh(D) antigen. Through the subsequent use of index panels of monoclonal anti-Rh(D) antibodies, a series of epitopes were defined of which the number and combination varied from one Rh(D) category to another. As new monoclonals were produced, their reactivity profiles against these partial Rh(D) RBCs became the standard method for determining Rh(D) antibody epitope specificity. Molecular analyses of partial Rh(D) phenotypes have shown that the Rh(D) genes in these individuals have either undergone intergenic recombination with the highly homologous Rh(CE) gene or, less commonly, have sustained point mutation(s).46
As noted earlier, to investigate the topological relationships among Rh(D) epitopes, Gorick et al37 performed competition experiments with Rh(D) MoAbs and observed varying degrees of inhibition. These results, when combined with those of Lomas et al,6 suggested a model for Rh(D) in which epitopes are spatially distinct yet demonstrate a certain degree of overlap, as shown in Fig 10A. This model explained how antibodies to two different Rh(D) epitopes (in this case epD2 and epD3) could inhibit each other's binding to wild-type Rh(D) and how a change in the structure of Rh(D) in category VI RBCs (asterisk, Fig10A) would cause the loss of epD2. However, based on this concept of Rh(D) epitopes as distinct domains, we would expect that antibodies against different epitopes of Rh(D) would be structurally and genetically distinct as well. Thus, it was surprising that our anti-Rh(D) clones demonstrated such marked restriction in gene usage. For example, only two superspecies of VH genes were used despite specificities for 4 of the original 6 Rh(D) epitopes described by Lomas et al.6 Furthermore, multiple specificities could arise from a single heavy chain depending on the light chain with which it was paired (eg, E1 with M2, M3, L3, or L4). In addition, other clones repeatedly demonstrated variable weak reactivity against certain Rh(D) category RBCs that would affect the epitope specificities to which they were assigned (eg, C1 with O1, M1, or J5).
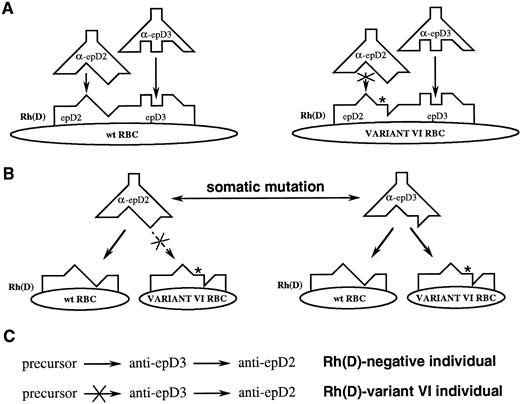
Conventional (A) and proposed (B) models for Rh(D) antigen/antibody binding. Note that the predicted combining sites and genetic relationships between antibodies differ between the two models. (C) If antibodies directed at different Rh(D) epitopes are clonally related, then the expressed repertoire will differ between Rh(D)-negative and partial Rh(D) individuals (see text for discussion).
Conventional (A) and proposed (B) models for Rh(D) antigen/antibody binding. Note that the predicted combining sites and genetic relationships between antibodies differ between the two models. (C) If antibodies directed at different Rh(D) epitopes are clonally related, then the expressed repertoire will differ between Rh(D)-negative and partial Rh(D) individuals (see text for discussion).
Several hypotheses could account for these findings. The most simplistic interpretation is that the heavy chain does not directly interact with the antigen, but rather is responsible for bringing the antibody in close proximity with the antigen. The specific interactions between the light chain and the antigen would then determine the epitope specificity for that antibody. In this regard, our data are consistent with the observations of Boucher et al10 on the relative cationic nature of anti-Rh(D) heavy chains. However, because we found that light chains become cationic during somatic mutation, the charge of the entire antibody may play a role in its ability to bind, resulting in the selection and expansion of particular B-cell clones.
A more compelling hypothesis is that Rh(D) epitopes do not differ spatially but differ only in the number and arrangement of contact residues presented (Fig 10B). In other words, the footprints of most, if not all, anti-Rh(D) antibodies are essentially identical to one another. The genetic events that produce partial Rh(D) molecules result in the loss of certain critical key points of contact necessary for some antibodies to bind; alternatively, they result in the formation of new structures that interfere with the binding of other anti-Rh(D) Igs. For example, the introduction of a ledge in Rh(D) category VI cells (asterisk, Fig 10B) does not interfere with the binding of an anti-epD3 antibody, but does prevent the binding of anti-epD2. Therefore, category VI RBCs are said to have epD3 but lack epD2.
This model is consistent with our inhibition experiments (Fig 9) and with those of Gorick et al37 and offers an explanation for the marked restriction in heavy chain gene usage. It also reconciles a mechanism by which one heavy chain (eg, E1) can confer binding to multiple epitopes and why some of our recombinant anti-Rh(D) antibodies, as well as some conventionally produced monoclonals,36 display variable reactivity against certain categories of partial Rh(D) RBCs. From the antigen's perspective, this model explains how a single point mutation in Rh(D) can result in the loss of multiple Rh(D) epitopes (such as T283I in category HMi RBCs47) and how the residues associated with the expression of some epitopes appear to be distributed among nearly all the extracellular loops of Rh(D).48 It also provides an understanding as to how ≥37 epitopes can fit on the relatively small extracellularly exposed surface of the Rh(D) molecule.3
This concept of coincident epitopes is best exemplified by comparing the E1/M2 and E1/M3 clones. The only difference between the reactivity of E1/M2 and E1/M3 is the ability of the latter antibody to agglutinate Rh(D) category VI cells (Fig 7). Hence, E1/M2 is classified as an anti-epD2 and E1/M3 as an anti-epD3 antibody. Light chains M2 and M3 differ by only 3 residues: D82A, G95aA, and W96V (Fig 5). Therefore, some combination of these residues confers reactivity against category VI cells. In other words, epD2 and epD3, as seen by the E1/M2 and E1/M3 antibodies, differ by the binding constraints imposed by at most three mutations. If the model depicted in Fig 10A were correct and the epitopes were independent, these mutations would have to cause enough structural alteration in the antibody combining site so that a completely separate epitope on the same antigen would be recognized. It would seem unlikely that these 3 mutations could cause such a change, especially given the lack of internal homology domains in Rh(D). Thus, we conclude that it is far more plausible that the footprints of these 2 antibodies are essentially identical and that one or more of these mutations (eg, the tryptophan in CDR3 of M2) prevent(s) the interaction of E1/M2 with category VI RBCs. Because other clones demonstrate that the light chain can confer specificity against epD1, epD2, or epD3 (with the E1 heavy chain); epD1 or epDX (with C5); and epD1, epD2, and epD6/7 (with D12), we suggest that all 5 of these epitopes have similar antibody combining sites.
Immunologic and Clinical Implications of Proposed Model
The model depicted in Fig 10B leads to additional predictions concerning the Rh(D) immune response beyond simply clarifying what is meant by an Rh(D) epitope. It is commonly stated in the transfusion medicine literature that individuals whose RBCs express partial Rh(D) antigens are free to make antibodies to the Rh(D) epitopes they lack.34 Therefore, an individual who produces category VI RBCs should be able to make anti-epD2 but not anti-epD3. If these epitopes were truly independent, then the immune repertoire of the anti-epD2 antibodies made by a category VI individual would be similar to those produced by an Rh(D)-negative person. However, to the immune system, epD2 and epD3 are not independent. We postulate that the somatic mutation of an anti-epD3 antibody can change its fine specificity to that of epD2 (or vice versa, Fig 10C). Suppose that the preferred way of making an anti-epD2 antibody is to go through an anti-epD3 intermediate. To an Rh(D)-negative individual, this process can take place unimpeded. However, in a category VI individual, this route would be unfavorable because an anti-epD3 antibody would be self-reactive. As a result, such an individual would have to make anti-epD2 antibodies by following alternative routes or by tolerating some degree of autoreactivity in the process. With respect to the latter point, it is of interest to note that a transient production of auto-anti-Rh(D) frequently precedes or accompanies the early production of allo-anti-Rh(D) in individuals who express partial Rh(D) antigens.49-54 We would predict, therefore, that the anti-epD2 antibodies from a category VI individual would be different in composition (ie, gene usage) and quite possibly quantitatively depressed as compared with an Rh(D)-negative individual. This may be analogous to the antibodies of the ABO blood group system in which it has been observed that anti-A and anti-B titers in blood group O individuals are significantly higher than in blood group B or A individuals, respectively.55 Blood group O individuals are unconstrained in creating their anti-A and anti-B immune repertoires, whereas individuals who produce A or B antigens (2 nearly identical structures) must do so in a manner that avoids self-reactivity.
In the case of antibodies E1/M2 and E1/M3, they appear to have arisen from a common precursor B cell rather than directly from each other (Fig 5). To test the framework of our hypothesis, ie, somatic mutation resulting in epitope migration of an antibody, we are constructing the precursors and potential intermediates between the M2 and M3 light chains and will then determine what Rh(D) epitope specificities (if any) they express. This concept of epitope migration has been previously reported for murine anti-cryptococcal56and anti-type II collagen57 antibodies.
If the proposed model for Rh(D) epitopes is correct, then the question of the number of epitopes may be obsolete. There may be as many epitopes as can be differentiated by the number of cell categories, ie, 2n epitopes, where n is the number of distinct partial Rh(D) RBCs. A more important question is the interrelationships between the various epitopes. For example, are some epitopes further away than others—not in the topological sense, but in terms of the number of mutational hits an antibody needs to receive to change its serologic reactivity. Furthermore, does the humoral immune response in a partial Rh(D) individual differ from that in an Rh(D)-negative individual in the manner predicted by this model? One may find that allo-anti-Rh(D) antibodies made by partial Rh(D) individuals are not as clinically significant, ie, capable of inducing hemolysis. This may explain why hemolytic disease of the newborn due to anti-Rh(D) produced by pregnant individuals with partial Rh(D) phenotypes is so rare even when taking into account the low prevalence of the partial Rh(D) phenotypes.34 A better understanding of the immune response to Rh(D) in these patients may alleviate concerns regarding the need to identify such individuals to ensure that they only receive Rh(D)-negative blood products for transfusion and Rh(D)-immune globulin during pregnancy.58 Furthermore, with respect to the design of recombinant Rh(D)-immune globulin for use in Rh(D)-negative patients, it may not be necessary to formulate cocktails of MoAbs containing multiple Rh(D) epitope specificities.
In summary, we have studied the genetic and immunological properties of a large array of anti-Rh(D) antibodies to elucidate this clinically significant human immune response on a molecular level. Our results show that anti-Rh(D) antibodies display a high degree of structural relatedness and the ability to inhibit each other's binding despite differences in epitope specificity. These findings suggest that Rh(D) epitopes are not spatially distinct and that Rh(D) antibodies may undergo epitope migration as a result of somatic mutation. The end result is that the prevalence of certain anti-Rh(D) specificities in the immune repertoire may be a function not only of what epitopes an individual lacks, but of the number of accessible pathways that the individual's immune system can use that avoid self-reactivity. This process may be a general feature of human immune responses to other clinically significant, closely related epitopes.
ACKNOWLEDGMENT
The authors thank Shari Russell and Vatinee Bunya for their excellent technical assistance and Christine Lomas-Francis, Marion Reid, Marilyn Moulds, and Peggy Spruell for providing samples of Rh(D) variant RBCs.
APPENDIX
Genbank accession numbers for anti-Rh(D) heavy chains are as follows: B01, AF044419; C01, AF044420; C03, AF044421; C04, AF044422; C05,AF044423; C08, AF044424; C10, AF044425; D01, AF044426; D03; AF044427; D04, AF044428; D05, AF044429; D07, AF044430; D08, AF044431; D09,AF044432; D10, AF044433; D11, AF044434; D12, AF044435; D13, AF044436; D14, AF044437; D15, AF044438; D16, AF044439; D17, AF044440; D18,AF044441; D20, AF044442; D30, AF044443; D31, AF044444; E01, AF044445; E03, AF044446. Genbank accession numbers for antiRh(D) κ light chains are as follows: F01, AF044447; G01, AF044448; H01, AF044449; I01,AF044450; I02, AF044451; I03, AF044452; I04, AF044453; I05, AF044454; I06, AF044455; I07, AF044456; I08, AF044457; I09, AF044458; I10,AF044459; I11, AF044460; I12, AF044461; I13, AF044462; I15, AF044463; I16, AF044464. Genbank accession numbers for anti-Rh(D) λ light chains are as follows: J01, AF044465; J02, AF044466; J04, AF044467; J06, AF044468; K01, AF044469; K02, AF044470; K03, AF044471; L01,AF044472; L03, AF044473; L04, AF044474; L05, AF044475; M01, AF044476; M02, AF044477; M03, AF044478; N01, AF044479; N02, AF044480; O01,AF044481; O02, AF044482; O03, AF044483; P01, AF044484; Q01, AF044485; R01, AF044486; S01, AF044487.
Supported in part by March of Dimes Birth Defects Foundation Basil O'Connor (Grant No. 5-FY94-0787) and Clinical Research (Grant No. 6-FY96-0367) Awards (D.L.S.), by a National Institutes of Health Specialized Center of Research (SCOR) in Transfusion Medicine and Biology Award (P50-HL54516; to D.L.S.), and by a grant from the National Blood Foundation (T.Y.C.).
Address reprint requests to Don L. Siegel, PhD, MD, Department of Pathology & Laboratory Medicine, 6-55 Founders Pavilion, Hospital of the University of Pennsylvania, 3400 Spruce St, Philadelphia, PA 19104.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal