ACUTE PROMYELOCYTIC leukemia (APL) is a distinct subtype of acute myelogenous leukemia (AML), identified by the French-American-British classification as AML-M31 and cytogenetically characterized by the balanced reciprocal translocation between chromosomes 15 and 17. Patients with the common hypergranular type of APL are most often leukopenic. However, a more aggressive form of APL, characterized by marked hyperleukocytosis and scarcely granulated blasts with bilobed or kidney-shaped nuclei, is described as the microgranular variant (M3v) and accounts for 25% of APL cases.2,3 The disease typically presents with a life-threatening hemorrhagic diathesis, which is worsened by cytotoxic chemotherapy. Recent studies report an incidence of early hemorrhagic deaths of about 10% to 20% in APL.4-7 In M3v APL the hemostatic disturbance and the inherent risk of early hemorrhagic death are particularly elevated.8 Improving the hemorrhagic complications is an important task in this disease, which shows an otherwise relatively favorable prognosis.9
The use of all-trans-retinoic acid (ATRA) for the remission induction therapy of APL has raised the complete remission (CR) rate to greater than 90%.6-8,10 ATRA promotes the terminal differentiation of leukemic promyelocytes. In these cells the fusion of the nuclear retinoic acid receptor (RARα) gene on chromosome 17 with part of the PML gene on chromosome 15 results in the expression of a chimeric PML/RARα protein, which is involved in both the leukemogenesis and the sensitivity to myeloid differentiation induced by ATRA.6
Clinicians soon noted that the ATRA-induced remission was accompanied by prompt improvement of the coagulopathy typical of this disease.10 11 Since then, a number of studies have confirmed that ATRA improves the hemostatic laboratory parameters and the bleeding complications.
This article reviews the effects of ATRA on the coagulopathy of APL and the mechanisms by which this drug affects the hemostatic system. We also focus on how ATRA influences fatal hemorrhagic events during the induction of remission in APL.
THE COAGULOPATHY OF APL: THE EFFECT OF ATRA
The coagulation/bleeding syndrome of the onset of APL is a complex disorder.8,12 Abnormalities of the laboratory coagulation tests compatible with the clinical picture of disseminated intravascular coagulation (DIC) are described in the majority of patients.12 DIC involves the rapid consumption of coagulation factors and platelets in the circulation for the massive intravascular clotting activation. The most common abnormalities of “routine” clotting tests in APL include hypofibrinogenemia, increased fibrinogen-fibrin degradation products (FDP), and prolonged prothrombin and thrombin times. Thrombocytopenia caused by the bone marrow (BM) invasion is further affected by the clotting mechanisms. All the laboratory parameters usually worsen when cytotoxic chemotherapy starts, resulting in severe hemorrhagic complications.13
The pathogenesis of this disorder in APL is intriguing and reflects the interaction of several pathophysiological processes. The activation of at least three different mechanisms, including the coagulation system, the fibrinolytic cascade and the pathways of nonspecific proteolysis, may lead to similar alterations of “routine” clotting tests. The use of new and more sensitive laboratory tests to detect enzyme-inhibitor complexes and byproducts of the coagulation/fibrinolysis reactions confirms the hyperactivity of all three systems in APL. Plasma markers of clotting activation, ie, thrombin-antithrombin complex (TAT), prothrombin fragment 1 + 2 (F1 + 2), and fibrinopeptide A (FPA), are all elevated.5,14-16 High circulating FDPs and urokinase-type (u-PA), associated with low plasminogen and α-2-antiplasmin levels, indicate hyperfibrinolysis.17-19 Plasma levels of leukocyte elastase and fibrinogen split products of elastase are also increased and demonstrate the activity of nonspecific proteases.16,19Each of these systems can potentially play a role in triggering the bleeding complications of APL. However, the new laboratory tests show that thrombin activity and fibrin generation in vivo are constant events in these patients. Of particular relevance is the detection of elevated levels of D-dimer, the lysis product of cross-linked fibrin, which definitely shows that hyperfibrinolysis occurs in response to clotting activation in APL.16,20 21
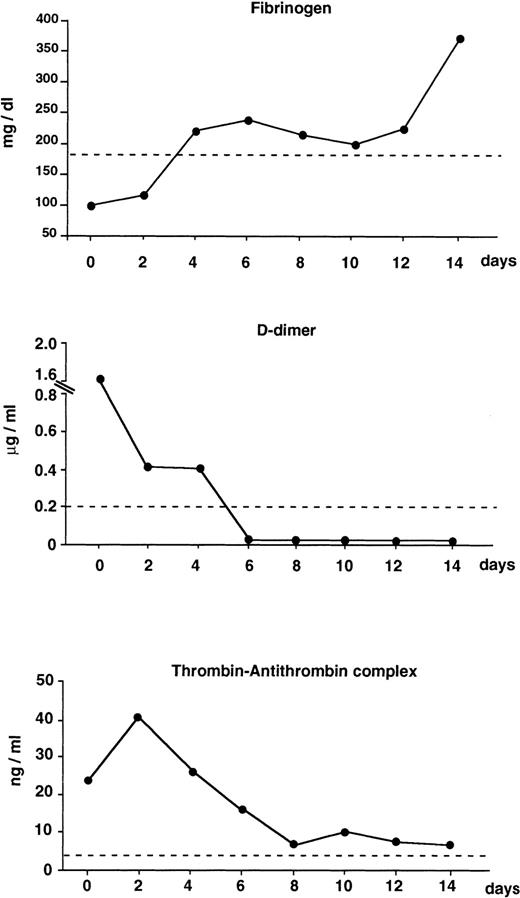
The advent of ATRA for the remission induction therapy of APL has opened new perspectives in the management of this complication. Clinicians promptly observed the rapid resolution of the bleeding symptoms in patients treated with ATRA.6-8,10,11 In an early French clinical study the correction of coagulation disorders was one of the early signs of ATRA's efficacy.11 A number of laboratory studies have subsequently confirmed the decrease or normalization of clotting and fibrinolytic variables during the first 1 or 2 weeks of therapy with ATRA.16,20,21 In our study,16 plasma hemostatic variables measured at the onset of APL showed the following: elevated hypercoagulability markers (TAT, F1 + 2, D-dimer), low mean protein C, normal fibrinolysis proteins, and increased elastase. After starting ATRA, all markers of clotting activation and fibrin degradation decreased within 4 to 8 days, protein C was increased, the overall fibrinolytic balance was unchanged, and elastase remained elevated. In addition, ATRA therapy was accompanied by reduced proteolysis of the von Willebrand factor.22 The beneficial effect on hypercoagulation/hyperfibrinolysis parameters (Fig1) paralleled the improvement of clinical signs of the coagulopathy in these patients. The benefit persisted when ATRA was given in combination with chemotherapy.16,22 This finding is of particular interest because the combination of ATRA with chemotherapy may prolong disease-free survival in APL and may therefore be a choice for treatment.7
Changes of coagulation markers in newly diagnosed APL patients (n = 9) receiving ATRA for induction therapy. Results are the median values of fibrinogen, D-dimer, and thrombin-antithrombin complex (TAT) at different intervals during the first 2 weeks of treatment. Dashed lines are: fibrinogen = the lower limit of normal control values; D-dimer and TAT = the mean + 2 SD (cut-off point) of normal control values. (Modified and reprinted with permission.15)
Changes of coagulation markers in newly diagnosed APL patients (n = 9) receiving ATRA for induction therapy. Results are the median values of fibrinogen, D-dimer, and thrombin-antithrombin complex (TAT) at different intervals during the first 2 weeks of treatment. Dashed lines are: fibrinogen = the lower limit of normal control values; D-dimer and TAT = the mean + 2 SD (cut-off point) of normal control values. (Modified and reprinted with permission.15)
HOW ATRA INTERACTS WITH THE HEMOSTATIC SYSTEM
Some of the mechanisms by which ATRA interacts with the hemostatic system have been elucidated and others are still under investigation. Retinoids mainly interfere with the hemostatic properties of different cells, including promyelocytic blast cells, normal human endothelial cells, and normal human monocytes.
ATRA and the Hemostatic Properties of the Leukemic Cell
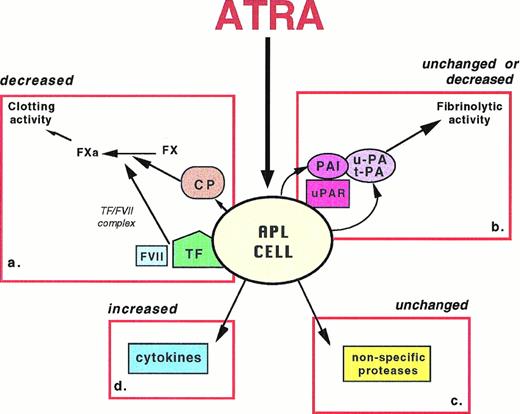
The principal hemostatic properties of the leukemic cell are schematically represented in Fig 2. They include the following: (1) the expression of procoagulant activities; (2) the expression of fibrinolytic and proteolytic activities; and (3) the secretion of inflammatory cytokines, ie, interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), which affect the hemostatic system at the vascular endothelium and leukocyte. ATRA can interfere with each of these properties.
Schematics of the principal pathways of APL cell interactions with the hemostatic system, which can be affected by ATRA. APL cell expresses: (a) cellular procoagulants (TF and CP) that activate the clotting cascade; ATRA decreases the expression of both TF and CP, thus reducing the procoagulant activity; (b) fibrinolysis proteins (u-PA, t-PA, PAI) and receptor (u-PAR); ATRA increases both plasminogen activators and inhibitors, resulting in unchanged or reduced fibrinolytic activity; (c) nonspecific proteases, including granule elastase, that proteolyze fibrinogen/fibrin and other coagulation factors; ATRA does not affect this cellular mechanism; and (d) cytokines, including IL-1β and TNF-α, that induce the endothelium thrombogenicity; ATRA increases the production of cytokines.
Schematics of the principal pathways of APL cell interactions with the hemostatic system, which can be affected by ATRA. APL cell expresses: (a) cellular procoagulants (TF and CP) that activate the clotting cascade; ATRA decreases the expression of both TF and CP, thus reducing the procoagulant activity; (b) fibrinolysis proteins (u-PA, t-PA, PAI) and receptor (u-PAR); ATRA increases both plasminogen activators and inhibitors, resulting in unchanged or reduced fibrinolytic activity; (c) nonspecific proteases, including granule elastase, that proteolyze fibrinogen/fibrin and other coagulation factors; ATRA does not affect this cellular mechanism; and (d) cytokines, including IL-1β and TNF-α, that induce the endothelium thrombogenicity; ATRA increases the production of cytokines.
Procoagulant activities.
At least three tumor cell procoagulants have been identified: (1) tissue factor (TF), which acts by forming a complex with factor VII (FVII) to activate factors X and IX and is the procoagulant of normal and malignant tissues23,24; (2) a membrane factor V receptor, which facilitates the assembly of prothrombinase complex, thus accelerating its activity up to 100,000 times25; (3) cancer procoagulant (CP), a cysteine proteinase that directly activates factor X, independently of the presence of FVII,26 and has been described in fetal and malignant tissues.26,27,28 Several studies have identified TF in leukemic cells.23,29,30 Our group has reported the finding of CP in blasts of various AML phenotypes, with a greatest expression in the AML-M3 type.31 CP levels appear to be related to the phase of the disease.32
Differentiating treatment with ATRA of cultured APL cells in vitro can influence their procoagulant activity (PCA). We have shown that the ATRA-induced cell differentiation is associated with the loss of capacity to express CP in the NB4 cell line,33 the first human APL line ever isolated, with the typical t(15;17) chromosomal balanced translocation. In the same cell line and in cultured blasts from APL patients, it has been reported that ATRA significantly depresses the expression of TF.34,35 We observed that ATRA exerts its inhibitory effect on the leukemic cell PCA in vivo as much as in vitro. Both TF and CP of APL marrow blasts were progressively reduced in patients given ATRA for remission induction therapy of APL.16 The demonstration that this effect parallels the improvement of the hypercoagulation parameters provides the first evidence in vivo of a role of tumor cell PCA in the clotting complications associated with malignancy. Reduction of leukemic cell PCA by ATRA appears to be one mechanism involved in the resolution of the coagulopathy.
Fibrinolytic and proteolytic properties.
Fibrinolytic and proteolytic activities of leukemic cells were first described by Gralnick and Abrell.36 Leukemic promyelocytes contain both the urokinase-type plasminogen activator (u-PA) and the tissue-type plasminogen activator (t-PA).37,38 Although the single-chain pro-urokinase (scu-PA) with little effect on plasminogen is predominant in cells from solid tumors, the two-chain active form (tcu-PA) is prevalent in various leukemic cells, including APL.39 Granulocytic proteases, such as elastase and chymotrypsin, are found in the granules of myeloid blasts as well. When released into the bloodstream these proteases are neutralized by their main inhibitor α-1-antitrypsin. Increased plasma levels of elastase-inhibitor complex have been described in acute leukemia.16,40 These enzymes degrade several clotting factors in vitro41 and can enhance the fibrinolytic system by proteolyzing the two plasmin inhibitors α-2-antiplasmin and C1 esterase inhibitor.42 Further, elastase can directly breakdown the fibrinogen molecule, producing a pattern of peptides (FDP) different from plasmin.43 44
The expression of these activities by APL cells is believed to play a major role in the pathogenesis of the bleeding syndrome. Freshly isolated APL blasts express lower levels of fibrinolytic and proteolytic activities than mature neutrophils and the granulocytic differentiation of APL cells induced by ATRA is not associated with a decrease or change in the expression of these activities in vitro.35 Similar results were obtained with ATRA treatment in the non-APL myeloid leukemia cell line HL60.45 A more detailed study with the APL cell line NB4 showed that the retinoids induce a prompt increase of u-PA activity on the cell surface, which is subsequently downregulated after 24 hours by the production of PA inhibitors.46 These results agree with our finding that, despite changes in some fibrinolysis proteins, the overall plasma fibrinolytic response, as measured by the “euglobulin lysis area,” is unaffected in APL patients receiving ATRA.16In these patients the initial signs of reactive hyperfibrinolysis (ie, elevated D-dimer), rapidly decreased and may reflect the activation of the fibrinolytic system at a cellular site, where specific receptors favor the assembly of all the fibrinolytic components. Thereafter, ATRA-induced synthesis of PA inhibitors may downregulate receptor-bound plasminogen activators as described in vitro.
Levels of circulating elastase are elevated at the onset of APL, possibly resulting from cell degranulation and lysis. These levels are not modified by ATRA therapy.16 Our study found no relation between the plasma elastase concentration and the levels of the D-dimer and other hemostatic variables during treatment with ATRA. This raises the question of whether this enzyme makes an important contribution to the bleeding disorders of APL.
Cytokine release.
Leukemic cells produce inflammatory cytokines, including TNF-α and IL-1β.47 A role for the blast cytokines in the pathogenesis of the acute leukemia coagulopathy was suggested by Cozzolino et al,48 who showed that leukemic promyelocytes from patients with DIC secrete more IL-1β than APL blasts from patients without DIC. They suggested the mechanism involves an interaction of cytokines with the hemostatic properties of the vascular endothelium. TNF-α, IL-1β, and endotoxin can induce the expression of the procoagulant TF by endothelial cells (EC).49,50These cytokines also downregulate the expression of EC thrombomodulin (TM), the surface high-affinity receptor for thrombin.51The TM-thrombin complex activates the protein C system, which in turn functions as a potent anticoagulant (see below). TF upregulation and TM downregulation lead to a prothrombotic condition of the vascular wall.52 In addition, TNF-α and IL-1β can stimulate the EC to produce the t-PA inhibitor PAI-1.53 Inhibition of fibrinolysis contributes to the prothrombotic potential of EC. Although endotoxin and TNF-α can also increase t-PA levels in vivo, when administered to patients or healthy volunteers they rapidly increase t-PA, followed by a much larger increase of PAI-1.54 55This shows that an initial increase of fibrinolytic activity is followed by a prolonged reduction of fibrinolysis.
ATRA upregulates the leukemic cells' ability to produce cytokines.56,57 In theory this effect should favor the prothrombotic potential of the endothelium, but this does not happen because of the protective role of ATRA on EC. ATRA counteracts both the TM downregulation and the TF upregulation of the endothelium induced by TNF-α.58 Recently we have shown that the TF expression induced on EC by the medium of APL NB4 cells treated with ATRA (containing IL-1β) is significantly prevented by the simultaneous presence of ATRA on the endothelium.59 Therefore, although ATRA increases cytokines synthesis by APL cells, it is also able to protect the endothelium against the prothrombotic assault of these mediators.
Other properties.
In addition to the above activities, leukemic cells possess other properties that play a role in the hemostatic balance and are affected by ATRA. Of particular importance is their ability to express TM, the surface receptor that binds and inactivates thrombin. ATRA increases the expression of TM in NB4 cells,34 HL60 cells,60 and in freshly isolated myeloid leukemia cells of different subtypes, including APL.61 This effect contributes to the cell membrane change from a procoagulant to an anticoagulant phenotype.
Another property is the APL cell capacity to synthesize and release the serine protease cathepsin G. This proteolytic enzyme (normally stored in the azurophil granules of mature granulocytes) cleaves fibrinogen and PAI-1, neutralizes the tissue factor pathway inhibitor, and is the mediator of neutrophil-induced platelet aggregation. ATRA treatment downregulates cathepsin G expression by APL cells.62 This finding adds further evidence for an overall antithrombotic effect of this drug on leukemic cells.
Finally, it has been reported that the annexin VIII gene is uniquely overexpressed in APL cells and is downregulated by ATRA.63Annexin VIII is one member of a family of Ca2+-dependent phospholipid-binding proteins. Like annexin V, it inhibits the phospholipid-dependent activation of blood coagulation and may therefore have a role in the bleeding diathesis of APL. However, it is also questioned whether annexin VIII exerts an anticoagulant role in APL, because it has no secretory peptide sequence, is not found extracellularly, and has never been detected in the plasma of APL patients.64
ATRA and EC Hemostatic Properties
EC can express procoagulant and anticoagulant as well as fibrinolytic and antifibrinolytic activities (Fig 3),thus playing an active role in the regulation of blood coagulation and fibrinolysis.
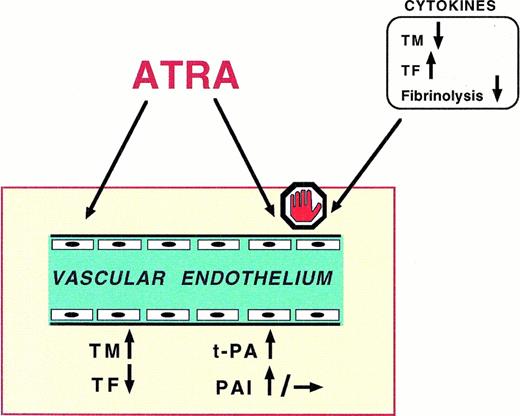
Schematics of the principal effects of ATRA on the vascular endothelium. (1) ATRA induces the expression of endothelial cells (EC) TM, the membrane receptor that binds and inactivates thrombin; the TM/thrombin complex activates the protein C/protein S system, a potent anticoagulant mechanism; (2) ATRA prevents the expression of TF, the tissue procoagulant exposed by EC upon appropriate stimuli; (3) ATRA induces the EC production of t-PA and, to minor extent, of PAI-1, favoring the fibrinolytic response of the endothelium; (4) finally, ATRA counteracts the prothrombotic effects of cytokines, ie, IL-1β and TNF-α, on EC.
Schematics of the principal effects of ATRA on the vascular endothelium. (1) ATRA induces the expression of endothelial cells (EC) TM, the membrane receptor that binds and inactivates thrombin; the TM/thrombin complex activates the protein C/protein S system, a potent anticoagulant mechanism; (2) ATRA prevents the expression of TF, the tissue procoagulant exposed by EC upon appropriate stimuli; (3) ATRA induces the EC production of t-PA and, to minor extent, of PAI-1, favoring the fibrinolytic response of the endothelium; (4) finally, ATRA counteracts the prothrombotic effects of cytokines, ie, IL-1β and TNF-α, on EC.
Procoagulant and anticoagulant properties.
Unlike leukemic cells, EC do not constitutively express PCA, but they can be stimulated to expose TF on their surface.50 As already described (see above), ATRA can counteract the cytokine-induced TF expression on EC membrane.58
Highly pertinent to the endothelium antithrombotic potential is the expression of TM, the surface high-affinity receptor for thrombin.51 The TM-thrombin complex activates circulating protein C, which in turn functions as a potent anticoagulant by proteolytically inactivating coagulation factors V and VIII. Protein C activity is accelerated by its cofactor protein S. TM plays a central role in the TM-thrombin-protein C-protein S system of clotting inhibition. ATRA protects the endothelium against the anti-TM effect of TNF-α and IL-1β,58 as described above. In addition, however, it can directly induce the synthesis of TM by EC. Treatment with ATRA significantly increases the expression of TM on cultured human EC by inducing TM mRNA and protein biosynthesis.65The fact that ATRA inhibits the effect of cytokines and directly stimulates TM anticoagulant activity by EC provides further evidence of its likely antithrombotic effect in tumors and inflammatory diseases.
Fibrinolytic and antifibrinolytic properties.
The vascular endothelium can produce all the components of the fibrinolytic system: t-PA, u-PA, PAI-1, and the receptors for plasminogen activators and plasminogen.66 Vasoactive substances such as bradykinin, platelet-activating factor, and thrombin can all induce the acute release of t-PA from a storage pool in the vessel wall. Activation of protein kinase C is implicated in the regulation of t-PA transcription and synthesis in human EC. This process stimulates t-PA production by histamine and thrombin. The retinoids are among pharmacological agents that can enhance t-PA synthesis by EC.67
ATRA can enhance EC fibrinolytic functions. Its mechanism of action is different from that of other known agonists, such as histamine, thrombin, and phorbol 12-myristate 13-acetate (PMA). These agonists act through cell-surface receptors, while retinoids act through intracellular receptors that belong to the family of nuclear receptors. ATRA potentiates the production of EC t-PA by histamine, thrombin, and PMA.68 The ATRA-induced stimulation of t-PA involves a distinct pathway, acting through binding to the nuclear retinoic acid receptors α and β.69 This evidence suggests that ATRA induces the vascular wall to protect against fibrin deposition. However, the fact that ATRA can, to a lesser extent, also induce the production of PAI-1 by EC66,67 protects against excess fibrinolysis.
ATRA and Normal Human Monocyte Hemostatic Properties
Retinoids modulate several functions of mononuclear phagocytes, such as IL-1 and IL-3 production, tumoricidal activity, phagocytosis, and Fc receptor expression. Relevant to this review is the fact that ATRA inhibits the PCA, namely TF, of human monocytes. Like EC, these cells do not constitutively express TF, but respond to different stimuli by generating and exposing this procoagulant on their surface. Monocyte/macrophage PCA generated in vivo may be implicated in the activation of blood coagulation seen in certain pathological conditions, including malignancy.70 ATRA dose-dependently inhibits the procoagulant response induced by endotoxin in human peripheral mononuclear cells.71 Our group found that the regulation of TF expression by retinoids is mediated by nuclear retinoic acid receptors and may be different from the regulation of TF in leukemic cells.72 The inhibition of monocyte PCA generation might help explain the retinoids' anticoagulant effect.
ATRA and Leukemic Cell/EC Adhesion Mechanisms
The influence of ATRA on the leukemic cell/EC interactions merits a separate analysis, because the attachment of the leukemic cell to the vessel wall is one potential mechanism of vascular complications.
ATRA can regulate the expression of surface adhesion molecules by leukemic cells,73-76 thus influencing the adhesion of these cells to the vascular endothelium. Leukocytes can adhere to EC and EC matrix (ECM) through membrane adhesion molecules.77 Members of the integrin family of cell adhesion receptors mediate cell-extracellular matrix and cell-cell adhesion. The very late antigen (VLA) integrins (β1 integrins) and leukocyte integrins (β2 integrins: CD11a/CD18, CD11b/CD18, and CD11c/CD18) are widely expressed within the hematopoietic system, including mature cells, myeloid progenitors, and myeloblastic cells. Ligands for these molecules are counter-receptors expressed on the endothelium, eg, vascular cell adhesion molecule-1 (VCAM-1), ligand for VLA4, and the intercellular adhesion molecules 1 and 2 (ICAM-1 and ICAM-2), ligands for the β2 integrins. EC activation by IL-1β or TNF-α increases the expression of EC adhesion molecules.
Retinoic acid upregulates genes that encode integrins.73Specifically, ATRA promotes the differential regulation of adhesion molecules on acute myeloid leukemia blasts.74,75 This may confer on blast cells new homotypic and heterotypic (blast/blast or blast/vascular cell) adhesion potential, which may facilitate cell migration and extravasation,78 and may also promote localized coagulation. We used a functional assay to study the effect of ATRA on the capacity of APL blasts to adhere to cultured human EC and EC matrix. ATRA treatment increased the adhesion of APL cells to the endothelium.76,79 However, more recently we observed that, in the same functional assay, pretreatment of the EC monolayer with ATRA impaired adhesion of the APL cells to EC. Therefore, ATRA may act as a pro-adhesive stimulus on the leukemic cell, but may also exert some anti-adhesive effects on the endothelium.80
THE IMPACT OF ATRA ON THE EARLY HEMOSTATIC EVENTS IN APL
Before the introduction of ATRA, most studies of APL patients treated with conventional chemotherapy reported CR rates from 60% to 80%.81 Fatal hemorrhages caused by the APL-associated coagulopathy were a major cause of failure. In a retrospective multicentric study of 268 consecutive APL patients treated between 1984 and 1987, the overall remission rate was 62% (167 of 268) and the prevalence of hemorrhagic deaths in induction 14% (37 of 268). Among the 37 patients who died from hemorrhage before the assessment of complete remission, 25 (67%) died within the first 10 days of treatment (early hemorrhagic deaths): three had gastrointestinal and the others cerebral hemorrhage. The rate of early hemorrhagic deaths was similar among patients given heparin, anti-fibrinolytics, or supportive therapy alone for management of the coagulopathy.4
More recent series of APL patients treated with intensive platelet transfusion support and new anthracyclines have yielded better results.3,82-84 Fenaux et al83 reported up to 86% CR in a small group of 21 APL patients given Rubidazone and Cytarabine (Ara C). A randomized clinical trial from the Italian GIMEMA group compared monochemotherapy (idarubicin, IDA) with combination chemotherapy (IDA plus Ara C) in 257 newly diagnosed APL patients.84 The CR rates were not significantly different in the two groups (76% in the IDA armv 67% in the combination chemotherapy arm), but fatal hemorrhages were significantly lower in patients given IDA alone (7 of 131, 5.3%, compared with 16 of 126, 12.7%, P < .05) (Dr G. Avvisati, personal communication, September 1997).
From the first clinical studies6 ATRA has produced a high rate of CR and rapid resolution of the coagulopathy without causing BM hypoplasia. Based on the study design, clinical experience with ATRA derives from nonrandomized trials (with or without historical controls) and randomized clinical trials.
Nonrandomized Trials
Groups all over the world have used ATRA, usually at a dose of 45 mg/m2/d, for the treatment of APL in phase II nonrandomized clinical trials.6,10,81 In many of these studies, an uncertain proportion of patients (probably 10% to 15%) were not found to have the (15;17) translocation by cytogenetic analysis or molecular testing. A noteworthy exception is the Italian study that considered genetic evidence of the specific t(15;17) lesion as a mandatory prerequisite for eligibility.85
All phase II trials confirmed that ATRA is very effective in inducing CR in APL patients at diagnosis and in relapse. Up to 95% of CR have been reported in patients for whom t(15;17) was documented.85 Compared with historical controls treated with conventional chemotherapy, patients administered ATRA showed a worthwhile improvement of the CR rate, ranging from 9% to 20%86-89 (Table 1). This increase reached the statistical significance in analyses including at least 50 patients per arm.88 The CR increase was usually associated with a variable reduction of total and hemorrhagic deaths in induction.88,89 A sub-analysis of the Italian trial compared the coagulopathy and transfusion requirements in patients treated with ATRA and historical controls. ATRA led to a significant reduction of early mortality, fatal and nonfatal bleeding, days with platelets <20 × 103/μL, days with fibrinogen <100 mg/dL, and platelet and red blood cell consumption.90 In some studies ATRA was also associated with a significant improvement of event-free and overall survival.86-89
CR and Early Mortality in APL Patients Treated With ATRA
| Reference . | No. Patients . | % CR . | Deaths in Induction (%) . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total . | Hemorrhagic . | Thrombotic . | ||||||||
| ATRA . | C . | ATRA . | C . | ATRA . | C . | ATRA . | C . | ATRA . | C . | |
| Nonrandomized Trials (with historical controls) | ||||||||||
| 86 | 26 | 29 | 96 | 76 | 4 | NR | 4 | NR | NR | NR |
| 87 | 51 | 80 | 86 | NR | 17 | 21 | NR | NR | NR | NR |
| 88 | 109 | 62 | 89 | 71-150 | 8 | 20-150 | NR | NR | NR | NR |
| 89 | 43 | 57 | 77 | 68 | 19 | 30 | 12 | 18 | NR | NR |
| 85 | 434 | 244 | 92 | 71-150 | 2 | 8-150 | 1 | 6-150 | 0.2 | 0 |
| Randomized Clinical Trials | ||||||||||
| 81 | 54 | 47 | 91 | 81 | 9 | 8 | 5.5 | 6.4 | 0 | 0 |
| 93 | 172 | 174 | 72 | 69 | 11 | 14 | 5.8 | 6.9 | 1.7 | 0 |
| Reference . | No. Patients . | % CR . | Deaths in Induction (%) . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total . | Hemorrhagic . | Thrombotic . | ||||||||
| ATRA . | C . | ATRA . | C . | ATRA . | C . | ATRA . | C . | ATRA . | C . | |
| Nonrandomized Trials (with historical controls) | ||||||||||
| 86 | 26 | 29 | 96 | 76 | 4 | NR | 4 | NR | NR | NR |
| 87 | 51 | 80 | 86 | NR | 17 | 21 | NR | NR | NR | NR |
| 88 | 109 | 62 | 89 | 71-150 | 8 | 20-150 | NR | NR | NR | NR |
| 89 | 43 | 57 | 77 | 68 | 19 | 30 | 12 | 18 | NR | NR |
| 85 | 434 | 244 | 92 | 71-150 | 2 | 8-150 | 1 | 6-150 | 0.2 | 0 |
| Randomized Clinical Trials | ||||||||||
| 81 | 54 | 47 | 91 | 81 | 9 | 8 | 5.5 | 6.4 | 0 | 0 |
| 93 | 172 | 174 | 72 | 69 | 11 | 14 | 5.8 | 6.9 | 1.7 | 0 |
Abbreviations: C, controls; GIMEMA, Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto (refs 85 and 90); NR, not reported.
P < .05.
However, phase II clinical trials showed the potential toxicity of ATRA. The most severe side effect is the so-called “ATRA syndrome,” which occurs in about a quarter of patients usually between the second day and the third week of treatment. It is a protean syndrome of fever, respiratory distress, pulmonary infiltrates, pleuropericardial effusions, and edema, more common among patients who present with a high white blood cell count or develop rapid leukocytosis. If not promptly recognized and treated, the ATRA syndrome can lead to death from progressive hypoxemia and multiorgan failure. There is no agreement as to the best treatment. Some investigators found chemotherapy after initial treatment with ATRA effective in preventing the syndrome,91 while others used high-dose intravenous corticosteroids (dexamethasone, 10 mg every 12 hours for at least 3 days) as soon as the first symptoms occur.78 The two approaches are not mutually exclusive. In two large multicenter studies where chemotherapy was rapidly added to ATRA and dexametasone also used at the earliest clinical signs, the incidence and severity of the syndrome was consistently decreased.85 92
Another intriguing complication of ATRA is thrombosis. In some series, fatal myocardial infarction and cerebral thrombosis have been reported.85,93 In another case, acute venous thrombosis and pulmonary embolism were observed in a patient with APL who developed hyperleukocytosis after 1 week of treatment with ATRA.94Interestingly, Escudier et al95 noted that thrombotic events were more common in patients suspected of having ATRA syndrome. Thromboembolic events were not characteristic features of APL before the ATRA era and the question arises whether this drug, which has multiple effects on hemostasis, also related to potentially dangerous prothrombotic conditions. An answer may possibly lie in the very subtle balance between procoagulant and anticoagulant mechanisms in cancer. For instance, high concentrations of circulating cytokines in the presence of low levels of ATRA in the bloodstream may definitely favor clotting activation and fibrin deposition at the vascular endothelium.
Summing up, nonrandomized studies indicated that ATRA was able to induce a rapid resolution of the coagulopathy and a limited, albeit not trivial, 5% to 6% reduction of early hemorrhagic deaths.89,90 Fatalities related to “ATRA syndrome” have represented a major problem in early studies, accounting for the lack of significant decrease in overall induction mortality. In the most recent series of patients, recognition and effective management of this complication contributed to reduce the total early deaths, as compared with historical controls.88 90
Randomized Clinical Trials
To date, only two randomized clinical trials comparing ATRA plus chemotherapy with chemotherapy alone in newly diagnosed APL patients have been completed.7,92 In both, the presence of t(15;17) was not considered mandatory for eligibility. The French trial was originally designed to include 215 patients, but accrual was stopped after the first interim analysis, which showed significantly better event-free survival in the ATRA group.7 At the time, 101 evaluable patients had been randomized, 54 in the ATRA and 47 in the chemotherapy arm. CR rate was higher in the ATRA group (91% v81%) but early termination of the trial meant that too few patients were admitted to reach statistical significance. Therefore, this point remains uncertain. The duration of coagulopathy was significantly reduced in the ATRA group (3 ± 3 v 6 ± 4 days) but no difference was observed in hemorrhagic deaths (three in both groups), number of platelet and red blood cell transfusions, and duration of hospital stay. Subsequent analyses of the results, after more prolonged follow-up, confirmed a significant improvement in overall survival in the ATRA group.96 97
The American trial comprised 346 evaluable APL patients.93CR rate was 72% in the group receiving ATRA (124 of 172) and 69% in the arm treated with chemotherapy alone (120 of 174) (P = .56). However, when the CR rate was recalculated on the 221 patients known to have the specific t(15;17) translocation, the CR rate became 76% (79 of 104) for ATRA and 69% (81 of 117) for chemotherapy (P = .29). These latter findings indicated a 7% absolute improvement of CR produced by ATRA, in agreement with the results of the French trial. Ten patients died of hemorrhage in the ATRA group (5.8%) compared with 12 (6.9%) in the chemotherapy group, confirming the tendency to a reduction of hemorrhagic mortality seen in nonrandomized studies. In any case, as in the French trial, the most important result was the demonstration that, compared with conventional chemotherapy, ATRA was associated with an improved disease-free survival and overall survival. Therefore, despite inconclusive evidence about the efficacy of the drug on CR and early mortality, both the French and American investigators concluded that ATRA should be included in the treatment of all patients with newly diagnosed APL.
CONCLUSIONS
In conclusion, the bulk of evidence reviewed herein clearly shows that ATRA has a profound impact on the hemostatic system, leading to rapid resolution of the APL-associated coagulopathy. ATRA can affect most of the leukemic cell functions in hemostasis, which are considered major pathogenetic determinants for the coagulopathy. These anticoagulant effects on tumor cells occur together with the drug's anticoagulant effects on normal endothelial and monocytic cells.
The resolution of the coagulopathy makes for better management of patients and avoids the potentially catastrophic consequences of starting chemotherapy in the acute phase of the disease. Nevertheless, the clinical impact of ATRA on CR and early hemorrhagic deaths remains uncertain. An approximately 10% absolute increase of CR and a variable reduction of hemorrhagic mortality in induction have been observed, but these limited improvements are difficult to confirm statistically in a rare disease such as APL. Nevertheless, there is general consensus that ATRA should be included in the management of APL because it is clear that the drug improves disease-free survival and overall survival.
ACKNOWLEDGMENT
We express our sincere appreciation to Drs Rossella Consonni and Marina Marchetti for their contribution to investigations performed in our laboratory. We are also grateful to J. Baggott for editorial assistance.
Address reprint requests to Anna Falanga, MD, Hematology Department, Ospedali Riuniti, Largo Barozzi 1, 24100 Bergamo, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal