Abstract
The endotoxin (lipopolysaccharide)-induced cytokine response is followed by a state of unresponsiveness to lipopolysaccharide (LPS) referred to as LPS tolerance or endotoxin desensitization. LPS tolerance, which can be experimentally induced in vitro and in vivo, is also known to occur in septic disease. Here, we evaluated whether dendritic cells (DC), the most potent antigen-presenting cells, are also subject to this phenomenon. Single doses of LPS added at the initiation of DC culture inhibited in a dose-dependent fashion the production of tumor necrosis factor-α (TNF-α), interleukin-10 (IL-10), and IL-12, but not the production of IL-8, in response to a second LPS challenge in day-5 DC. In addition, the LPS-induced expression of the CD83 maturation antigen was inhibited in these cells. Moreover, the endocytic activity of DC generated in the presence of LPS was dramatically reduced. DC desensitized with LPS were potent stimulators of T-cell proliferation but poor inducers of interferon-γ (IFN-γ) production in the allogeneic mixed leukocyte reaction. TNF-α and prostaglandin E2, two major products of LPS stimulation, could replace LPS for the induction of tolerance to LPS. Moreover, treatment of desensitized DC with TNF-α plus prostaglandin E2 fully restored CD83 expression and partially restored IL-12 production as well as the IFN-γ–inducing activity of DC in the mixed leukocyte reaction. Our data show that human DC are highly susceptible to the induction of LPS tolerance, which seems to be a state of differential deactivation in which some functions are impaired whereas others are retained. Tolerization at the level of the professional antigen-presenting cell by inflammatory mediators may play an important role in septic disease and in the origin of cancers associated with chronic inflammation.
THE INNATE IMMUNE system rapidly responds to the invasion of gram-negative bacteria with an acute inflammatory response.1 A major signal of infection is lipopolysaccharide (LPS), a common constituent of the outer membranes of gram-negative bacteria.1,2 Macrophages but also dendritic cells (DC) are highly sensitive to even low concentrations of LPS and respond by releasing inflammatory mediators. The activation of DC by LPS3,4 or by LPS-induced inflammatory products5 eventually results in the development of an antigen-specific T-cell response.6 7
Although monocytes respond to primary encounter with LPS by rapidly producing cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-10 (IL-10) exposure to LPS also renders monocytes temporarily hyporesponsive to LPS. Thus, monocytes preexposed to even low concentrations of LPS will produce scarce amounts of these cytokines in response to a subsequent secondary challenge with high-dose LPS. This phenomenon has been referred to as endotoxin desensitization or LPS tolerance.8 In animal models, LPS tolerance could be induced in vivo by administering a single sublethal injection of LPS.9 Moreover, monocytes from patients who survived the acute phase of septic shock often exhibit a strongly reduced capacity to secrete TNF-α in response to LPS in vitro.10 Most importantly, these patients have a poor prognosis and may die weeks or months later with signs of persistent infections,10 suggesting that T-cell responses are impaired in these patients.
DC are the most professional antigen-presenting cells specifically adapted to initiate T-cell responses.6,7 To evaluate a potential role of DC in septic disease, we investigated the effects of LPS on DC development in vitro. We have previously shown that prostaglandin E2 (PGE2) and TNF-α, both products of LPS stimulation, cooperate to activate human DC.11 The combination of PGE2 and TNF-α synergistically induced IL-12 synthesis in DC as well as, in an additive manner, the upregulation of various surface antigens including the maturation antigen CD83. These data suggested that PGE2 and TNF-α mediate the maturation-inducing effects of LPS. Therefore, we also investigated the effects of TNF-α and PGE2 on DC development.
MATERIALS AND METHODS
Media and reagents.
The medium used in this study was RPMI 1640 supplemented with 1% heat-inactivated (30 minutes, 56°C) pooled human AB serum, 50 U/mL penicillin, 50 μg/mL streptomycin, 2.5 μg/mL fungizone, 2 mmol/L L-glutamine, 10 mmol/L Hepes, 0.1 mmol/L nonessential amino acids, 1 mmol/L pyruvate, and 5 × 10−5 mol/L 2-mercaptoethanol (all from Boehringer Ingelheim Bioproducts, Vienna, Austria). Human albumin (for intravenous use; Octapharma, Vienna, Austria) was added to a final concentration of 2 mg/mL (complete medium). Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) (Leucomax; 1.11 × 107 U/mg) was from Novartis (Basel, Switzerland). Recombinant human IL-4 (2 × 107 U/mg) was kindly supplied by the Schering-Plough Research Institute (Kenilworth, NJ). LPS from Salmonella abortus equi was purchased from Sigma Chemical Company (St Louis, MO). Recombinant human TNF-α (1 × 107 U/mg) was purchased from Genzyme (Cambridge, MA). PGE2 was purchased from Sigma and from Calbiochem-Novabiochem International (San Diego, CA). Fluoresceinated Dextran (FITC-DX; molecular weight 70.000) and fluoresceinated bovine serum albumin (FITC-BSA) were from Sigma.
Culture of human DC.
DC were generated from peripheral blood mononuclear cells (PBMC) similarly as described.5 Briefly, PBMC were isolated from leukocyte-enriched buffy coats by standard density gradient centrifugation on Ficoll-Paque (Pharmacia, Uppsala, Sweden). Monocytes were isolated from PBMC by centrifugal elutriation (greater than 95% purity) and cultured in complete medium containing 1,000 U/mL of each GM-CSF and IL-4 at a density of 106 cells/mL. DC developed under essentially endotoxin-free conditions as indicated by the absence of spontaneous TNF-α production (less than 5 pg/mL of TNF-α per 1 × 106 DC). To tolerize DC, LPS (0.2 ng/mL to 10 ng/mL), TNF-α (100 or 1,000 U/mL), or PGE2 (10 nmol/L or 10 μmol/L) were added as a single dose at the onset of DC culture. On day 2, 0.5 volumes of fresh medium containing 1,000 U/mL of GM-CSF and IL-4 were added. After 5 days of culture (primary culture), the cells were harvested. The cells were washed extensively and recultured in cytokine-containing medium at 3 to 4 × 105 cells/mL with or without stimuli (reculture). After 48 hours, supernatants were assayed for the presence of cytokines and cells for surface antigen expression.
Flow cytometric measurement of surface antigen expression and endocytic activity.
To determine surface Ag expression, cells (105 DC in 50 μL) were labeled with primary monoclonal antibody (MoAb) in complete medium followed by FITC-conjugated F(ab′)2 fragments of goat anti-mouse Ig (Dako, Glostrup, Denmark). The following MoAbs were used: VIM-13 (IgM, anti-CD14, was a kind gift of Dr W. Knapp, Vienna, Austria), G46-2.6 (IgG1, anti-HLA-ABC), L243 (IgG2a, anti-HLA-DR), HB-15a (IgG2b, anti-CD83), 84H10 (IgG1, anti-CD54), BB1 (IgM, anti-CD80), BU63 (IgG1, anti-CD86), and 5C3 (IgG1, anti-CD40). Washes were in Hanks' Balanced Salt Solution (HBSS) containing 0.2% albumin. After the last wash, the cells were stored in HBSS containing 0.2% albumin and 2% formaldehyde. The samples were analyzed on a FACScan (Becton-Dickinson, San Jose, CA). Data were analyzed and presented using CellQuest software (Becton-Dickinson).
The endocytic activity of DC was measured as described previously.3 FITC-DX was used to measure mannose-receptor-mediated endocytosis and FITC-BSA to assess macropinocytosis. Cells (105) were incubated with FITC-DX or FITC-BSA (0.5 mg/mL) for 30 minutes at 37°C (control at 0°C) and then washed extensively with ice-cold buffer. The samples were analyzed on a FACScan. Data were analyzed and presented using CellQuest software.
Quantitation of DC cytokines.
TNF-α, IL-10, and IL-8 were measured in culture supernatants by specific enzyme-linked immunosorbent assay (ELISA) using commercially available kits from Medgenix, (Fleurus, Belgium) and Central Laboratory of The Netherlands Red Cross Blood Transfusion Service (Amsterdam, The Netherlands). IL-12 was measured using a commercially available kit from Genzyme (Cambridge, MA) which detects both IL-12 p40 and the bioactive IL-12 p70 heterodimer consisting of p40 and p35. Cytokines were quantitated using a microtiter plate reader.
Determination of the allostimulatory potential of DC.
DC generated in the presence or absence of LPS were used as stimulators in an allogeneic mixed leukocyte reaction (MLR). T cells from normal adult blood were used as responders. Graded doses (103 to 105) of irradiated (30 Gy) DC were added to a constant number of T cells (2 × 105/well) in 96-well flat-bottomed tissue culture plates in medium containing 5% pooled human AB serum. After 5 days, T-cell proliferation was measured as [3H]thymidine incorporation (16-hour pulse with 1 μCi/well; NEN, Boston, MA) and culture supernatants (50 μL) were collected for interferon-γ (IFN-γ) determinations. IFN-γ levels were assessed by use of a specific ELISA from Genzyme.
RESULTS
DC generated in the presence of LPS exhibit a reduced capacity to produce TNF-α and IL-10.
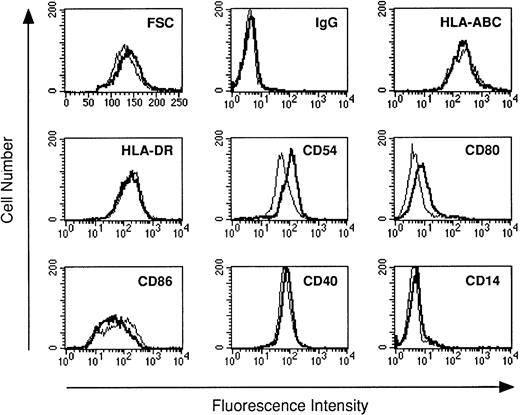
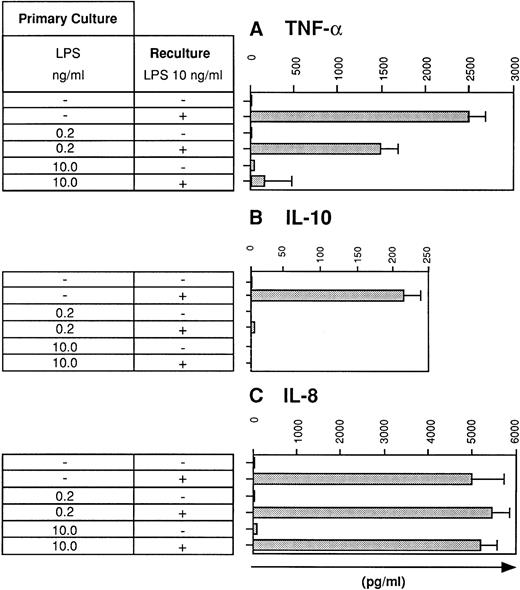
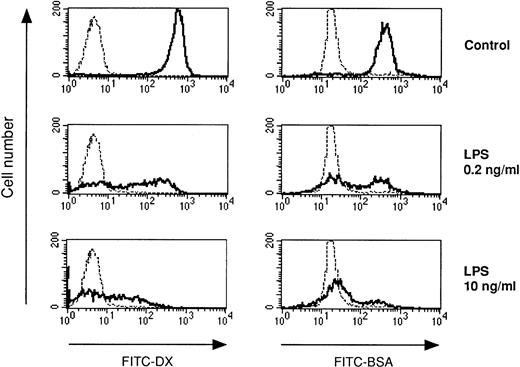
Previous studies have shown that human monocytes preexposed to LPS exhibit a strongly diminished production of TNF-α and IL-10 in response to secondary stimulation with LPS.8 We wondered whether DC are also subject to the phenomenon of LPS tolerance. DC were generated using the method of Sallusto and Lanzavecchia.5This protocol generates a single population of DC which exhibited homogenous forward scatter properties (Fig1, first histogram) and homogenously stained for major histocompatibility complex (MHC) class I and II molecules as well as for adhesion and costimulatory molecules. In addition, the cells lacked CD14 expression (Fig 1). To study LPS tolerance of DC, a single dose of LPS was added at the initiation of DC culture. Addition of LPS had relatively little influence on the phenotype of the cells (Fig 1). The cells were then restimulated with LPS on day 5 and cytokines were determined in cell culture supernatants after 48 hours. Figure 2A shows that the production of TNF-α by DC generated in the presence of low-dose LPS (0.2 ng/mL) was diminished and was almost completely prevented in DC generated in the presence of high-dose LPS (10 ng/mL). In addition, IL-10 production by DC preexposed to low-dose LPS was dramatically reduced and was virtually abolished at the higher LPS dose (Fig 2B). These results suggested that LPS tolerance can be induced in DC and that the degree of inhibition depended on the LPS dose administered at the beginning of DC culture. In contrast to TNF-α and IL-10, IL-8 production was not inhibited in LPS-desensitized DC (Fig 2C).
The phenotype of DC generated with or without LPS. DC were cultured for 5 days in the absence (standard lines) or presence of LPS (bold lines). Day-5 DC were analyzed by flow cytometry for the surface expression of the antigens indicated using the antibodies listed in Materials and Methods. Cells were analyzed without scatter gating. In the first histogram, forward scatter (FSC, x-axis) is plotted against the number of events (y-axis).
The phenotype of DC generated with or without LPS. DC were cultured for 5 days in the absence (standard lines) or presence of LPS (bold lines). Day-5 DC were analyzed by flow cytometry for the surface expression of the antigens indicated using the antibodies listed in Materials and Methods. Cells were analyzed without scatter gating. In the first histogram, forward scatter (FSC, x-axis) is plotted against the number of events (y-axis).
LPS desensitization diminishes TNF-α and IL-10 production by human DC. DC were cultured for 5 days in the absence or presence of LPS at the concentrations indicated (primary culture). Day-5 DC were recultured for 48 hours with or without LPS (10 ng/mL) and supernatants were analyzed for (A) TNF-α, (B) IL-10, and (C) IL-8 levels using specific ELISAs. Data represent mean values of triplicate measurements with standard deviation (SD) from one experiment representative of eight independent experiments.
LPS desensitization diminishes TNF-α and IL-10 production by human DC. DC were cultured for 5 days in the absence or presence of LPS at the concentrations indicated (primary culture). Day-5 DC were recultured for 48 hours with or without LPS (10 ng/mL) and supernatants were analyzed for (A) TNF-α, (B) IL-10, and (C) IL-8 levels using specific ELISAs. Data represent mean values of triplicate measurements with standard deviation (SD) from one experiment representative of eight independent experiments.
IL-12 production is also downregulated in LPS-tolerant DC.
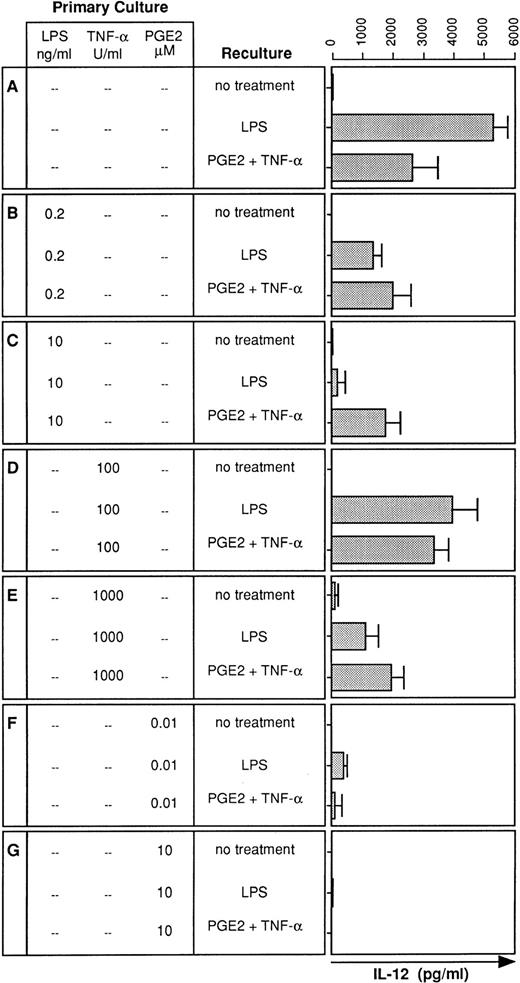
Although IL-12 is an important proinflammatory cytokine,12its expression in LPS tolerance has not been investigated. We therefore analyzed the LPS-induced IL-12 production by DC generated in the presence or absence of LPS. Whereas DC generated in the absence of LPS produced substantial amounts of IL-12 (Fig3A), presence of LPS during DC development profoundly downregulated IL-12 production in response to LPS restimulation (Fig 3B and C). Similar to TNF-α (Fig 2A), the degree of downregulation of IL-12 production depended on the LPS dose added at the onset of DC culture (Fig 3B and C).
IL-12 production is also diminished in desensitized DC: restoration by PGE2 plus TNF-α. DC were cultured for 5 days in the presence or absence of LPS, TNF-α, or PGE2 at the concentrations indicated (primary culture). Day-5 DC were recultured with or without LPS (10 ng/mL) or PGE2 (10 μmol/L) plus TNF-α (1,000 U/mL) for 48 hours and supernatants were analyzed for IL-12 levels using a specific ELISA. Data represent mean values of triplicate measurements with SD from one experiment representative of four independent experiments.
IL-12 production is also diminished in desensitized DC: restoration by PGE2 plus TNF-α. DC were cultured for 5 days in the presence or absence of LPS, TNF-α, or PGE2 at the concentrations indicated (primary culture). Day-5 DC were recultured with or without LPS (10 ng/mL) or PGE2 (10 μmol/L) plus TNF-α (1,000 U/mL) for 48 hours and supernatants were analyzed for IL-12 levels using a specific ELISA. Data represent mean values of triplicate measurements with SD from one experiment representative of four independent experiments.
DC generated in the presence of LPS fail to acquire CD83 expression in response to LPS.
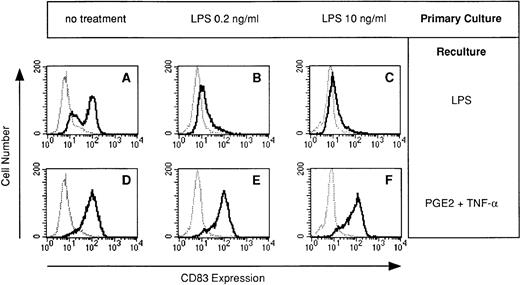
The terminal maturation step of DC is characterized by the neoexpression of CD83.13-15 Because synthesis of proinflammatory cytokines by DC is usually associated with terminal maturation, we speculated that the failure of LPS-desensitized DC to produce cytokines reflects the failure of DC to mature. We therefore analyzed CD83 expression in DC generated in the presence of LPS. The LPS-induced expression of CD83 during restimulation was dramatically impaired in DC preexposed to LPS (Fig 4B and C).
LPS-desensitized DC fail to acquire CD83 expression: restoration by TNF-α plus PGE2. DC were cultured for 5 days in the presence or absence of LPS at the concentrations indicated (primary culture). Day-5 DC were recultured with or without LPS (10 ng/mL) or PGE2 (10 μmol/L) plus TNF-α (1,000 U/mL) for 48 hours and cells were analyzed for CD83 expression (bold line) by flow cytometry. The isotype control (IgG2b) is also presented (dotted line).
LPS-desensitized DC fail to acquire CD83 expression: restoration by TNF-α plus PGE2. DC were cultured for 5 days in the presence or absence of LPS at the concentrations indicated (primary culture). Day-5 DC were recultured with or without LPS (10 ng/mL) or PGE2 (10 μmol/L) plus TNF-α (1,000 U/mL) for 48 hours and cells were analyzed for CD83 expression (bold line) by flow cytometry. The isotype control (IgG2b) is also presented (dotted line).
TNF-α and PGE2 can replace LPS for the induction of LPS tolerance.
Two major products of LPS stimulation are TNF-α and PGE2.16,17 Therefore, we tested whether TNF-α or PGE2 can replace LPS for the induction of LPS tolerance. DC cultures were initiated either in the presence of TNF-α (100 or 1,000 U/mL) or PGE2 (10 nmol/L or 10 μmol/L), stimulated on day 5 with LPS, and analyzed for IL-12 production. DC cultured with TNF-α exhibited reduced IL-12 production in response to LPS stimulation (Fig 3D and E). Downregulation occurred again in a dose-dependent manner. In contrast to high-dose LPS, which caused an almost complete downregulation of IL-12 production (Fig 3C), high-dose TNF-α was less potent in inhibiting IL-12 production (Fig 3E). Consistent with previous observations,18 PGE2 was most potent in preventing IL-12 production by DC. A single dose of PGE2 at 10 nmol/L was sufficient to induce an almost complete downregulation of the LPS-induced IL-12 production (Fig 3F), and IL-12 production was virtually abolished by PGE2 at 10 μmol/L (Fig 3G).
Treatment with TNF-α plus PGE2 restores IL-12 production and CD83 expression in DC desensitized with LPS.
We have previously shown that PGE2 stimulates low-level IL-12 production in human DC and that TNF-α strongly synergizes with PGE2 to induce high-level IL-12 synthesis.11 Therefore, we wondered whether the combination of PGE2 and TNF-α is able to restore IL-12 synthesis in desensitized DC. Figure 2B and C show that PGE2 in combination with TNF-α partially restored IL-12 production in LPS-desensitized DC. Moreover, this combination also restored IL-12 production in DC desensitized with high-dose TNF-α (Fig 3E). The synergistic effect of the combination of PGE2 and TNF-α was required for partial restoration of IL-12 synthesis and either substance alone failed to restore IL-12 production (data not shown). IL-12 production could not be restored in DC tolerized with PGE2 alone (Fig 3F and G).
In addition to the stimulation of IL-12 synthesis, TNF-α plus PGE2 fully restored CD83 expression in DC desensitized with LPS (Fig 4E and F). TNF-α plus PGE2 also restored CD83 expression in DC desensitized with either TNF-α or PGE2 (data not shown).
Desensitized DC exhibit reduced endocytic activity, are potent in stimulating allogeneic T-cell proliferation but less efficient at inducing IFN-γ production by allogeneic T cells.
We also investigated the accessory cell potential of DC in LPS tolerance. A prerequisite for antigen presentation is antigen uptake. DC have high-capacity mechanisms for the uptake of soluble proteins. Antigens are interalized by DC either via fluid phase (macropinocytosis) or via receptor-mediated endocytosis (eg, through the mannose receptor). Figure 5 shows that either pathway for antigen uptake was strongly inhibited in DC generated in the presence of LPS. Similar results were obtained when DC developed in the presence of TNF-α or PGE2 (data not shown).
LPS-desensitized DC exhibit reduced endocytic capacity. DC were cultured in the presence or absence of LPS at the concentrations indicated. The endocytic activity of day-5 DC was measured using FITC-DX (mannose receptor-mediated endocytosis) and FITC-BSA (macropinocytosis). The cells were incubated with FITC-DX or FITC-BSA for 30 minutes at 37°C (controls at 0°C, dotted lines), washed, and analyzed by flow cytometry.
LPS-desensitized DC exhibit reduced endocytic capacity. DC were cultured in the presence or absence of LPS at the concentrations indicated. The endocytic activity of day-5 DC was measured using FITC-DX (mannose receptor-mediated endocytosis) and FITC-BSA (macropinocytosis). The cells were incubated with FITC-DX or FITC-BSA for 30 minutes at 37°C (controls at 0°C, dotted lines), washed, and analyzed by flow cytometry.
Next, LPS-sensitive and LPS-desensitized DC were compared as stimulators of allogeneic T-cell proliferation. Figure6 shows that LPS-desensitized DC were as efficient as LPS-sensitive DC in stimulating T-cell proliferation in an allogeneic MLR. Phenotypic analysis of DC generated in the presence of LPS revealed normal or modestly increased expression of MHC class I and II molecules, and adhesion (CD54) and costimulatory molecules (CD80, CD86) (Fig 1).
LPS-desensitized DC are potent stimulators of allogeneic T-cell proliferation. DC were cultured in the presence or absence of LPS at the concentrations indicated. Day-5 DC were irradiated and used as stimulators of allogeneic T-cell proliferation. Proliferation was monitored by measuring [methyl-3H]thymidine uptake on day 5 of coculture. Each value represents the mean cpm of triplicate cultures with SD.
LPS-desensitized DC are potent stimulators of allogeneic T-cell proliferation. DC were cultured in the presence or absence of LPS at the concentrations indicated. Day-5 DC were irradiated and used as stimulators of allogeneic T-cell proliferation. Proliferation was monitored by measuring [methyl-3H]thymidine uptake on day 5 of coculture. Each value represents the mean cpm of triplicate cultures with SD.
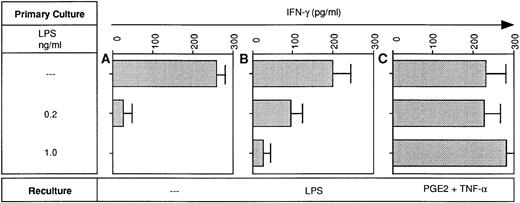
Because LPS-desensitized DC were relatively poor sources of IL-12 (Fig3), it was tempting to assume that they were poor stimulators of IFN-γ production by allogeneic T cells. However, at high DC input (greater than or equal to 5 × 103DC/2 × 105 T cells), LPS-desensitized and LPS-sensitive DC were equally potent at inducing IFN-γ production in allogeneic T cells (data not shown). In contrast, at lower DC:T ratios (1 × 103 DC/2 × 105 T cells), LPS-tolerant DC were clearly less effective as inducers of IFN-γ production (Fig 7A). Restimulation of tolerant DC with LPS modestly improved IFN-γ production in the MLR (Fig 7B). Most importantly, pretreatment of desensitized DC with TNF-α in combination with PGE2 fully restored their IFN-γ inducing activity in the MLR (Fig 7C).
LPS-desensitized DC are poor inducers of IFN-γ production in the MLR: restoration of the IFN-γ inducing activity by pretreatment of desensitized DC with PGE2 plus TNF-α. DC were cultured in the presence or absence of LPS at the concentrations indicated (primary culture). Day-5 DC were recultured with or without LPS (10 ng/mL) or PGE2 (10 μmol/L) plus TNF-α (1,000 U/mL) for 48 hours and then 1 × 103 DC were used as stimulators of 2 × 105 allogeneic T cells. On day 5 of coculture supernatants were obtained and analyzed for IFN-γ levels using a specific ELISA. Data represent mean values of triplicate measurements with SD from one experiment representative of two independent experiments.
LPS-desensitized DC are poor inducers of IFN-γ production in the MLR: restoration of the IFN-γ inducing activity by pretreatment of desensitized DC with PGE2 plus TNF-α. DC were cultured in the presence or absence of LPS at the concentrations indicated (primary culture). Day-5 DC were recultured with or without LPS (10 ng/mL) or PGE2 (10 μmol/L) plus TNF-α (1,000 U/mL) for 48 hours and then 1 × 103 DC were used as stimulators of 2 × 105 allogeneic T cells. On day 5 of coculture supernatants were obtained and analyzed for IFN-γ levels using a specific ELISA. Data represent mean values of triplicate measurements with SD from one experiment representative of two independent experiments.
DISCUSSION
In the present work we show that exposure of developing DC to relatively low doses of LPS leads to a lack of DC responsiveness to LPS. Desensitized DC failed to acquire CD83 expression (Fig 4) and to produce the important proinflammatory cytokines TNF-α (Fig 2) and IL-12 (Fig 3) in response to LPS. Desensitized DC were also characterized by a significantly reduced ability to endocytose soluble antigens (Fig 5). In addition, desensitized DC deficient in IL-12 synthesis were less efficient inducers of IFN-γ production in the allogeneic MLR (Fig 7). PGE2 and TNF-α, two major products of LPS stimulation, could replace LPS for the induction of tolerance to LPS (Fig 3). Most importantly, the combination of these two factors when added exogenously fully restored CD83 expression (Fig 4) and partially restored IL-12 production in DC desensitized with LPS (Fig 3B and C). IL-12 production could also be restored in DC desensitized with TNF-α alone (Fig 3E) but not in DC desensitized with PGE2 alone (Fig 3F and G), indicating that PGE2 when added alone exerts additional and possibly irreversible inactivation effects. Restoration of IL-12 synthesis in desensitized DC also restored their IFN-γ–inducing activity in the MLR (Fig 7). However, desensitization was not a state of complete deactivation because functions such as IL-8 production (Fig2C) and stimulation of allogeneic T cell proliferation (Fig 6) were retained.
DC generated from monocytes with GM-CSF and IL-4 are immature and can be induced by inflammatory stimuli such as LPS to undergo a terminal maturation step.7 Terminal maturation is characterized by the production of cytokines such as TNF-α4,19 and IL-124,20 as well as by the neoexpression of the CD83 antigen.13-15 LPS tolerance in DC seems to be the inability to perform this terminal maturation step because desensitized DC failed to acquire CD83 expression (Fig 4) and to produce TNF-α and IL-12 (Figs 2 and 3). We have recently shown that a combination of PGE2 and TNF-α, both products of LPS stimulation,16,17 induces terminal maturation of DC, which was accompanied by efficient IL-12 synthesis in DC.11 PGE2 alone was a weak stimulus of IL-12 production, but in synergy with TNF-α, induced high-level IL-12 production in DC.11 These data suggested that TNF-α and PGE2 endogenously produced by DC mediate the stimulatory effects of LPS. This contention is further supported by our previous finding that DC maturation induced by Bacillus Calmette-Guérin mycobacteria could partially be prevented by neutralization of endogenously produced TNF-α.19 The reduced ability of desensitized DC to produce IL-12 in response to LPS restimulation (Fig 3) may therefore be attributible to the deficient synthesis of TNF-α in desensitized DC (Fig 2) and thus to the inability to create a strong synergy between endogenously produced PGE2 and TNF-α. However, stimulation of desensitized DC with LPS plus TNF-α could not restore IL-12 synthesis (data not shown). Only the combination of PGE2 and TNF-α restored IL-12 production (Fig 3B, C, and E), strongly suggesting that synthesis of PGE2 is also impaired in desensitized DC.
We have repeatedly observed that individual DC cultures inefficiently perform maturation in response to LPS stimulation (Fig 4A). This might reasonably be due to desensitization of their monocytic precursors in vivo. Recent encounter with inflammatory mediators in vivo (eg, during bacterial or viral infection) would downmodulate the cytokine-producing capacity of monocytes that are still capable of differentiating into DC in vitro but fail to mature in response to LPS. Importantly, stimulation of such cultures by the addition of PGE2 plus TNF-α resulted in complete maturation of DC (Fig 4D).
Desensitized DC were potent stimulators of T-cell proliferation (Fig6). Desensitized DC expressed high levels of MHC and adhesion and costimulatory molecules (data not shown), thus providing the signals required for the induction of allogeneic T-cell proliferation.21 DC-derived cytokines are not essential for T-cell proliferation but rather influence T-cell differentiation. DC-derived IL-12 is required for the optimal generation of type 1 T-helper cells.12,22 IL-12 acts by strongly enhancing IFN-γ production by T-helper cells and thereby skews the balance towards Th1-dominated T-cell responses. At high DC numbers, desensitized DC were also efficient in inducing IFN-γ production in the MLR (data not shown), confirming that under conditions of optimal T-cell receptor stimulation and costimulation, IL-12 is not essential for IFN-γ production.23 However, when these signals become limiting (ie, at low DC numbers), IL-12 becomes essential for efficient IFN-γ production.23 Under such conditions, which more likely ressemble the in vivo situation, the ability of desensitized DC to induce IFN-γ production in the MLR was clearly impaired (Fig 7A). Restoration of IL-12 synthesis in desensitized DC also restored the IFN-γ–inducing activity of these cells (Fig 7C).
Monocyte deactivation by endotoxin desensitization occurs in patients with septic disease and is associated with persistent infections and increased mortality.10 The fact that many of these patients die with signs of opportunistic infections suggests that antigen presentation is impaired, resulting in a failure to elicit productive antigen-specific T-cell responses. Provided that deactivation of DC, which are the most potent antigen-presenting cells, by endotoxin desensitization also occurs in vivo, it would reasonably explain the deficit of these patients in antigen presentation. The strongly reduced endocytic activity of desensitized DC as shown in Fig 5 suggests that these cells will inefficiently internalize and thus inefficiently present infectious antigens. Moreover, inhibition of IL-12 production in desensitized DC (Fig 3) resulted in the failure to induce IFN-γ production in allogeneic T cells (Fig 7). These findings would suggest that septic patients who struggle with persistent infections suffer from the inability to efficiently elaborate IFN-γ due to the lack of help usually provided by IL-12. In fact, Döcke et al10 recently reported that treatment of such patients with IFN-γ resulted in recovery of monocyte function and clearance of sepsis in most of the patients.
Chronic inflammation has also been implicated in the origin of cancer. Chronic atrophic gastritis, as induced by Helicobacter pylori,is considered an inflammatory precursor of gastric adenocarcinoma.24 Based on our in vitro study, the persistence of inflammatory stimuli in gastric tissue should induce tolerance in DC maturing in such inflammatory lesions. DC deactivation by desensitization would then impair immune surveillance and thus facilitate tumor outgrowth. Berman et al25 recently reported beneficial effects of the systemic administration of IL-10 in a murine tumor therapy model. Among several possibilities, one reasonable interpretation of these findings is that the systemic suppression of inflammatory mediators by IL-10 subsequently allows DC development in a noninflammatory environment, resulting in the restoration of DC responsiveness.
In conclusion, we show that human DC are subject to the phenomenon of endotoxin desensitization or LPS tolerance. Prolonged exposure of developing DC to LPS reduces their capacity to pick up microbial antigens and obviously depletes DC of endogeneous factors (TNF-α and PGE2) needed for IL-12 production and DC maturation. Deficient IL-12 synthesis results in a lack of IFN-γ, which is important for antigen presentation and for the development of Th1 T-cell responses. Deactivation of DC by desensitization in vivo may contribute to the immunodeficiencies that can be observed in septic disease and cancers associated with chronic inflammation.
ACKNOWLEDGMENT
We thank Karin Salzmann for excellent technical assistance.
Supported by the Austrian Science Fund (FWF) Grant No. 11758MED to M.T.
Address reprint requests to Martin Thurnher, PhD, The Department of Urology, Anichstrasse 35, 6020 Innsbruck, Austria.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 6. LPS-desensitized DC are potent stimulators of allogeneic T-cell proliferation. DC were cultured in the presence or absence of LPS at the concentrations indicated. Day-5 DC were irradiated and used as stimulators of allogeneic T-cell proliferation. Proliferation was monitored by measuring [methyl-3H]thymidine uptake on day 5 of coculture. Each value represents the mean cpm of triplicate cultures with SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/9/10.1182_blood.v91.9.3112/3/m_blod40948006x.jpeg?Expires=1769085182&Signature=qXuYIsihBVxNQdQTwUcXCas98tEtTnxKyOWkZL1Z757gH06ujSv76Z9ysa7j0tCMbxzMTfmQ0n9AtC3YSdyNl3KOAzMNg5IMLGtGabS4EPREp8X1WuQsQtIexb5-H7sL1nlVZllTuupX3hB~tLnAHq3un3DZ~ahtw8gwdJRZg0uryggzvdUWp9P8J8cIsHoAq0-UWhIBF-bMFyRe~4rfN86sqivIDj2pJ~fppwMDXumR4PvlpWSuvpyJyXyCzpuNt4jfkukAmaGQG3pRoGwBEAhfHhJoR7TDKlW6pEHQo0xpHKBG-ia7oQOqhLQdx5aFgQSeu4D11oCviNCq4Q0dqA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal