Abstract
Highly regulated interactions between adhesion receptors on progenitor cells and their extracellular matrix ligands are essential for the control of hematopoiesis in bone marrow stroma. We have examined the relationship between α4β1-integrin–mediated adhesion and growth of CD34+ cells by assessing their adhesive and migratory patterns of proliferation in a mixture of hematopoietic growth factors in the presence of different recombinant fragments of theHepII/IIICS region of fibronectin. CD34+ cells were isolated from cord blood and placed in culture wells containing serum-free medium and growth factors. Wells were precoated with either the H120 fragment of fibronectin, which contains three α4β1-integrin binding sites, or the H0 fragment, which lacks the two highest affinity α4β1 binding sequences. Proliferation of single cells of CD34+38+DR+and CD34+38−DR+ phenotypes occurred in contact with the H120 substrate and was associated with migration. Larger numbers of cells were used to quantitate proliferative responses. Cells growing in wells coated with H120 formed attachments to the base of the wells throughout the culture period. Higher total cell counts were consistently found in wells coated with H120 compared with H0 and bovine serum albumin controls. The difference was first apparent at day 8 of culture and reached a maximum at days 11 through 13, when expansion with H120 was a mean of 1.8-fold higher than that seen with H0 (P≤ .0001). The greatest expansion (2.25-fold) with H120 compared with H0 was seen when the growth factor concentrations were reduced to 1/16 of the standard levels (P ≤ .001). The increase in total cell numbers was not at the expense of CD34+ cells as numbers of these were similar in H120 and control cultures. These results provide evidence for synergy between growth factors and integrins that may be relevant to understanding hematopoiesis in marrow stroma.
HEMATOPOIETIC CELL development occurs within the bone marrow stroma where interactions between progenitor cells and their extracellular matrix (ECM) ligands are recognized to be of importance not only for normal differentiation and proliferation but also for maintenance of the hematopoietic stem cell.1 It is likely that highly regulated interactions between adhesion receptors on the progenitor cells and their ECM ligands play key roles in these processes. Foremost among adhesion receptors are the integrins that control cell attachment to specific matrix proteins and that can mediate transfer of information to the intracellular compartment. Recent findings linking signaling pathways from integrins and growth factor receptors suggest that cooperation between these pathways is essential for normal cell development.2 3
Most cells require attachment to a substrate to enter the cell cycle and grow normally. For example, in fibroblasts cell anchorage is essential for cyclin E-CDK2 kinase activity,4 and the synthesis of cyclin A is enhanced by adhesion-dependent signals.5 Additional links between growth and adhesion in nonhematopoietic cells include an association between insulin-mediated pathways and the αvβ3-integrin receptor, which results in a 2.5-fold increase in DNA synthesis when cells are plated on vitronectin6 and between integrin-mediated signal transduction and the Ras pathway that is likely to impact on cell growth.7 Although hematopoietic cells will grow in culture without adherence, a link between adhesion and growth has been shown in T cells where costimulation of the T-cell receptor and the β2-integrin receptor lymphocyte function antigen-1 (LFA-1) causes a synergistic enhancement of T-cell proliferation,8 and in the CD4 subset of T cells the interaction of α4β1 and α5β1 integrins with fibronectin facilitates CD3-mediated cell growth.9,10 Fibronectin has also been shown to promote a twofold enhancement of proliferation of erythroid colony-forming units (CFU-Es) and erythroid burst-forming units (BFU-Es) and of granulocyte, erythroid, monocyte, megakaryocyte colony-forming unit (CFU-GEMM)–derived colonies from human bone marrow11 and to potentiate the effect of interleukin-3 (IL-3) on the growth of CFU-GEMM–, BFU-E–, CFU-E–, and macrophage colony-forming unit (CFU-M)–derived colonies from CD34+cells, an effect that was reversed by blocking the fibronectin α5β1-integrin interaction with RGD-containing peptides.12 More recently it has been reported that cytokines that stimulate the proliferation of CD34+ cells enhance the adhesion of these cells to a fibronectin substrate.13 However, adhesion of hematopoietic progenitors to stroma is associated with inhibition of proliferation that occurs through fibronectin receptors.14
The integrins α4β1 (very late activation antigen-4 [VLA-4]) and α5β1 (VLA-5), which are present on CD34+ cells, mediate adhesion to different domains of fibronectin.15,16 Long-term culture-initiating cells and CFU-mix progenitors adhere to the fibronectin COOH-terminal heparin-binding domain,17 and primitive murine spleen colony-forming unit (CFU-S) day-12 colony-forming cells adhere to the α4β1binding sequence in fibronectin.18 We have recently shown that IL-3 modulates α4β1-integrin function on CD34+ cells, resulting in a reduction in cell adhesion to surfaces coated with the HepII/IIICS domain of fibronectin (the specific ligand for α4β1 integrin) and an increase in cell migration on the same substrate.19 The importance of α4β1 integrins in hematopoiesis is further highlighted by a study that showed that the addition of anti-α4β1 antibodies to long-term bone marrow cultures abrogated the production of lymphoid cells and retarded myelopoiesis20 and by the finding that antibodies to α4β1 selectively mobilized hematopoietic progenitors into the blood.21
In the present study we set out to explore the adhesive and migratory patterns of growth of CD34+ progenitor cells isolated from cord blood by assessing their proliferation in a mixture of hematopoietic growth factors in the presence of different recombinant proteins of the IIICS region of fibronectin. The α4β1 integrin binds to two main sites in the alternatively spliced IIICS region that are represented by the CS1 sequence, the highest affinity α4β1 binding site, and the CS5 sequence, which binds more weakly.22-24The lowest affinity α4β1 binding site resides in the adjacent HepII domain and is represented by the peptide sequence designated H1.25 Minimal active peptide sequences have been defined for the three sites: LDV for CS1, REDV for CS5, and IDAPS for H1. We used two different recombinant proteins from the IIICS region as ligands for α4β1: H120, which contains all three binding sites, and HO, which contains H1 alone thus enabling assessment of the effect of strong and weak α4β1 binding activity on adhesion and proliferation.
We initially assessed adhesive and migratory patterns of growth with H120 and H0 for two different subsets of CD34+ cells representing different stages of maturity, CD34+38+DR+ (more mature) and CD34+38−DR+ (less mature), which were isolated as single cells.26-28 For comparison of proliferative responses, larger numbers of CD34+ cells were expanded in wells coated with the H120 and H0 fragments.
MATERIALS AND METHODS
Cloning and expression of recombinant HepII/IIICS variants.
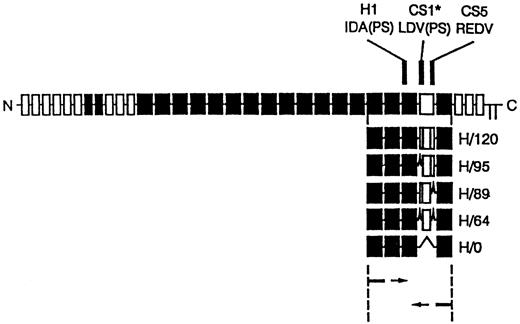
cDNA clones for the five different variants of the HepII /IIICS region of human fibronectin were synthesised using reverse transcription polymerase chain reaction (RT-PCR) amplification of primary human skin fibroblast mRNA as described by Mould et al.29 The PCR cloning strategy and the resulting recombinant proteins are shown in Fig 1. The PCR products were ligated into pUC119 and transformed intoEscherichia coli JM109. Individual clones containing each of the variants were identified by restriction analysis and sequenced. Inserts were subcloned into the EcoRI site of the pGEX-2T expression vector and used to transform E coli strain BLR. Recombinant clones were expressed as glutathione-S-transferase fusion proteins. These were isolated by glutathione affinity chromatography, thrombin digestion, and heparin-agarose affinity chromatography. The H120 (containing H1, CS1, and CS5 sequences) and HO (containing the H1 sequence only) variants were used to coat the wells in CD34+ expansion experiments. Enzyme-linked immunosorbent assay (ELISA) experiments using a monoclonal antibody to the HepII domain showed that each of the variants bound equally well to microtiter plates used for adhesion assays.29
Diagram of recombinant proteins containing the five different splice variants of the HepII/IIICS region obtained by RT-PCR expression cloning. The 3′ primer was complementary to the end of the 15th type III repeat and the 5′ primer to the start of the 12th type III repeat of human fibronectin. The full-length fibronectin subunit is shown together with the alternatively spliced IIICS region, which is represented as the open box. The numbers assigned to the H variants refer to the number of amino acids in the IIICS. The locations of the three recognition sequences for α4β1, HI, CSI, and CS5, are indicated. Note that H120 contains all three sites and H0 contains HI alone.
Diagram of recombinant proteins containing the five different splice variants of the HepII/IIICS region obtained by RT-PCR expression cloning. The 3′ primer was complementary to the end of the 15th type III repeat and the 5′ primer to the start of the 12th type III repeat of human fibronectin. The full-length fibronectin subunit is shown together with the alternatively spliced IIICS region, which is represented as the open box. The numbers assigned to the H variants refer to the number of amino acids in the IIICS. The locations of the three recognition sequences for α4β1, HI, CSI, and CS5, are indicated. Note that H120 contains all three sites and H0 contains HI alone.
Isolation and analysis of CD34+ cells.
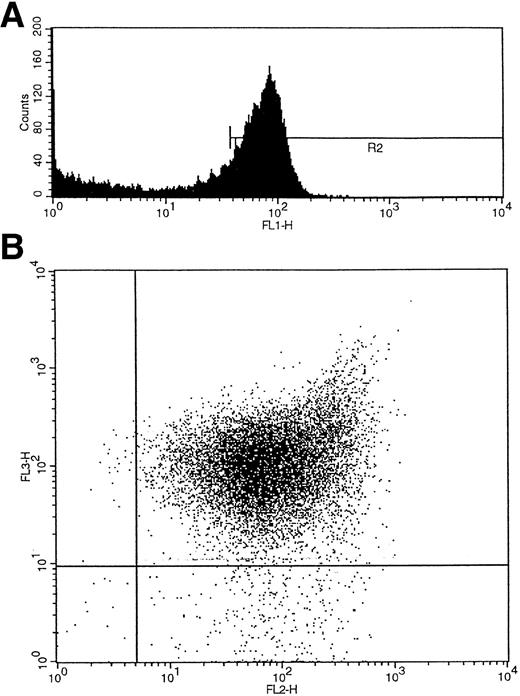
Umbilical cord blood collected into heparin was layered over Ficoll-Hypaque (Lymphoprep; Nycomed, Birmingham, UK), and the interface mononuclear cells were collected following density gradient centrifugation. CD34+ cells were isolated using a CD34 antibody (QBEND/10) and a secondary anti-mouse antibody conjugated to magnetic beads according to the manufacturer's instructions (mini-MACS CD34 isolation kit; Miltenyi Biotec, Bergisch Gladbach, Germany). For maximum purity the isolated cells were also sorted on a fluorescence-activated cell sorter (FACS) flow cytometer (FACS Vantage flow cytometer; Becton Dickinson, San Jose, CA) using a second fluorochrome-conjugated (fluorescein isothiocyanate [FITC] or phycoerythrin [PE]) CD34 antibody (anti–HPCA-2) and an isotype-matched IgG control (Becton Dickinson). For three-color FACS analysis and single-cell sorting, a CD38 antibody conjugated to PE (Becton Dickinson) and an HLA-DR antibody conjugated to tricolor (CALTAG; San Francisco, CA) were added to the cells with the CD34 FITC-conjugated antibody, and control cells were labeled with the corresponding fluorochrome-conjugated isotype-matched IgGs. The cells were washed twice with phosphate-buffered saline (PBS)/0.5% bovine serum albumin (BSA) before sorting. CD34+ cells were subdivided into CD34+38+DR+ and CD34+CD38−DR+ populations after first gating by size on the lymphocyte population and by fluorescence (f11) on the CD34+ cell population (R2) (Fig 2A). The two additional fluorescence parameters, fl2 (CD38-PE) and fl3 (HLA-DR–tricolor), were used to analyze subpopulations of CD34+ cells that were CD38+DR+ and CD38−DR+ (Fig 2B).28 Single cells of each of the two subtypes were deposited into single wells containing serum-free medium (X-vivo 10; Bio-Whittaker, UK) and growth factors.
(A) Initial gating of CD34+ cells by fluorescence (fl1) shown as R2. (B) Two additional fluorescence parameters, fl2 (CD38-PE) and fl3 (HLA-DR-tricolor), were used to analyze subpopulations of CD34+ cells that were CD38+DR+ and CD38−DR+.
(A) Initial gating of CD34+ cells by fluorescence (fl1) shown as R2. (B) Two additional fluorescence parameters, fl2 (CD38-PE) and fl3 (HLA-DR-tricolor), were used to analyze subpopulations of CD34+ cells that were CD38+DR+ and CD38−DR+.
Migration of CD34+38+DR+ and CD34+38−DR+ cells within U-shaped wells: Effect of α4β1 binding sequences.
Single cells of CD34+38+DR+ and CD34+38−DR+ phenotype were deposited by the automated cell deposition unit (ACDU) of the FACS Vantage cell sorter into individual U-bottomed wells of a 96-well plate (Falcon, Oxford, UK) that contained 100-μL aliquots of serum-free medium and that had been precoated with the fibronectin fragments H120 and H0 at 15 μg/mL (3.75 μg/cm2) as described later. A combination of growth factors was added to the cultures: recombinant human IL-3 (rhIL-3; Sandoz, Basel, Switzerland) at 10 ng/mL, rhIL-6 (Sandoz) at 200 U/mL, rh granulocyte colony-stimulating factor (G-CSF; Amgen, Thousand Oaks, CA) at 500 ng/mL, rh stem cell factor (SCF; Amgen) at 100 ng/mL, and rh erythropoietin at 2 U/mL (Boehringer, Mannheim, Germany). These growth factor concentrations were established by determining plateau levels of growth in colony assays in our laboratory. The cells were incubated at 37°C in a humidified atmosphere of 5% CO2 and 5% O2 in air, and growth and migratory patterns were assessed after 1 week and 2 weeks.
Expansion of CD34+ cells in liquid culture: Effect of α4β1 binding sequences.
Twenty-four–well plates (Costar, Cambridge, MA) were coated for 60 minutes at room temperature with 1 mL aliquots of recombinant H120 or H0 proteins diluted with Dulbecco's PBS to a concentration of 15 μg/mL (7.5 μg/cm2). Nonspecific binding sites were blocked for 30 minutes at room temperature with 1 mL of 10 mg/mL heat-denatured BSA. Control plates were prepared with BSA only or left uncoated. A total of 103 to 2.5 × 104 purified CD34+ cells were added to individual wells in serum-free medium together with the standard concentration of growth factors described previously. Cells were incubated at 37°C in a humidified atmosphere of 5% CO2and 5% O2 in air for varying lengths of time. Viable cells were counted with a hematocytometer after staining with Trypan blue. Where appropriate, adherent and nonadherent fractions were separated for counting; nonadherent cells were removed by shaking the wells and washing twice with 1 mL PBS, and bound cells were removed by aspiration with PBS. Cell morphology was assessed by staining with May-Grünwald-Giemsa after cytocentrifugation.
RESULTS
Adhesion and migration patterns of single CD34+38+DR+ and CD34+38−DR+ cells within U-shaped wells: Effect of α4β1 binding sequences.
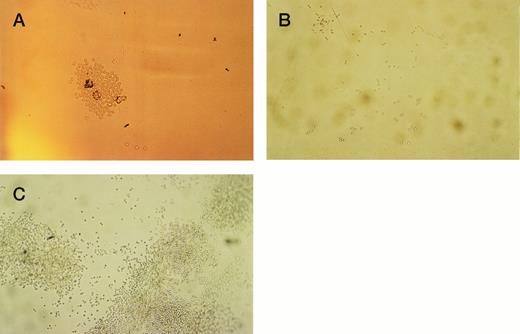
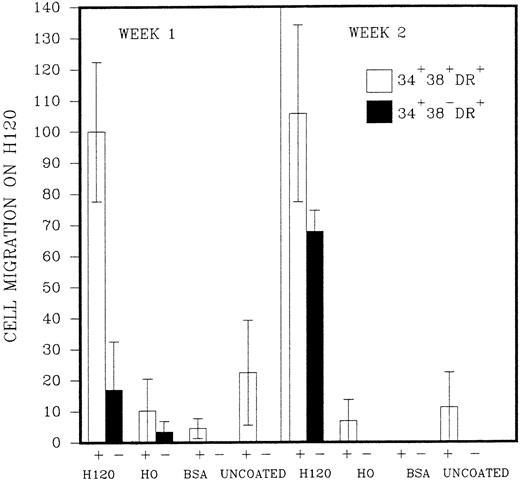
The initial purpose of the study was to assess the pattern of cell adhesion and migration with different coating conditions using single cells of defined phenotype isolated by FACS (Fig 2). Microscopic examination revealed that single cells of CD34+38+DR+ phenotype started to divide in most wells after 2 or 3 days, and a proportion of these continued to proliferate to reach maximum growth at 2 to 3 weeks without a change in medium. Cells of the more immature CD34+38−DR+ phenotype took longer to divide, in some cases up to 2 weeks. Deposition of single cells into a U-shaped well enabled individual cells to be followed as they divided in the presence or absence of the H120 or H0 fibronectin fragments. Cell division in wells coated with H0 or BSA or in uncoated wells took place at the base of the well where cells settled by gravity (Fig 3A), whereas cells dividing in wells coated with the H120 fragment could be seen to have migrated around the base and up the sides of the well (Fig 3B). This pattern of single-cell migration and growth could easily be observed from the initial cell divisions, and as proliferation increased growth took place in focal sites around the well (Fig 3C). In the H0, BSA or uncoated-well proliferation continued to occur at the base of the well. Both CD34+38+DR+ and CD34+38−DR+ phenotypes showed the same migratory pattern of behavior in the presence of H120 from the earliest cell divisions. Figure 4 shows the mean number of wells in which a migratory pattern was seen for the two phenotypes with different coating conditions at weeks 1 and 2. Migration was significantly increased in H120 wells compared with control wells. The mean number of wells in which migratory growth on H120 occurred from cells of 34+38+DR+ phenotype did not change between weeks 1 and 2. Migration and growth occurred less frequently in the more immature 34+38−DR+cells after 1 week when overall growth was minimal but had increased by week 2 (Fig 4). The proliferation efficiency of single cells (ie, number of wells in which growth occurred) did not vary significantly with coating conditions. Wells in which migration did not occur with H120 were usually those in which proliferation did not subsequently increase beyond a few cells. Occasionally a migratory pattern was seen in cells grown in uncoated wells, but this took the form of streaks or lines of cells rather than the spreading pattern seen with H120. Virtually no migration occurred in wells coated with BSA or H0.
Single cells of CD34+38+DR+ and CD34+38−DR+ phenotypes were deposited into single U-shaped wells into serum-free medium and standard growth factors. The wells had been precoated with H120, H0, or BSA or left uncoated. Wells were photographed at 1 and 2 weeks. (A) Proliferation from a CD34+38+DR+ cell at 1 week in a BSA-coated well. Cells are growing in the base of the well. (B) Proliferation from a CD34+38−DR+ cell at 1 week in a well coated with H120. Cells have migrated around and up the sides of the well. (C) Proliferation from a CD34+38+DR+ cell at 2 weeks in a well coated with H120. Cells have proliferated in focal sites around and up the sides of the well.
Single cells of CD34+38+DR+ and CD34+38−DR+ phenotypes were deposited into single U-shaped wells into serum-free medium and standard growth factors. The wells had been precoated with H120, H0, or BSA or left uncoated. Wells were photographed at 1 and 2 weeks. (A) Proliferation from a CD34+38+DR+ cell at 1 week in a BSA-coated well. Cells are growing in the base of the well. (B) Proliferation from a CD34+38−DR+ cell at 1 week in a well coated with H120. Cells have migrated around and up the sides of the well. (C) Proliferation from a CD34+38+DR+ cell at 2 weeks in a well coated with H120. Cells have proliferated in focal sites around and up the sides of the well.
Single cells of CD34+38+DR+(represented as +) and CD34+38−DR+ (represented as −) phenotypes were deposited into single U-shaped wells into serum-free medium and standard growth factors. The wells had been precoated with H120, H0, or BSA or left uncoated. The figure shows the mean number of wells (n = 2,3, or 4) ± SD in which a migratory pattern was seen expressed as a percentage of the result with H120 at week 1 for the CD34+38+DR+ (+) phenotype. Migration was defined as movement of cells against gravity up and around the sides of the wells.
Single cells of CD34+38+DR+(represented as +) and CD34+38−DR+ (represented as −) phenotypes were deposited into single U-shaped wells into serum-free medium and standard growth factors. The wells had been precoated with H120, H0, or BSA or left uncoated. The figure shows the mean number of wells (n = 2,3, or 4) ± SD in which a migratory pattern was seen expressed as a percentage of the result with H120 at week 1 for the CD34+38+DR+ (+) phenotype. Migration was defined as movement of cells against gravity up and around the sides of the wells.
Effect of α4β1 binding sequences on CD34+ cell growth and adhesion.
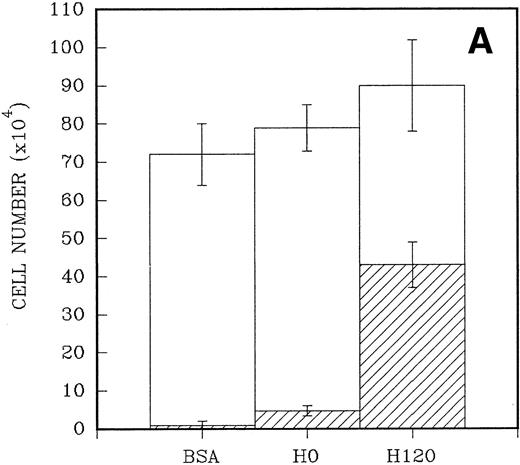
To quantitate the proliferative responses of CD34+ cells to the H120 and H0 fragments, larger numbers of cells were cultured initially for a mean of 7 days. A total of 2.5 × 104CD34+ cells were placed in triplicate into wells that had been precoated with H120 and H0 fragments and contained serum-free medium and the standard growth factor concentrations. Control wells were coated with BSA. When the cells were counted the adherent cells were counted separately from the nonadherent cells in suspension. A 10-fold increase in numbers of adherent cells (mean = 43 × 104 ± 6 × 104) was found in H120 wells compared with H0 wells (mean, 4.7 ± 1.4 × 104; P < .001). Only a small number of adherent cells were present in wells coated with BSA (mean, 1.0 ± 1 × 104; P < .001). At the end of the culture period total cell numbers were higher in H120 wells (mean, 90.3 ± 10.3 × 104) compared with H0 wells (mean, 79.6 ± 4.9 × 104) and BSA wells (mean, 72.0 ± 6.6 × 104), but the differences were not statistically significant in these relatively short-term cultures (Fig 5A).
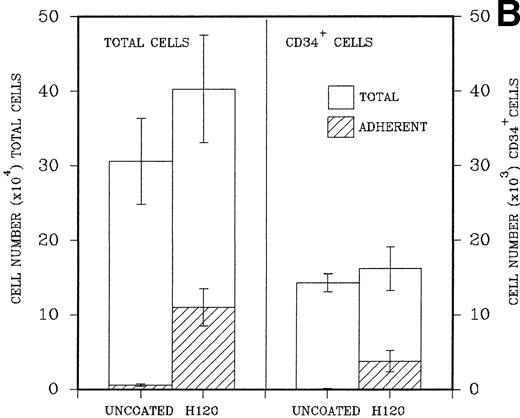
(A) A total of 2.5 × 104CD34+ cells isolated from cord blood were placed in triplicate into wells precoated with H120, H0, or BSA containing serum-free medium and standard growth factors. Adherent and nonadherent cells were counted after 6 days. Hatched areas represent the adherent cell component. (B) A total of 5 × 103CD34+ cells were placed in triplicate into wells precoated with H120 fragment or left uncoated, containing serum-free medium and the standard concentration of growth factors. Adherent and nonadherent cells were counted after 8 days and analyzed by FACS for the proportion of CD34+ cells in each fraction. Hatched areas represent the adherent cell component.
(A) A total of 2.5 × 104CD34+ cells isolated from cord blood were placed in triplicate into wells precoated with H120, H0, or BSA containing serum-free medium and standard growth factors. Adherent and nonadherent cells were counted after 6 days. Hatched areas represent the adherent cell component. (B) A total of 5 × 103CD34+ cells were placed in triplicate into wells precoated with H120 fragment or left uncoated, containing serum-free medium and the standard concentration of growth factors. Adherent and nonadherent cells were counted after 8 days and analyzed by FACS for the proportion of CD34+ cells in each fraction. Hatched areas represent the adherent cell component.
In a similar experiment to determine the effects of H120 on the maintenance of CD34+ cells in culture, 5 × 103 FACS-purified CD34+ cells were placed in precoated wells in triplicate. Control wells were left uncoated. After expansion in culture cells in both, nonadherent and adherent layers were counted, resuspended in 0.5% BSA/PBS, and labeled with a CD34 antibody for FACS analysis. H120 wells again contained increased total numbers of cells (mean, 40.3 ± 7.2 × 104) compared with uncoated wells (mean, 30.8 ± 5.6 × 104). There were increased numbers of cells in the adherent layer of H120 wells (mean, 11.1 ± 2.5 × 104) compared with the uncoated wells (mean, 0.4 ± 0.32 × 104; P < .001). The percentage of CD34+ cells analyzed by FACS was expressed as total numbers of cells. The mean total number of CD34+ cells was similar in both H120 wells (16.2 ± 3.0 × 103) and uncoated wells (14.3 ± 1.2 × 103), and again the adherent layer in H120 wells contained significantly increased numbers of cells (3.8 ± 1.4 × 103) compared with noncoated wells (0.07 ± 0.06 × 103;P < .001; Fig 5B).
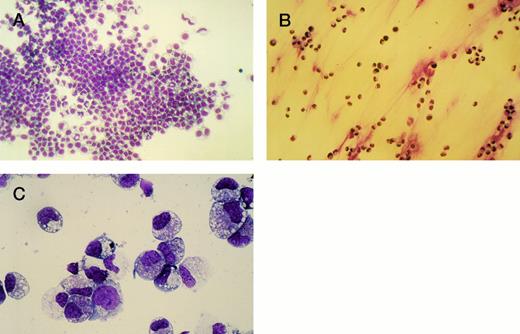
To investigate the effects of H120 on the morphological phenotype of cells during a longer period in culture, CD34+ cells (103) were added to wells containing serum-free medium and growth factors with and without the H120 fragment. After 2 weeks, the nonadherent cells growing in suspension were removed, counted, and stained with May-Grünwald-Giemsa and the adherent cells stained in situ on the well bases (Fig 6A through C). The adherent layers were of the same appearance in all H120 wells and contained aggregates of mainly blasts and promyelocytes with more mature myelocytes and metamyelocytes clearly seen at the edge of the main cell mass (Fig 6A). Cells grown on uncoated surfaces did not form an adherent layer, although a few cells were attached (Fig 6B). A differential count of the nonadherent cells was the same from all wells (Fig 6C). The majority of cells (mean of 58%) was predominantly of blast or promyelocyte morphology together with 16% myelocytes, 12% metamyelocytes, 7% neutrophils, and 7% erythroblasts.
(A) Adherent layer of cells in H120-coated wells stained in situ with May-Grünwald-Giemsa after removing cells in suspension. (B) Cells adherent to uncoated wells stained in situ with May-Grünwald-Giemsa after removal of suspension cells. (C) Photograph of cytospin of suspension cells stained with May-Grünwald-Giemsa. For differential cell count see text.
(A) Adherent layer of cells in H120-coated wells stained in situ with May-Grünwald-Giemsa after removing cells in suspension. (B) Cells adherent to uncoated wells stained in situ with May-Grünwald-Giemsa after removal of suspension cells. (C) Photograph of cytospin of suspension cells stained with May-Grünwald-Giemsa. For differential cell count see text.
Effect of α4β1 binding sequences on CD34+ cell growth with reducing concentration of growth factors.
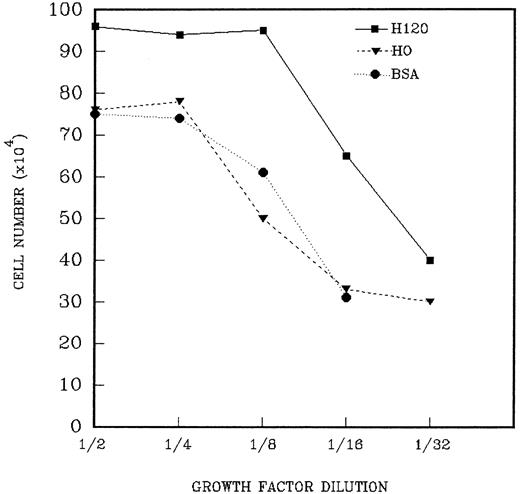
The foregoing results indicated that the H120 substrate supported adhesion, migration, and focal patterns of growth of CD34+cells. To assess the relative contribution of α4β1-integrin engagement by H120 on cell proliferation, a range of growth factor concentrations was used for cell expansion and cells cultured for periods of 9 to 13 days. CD34+ cells (2.5 to 6.5 × 103) were added to wells precoated with H120 or H0 fragments to which a mixture of growth factors in serially diluted concentrations in serum-free medium was added. Figure 7 shows the results from one experiment in which the cells proliferating from 2.5 × 103 input CD34+ cells were counted on day 13. Higher total cell counts were seen with H120 compared with H0 and BSA controls at all dilutions of growth factors. In the presence of H120 cell growth was not affected until growth factors were diluted by 1 in 16, whereas in the H0- and BSA-coated wells growth was reduced at one-eighth dilution of growth factors (Fig 7). At this dilution growth in H120 cultures yielded a 413-fold expansion of cells, whereas growth was 40% to 50% below this value in BSA- and H0-coated wells (265-fold and 217-fold, respectively). Cell numbers with H0 were expressed as a percentage of expansion with H120 for each of three separate experiments, and the relative fold expansion with H120 is shown in Table 1. Significant increases in proliferation occurred with H120 compared with H0 at all growth factor dilutions, with the greatest mean difference in expansion (2.25-fold) occurring at a dilution of one sixteenth (P < .001). A similar increase occurred when growth with H120 was compared with that in control wells coated with BSA in two experiments, where the greatest differential expansion (2.5-fold) between H120 and BSA cultures also occurred at a one-sixteenth–growth factor dilution.
A total of 2.5 × 103 CD34+cells were added to wells precoated with H120 or H0 fragments to which the standard mixture of growth factors in serially diluted concentrations was added. Control wells were coated with BSA. Total cells were counted after 13 days. The figure shows the result of one of three similar experiments whose combined results are given in Table1.
A total of 2.5 × 103 CD34+cells were added to wells precoated with H120 or H0 fragments to which the standard mixture of growth factors in serially diluted concentrations was added. Control wells were coated with BSA. Total cells were counted after 13 days. The figure shows the result of one of three similar experiments whose combined results are given in Table1.
CD34+ Cell Growth With H120 and H0 Fragments: Effect of Reducing Growth Factor Concentrations
| Growth Factor Concentration . | P Value . | Fold Expansion With H120 Compared With H0 . |
|---|---|---|
| 1/1 | <.01 | 1.53 |
| 1/2 | <.05 | 1.63 |
| 1/4 | .01 | 1.57 |
| 1/8 | <.01 | 1.69 |
| 1/16 | <.001 | 2.25 |
| Growth Factor Concentration . | P Value . | Fold Expansion With H120 Compared With H0 . |
|---|---|---|
| 1/1 | <.01 | 1.53 |
| 1/2 | <.05 | 1.63 |
| 1/4 | .01 | 1.57 |
| 1/8 | <.01 | 1.69 |
| 1/16 | <.001 | 2.25 |
In three separate experiments CD34+ cells were cultured in serum-free medium and a serially diluted mixture of growth factors in wells coated with H120 or H0 fragments. Growth factor concentrations ranged from standard concentration (IL-3 10 ng/mL, IL-6 200 U/mL, G-CSF 500 ng/mL, SCF 100 ng/mL, erythropoietin 2 U/mL) to concentration (IL-3 0.625 ng/mL, IL-6 12.5 U/mL, G-CSF 31.25 ng/mL, SCF 6.25 ng/mL, erythropoeitin 0.125 U/mL). Cells were counted at a mean of 11 days. Cell numbers with H0 were expressed as the mean percentage of expansion with H120 and the overall fold expansion with H120 compared with H0 is shown. Student'st-test was used to calculate P values.
Effect of α4β1 binding sequences on CD34+ cell growth with time in culture.
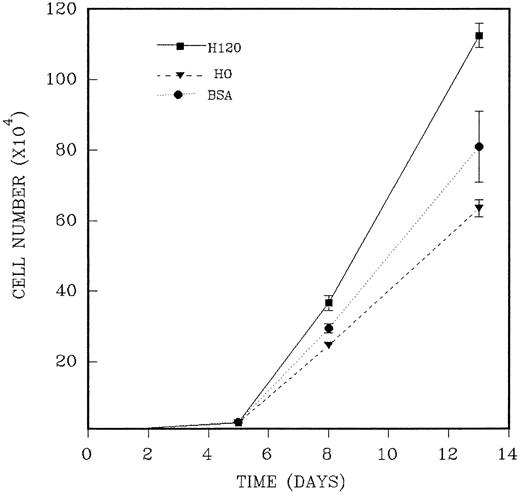
CD34+ cells were isolated as previously described and added in duplicate to wells precoated with H120 or H0 fragments to which a mixture of growth factors at one fourth of the standard concentration in serum-free medium was added. The reduced growth factor concentration was shown to maintain plateau growth in all conditions and was used to facilitate any contribution to long-term growth provided by the H120 fragment. The medium was not renewed during the period of culture. In three separate experiments, total cells were counted at three or four time points between 5 and 14 days of culture. As previously shown, the H120-coated wells supported an adherent cell component throughout the growth period, and total cell counts from these wells were the combined adherent and nonadherent fractions. An example of the course of growth over 13 days from an input of 2.5 ×103CD34+ cells is shown in the experiment described in Fig 8. Cell expansion was 10-fold at day 5 for all wells, and this was followed by marked proliferation between days 5 and 8 with differences between H120, H0, and BSA wells first appearing on day 8. On day 13 mean cell expansion was 450-fold with H120, 254-fold with H0, and 324-fold with BSA. Mean total cell numbers were significantly increased on day 8 (P < .05) and on day 13 (P < .01) in the H120 wells compared with the H0 wells. In the absence of any change in culture medium, cell numbers declined after this time in two experiments. The results of three time course experiments are shown in Table 2. The mean increase in cell expansion is shown for H120 compared with H0 (mean of 1.80-fold expansion, P ≤ .0001) and for H120 compared with BSA (mean of 1.45-fold expansion, P ≤ .001).
A total of 2.5 × 103 CD34+cells were added in duplicate to wells precoated with H120 or H0 fragments to which the standard mixture of growth factors at one fourth of the standard concentration in serum-free medium was added. Control wells were coated with BSA. Total cells were counted on different days of culture. The figure shows one of three similar experiments whose combined results are given in Table 2.
A total of 2.5 × 103 CD34+cells were added in duplicate to wells precoated with H120 or H0 fragments to which the standard mixture of growth factors at one fourth of the standard concentration in serum-free medium was added. Control wells were coated with BSA. Total cells were counted on different days of culture. The figure shows one of three similar experiments whose combined results are given in Table 2.
Growth of CD34+ Cells With H120, H0, and BSA: Effect of Time in Culture
| Day of Maximum Expansion . | Fold Expansion With H120 Compared With H0 . | Fold Expansion With H120 Compared With BSA . |
|---|---|---|
| 13 | 1.77 | 1.38 |
| 11 | 1.89 | 1.45 |
| 11 | 1.76 | 1.52 |
| Mean, 1.80 ± 0.05 | Mean, 1.45 ± 0.05 | |
| P ≤ .0001 | P ≤ .001 |
| Day of Maximum Expansion . | Fold Expansion With H120 Compared With H0 . | Fold Expansion With H120 Compared With BSA . |
|---|---|---|
| 13 | 1.77 | 1.38 |
| 11 | 1.89 | 1.45 |
| 11 | 1.76 | 1.52 |
| Mean, 1.80 ± 0.05 | Mean, 1.45 ± 0.05 | |
| P ≤ .0001 | P ≤ .001 |
In three separate experiments CD34+ cells were cultured up to 14 days in serum-free medium and a mixture of growth factors at ¼ the standard concentration in wells coated with H120, H0, or BSA. Cells were counted on different days of culture. The day of maximum fold expansion with H120 compared with H0 and BSA is shown. Student's t-test was used to calculate Pvalues.
DISCUSSION
In most cells, adhesion is an essential process in the control of growth and differentiation and, although hematopoietic progenitor cells can grow in suspension and therefore can bypass this requirement, they have a close association with their stromal extracellular matrix which, under physiological conditions, may regulate interactions with locally available growth factors.30 Fibronectin is a component of the marrow extracellular matrix,31 and the CS1 sequence has been identified previously in a mouse stromal cell line.18We have found (unpublished data, October 1997) that the alternatively spliced high-affinity CS1 sequence, present in H120, is expressed by human bone marrow stromal cells as is the sequence equivalent to H0. We have previously shown that CD34+ cell adhesion and migration on fibronectin through α4β1 integrins is regulated by hematopoietic growth factors, probably by an inside-out signaling mechanism, and we show here that similar ligation of α4β1 integrins can also synergize with signaling pathways from growth factors resulting in increased cell proliferation that is associated with cell attachment and migration during the growth period.
The proliferation of cells in contact with H120 was associated with their adhesion during growth (Figs 5A and B), but this attachment was not fixed as studies with single cells showed that cell migration around and up the sides of the wells also occurred (Figs 3B and C). We have recently shown that IL-3 can provide a stimulus to CD34+ cells to transiently reduce adhesion and promote migration on H120 through α4β1 integrins. Here we confirm that a migratory stimulus occurs with H120 but not H0 during a period of growth from single cells originating from different subsets of CD34+ progenitor cells. IL-3 is a component of the growth factor mixture and may provide the major stimulus responsible for the migration patterns seen. It has been suggested that hematopoietic stem cells in fetal bone marrow reside within the CD34+38−DR+ cell population,26 and cord blood CD34+DR+ cells appear to contain the majority of primitive hematopoietic progenitor cells.27 Our use of the CD34+38−DR+ population from cord blood is thus representative of an immature subset. However, it has been shown more recently that the CD34+38−DR− population in cord blood is enriched in long-term culture-initiating cells (LTC-ICs),28 and our use of CD34+38−DR+ cells may therefore not reflect properties of the most primitive subset. Single cells from the CD34+38−DR+subset showed a migratory pattern of growth on an H120 substrate from the first cell division implying a prerequisite degree of adhesion to H120 and suggesting that mobilization of at least this progenitor subset in response to growth factors may occur using the same cellular mechanisms.
CD34+ cells grown in liquid culture adhere to the H120 fragment during the period of growth, and only a few cells were attached to the H0 fragment or to BSA-coated or BSA-uncoated wells (Figs 5 and 6A and B). An increased number of cells proliferating from input CD34+ cells was consistently found in wells coated with H120 compared with H0 and BSA (Fig 7 and 8; Tables 1 and 2). In time-course experiments this difference was first apparent at day 8 and reached a maximum at days 11 to 13 (Fig 8 and Table 2) when expansion with H120 was a mean of 1.8-fold that seen with H0 and 1.45-fold over that with BSA. The greatest mean relative expansion (2.25-fold) with H120 compared with H0 was seen when the growth factor concentration was reduced to one sixteenth of the standard mixture (Table 1). Thus, with limiting availability of growth factors, the contribution of H120 to proliferation is increased.
The above results show that cells growing in contact with H120 received an additional proliferative stimulus from its highest affinity α4β1 binding site. Wells coated with H0 were a good control as this fragment is the same as the H120 fragment, apart from lacking the two highest affinity (CS1 and CS5) α4β1 sequences. The increase in cell proliferation between H120- and BSA-coated control wells was not as marked as between H120 and H0 wells (Fig 8 and Table 2). The reasons for this are not clear, although it is possible that BSA has no effect on growth, whereas the H1 sequence of H0 could have an inhibitory effect in the absence of the CS1 sequence. This possibility has been discussed previously.25 A previous report of the inhibitory roles of fibronectin and stroma for hematopoietic progenitors14 is difficult to reconcile with our results with H120, but the methods used by this group differ considerably to those of our study, eg, the degree of inhibition of proliferation due to fibronectin was asssessed over a relatively short time period by a thymidine suicide technique that contrasts with our direct measurement of longer term growth (up to 14 days) of the progeny of CD34+ cells with a mixture of growth factors in contact with H120 and H0.
Most cell amplification in cultures of CD34+ cells is associated with differentiation of progenitors and stem cells in response to growth factors,32 and the number of the more primitive LTC-ICs usually declines,33,34 although more recent studies have shown that LTC-ICs can undergo expansion in liquid cultures.35 Growth of total nucleated cells from CD34+ cells varies with the source of input cells,36 the number of input cells,37 and the combination of growth factors used. Increases in cell numbers from cord blood CD34+ cells vary from an 85-fold increase at 10 days36 to a 791-fold total expansion.37 We showed an overall expansion in cell numbers using cord blood CD34+ cells of 160-fold with H0 to 260-fold with H120 (means of six experiments with one-fourth growth factor concentrations) over 11 to 13 days. Loss of stem cells has been attributed to removal of stromal cells,38 and the addition of components of the marrow stroma such as fibronectin may provide a more physiological environment in which to induce expansion. In this respect, stromal-conditioned media have been found to significantly enhance expansion of primitive hematopoietic stem cells and progenitor cells from CD34+ cells taken from mobilized peripheral blood,39 and stromal-derived heparan sulphate has recently been shown to have a role in the maintenance of LTC-ICs.40There was no difference in the morphological maturation stage of cells taken from cultures grown with or without H120, and the increase in total cell numbers with H120 was not at the expense of loss of CD34+ cells as numbers of these were similar in all culture conditions (Fig 5B). Preliminary results (not shown) also indicate that numbers of granulocyte-macrophage colony-forming cell– and BFU-E–derived colonies are maintained with H120.
We have shown here that ligation of α4β1integrins provides a stimulus to CD34+ cell growth, and more information is now needed about the effect of H120 on the self-renewal versus differentiation decisions of earlier progenitors, together with any effects on cell survival and cell cycling. The results shown in Table 1 suggest that this probably needs to be performed with limiting concentrations of growth factors to enable the effects of H120 to be clearly expressed. Under such circumstances, which may more closely resemble steady-state conditions in the marrow, integrins may play a part in maintaining survival of stem cells as they do in epithelial and endothelial cells.42 42
In conclusion our findings show that ligation of α4β1 integrin by the IIICS region of fibronectin can synergize with growth factors resulting in an enhanced growth of CD34+ cells occurring over a prolonged period in liquid culture. Growth occurs in an adhesion-related manner and is accompanied by cell migration. The use of H120 substrata in the ex vivo expansion of CD34+ cells provides a model for understanding the role of stromal control of hematopoiesis, and in future studies we will hope to perform a detailed analysis of the effects of H120 on early progenitor and stem cells.
ACKNOWLEDGMENT
The authors thank M Hughes and J Barry for technical assistance with FACS sorting, Suzanne Bridge for excellent assistance in the preparation of this manuscript, and Professor M Dexter for helpful advice and valuable discussions.
K.P.S. is a clinical research fellow of the Cancer Research Campaign.
Address reprint requests to Karen P. Schofield, MD, CRC Department of Medical Oncology, Paterson Institute for Cancer Research, Wilmslow Road, Manchester, M20 4BX, UK.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal