Abstract
One obstacle to retrovirus-mediated gene therapy for human hematopoietic disorders is the low efficiency of gene transfer into pluripotent hematopoietic stem cells (HSC). We have previously shown a direct correlation between retrovirus receptor mRNA levels in mouse HSC and the efficiency with which they are transduced. In the present study, we assayed retrovirus receptor mRNA levels in a variety of mouse and human HSC populations to identify HSC which may be more competent for retrovirus transduction. The highest levels of amphotropic retrovirus receptor (amphoR) mRNA were found in cryopreserved human cord blood HSC. The level of amphoR mRNA in Lin−CD34+ CD38− cells isolated from frozen cord blood was 12-fold higher than the level in fresh cord blood Lin− CD34+ CD38− cells. In mice, the level of amphoR mRNA in HSC from the bone marrow (BM) of mice treated with stem cell factor and granulocyte-colony stimulating factor was 2.8- to 7.8-fold higher than in HSC from the BM of untreated mice. These findings suggest that HSC from frozen cord blood and cytokine-mobilized BM may be superior targets for amphotropic retrovirus transduction compared with HSC from untreated adult BM.
PLURIPOTENT HEMATOPOIETIC stem cells (HSC) are ideal targets for gene therapy because of their capacity to self-renew and reconstitute all lineages of the hematopoietic system after myeloablation and transplantation.1,2 If retroviruses carrying a therapeutic gene can be introduced into the genome of HSC, the progeny of the transduced HSC would all carry the transferred gene.3 Expression of the therapeutic gene at appropriate levels should lead to a permanent correction of the specific hematologic disorder.1,2 The host range for retroviruses is determined by the gp70 glycoprotein of their envelope.2Ecotropic gp70 binds to an amino acid transporter protein.4The gp70 binding site is not conserved on the amino acid transporter of other mammals, hence ecotropic viruses transduce only mouse cells.5 Under appropriate conditions, ecotropic retrovirus transduction of mouse HSC leads to marking of 20% to 60% of the mature circulating blood cells.6-12
Two different retroviruses are currently being used to transduce mouse and human HSC. The gp70 envelope proteins of amphotropic retroviruses and the Gibbon Ape Leukemia Virus (GaLV) use homologous but distinct phosphate transport proteins as receptors.13-16 The amphotropic and GaLV gp70 binding sites are conserved among most mammals, which has led to the development of amphotropic and GaLV retroviruses as vehicles for gene transfer into human HSC. In contrast to the efficient transduction of mouse HSC with ecotropic retroviruses, transduction of Rhesus monkey and human HSC with amphotropic or GaLV retroviruses has been relatively inefficient, with fewer than 1% of the circulating blood cells containing proviral DNA sequences.17-21
We have previously reported that enriched populations of mouse and human HSC from bone marrow (BM) contained relatively low levels of amphoR mRNA.22 Using counterflow centrifugal elutriation (CCE), we identified a subpopulation of murine HSC (FR25 Lin− c-kitHI) with a low level of amphoR mRNA and a subpopulation (FR35 Lin− c-kitHI) with a higher level of amphoR mRNA.23 We simultaneously transduced both subpopulations of HSC with ecotropic and amphotropic retrovirus vectors of similar titers. Our results showed that HSC containing high levels of amphoR mRNA were transduced by amphotropic retroviruses with an efficiency comparable with that achieved with ecotropic viruses. HSC with low levels of amphoR mRNA were transduced 20-fold less efficiently by amphotropic retroviruses compared with ecotropic viruses.22
In this study, we measured retrovirus receptor mRNA levels in different populations of human and mouse HSC. Based on our previous results, we hypothesized that identification of subpopulations of HSC with high levels of amphoR and/or GaLVR mRNA would lead to improved retrovirus transduction efficiency. We found a high level of amphoR mRNA in the HSC-enriched population from cryopreserved human cord blood. We also found a high level of amphoR mRNA in several HSC populations from the BM of mice treated with granulocyte-colony stimulating factor (G-CSF) and stem cell factor (SCF).
MATERIALS AND METHODS
Purification of HSC from human fetal liver.
CD34+ fetal liver cells (purity greater than 80%) were purchased from Poietic Technologies, Inc (Germantown, MD). The cells were stained with fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody (MoAb) that recognized the lineage markers CD3, CD15, and CD20 (Becton Dickinson, San Jose, CA); glycophorin A (Coulter Immunology, Hialeah, FL); and anti–CD34-PECy5 (PECy5 = phycoerythrin cyanogen 5) and anti–CD38-PE MoAbs, which are specific for primitive cell markers (Becton Dickinson). The Lin−CD34+ CD38+ (FITC−PECy5+ and PE+) and Lin− CD34+ CD38−(FITC− PECy5+ and PE−) cells were isolated by flow cytometry. Reanalysis of the sorted samples indicated purities of greater than 95%.
Purification of HSC from human cord blood.
Human cord blood was obtained after normal delivery with informed consent and with the approval of the Institutional Review Board of the Indiana University School of Medicine. Samples were provided as fresh and cryopreserved cord blood. Mononuclear cells (MNCs) were isolated by centrifugation on a layer of Lymphocyte Separation Medium (Organon Teknika Corp, Durham, NC) followed by red blood cell lysis in ACK Lysing Buffer (Bio Whittaker, Walkersville, MD) at 4°C for 30 minutes. The cells were then stained with MoAb for flow cytometry as described above. The Lin− CD34+CD38− population was defined as the lowest 1% to 2% of the FITC− PECy5+ PE− cells. The Lin− CD34+ CD38+ cell population was isolated from the highest 1% to 2% of the PE-stained FITC− PECy5+ PE+ cells.
Purification of HSC from human adult BM.
Human BM (25-mL samples) was purchased from Poietic Technologies. The cells were fractionated by CCE into FR15 (discarded), FR20, FR25, FR30, FR35, and FR40 fractions. The cells in each fraction were stained with MoAb and sorted by flow cytometry as described above.
Retrovirus binding assay.
The virus binding assay was performed exactly as described by Kadan et al24 and Crooks and Kohn.25 Human adult BM MNCs were incubated with media containing amphotropic retrovirus and 8 μg polybrene/mL media at 37°C for 40 minutes. After washing with 10% heat-inactivated goat serum in phosphate-buffered saline (PBS) to remove unbound virus, the cells were incubated with FITC-conjugated 83A25, a rat MoAb specific for gp70, at 4°C for 60 minutes. Control cells were incubated with FITC-conjugated isotype antibodies. The cells were washed with a 10% solution of heat-inactivated newborn calf serum in PBS. Finally, the cells were stained with anti–CD34-PECy5 and anti–CD38-PE MoAbs as described above. The CD34+(PECy5+) cells were analyzed for FITC (virus binding) and PE (CD38 expression) by flow cytometry.
Purification of HSC from mouse yolk sac.
Yolk sac cells were obtained as previously described.26 27Briefly, 9.5-day embryos were dissected from the uterus of timed-mated C57BL/6J female mice. The yolk sacs were dissected free of the embryo, dispersed by drawing through a 23-gauge needle, and cultured for 60 minutes at 37°C in Hank's buffered saline solution (HBSS) containing 0.1% collagenase (Sigma, St Louis, MO) and 20% fetal calf serum (FCS). The yolk sac cells were collected by centrifugation and incubated at 4°C for 20 minutes sequentially with rat anti-mouse FITC-conjugated CD16/CD32 (Pharmingen, San Diego, CA), a MoAb which is specific for FcγII/III receptors that are expressed on natural killer cells, monocytes, macrophages, granulocytes, B-lymphocytes, and most fetal T lymphocytes before expression of CD4, CD8, and TCR; rat anti-mouse FITC-conjugated TER-119 (Pharmingen), specific for red cells; and rat anti-mouse biotin-conjugated CD34-biotin (Pharmingen) followed by streptaviden-conjugated phycoerythrin. The cells were washed, resuspended in HBSS with 5% FCS, and Lin−CD34+ (FITC− PE+) or Lin− CD34− (FITC−PE−) cells were enriched by flow cytometry using a FACStar instrument (Becton Dickinson).
Purification of murine HSC from the BM of mice treated with G-CSF and SCF.
Adult splenectomized mice were injected subcutaneously for 5 consecutive days with 200 μg/kg/d G-CSF and 50 μg/kg/d SCF. BM cells were collected 14 days after the final injection of cytokines. MNCs were isolated by centrifugation on a layer of Lymphocyte Separation Medium (Organon Teknika) and were fractionated by CCE as described previously.28 Briefly, MNCs were separated at flow rates of 15 (FR15, discarded), 25 (FR25), 30 (FR30), and 35 (FR35) mL/min. The Lin+ cells were subtracted by immunobead selection and the Lin− cells were sorted based on c-kitHi expression.28
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of retrovirus receptor mRNA in mouse and human HSC.
Total cellular RNA from enriched mouse and human HSC populations was purified using RNAzol (Tel-Test, Inc, Friendswood, TX) or Trizol (Tel-Test) following the manufacturer's instructions. RNA levels were analyzed by RT-PCR as described previously.29 Briefly, mRNA from different cell populations was reverse transcribed and the cDNAs were amplified using primer pairs specific for mouse and human β2-microglobulin (β2-m), ecoR, amphoR, and GaLVR (Table1). The PCR was performed at 94°C for 1 minute (denaturing), 58°C or 65°C for 1 minute (annealing), and 72°C for 2 minutes (extension) using a DNA Thermal Cycler (Perkin Elmer Cetus, Norwalk, CT) for 35 cycles. β2-m mRNA was used in a limiting dilution RT-PCR assay to identify a set point on the linear phase of the PCR amplification curve, which was used to quantify the level of mRNA for each retrovirus receptor relative to β2-m.
Primer Sequences Used in the RT-PCR Assay
| Mouse β2-m | Sense, 5′ TGC TAT CCA GAA AAC CCC TC 3′ |
| Antisense, 5′ GTC ATG CTT AAC TCT GCA GG 3′ | |
| Fragment size, 258 bp | |
| Mouse amphoR | Sense, 5′ CGG GCG GAA GAC GAG AAG GA 3′ |
| Antisense, 5′ GAA GCC ACT GGA CGG TGT GA 3′ | |
| Fragment size, 309 bp | |
| Mouse ecoR | Sense, 5′ CTG CCT CAA CAC CTA TGA CC 3′ |
| Antisense, 5′ TGC TGA CGT GAG AAC TCT CC 3′ | |
| Fragment size, 363 bp | |
| Human β2-m | Sense, 5′ CTC GCG CTA CTC TCT CTT TC 3′ |
| Antisense, 5′ CAT GTC TCG ATC CCA CTT AAC 3′ | |
| Fragment size, 330 bp | |
| Human amphoR | Sense, 5′ CGG AAC ATC TTC GTG GCC TG 3′ |
| Antisense, 5′ GCT GGT CAT GAG AGA GCC GTG 3′ | |
| Fragment size, 220 bp | |
| Human GaLVR | Sense, 5′ GTG TGG CAA CTC GTG GCT TC 3′ |
| Antisense, 5′ CAG CAA CGG TGC TCC AG 3′ | |
| Fragment size, 321 bp |
| Mouse β2-m | Sense, 5′ TGC TAT CCA GAA AAC CCC TC 3′ |
| Antisense, 5′ GTC ATG CTT AAC TCT GCA GG 3′ | |
| Fragment size, 258 bp | |
| Mouse amphoR | Sense, 5′ CGG GCG GAA GAC GAG AAG GA 3′ |
| Antisense, 5′ GAA GCC ACT GGA CGG TGT GA 3′ | |
| Fragment size, 309 bp | |
| Mouse ecoR | Sense, 5′ CTG CCT CAA CAC CTA TGA CC 3′ |
| Antisense, 5′ TGC TGA CGT GAG AAC TCT CC 3′ | |
| Fragment size, 363 bp | |
| Human β2-m | Sense, 5′ CTC GCG CTA CTC TCT CTT TC 3′ |
| Antisense, 5′ CAT GTC TCG ATC CCA CTT AAC 3′ | |
| Fragment size, 330 bp | |
| Human amphoR | Sense, 5′ CGG AAC ATC TTC GTG GCC TG 3′ |
| Antisense, 5′ GCT GGT CAT GAG AGA GCC GTG 3′ | |
| Fragment size, 220 bp | |
| Human GaLVR | Sense, 5′ GTG TGG CAA CTC GTG GCT TC 3′ |
| Antisense, 5′ CAG CAA CGG TGC TCC AG 3′ | |
| Fragment size, 321 bp |
The fragments amplified by PCR were resolved by polyacrylamide gel electrophoresis. The specificity of the primer pairs was confirmed by restriction enzyme digestion of the amplified fragment at sites predicted by the DNA sequence. The intensity of the bands was quantified using a Molecular Dynamics Densitometer System (Sunnyvale, CA). HeLa and NIH-3T3 cells are efficiently transduced by amphotropic retroviruses.30 31 We have shown that HeLa and NIH-3T3 cells have higher levels of amphoR and GaLVR mRNA than human or mouse BM HSC, respectively. The levels of amphoR and GaLVR mRNA in HSC-enriched fractions were normalized to the level of amphoR and GaLVR mRNA in HeLa or NIH-3T3 cells by the following formula:
The levels of receptor mRNA in the human HSC-enriched Lin− CD34+ CD38− fractions were then compared with the levels in the progenitor-enriched Lin− CD34+ CD38+ fractions.
RESULTS
Retrovirus binding assay.
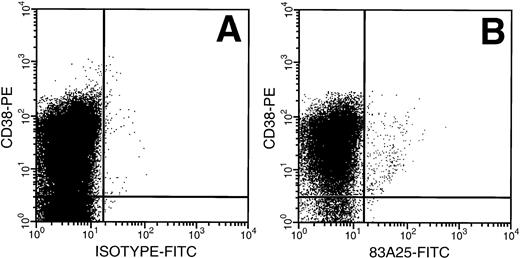
MNCs from untreated adult BM were exposed to amphotropic retrovirus and the binding capacity of the CD34+ cells was determined using a FITC-labeled antibody specific for the retrovirus envelope protein gp70. The highest FITC fluorescence intensity was seen on the CD34+ CD38+ cells (Fig1), a population enriched for progenitor cells. There was a relative decrease in retrovirus binding in cells with decreasing CD38 expression and the HSC-enriched CD34+CD38− subpopulation bound less than 10% of the virus bound by CD34+ CD38+ cells.
Retrovirus binding to CD34+CD38+ and CD34+ CD38− cells from adult BM. (A) Cells isolated from a Cell Pro CD34 column were incubated with a virus containing medium and stained with anti–CD34 PECy5 for gating and anti–CD38-PE MoAb and isotype-FITC Ab. (B) Cells incubated as above with FITC-conjugated 83A25 MoAb (anti-mouse retrovirus gp70).
Retrovirus binding to CD34+CD38+ and CD34+ CD38− cells from adult BM. (A) Cells isolated from a Cell Pro CD34 column were incubated with a virus containing medium and stained with anti–CD34 PECy5 for gating and anti–CD38-PE MoAb and isotype-FITC Ab. (B) Cells incubated as above with FITC-conjugated 83A25 MoAb (anti-mouse retrovirus gp70).
RT-PCR analysis of retrovirus receptor mRNA levels in human HSC.
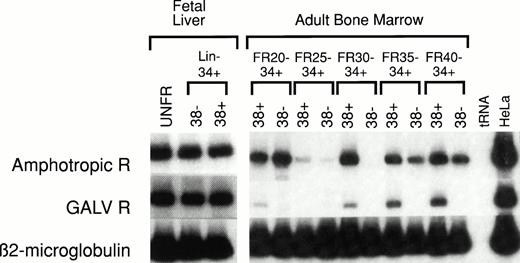
Human HSC-enriched Lin− CD34+CD38− cells from fetal liver and BM confer long-term engraftment in fetal sheep32 and severe combined immunodeficiency (SCID)- hu mice.33 We measured the relative level of retrovirus receptor mRNA in Lin−CD34+ CD38− HSC and Lin−CD34+ CD38+ progenitor cells from both of these hematopoietic organs. The level of receptor mRNA in HSC and progenitor cells was compared with the level of receptor mRNA in HeLa cells, which served as the reference standard. AmphoR and GaLVR mRNA levels were uniformly low in HSC-enriched Lin− CD34+CD38− and progenitor-enriched Lin−CD34+ CD38+ cells from fetal liver (Fig2 and Table 2).
RT-PCR analysis of amphotropic retrovirus receptor (Amphotropic R) and Gibbon Ape Leukemia Virus receptor (GALV R) mRNA levels in human fetal liver and adult BM. RNA was extracted from unfractionated (UNFR), HSC-enriched Lin−CD34+ CD38−, and progenitor-enriched Lin− CD34+ CD38+ fetal liver and adult BM cells. The levels of Amphotropic R (upper bands) and GALV R mRNA (middle bands) were quantified based on the level of β2-microglobulin mRNA (lower bands) in the same sample. These values were then compared with the levels of Amphotropic R or GALV R mRNA in HeLa cells (right column) and compared with the mRNA levels in unfractionated control BM (see Table 2). Adult human BM cells were fractionated by CCE at flow rates (FR) of 20, 25, 30, 35, and 40 mL/min (FR20 to FR40). Samples from five separate experiments were analyzed (see Table 2).
RT-PCR analysis of amphotropic retrovirus receptor (Amphotropic R) and Gibbon Ape Leukemia Virus receptor (GALV R) mRNA levels in human fetal liver and adult BM. RNA was extracted from unfractionated (UNFR), HSC-enriched Lin−CD34+ CD38−, and progenitor-enriched Lin− CD34+ CD38+ fetal liver and adult BM cells. The levels of Amphotropic R (upper bands) and GALV R mRNA (middle bands) were quantified based on the level of β2-microglobulin mRNA (lower bands) in the same sample. These values were then compared with the levels of Amphotropic R or GALV R mRNA in HeLa cells (right column) and compared with the mRNA levels in unfractionated control BM (see Table 2). Adult human BM cells were fractionated by CCE at flow rates (FR) of 20, 25, 30, 35, and 40 mL/min (FR20 to FR40). Samples from five separate experiments were analyzed (see Table 2).
RT-PCR Analysis of Retrovirus Receptor mRNA in Human HSC
| . | AmphoR mRNA . | GaLVR mRNA . |
|---|---|---|
| Fetal liver | ||
| Lin− CD34+ CD38+ (2) | 0.31 ± 0.04* | 0.38 ± 0.41 |
| Lin−CD34+ CD38− (2) | 0.22 ± 0.13 | 0.28 ± 0.28 |
| Cord blood | ||
| Lin−CD34+ CD38+ (Fresh) (3) | 0.38 ± 0.34 | 0.08 ± 0.04 |
| Lin− CD34+CD38− (Fresh) (3) | 0.13 ± 0.05 | 0.07 ± 0.03 |
| Lin− CD34+ CD38+ (Frozen) (3) | 1.54 ± 1.61 | Not detected |
| Lin− CD34+ CD38− (Frozen) (3) | 1.55 ± 0.61†‡ | Not detected |
| Bone marrow | ||
| Lin− CD34+CD38+ (5) | 0.20 ± 0.20 | 0.22 ± 0.22 |
| Lin− CD34+ CD38− (4) | 0.07 ± 0.10 | 0.15 ± 0.14 |
| FR20 Lin−CD34+ CD38− (5) | 0.10 ± 0.10 | 0.01 ± 0.01 |
| FR25 Lin− CD34+CD38− (5) | 0.03 ± 0.02 | 0.02 ± 0.02 |
| FR30 Lin− CD34+ CD38− (5) | 0.05 ± 0.06 | 0.03 ± 0.03 |
| FR35 Lin−CD34+ CD38− (5) | 0.07 ± 0.06 | 0.05 ± 0.04 |
| FR40 Lin− CD34+CD38− (5) | 0.12 ± 0.15 | 0.10 ± 0.20 |
| HeLa cells (5) | 1.0 | 1.0 |
| . | AmphoR mRNA . | GaLVR mRNA . |
|---|---|---|
| Fetal liver | ||
| Lin− CD34+ CD38+ (2) | 0.31 ± 0.04* | 0.38 ± 0.41 |
| Lin−CD34+ CD38− (2) | 0.22 ± 0.13 | 0.28 ± 0.28 |
| Cord blood | ||
| Lin−CD34+ CD38+ (Fresh) (3) | 0.38 ± 0.34 | 0.08 ± 0.04 |
| Lin− CD34+CD38− (Fresh) (3) | 0.13 ± 0.05 | 0.07 ± 0.03 |
| Lin− CD34+ CD38+ (Frozen) (3) | 1.54 ± 1.61 | Not detected |
| Lin− CD34+ CD38− (Frozen) (3) | 1.55 ± 0.61†‡ | Not detected |
| Bone marrow | ||
| Lin− CD34+CD38+ (5) | 0.20 ± 0.20 | 0.22 ± 0.22 |
| Lin− CD34+ CD38− (4) | 0.07 ± 0.10 | 0.15 ± 0.14 |
| FR20 Lin−CD34+ CD38− (5) | 0.10 ± 0.10 | 0.01 ± 0.01 |
| FR25 Lin− CD34+CD38− (5) | 0.03 ± 0.02 | 0.02 ± 0.02 |
| FR30 Lin− CD34+ CD38− (5) | 0.05 ± 0.06 | 0.03 ± 0.03 |
| FR35 Lin−CD34+ CD38− (5) | 0.07 ± 0.06 | 0.05 ± 0.04 |
| FR40 Lin− CD34+CD38− (5) | 0.12 ± 0.15 | 0.10 ± 0.20 |
| HeLa cells (5) | 1.0 | 1.0 |
The number of samples are shown in parentheses. Receptor mRNA levels were normalized to the level of β2-m mRNA in the same sample. For quantification, the receptor mRNA levels were compared with the level of the receptor mRNA in unfractionated BM.
Mean ± SD of the mRNA levels relative to the level in HeLa cells.
P < .005 compared with Lin− CD34+CD38− BM cells.
P < .005 compared with fresh Lin−CD34+ CD38− cord blood cells.
The mean level of amphoR and GaLVR mRNA in unfractionated human BM was 20% and 22%, respectively, of the mean level of amphoR and GaLVR mRNA in five different preparations of HeLa cell RNA (Table 2). We have previously shown that subpopulations of mouse BM HSC separated by CCE vary in the level of amphoR mRNA. We analyzed amphoR and GaLVR mRNA levels in five different human adult BM HSC populations separated on the basis of size using CCE. For each CCE fraction, a progenitor-enriched Lin− CD34+CD38+ population and a HSC-enriched Lin−CD34+ CD38− population was isolated by flow cytometry. Discrete populations of Lin− CD34+CD38− BM cells expressing generally low levels of amphoR mRNA compared with HeLa cells could be identified in each of the five experiments (Figure 2). However, the population(s) of Lin− CD34+ CD38− BM cells expressing the lowest and highest levels of amphoR mRNA appeared in different CCE fractions in the five separate experiments. Analysis of pooled data from all five experiments showed no significant difference in the level of amphoR mRNA between any of the CCE fractions (Table 2). The levels of GaLVR mRNA in human HSC separated by CCE followed the same general patterns as the amphoR mRNA levels.
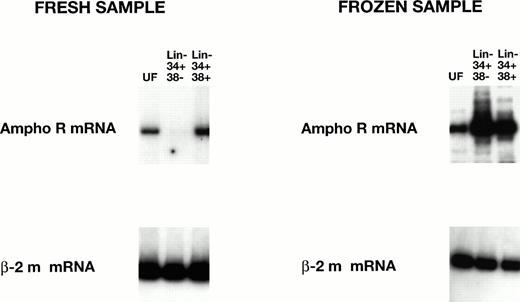
Preliminary studies showed strikingly higher levels of amphoR mRNA in both Lin− CD34+ CD38+ and Lin− CD34+ CD38− cord blood cells that had been cryopreserved before processing. To test this directly, three cord blood samples were divided at the time of collection, one half for immediate RT-PCR analysis and the other half for analysis after a freeze/thaw cycle (Fig3). We found a significant increase (12-fold, P < .005; 22-fold, P < .005) in the level of amphoR mRNA in the cryopreserved Lin−CD34+ CD38− cells compared with the amphoR mRNA level in Lin− CD34+ CD38−cells in fresh cord blood and adult BM, respectively (Table 2). We were not able to detect GaLVR mRNA in either the Lin−CD34+ CD38+ or Lin−CD34+ CD38− fraction from cryopreserved cord blood.
RT-PCR analysis of amphotropic receptor (ampho R) mRNA levels in fresh and frozen human cord blood cells. The levels of mRNA encoding ampho R mRNA and β2-microglobulin mRNA in unfractionated (UF) Lin− CD34+ CD38− and Lin− CD34+ CD38+ cells were analyzed as in Fig 2. The level of ampho R mRNA in the HSC-enriched Lin− CD34+ CD38− fraction from frozen cord blood is significantly increased (P < .005) over the level of ampho R mRNA in the HSC-enriched Lin− CD34+ CD38−fraction from fresh cord blood (see Table 2).
RT-PCR analysis of amphotropic receptor (ampho R) mRNA levels in fresh and frozen human cord blood cells. The levels of mRNA encoding ampho R mRNA and β2-microglobulin mRNA in unfractionated (UF) Lin− CD34+ CD38− and Lin− CD34+ CD38+ cells were analyzed as in Fig 2. The level of ampho R mRNA in the HSC-enriched Lin− CD34+ CD38− fraction from frozen cord blood is significantly increased (P < .005) over the level of ampho R mRNA in the HSC-enriched Lin− CD34+ CD38−fraction from fresh cord blood (see Table 2).
RT-PCR analysis of retrovirus mRNA levels in mouse yolk sac HSC.
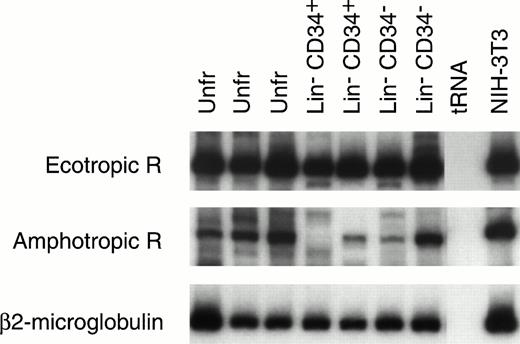
Mouse HSC capable of reconstituting the entire hematopoietic system have been variously identified by ourselves28 and others34-37 as c-kit+ CD34+and/or CD34− cells. The Lin−CD34+ and Lin− CD34− cells from 9.5-day yolk sac were enriched by flow cytometry and their RNA was extracted for RT-PCR analysis (Fig 4). The amphotropic and ecotropic retrovirus receptor (ecoR) mRNA values were compared with the level of expression in NIH-3T3 cells, which can be efficiently transduced with both amphotropic and ecotropic vectors. AmphoR and ecoR mRNAs were present in unfractionated Lin−CD34+, and Lin− CD34− yolk sac cells. The relative level of amphoR and ecoR mRNA in most yolk sac samples was equal to or greater than the level of amphoR and ecoR mRNA in NIH-3T3 cells.
RT-PCR analysis of ecotropic retrovirus receptor (Ecotropic R; top panel) and amphotropic retrovirus receptor (Amphotropic R; middle panel) mRNA levels in mouse 9.5-day yolk sac cells. Receptor mRNA levels are shown for two or three separate samples of RNA isolated from unfractionated (Unfr) yolk sac and sorted Lin− CD34+ and Lin−CD34− yolk sac cells. The levels of mRNA encoding Ecotropic R and Amphotropic R can be estimated based on the level of β2-microglobulin mRNA (lower bands) in the same sample. The levels of Ecotropic R and Amphotropic R mRNA in NIH-3T3 cells are also seen (right column).
RT-PCR analysis of ecotropic retrovirus receptor (Ecotropic R; top panel) and amphotropic retrovirus receptor (Amphotropic R; middle panel) mRNA levels in mouse 9.5-day yolk sac cells. Receptor mRNA levels are shown for two or three separate samples of RNA isolated from unfractionated (Unfr) yolk sac and sorted Lin− CD34+ and Lin−CD34− yolk sac cells. The levels of mRNA encoding Ecotropic R and Amphotropic R can be estimated based on the level of β2-microglobulin mRNA (lower bands) in the same sample. The levels of Ecotropic R and Amphotropic R mRNA in NIH-3T3 cells are also seen (right column).
RT-PCR analysis of retrovirus mRNA levels in mouse HSC populations from mice treated with G-CSF and SCF.
We have previously shown that five daily injections of G-CSF and SCF into adult splenectomized mice causes a mobilization of HSC into the peripheral blood. Peripheral blood HSC levels decline after cytokine treatment is stopped, but 14 days after treatment we showed a greater than 10-fold increase in the number of HSC in the BM.38
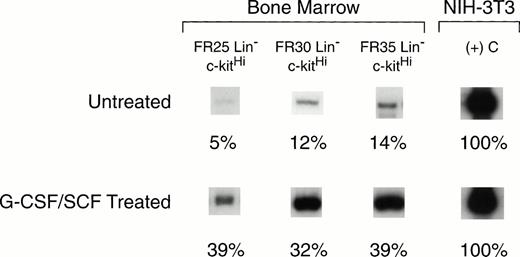
BM cells collected on day 14 after cytokine treatment were fractionated by CCE into FR25, FR30, and FR35 cell populations. Lin−cells were sorted by flow cytometry to isolate c-kitHIcells for RNA analysis. These values were compared with those for the same cell fractions from untreated control mouse BM (Fig5). AmphoR mRNA levels in the HSC-enriched FR25, FR30, and FR35 Lin− c-kitHi populations from untreated BM were 5%, 12%, and 14%, respectively, of the level of amphoR mRNA in NIH-3T3 cells (Table 3).This level was significantly lower than the amphoR mRNA level in unfractionated control BM cells (P < .01). The level of amphoR mRNA in unfractionated BM collected 14 days after cytokine treatment was nearly equivalent to the level in NIH-3T3 cells and was 1.7-fold higher than the level in unfractionated control BM from untreated mice (P < .01). In contrast to the low level of amphoR mRNA in FR25, FR30, and FR35 Lin−c-kitHI cells from untreated BM, the mean level of amphoR mRNA in FR25, FR30, and FR35 Lin− c-kitHIcells from mice treated with G-CSF and SCF was increased to a level equivalent to that of unfractionated control BM (Table 3). There was no increase in the levels of ecoR mRNA when the elutriated Lin− c-kitHI cell fractions from cytokine-treated mice were compared with the values from untreated mouse BM.
RT-PCR analysis of amphotropic retrovirus receptor mRNA levels in BM hematopoietic stem cells from untreated mice (top panel), and from mice injected for 5 consecutive days with G-CSF and SCF (bottom panel). BM cells were collected from mice treated with G-CSF and SCF at 14 days after the last cytokine treatment. The cells were fractionated by CCE at flow rates of 25, 30, and 35 mL/min (FR25, FR30, FR35), and Lin− c-kitHI cells were isolated from each fraction by flow cytometry. The levels of amphotropic receptor mRNA were quantified based on the level of β2-microglobulin mRNA (not shown) in the same sample. These values were normalized to the level of amphotropic receptor mRNA in NIH-3T3 cells (right column) and compared with the mRNA level in unfractionated BM (see Table 3).
RT-PCR analysis of amphotropic retrovirus receptor mRNA levels in BM hematopoietic stem cells from untreated mice (top panel), and from mice injected for 5 consecutive days with G-CSF and SCF (bottom panel). BM cells were collected from mice treated with G-CSF and SCF at 14 days after the last cytokine treatment. The cells were fractionated by CCE at flow rates of 25, 30, and 35 mL/min (FR25, FR30, FR35), and Lin− c-kitHI cells were isolated from each fraction by flow cytometry. The levels of amphotropic receptor mRNA were quantified based on the level of β2-microglobulin mRNA (not shown) in the same sample. These values were normalized to the level of amphotropic receptor mRNA in NIH-3T3 cells (right column) and compared with the mRNA level in unfractionated BM (see Table 3).
RT-PCR Analysis of Retrovirus Receptor mRNA in BM HSC From Untreated and G-CSF– and SCF-Treated Mice
| . | amphoR . | ecoR . |
|---|---|---|
| Untreated mice | ||
| Unfractionated (9) | 0.49 ± 0.12* | 1.16 ± 0.47 |
| FR25 Lin− c-kitHI (3) | 0.05 ± 0.04† | 0.49 ± 0.25 |
| FR30 Lin− c-kitHI(2) | 0.12 ± 0.04† | Not done |
| FR35 Lin−c-kitHI (7) | 0.14 ± 0.02† | 1.10 ± 0.33 |
| Cytokine-injected mice‡ | ||
| Unfractionated (3) | 0.86 ± 0.23† | 1.53 ± 0.51 |
| FR25 Lin−c-kitHI (2) | 0.39 ± 0.37 | 0.61 ± 0.33 |
| FR30 Lin− c-kitHI (2) | 0.30 ± 0.20 | 0.67 ± 0.35 |
| FR35 Lin−c-kitHI (2) | 0.39 ± 0.06 | 0.81 ± 0.05 |
| NIH-3T3 | 1.0 | 1.0 |
| . | amphoR . | ecoR . |
|---|---|---|
| Untreated mice | ||
| Unfractionated (9) | 0.49 ± 0.12* | 1.16 ± 0.47 |
| FR25 Lin− c-kitHI (3) | 0.05 ± 0.04† | 0.49 ± 0.25 |
| FR30 Lin− c-kitHI(2) | 0.12 ± 0.04† | Not done |
| FR35 Lin−c-kitHI (7) | 0.14 ± 0.02† | 1.10 ± 0.33 |
| Cytokine-injected mice‡ | ||
| Unfractionated (3) | 0.86 ± 0.23† | 1.53 ± 0.51 |
| FR25 Lin−c-kitHI (2) | 0.39 ± 0.37 | 0.61 ± 0.33 |
| FR30 Lin− c-kitHI (2) | 0.30 ± 0.20 | 0.67 ± 0.35 |
| FR35 Lin−c-kitHI (2) | 0.39 ± 0.06 | 0.81 ± 0.05 |
| NIH-3T3 | 1.0 | 1.0 |
The number of samples are shown in parentheses. Receptor mRNA levels were quantified based on the level of β2-m mRNA in the same sample. They were then normalized to the levels in NIH-3T3 receptor mRNA and compared with the level of the receptor mRNA in unfractionated BM.
Mean ± SD of the mRNA levels relative to the level in NIH-3T3 cells.
P < .01 compared with unfractionated control BM.
Cytokine-injected mice received 200 μg G-CSF/kg/d and 50 μg SCF/kg/d for 5 days. BM cells were collected 14 days later.
DISCUSSION
Infrequent transduction of canine, primate, and human HSC with amphotropic and GaLV vectors has been shown in gene marking studies.17-20 The inefficient gene transfer in these trials may be the result of low virus titer,2 inability of the retrovirus genome to integrate into the DNA of quiescent HSC,39-44 and/or a low number of amphotropic receptors in HSC. Several earlier studies, including our own, showed that amphotropic retrovirus transduction of HSC from mouse BM was inefficient, with less than 1% of the peripheral blood cells containing a provirus.22,45 In previous studies we have shown low levels of amphoR mRNA in mouse and human HSC. We have also shown that the level of ecotropic receptor mRNA is relatively high in mouse HSC. Under appropriate culture conditions we have observed relatively efficient transduction of mouse HSC using ecotropic retrovirus vectors, with 35% to 60% of the peripheral blood cells containing the provirus. The relatively efficient transduction of HSC with ecotropic retrovirus vectors indicates the cell cycle status of the target HSC permits proviral integration.6-12 39
We have shown a direct correlation between amphoR mRNA levels and transduction efficiency of mouse HSC22 and human cultured cell lines.31 Other groups have shown that transient expression of the amphotropic receptor gene in cultured cells increases transduction with amphotropic retroviral vectors.45-47Although we cannot exclude the possibility that canine, primate, and human HSC remain quiescent under conditions which allow mouse HSC to cycle, we hypothesize that the inefficient transduction of HSC using amphotropic retrovirus vectors is a result of limiting numbers of amphotropic receptors.
To identify human HSC which might be more efficiently transduced by amphotropic or GaLV vectors, we examined retrovirus receptor mRNA levels in several sources of HSC which have been successfully used for transplantation into humans,19-21 fetal sheep,32 or nonobese diabetic (NOD)/SCID mice.33,48 We found low levels of amphoR and GaLVR mRNA in human fetal liver Lin− CD34+CD38− cells equivalent to the low levels found in fractionated and unfractionated BM. This observation is consistent with the findings of a previous study49 which reported low levels of amphoR mRNA and low levels of transduction in unfractionated and HSC-enriched mouse fetal liver cells.
In contrast to fetal liver and adult BM, relatively high levels of amphoR mRNA were detected in cord blood HSC that had been previously cryopreserved. Cryopreserved cord blood has been used successfully for transplantation,50-55 including the repopulation of myeloablated adult recipients. Kohn et al21 have shown that fresh human cord blood HSC can be marked with amphotropic retrovirus vectors containing an adenosine deaminase cDNA. These authors concluded that the low level of marked cells observed in their patients' peripheral blood was caused in part by the fact that the transduced cord blood cells were transplanted into nonmyeloablated recipients. On the basis of our findings, we propose that previously cryopreserved umbilical cord blood HSC are excellent candidates for gene therapy protocols involving myeloablation.21,56 57
We are extending our investigation of the effect of cryopreservation on amphoR mRNA expression in HSC. One ongoing study involves analysis of receptor mRNA levels in cryopreserved mouse, monkey, and human HSC. In another study, we will specifically test the effect of a brief in vitro exposure of HSC to dimethyl sulfoxide as a possible basis for enhanced amphoR mRNA expression.
In the mouse, we detected relatively high levels of ecoR and amphoR mRNA in 9.5-day unfractionated mouse yolk sac cells and enriched Lin− CD34+ and Lin−CD34− cells. This contrasts with a previous study49 that failed to detect any amphoR mRNA in unfractionated yolk sac cells from days 9.5 through 13.5 of ontogeny. The Lin− CD34+ cells from 9.5-day yolk sac can repopulate neonatal mice26 27 and, based on our observation that these cells express both ecoR and amphoR mRNA, we suggest that yolk sac HSC may be excellent targets for gene marking studies, particularly with ecotropic retroviruses, to show the ability of yolk sac HSC to repopulate all of the hematopoietic lineages of adult mice.
We found high levels of amphoR mRNA in BM from mice treated with G-CSF and SCF. This finding may account for the more efficient gene transfer (10% to 30% positive peripheral blood cells) we recently observed with HSC from the peripheral blood or BM of mice, monkeys, and dogs58-60 treated with injections of G-CSF and SCF. This finding is consistent with an earlier observation by Crooks and Kohn25 that addition of interleukin-3 (IL-3), IL-6, and SCF to CD34+ cells in culture results in an increase in amphotropic retrovirus binding. We propose that cytokine treatment of HSC leads to induction of mRNA expression by the gene encoding the amphoR.
In summary, we have identified several subpopulations of murine and human HSC with high levels of amphoR mRNA. From these findings we predict that HSC from cryopreserved human cord blood may be desirable targets for retrovirus transduction. We also predict that HSC from patients treated with cytokines may be improved targets for amphotropic retrovirus transduction.
ACKNOWLEDGMENT
We thank Dr S-I. Nishikawa of Kyoto University (Kyoto, Japan) for providing the anti c-kit MoAb; Dr Paul Tolstoshev of Genetic Therapy, Inc (Gaithersburg, MD) for providing the 83A25 MoAb; and Dr Larry M. Lantz of the National Institute of Allergy and Infectious Diseases, NIH (Bethesda, MD) for conjugating biotin to the c-kit MoAb.
Supported in part by grants from the NIH RO1 HL 54037 and RO1 HL 46416, and a project in PO1 HL 53586 from the NIH (to H.E.B.).
Address reprint requests to Donald Orlic, PhD, Hematopoiesis Section/Laboratory of Gene Transfer, National Human Genome Research Institute/NIH, Bldg 49/Room 3A11, 49 Convent Dr, Bethesda, MD 20892-4442.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal