Abstract
Hemophilia A is caused by a deficiency of blood coagulation factor VIII (FVIII) and has been widely discussed as a candidate for gene therapy. While the natural canine model of hemophilia A has been valuable for the development of FVIII pharmaceutical products, the use of hemophiliac dogs for gene therapy studies has several limitations such as expense and the long canine generation time. The recent creation of two strains of FVIII-deficient mice provides the first small animal model of hemophilia A. Treatment of hemophiliac mice of both genotypes with potent, human FVIII-encoding adenoviral vectors resulted in expression of biologically active human FVIII at levels, which declined, but remained above the human therapeutic range for over 9 months. The duration of expression and FVIII plasma levels achieved were similar in both hemophiliac mouse strains. Treated mice readily survived tail clipping with minimal blood loss, thus showing phenotypic correction of murine hemophilia A by in vivo gene therapy.
HEMOPHILIA A, A COMMON bleeding disorder affecting one in 5 to 10,000 males in all populations,1 is caused by a deficiency of blood coagulation factor VIII (FVIII). Hemophilia A is categorized into severe, moderate, or mild forms, with over half of the patients manifesting the severe disease.1Severe hemophiliacs, defined as having less than 1% of normal FVIII levels, suffer from episodes of spontaneous and prolonged bleeding into muscles, internal organs, and joints and frequently develop a disabling arthropathy.1 Current treatment is directed toward replacing the missing clotting factor in response to bleeding crises with infusions of plasma-derived or recombinant FVIII.2While prophylactic treatment of hemophilia A has been shown to reduce the frequency and severity of bleeding, such therapy is limited by the availability and high cost of purified FVIII, and the short half-life of FVIII in vivo.2
Somatic cell gene therapy, which would provide constant blood levels of FVIII, would be a significant treatment improvement.3However, expression of FVIII is problematic. The accumulation of FVIII mRNA is inhibited by sequences present in the coding region,4-8 and secretion of FVIII is inefficient.9-11 In addition, the FVIII protein must be secreted directly into the vasculature4,12,13 where the protein is stabilized by formation of a complex with von Willebrand factor (vWF).4
Previous studies in gene therapy for hemophilia A had used ex vivo retroviral transduction approaches. In general, expression of FVIII protein in genetically modified cells in vitro was low5,8,14-17 and undetectable in vivo.15,16Short-term expression of human FVIII in mice was achieved via ex vivo gene transfer strategies,12 13 although the necessity for ex vivo cell manipulation and reimplantation is a limitation to the application of gene therapy in large animal models and humans.
Considerable progress has been made recently in the development of adenoviral-mediated in vivo gene therapy of hemophilia A.18-21 Adenoviral vectors are an efficient system for in vivo FVIII gene delivery because a peripheral vein injection in mice18,22,23 and dogs21 results in efficient transduction of hepatocytes, cells capable of secreting FVIII directly into the blood.4 A vector encoding an albumin-promoted FVIII cDNA resulted in high-level, liver-specific expression of human FVIII in normal adult mice,19 which was sustained for over 5 months at levels fourfold above the human therapeutic range.20 Furthermore, administration of a potent FVIII-encoding adenoviral vector to FVIII-deficient hemophiliac dogs, a well characterized, large hemophilia A animal model,24,25resulted in high-level human FVIII expression and complete correction of the coagulation deficiency.21 However, phenotypic correction in the treated dogs was transient, as the animals developed a strong antibody response directed to the human protein.21Therefore, the canine hemophilia A model is not amenable to long-term expression of human FVIII.
The recent generation of FVIII-deficient mice, by gene disruption techniques, provides the first small animal model of hemophilia A.26 Two distinct hemophiliac genotypes were developed by insertion of a neomycin expression cassette into exon 16 or exon 17 of the murine FVIII gene.26 Similar mutations in humans are known to cause severe hemophilia A.27 Affected mice of both genotypes have FVIII activity levels less than 1% of normal and display lethal bleeding after trauma.26 Therefore, the phenotype of these mice is similar to that of human hemophiliacs.1 The murine hemophilia A model provides a novel tool for the development of hemophilia gene therapy.
In this work, we evaluated adenoviral vectors for the treatment of murine hemophilia A. Studies were conducted in both hemophiliac mouse strains and used vectors prepared in two distinct adenoviral vector backbones. The level and duration of FVIII expression, phenotypic correction, and the antigenicity of human FVIII in the mouse model was assessed. We showed expression of functional human FVIII with levels, which declined, but remained above therapeutic for at least 9 months and achieved complete phenotypic correction.
MATERIALS AND METHODS
Construction of recombinant adenoviruses.
The recombinant adenovirus encoding human FVIII, Av1H8101 (previously named Av1ALAPH81)19 has been described.19 The vector was checked for the presence of replication-competent adenovirus contamination by polymerase chain reaction (PCR) directed at E1a sequences,28 and all vector preparations contained less than 10 plaque-forming units (pfu) of E1a-containing vector per 108 pfu. The β-galactosidase-encoding vector, Av3nBg, has been described.29 Av3H8101 was generated by cotransfection of the plasmid, pAvALAPH81,19 and viral DNA from the vector, Av3nBg,29 into AE1-2a cells29 as described.29 The Av1H8101 vector concentration was determined by spectrophotometric analysis30 and by plaque assay31 on 293 cells. The particle to pfu ratio was 80. Av3H8101 vector concentrations were determined by spectrophotometric analysis.30 Titers are given as particles per milliliter.
Murine hemophilia A pathophysiology, animal breeding, genotyping, and manipulations.
Breeding colonies of both genotypes of FVIII knockout mice have been established.26 Initially, carrier females were bred with normal C57BL/6 male mice. The genotypes of 4-week-old pups were determined by PCR analysis of genomic DNA isolated from tail clips. Briefly, mice were lightly anesthetized with isofluorane, and 1-cm sections of the tail were collected and frozen on dry ice. The tails were immediately cauterized to stop bleeding. Notably, approximately 30% of affected mice did not survive tail clipping with cautery. DNA was isolated from the tail clips using the QIAmp Tissue Kit (Qiagen, Chatsworth, CA) following the suggested protocol. PCR analysis was performed as described26,32 using 400 ng of each genomic DNA and primers specific for the inserted neo gene, exon 16 or exon 17.32 Subsequently, hemizygous affected males and homozygous affected females were mated.
The hemophiliac mice are occasionally anemic and bleed severely from scratches and routine procedures such as ear tagging. Furthermore, the knockout mice suffer from joint bleeds, subcutaneous bleeding, and spontaneous death indicating a similarity to the pathophysiology of human hemophilia A.1 Surprisingly, however, hemophiliac females of both genotypes survive pregnancy, birth, and the nursing of pups32 (S.C. unpublished). Breeding of affected males and females has eliminated the need to genotype the pups by PCR analysis of genomic DNA.26 32 All mice were confirmed FVIII-deficient by analysis of plasma levels of functional FVIII using the Coatest bioassay (see below).
Mice were ear tagged for identification and housed in cages of five to six each. Occasionally, mice would bleed profusely from ear tagging, scratches, and injuries incurred from fighting. Topical thrombin (Thrombostat, Parke-Davis, Morris Plains, NJ) was applied to halt the bleeding. Approximately 10% of the affected mice died spontaneously.
The tail clip survival study involved lightly anesthetizing the mice and clipping 2-cm sections of the tail, without subsequent cauterization. After the procedure, mice were checked every 4 hours. The surviving mice at 24 hours after the procedure were recorded. Alternative assays to assess bleeding time and/or blood loss over time were performed before the tail clip survival study. However, such assays did not distinguish definitively between normal and hemophiliac mice. Similarly, assessment of the activated partial thromboplastin time (APTT) of mouse plasma samples did not distinguish normal from hemophiliac mice (unpublished data). All experiments involving mice adhered to protocols approved by the Institutional Animal Care and Use Committee in accordance with the Animal Welfare Act.
Adenoviral vector administration via tail vein injections and retroobital phlebotomy were performed as described.18 Mice injected with Av1H8101 received a dose of 4 × 1010particles per mouse, and mice injected with the Av3 vectors, Av3H8101 and Av3nBg received a dose of 6 × 1010 particles per mouse. These vector doses yielded equal liver transduction as determined by Southern analysis (data not shown). Topical thrombin (Thrombostat, Parke-Davis) was applied to the injection site to halt the bleeding after vector administration. Notably, the hemophiliac mice did not show any adverse effects from retroorbital bleeding.
FVIII assays, Southern blot, and RNAse protection analyses.
Biologically active human FVIII was measured using the Coatest chromogenic bioassay (Chromogenix, Mölndal, Sweden) as directed. Coatest measures the FVIII-dependent generation of factor Xa from factor X, with one unit defined as the amount of FVIII activity in 1 mL of pooled human plasma, 100 to 200 ng/mL.33 Pooled human plasma (George King Bio-Medical, Inc, Overland Park, KS) was used as the FVIII activity standard to generate a standard curve. Pooled mouse plasma collected from normal C57BL/6 mice served as the normal mouse FVIII activity positive control. When compared with human plasma, normal C57BL/6 plasma FVIII levels showed a median value of 2,500 mU/mL. Pooled plasma isolated from exon 17-disrupted affected mice served as the negative control (<1% of normal mouse levels) in each assay performed. FVIII activity values are reported as the mean value and the standard error of the mean.
The enzyme-linked immunosorbent assay (ELISA) assay, designed to measure human FVIII-specific antibodies, was performed as follows. Recombinant human FVIII (Hyland Division of Baxter Healthcare, Glendale, CA), 100 μL/well at 3 U/mL, in 0.05 mol/L carbonate-bicarbonate buffer pH 9.0, was incubated on 96-well Immunlon 1 pates (Dynatech, Chantilly, VA) at 4°C, overnight. The plate was washed one time with TBS (20 mmol/L Tris-Cl in 0.9% saline) and 200 μL of blocking buffer (0.17 mol/L H3BO3, 0.12 mol/L NaCl, and 0.5% bovine serum albumin [BSA]) was added for 5 hours at room temperature (RT). Plates were washed three times with TBS, and 100 μL mouse plasma samples, appropriately diluted in TBS plus 0.5% BSA, were added to each well and incubated overnight at 4°C. Plates were washed three times with TBS, and the detection antibody (100 μL), alkaline-phosphatase conjugated goat antimouse IgG (Southern Biotechnology Associates, Inc, Birmingham AL) diluted 1:3,000 in TBS with 0.5% BSA, was added to each well and incubated at RT for 2 hours. p-Nitrophenyl phosphate (P-NPP; Calbiochem, La Jolla, CA) 2 mg/mL, in buffer containing 0.1 mol/L glycine, 1 mmol/L MgCl2, 2 mmol/L ZnCl, pH 10.4, was added to each well (100 μL) and the absorbance was read at 405 nm using the Dynatech MR5000 (Dynatech) automated microplate ELISA reader. The concentration of anti-FVIII antibody was calculated from a standard curve using a monoclonal mouse antihuman FVIII antibody (MoAb) 413.34 The limit of sensitivity of the ELISA was 50 ng/mL for mouse plasma samples diluted 1:10.
DNA was isolated from mouse livers using the QIAmp Tissue Kit (Qiagen). A total of 10 μg of each DNA sample was digested with BamHI and subjected to Southern analysis.18 The probe, prepared by random oligonucleotide priming, contained FVIII cDNA sequences from +73 to +1,345.35 36 The copy number control standards were prepared by adding 600 pg and 60 pg of viral DNA, equivalent to 10 and 1 vector copies per cell, respectively, to 10 μg of control mouse liver genomic DNA and digesting with BamHI. No vector was detected in uninjected control mouse liver DNA (data not shown). The band intensities were quantitated with a Molecular Dynamics' PhosphorImager SF (Sunnydale, CA).
RNA was isolated from mouse livers using the RNAzole B (Tel-Test, Friendswood, TX) extraction method. RNAse protection analyses were performed using the RNAse Protection Kit II (Ambion, Austin, TX). For each sample, 20 to 50 μg of total cellular RNA were hybridized with an excess of a gel-purified RNA probe (see below), digested with the RNAse A/T1 solution provided with the kit diluted 1:100, processed as directed, and analyzed on an 8% polyacrylamide-8 mol/L urea gel (SequaGel, National Diagnostics, Atlanta, GA). 32P-labeled fragments from HpaII-digested pBR322 were used as the DNA size markers. The FVIII probe template, pGemSRpr,19 contains FVIII coding region sequences from (+9) to (+214) and was linearized with HindIII. The mouse glyceraldehyde-3-phosphodehydrogenase (GAPDH)-specific probe template was generated from the pTRI-GAPDH mouse plasmid (Ambion, Austin, TX) digested with StyI. All antisense RNA probes were synthesized with SP6 polymerase and α-32P-CTP (3,000 Ci/mmole, Amersham, Arlington Heights, IL).
RESULTS
Expression of functional human FVIII in hemophiliac mice.
The first generation, recombinant human FVIII adenoviral vector, Av1H8101 (previously referred to as Av1ALAPH81)19 contains a mouse albumin promoter, an intron from the human apoliprotein A1 gene, and a human B-domain deleted (BDD) FVIII cDNA. Absence of the B-domain has no effect on FVIII function, activity, or immunogenicity.11,37,38 The vector backbone was derived from adenovirus serotype 5 (Ad5) and is devoid of the E1 and E3 regions. A second human FVIII-encoding adenoviral vector, Av3H8101, was generated with the identical FVIII expression cassette and an Ad5 viral backbone with an additional deletion of the E2a region.29Attenuation of E2a gene expression has been reported to prolong transgene expression in some model systems.39-41
A breeder colony of FVIII knockout mice has been established, and we confirmed that the affected mice exhibit FVIII activity levels less than 1% of normal and bleed acutely in response to trauma.26 To assess human FVIII expression in the hemophiliac mice, Av1H8101 and Av3H8101 were administered via tail vein injection to groups of five exon 17–disrupted mice (see Materials and Methods). Plasma levels of biologically active human FVIII were measured using the Coatest chromogenic bioassay. Before vector treatment, all mice showed FVIII plasma levels at less than 1% the amount detected in a normal, C57BL/6 mouse plasma sample, 2500 mU/mL. Two weeks after vector treatment, FVIII plasma levels were increased to mean values of 960 ± 68 and 946 ± 170 mU/mL in the Av1 and Av3 vector-treated groups, respectively. Human physiologic levels of FVIII are defined as 1,000 mU/mL, and therapeutic levels, the amount of FVIII necessary to convert a severe hemophiliac to a mild or moderate hemophiliac condition, are 50 mU/mL.33 Therefore, the mice treated with either the Av1 and Av3 vectors expressed human physiologic levels of biologically active FVIII, showing directly that adenoviral vector-mediated expression of FVIII resulted in secretion of functional FVIII protein. Furthermore, removal of the E2a region had no effect on vector transduction efficiency or transgene transcriptional activity (data not shown), indicating that the Av1 and Av3 vectors were equally efficacious.
Time course of human FVIII expression in the hemophiliac mice.
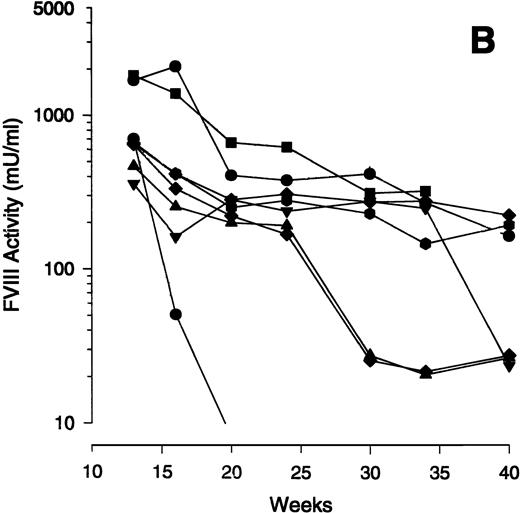
To assess the time course of human FVIII expression with Av1 and Av3 vectors in exon 17–disrupted hemophiliac mice, four animals were treated with Av1H8101 and eight were treated with Av3H8101. Plasma was obtained at the indicated times and assayed for FVIII activity (Fig 1A). The Av1 and Av3 vectors mediated expression of supraphysiologic levels of FVIII with peaks of 2,100 and 2,550 mU/mL, respectively, at 1 to 2 weeks postinjection. FVIII expression was maintained at levels above the human therapeutic range for at least 40 weeks. With both vectors, the decline in FVIII expression was greatest between 10 to 20 weeks with more stable expression observed in some animals thereafter (Fig 1A).
Time course of FVIII expression in hemophiliac mice. The adenoviral vectors Av1H8101 (4 × 1010 particles/mouse) or Av3H8101 (6 × 1010 particles/mouse) were administered via tail vein injection to groups of 4 or 8 exon 17–disrupted hemophiliac mice, respectively. These vector doses yielded equal liver transduction as determined by Southern analysis (data not shown). At the indicated time points, plasma samples were collected and FVIII biological activity was quantitated. (A) Mean plasma levels of biologically active FVIII. (•) Mice that received Av1H8101. (▪) Mice that received Av3H8101. Data are plotted as a mean value and the standard error of the mean at each time point. The dotted line represents the human therapeutic level of FVIII, 50 mU/mL.33 (B) FVIII plasma levels of individual Av3 vector-treated mice. One mouse (▪) died between 34 and 40 weeks.
Time course of FVIII expression in hemophiliac mice. The adenoviral vectors Av1H8101 (4 × 1010 particles/mouse) or Av3H8101 (6 × 1010 particles/mouse) were administered via tail vein injection to groups of 4 or 8 exon 17–disrupted hemophiliac mice, respectively. These vector doses yielded equal liver transduction as determined by Southern analysis (data not shown). At the indicated time points, plasma samples were collected and FVIII biological activity was quantitated. (A) Mean plasma levels of biologically active FVIII. (•) Mice that received Av1H8101. (▪) Mice that received Av3H8101. Data are plotted as a mean value and the standard error of the mean at each time point. The dotted line represents the human therapeutic level of FVIII, 50 mU/mL.33 (B) FVIII plasma levels of individual Av3 vector-treated mice. One mouse (▪) died between 34 and 40 weeks.
Examination of the time course data from individual mice showed an initial decline between 10 to 20 weeks. Subsequently, FVIII levels plateaued in some animals for at least 40 weeks. Interestingly, some mice showed a sudden, sporadic decline in FVIII expression. To illustrate this observation, the FVIII expression levels of the individual mice that received the Av3 vector are plotted from weeks 12 to 40 (Fig 1B). One mouse of the four that received Av1 (data not shown) and three of the eight that received Av3 expressed constant levels of FVIII from weeks 12 to 40 (Fig 1B).
Comparison of FVIII expression in exon 16 and exon 17–disrupted hemophiliac mice.
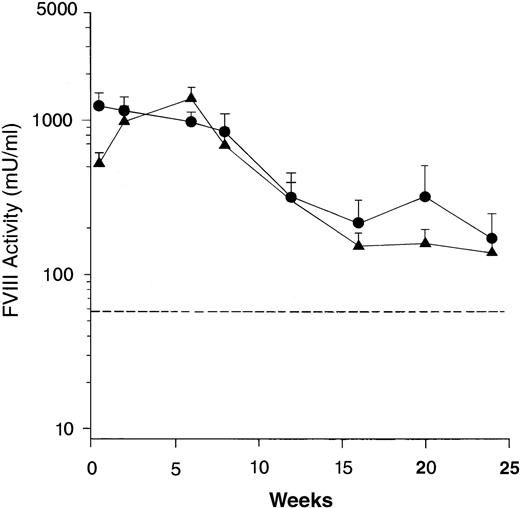
Two separate genotypes of the FVIII knockout mice were generated by disruption of either exon 16 or exon 17.26 32 To show that long-term expression of human FVIII in the mice was not dependent on a specific murine FVIII mutation, groups of 12 exon 16– and 11 exon 17–disrupted mice were treated with Av3H8101. High level expression of biologically active FVIII was detected in both mouse genotypes with expression sustained for at least 24 weeks at levels well above the human therapeutic range (Fig 2). There was no difference in the level or duration of FVIII expression between the two genotypes.
Comparison of human FVIII expression in exon 16 and exon 17–disrupted hemophiliac mice. The adenoviral vector, Av3H8101 (6 × 1010 particles/mouse) was administered via tail vein injection to groups of 12 exon 16– or 11 exon 17–disrupted hemophiliac mice. At the indicated time points, plasma samples were collected and FVIII biological activity was quantitated. Mice that were expressing below less than 1% of normal FVIII levels (<25 mU/mL) were killed at 24 weeks. The FVIII levels in the remaining mice were assayed for an additional 16 weeks. (A) Mean plasma levels of biologically active FVIII. (•) Exon 16-disrupted mice. (▴) Exon 17–disrupted mice. Data are plotted as a mean value and the standard error of the mean at each time point. The dotted line represents the human therapeutic level of FVIII, 50 mU/mL.33
Comparison of human FVIII expression in exon 16 and exon 17–disrupted hemophiliac mice. The adenoviral vector, Av3H8101 (6 × 1010 particles/mouse) was administered via tail vein injection to groups of 12 exon 16– or 11 exon 17–disrupted hemophiliac mice. At the indicated time points, plasma samples were collected and FVIII biological activity was quantitated. Mice that were expressing below less than 1% of normal FVIII levels (<25 mU/mL) were killed at 24 weeks. The FVIII levels in the remaining mice were assayed for an additional 16 weeks. (A) Mean plasma levels of biologically active FVIII. (•) Exon 16-disrupted mice. (▴) Exon 17–disrupted mice. Data are plotted as a mean value and the standard error of the mean at each time point. The dotted line represents the human therapeutic level of FVIII, 50 mU/mL.33
Examination of the time courses of FVIII expression for the individual mice showed the same pattern of stable expression previously shown in Fig 1B. At 40 weeks, four of the exon 16 mice, and four of the exon 17 mice displayed stable, sustained FVIII expression (data not shown). Notably, by 52 weeks, three exon 16 mice and one exon 17 mouse continued to display constant, stable FVIII plasma levels (data not shown).
Phenotypic correction of murine hemophilia A.
Mice expressing therapeutic levels of FVIII appeared phenotypically normal, as they were no longer anemic, did not die spontaneously, and did not bleed profusely from scratches or ear tagging. To show directly that expression of biologically active human FVIII in the treated mice resulted in correction of the bleeding defect, a tail clip survival study was performed (Table 1). Groups of exon 16– and exon 17–disrupted hemophiliac mice were treated with Av3H8101, a β-galactosidase-encoding adenoviral vector, Av3nBg,29 or were untreated. All of the Av3H8101-treated animals expressed therapeutic levels of FVIII 5 days after vector administration. On day 6, tail clips were performed on the treated and untreated hemophiliac mice, as well as normal, age-matched C57BL/6 mice. All of the normal C57BL/6 mice readily survived tail clipping with no evidence of distress. Only 5% to 30% of the untreated and Av3nBg-treated hemophiliac mice survived tail clipping and the survivors were moribund at 24 hours. In contrast, all mice that received Av3H8101 readily survived tail clipping with no evidence of distress. These data show phenotypic correction of murine hemophilia A.
Phenotypic Correction of Murine Hemophilia A
| Mouse Strain . | Vector . | Survivors . |
|---|---|---|
| C57BL/6 | None | 20/20 |
| Ex 16 KO | 2/10 | |
| None | ||
| Ex 17 KO | 4/10-150 | |
| Ex 16 KO | 1/10-151 | |
| Av3nBg | ||
| Ex 17 KO | 0/10-150 | |
| Ex 16 KO | 11/11 | |
| Av3H8101 | ||
| Ex 17 KO | 12/12 |
| Mouse Strain . | Vector . | Survivors . |
|---|---|---|
| C57BL/6 | None | 20/20 |
| Ex 16 KO | 2/10 | |
| None | ||
| Ex 17 KO | 4/10-150 | |
| Ex 16 KO | 1/10-151 | |
| Av3nBg | ||
| Ex 17 KO | 0/10-150 | |
| Ex 16 KO | 11/11 | |
| Av3H8101 | ||
| Ex 17 KO | 12/12 |
A group of 20 age-matched, normal C57BL/6 mice and groups of 10-12 exon 16–(Ex 16 KO) or exon 17–disrupted (Ex 17 KO) hemophiliac mice were treated with the indicated vectors (6 × 1010particles per mouse) or were untreated. FVIII plasma levels were measured before vector administration and 5 days after treatment. All mice treated with Av3H8101 showed expression of functional FVIII at levels well above therapeutic. On day 6, a 2-cm section of the mouse tail was clipped and the mouse survival rate recorded 24 hours later.
One mouse died before tail clipping.
Two mice died before tail clipping.
Assessment of vector persistence and antihuman FVIII antibody response.
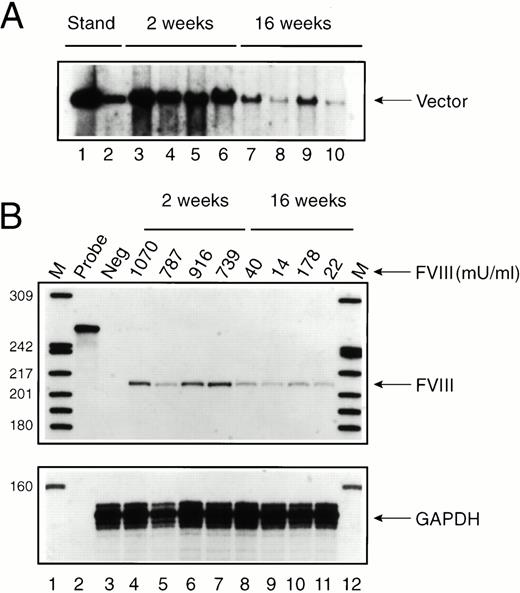
To evaluate the mechanism by which FVIII expression was attenuated in some mice (see Fig 1), Av1H8101-treated exon 17–disrupted mice were killed 2 and 16 weeks after vector administration and liver DNA and RNA were analyzed (Fig 3). Southern analysis showed an average of six vector copies per cell at 2 weeks and 0.8 vector copies per cell at 16 weeks (Fig 3A). Despite the substantial drop in vector copy number, FVIII RNA levels decreased only twofold to threefold (Fig 3B). Furthermore, FVIII RNA levels did not parallel or predict FVIII protein expression in the plasma (Fig 3B). Therefore, loss of FVIII expression at 16 weeks in three of the four mice analyzed was not due to a complete loss of vector DNA or to complete inactivation of the albumin promoter.
Time course of vector persistence in exon 17–disrupted hemophiliac mice. Av1H8101 (4 × 1010 particles/mouse) was administered via tail vein injection to a group of 8 exon 17–disrupted hemophiliac mice. FVIII biological activity was measured before and at 2 and 16 weeks after vector administration. Groups of four mice each were killed at 2 or 16 weeks, and DNA and RNA were isolated from each mouse liver. (A) Southern analysis. Each DNA sample (10 μg) was digested with BamHI. The arrow designates a 3.4-kb fragment containing the vector-derived FVIII sequence. The standards (lanes 1 and 2) were generated by digesting purified Av1H8101 viral DNA in amounts equivalent to 10 and 1 vector copies per cell. Lanes 3 through 6 and 7 through 10 represent liver DNA from mice treated with Av1H8101 or Av3H8101, respectively. No vector was detected in uninjected control mouse liver DNA (data not shown). (B) FVIII protein expression and RNAse protection analysis. Plasma levels of biologically active FVIII protein measured in each mouse are displayed above the lanes. FVIII levels at 2 weeks and 16 weeks are displayed above lanes 4 through 7, and 8 through 11, respectively. For the RNAse protection analysis, 50 μgs of total cellular RNA isolated from the mouse livers were used in each reaction. The arrow labeled FVIII designates the 212-nt human FVIII-specific protected probe fragment. Lane 2 contains undigested full-length probe. Lane 3 contains liver RNA isolated from an uninjected control exon 17–disrupted hemophiliac mouse. Lanes 4 through 7 and 8 through 11 represent RNA from mice at 2 or 16 weeks after vector treatment, respectively. Lanes 1 and 12 contain32P-labeled DNA molecular-weight markers. The lower panel displays a separate RNAse protection assay using 20 μg of total cellular mouse liver RNA and an antisense RNA probe encoding a portion of the mouse glyceraldehyde 3-phosphodehydrogenase (GAPDH) cDNA. The arrow labeled GAPDH designates the 134-nt mouse GAPDH-specific protected probe fragment.
Time course of vector persistence in exon 17–disrupted hemophiliac mice. Av1H8101 (4 × 1010 particles/mouse) was administered via tail vein injection to a group of 8 exon 17–disrupted hemophiliac mice. FVIII biological activity was measured before and at 2 and 16 weeks after vector administration. Groups of four mice each were killed at 2 or 16 weeks, and DNA and RNA were isolated from each mouse liver. (A) Southern analysis. Each DNA sample (10 μg) was digested with BamHI. The arrow designates a 3.4-kb fragment containing the vector-derived FVIII sequence. The standards (lanes 1 and 2) were generated by digesting purified Av1H8101 viral DNA in amounts equivalent to 10 and 1 vector copies per cell. Lanes 3 through 6 and 7 through 10 represent liver DNA from mice treated with Av1H8101 or Av3H8101, respectively. No vector was detected in uninjected control mouse liver DNA (data not shown). (B) FVIII protein expression and RNAse protection analysis. Plasma levels of biologically active FVIII protein measured in each mouse are displayed above the lanes. FVIII levels at 2 weeks and 16 weeks are displayed above lanes 4 through 7, and 8 through 11, respectively. For the RNAse protection analysis, 50 μgs of total cellular RNA isolated from the mouse livers were used in each reaction. The arrow labeled FVIII designates the 212-nt human FVIII-specific protected probe fragment. Lane 2 contains undigested full-length probe. Lane 3 contains liver RNA isolated from an uninjected control exon 17–disrupted hemophiliac mouse. Lanes 4 through 7 and 8 through 11 represent RNA from mice at 2 or 16 weeks after vector treatment, respectively. Lanes 1 and 12 contain32P-labeled DNA molecular-weight markers. The lower panel displays a separate RNAse protection assay using 20 μg of total cellular mouse liver RNA and an antisense RNA probe encoding a portion of the mouse glyceraldehyde 3-phosphodehydrogenase (GAPDH) cDNA. The arrow labeled GAPDH designates the 134-nt mouse GAPDH-specific protected probe fragment.
A second hypothesis to explain the sudden, sporadic decline in FVIII plasma levels observed in some mice (see Fig 1) was the development of a humoral immune response directed against the human FVIII protein. An ELISA designed to detect antihuman FVIII antibodies was used to assay plasma from the exon 16 and exon 17 mice represented in Fig 2 before vector administration and at various time points thereafter (Table 2). In all cases, antibody levels were either undetectable or low, ranging from below the limit of sensitivity, 50 ng/mL, up to 97 ng/mL. There was no case in which the drop in FVIII plasma levels could be attributed to the generation of a strong antibody response. Interestingly, Qian et al42showed that hemophiliac mice of both genotypes treated with a single intravenous administration of purified full-length FVIII protein rapidly developed a strong immune response (approximately 40,000 ng/mL) to the human protein.
Hemophiliac Mouse Plasma Levels of Antihuman FVIII Antibodies
| Genotype . | Mouse No. . | Time of Sample Collection (wk) . | Factor VIII Levels (mU/mL) . | Antihuman FVIII Antibody Levels (ng/mL) . |
|---|---|---|---|---|
| Ex 16 KO | Prebleed | (−) | <50 | |
| 1 | 12 | 277 | 52 | |
| 16 | (−) | 54 | ||
| Prebleed | (−) | <50 | ||
| 2 | 12 | 245 | 51 | |
| 16 | 26 | 60 | ||
| Prebleed | (−) | <50 | ||
| 3 | 16 | 208 | <50 | |
| 20 | 33 | 62 | ||
| Prebleed | (−) | <50 | ||
| 4 | 8 | 1983 | 53 | |
| 24 | 300 | 79 | ||
| Prebleed | (−) | <50 | ||
| 5 | 12 | 184 | 62 | |
| 16 | (−) | 93 | ||
| Ex 17 KO | Prebleed | (−) | <50 | |
| 6 | 2 | 136 | 53 | |
| 6 | 33 | 51 | ||
| Prebleed | (−) | <50 | ||
| 7 | 12 | 240 | 97 | |
| 16 | (−) | 76 | ||
| Prebleed | (−) | <50 | ||
| 8 | 12 | 554 | 58 | |
| 24 | 318 | 84 | ||
| Prebleed | (−) | 62 | ||
| 9 | 20 | 242 | <50 | |
| 24 | 24 | 87 |
| Genotype . | Mouse No. . | Time of Sample Collection (wk) . | Factor VIII Levels (mU/mL) . | Antihuman FVIII Antibody Levels (ng/mL) . |
|---|---|---|---|---|
| Ex 16 KO | Prebleed | (−) | <50 | |
| 1 | 12 | 277 | 52 | |
| 16 | (−) | 54 | ||
| Prebleed | (−) | <50 | ||
| 2 | 12 | 245 | 51 | |
| 16 | 26 | 60 | ||
| Prebleed | (−) | <50 | ||
| 3 | 16 | 208 | <50 | |
| 20 | 33 | 62 | ||
| Prebleed | (−) | <50 | ||
| 4 | 8 | 1983 | 53 | |
| 24 | 300 | 79 | ||
| Prebleed | (−) | <50 | ||
| 5 | 12 | 184 | 62 | |
| 16 | (−) | 93 | ||
| Ex 17 KO | Prebleed | (−) | <50 | |
| 6 | 2 | 136 | 53 | |
| 6 | 33 | 51 | ||
| Prebleed | (−) | <50 | ||
| 7 | 12 | 240 | 97 | |
| 16 | (−) | 76 | ||
| Prebleed | (−) | <50 | ||
| 8 | 12 | 554 | 58 | |
| 24 | 318 | 84 | ||
| Prebleed | (−) | 62 | ||
| 9 | 20 | 242 | <50 | |
| 24 | 24 | 87 |
Plasma was collected at the indicated times from the exon 16–(Ex 16 KO) and exon 17–disrupted (Ex 17 KO) mice treated with Av3H8101 (6 × 1010 particles/mouse) represented in Fig 3. Plasma was analyzed for FVIII biological activity and for the presence of antihuman FVIII antibodies by ELISA. For the antibody analysis, plasma samples were diluted 1:10, and the limit of sensitivity of the assay was 50 ng/mL. (−) represents a FVIII activity level of less than 1% of that detected in a normal mouse plasma sample analyzed in parallel in each assay.
DISCUSSION
In this work, we showed long-term phenotypic correction of hemophilia A by in vivo gene therapy in a clinically relevant small hemophilia A animal model. Whereas sustained human FVIII expression was demonstrated previously in normal mice20 and short-term phenotypic correction was achieved in hemophiliac dogs,21 the FVIII-deficient hemophiliac mouse26 has several advantages over these animal models for the study of hemophilia A gene therapy and FVIII in vivo function. In contrast to normal mice, the biologic activity and function of the human FVIII protein expressed in hemophiliac mice can be measured directly, and phenotypic correction of the bleeding defect assessed. In addition, both FVIII-deficient mouse genotypes were developed as a mixture of two mouse strains, C57BL/6 and 129SV,26 providing a more diverse genetic background than inbred strains for gene therapy studies. Compared with the canine hemophilia A model, the knockout mice provide the only available small animal model of hemophilia A. Small animal models facilitate the performance of multiple studies with large cohorts to better characterize vector function and FVIII protein expression.
An important attribute that distinguishes the hemophiliac mouse model from that of normal mice is the ability to show phenotypic correction. The murine hemophiliac phenotype is characterized by subcutaneous, intraperitoneal, intrathoracic, and joint bleeds. The mice bleed extensively after minor lacerations, are occasionally anemic, and exhibit a high spontaneous mortality rate. We initially observed that, after vector treatment, the mice appeared normal and the bleeding phenotype resolved. To obtain an objective assessment of phenotypic correction, a tail clip survival study, which clearly distinguished hemophiliac from normal mice, was used. This assay showed that human FVIII expressed by an intravenously administered adenoviral vector is biologically active, functional in mice, and therapeutic.
The time course of FVIII expression in individual mice showed an intriguing pattern. With both the Av1 and Av3 vectors, treated mice displayed an initial drop in FVIII plasma levels, probably due to a low level of vector-mediated toxicity as previously described with normal mice.20 FVIII expression in several of the mice subsequently stabilized, showing that a single adenoviral vector administration has the potential to achieve long-term, constant-level expression. Furthermore, long-term FVIII expression was not dependent on a specific murine FVIII mutation, as a similar expression pattern was observed in both hemophiliac mouse genotypes. However, sustained expression was not uniformly achieved, as FVIII levels rapidly and sporadically declined in some mice.
The mechanism by which FVIII expression levels declined was investigated by DNA, RNA, and human FVIII-specific antibody analyses. Over time, vector DNA levels declined substantially, while FVIII RNA levels showed only a minimal decrease. Furthermore, FVIII RNA levels were similar in mice with widely divergent FVIII plasma levels. Thus, the decline in plasma levels could not be completely attributed to either vector loss or transcriptional inactivation. The development of an immune response to human FVIII seemed a likely explanation for the loss of FVIII expression. Little or no human FVIII-specific antibody response was detected in either strain of hemophiliac mice before or after attenuation of expression, suggesting that a humoral immune response was not responsible for the loss of FVIII. However, we cannot rule out the possibility that loss of expression resulted from a shift in the subclass or quality of a low titer antibody. A cell-mediated immune response directed toward hepatocytes expressing human FVIII could also explain the decline in FVIII expression, but the elimination of hepatocytes would have resulted in a parallel loss of FVIII RNA, which was not observed. Presently, the explanation for the rapid loss of expression in some mice remains unclear. Further investigation of this phenomenon may lead to a better understanding of vector and FVIII physiology and to the development of vectors which more uniformly remain efficacious.
A major issue broadly relevant to the field of gene therapy is the potential immunogenicity of endogenously expressed foreign proteins. Ten percent to 30% of human hemophiliacs treated with intermittent, intravenous FVIII protein administration develop FVIII inhibitory antibodies.1,43 Hemophiliac mice injected intravenously with human full-length FVIII develop a potent anti-FVIII antibody response,42 and a T-cell response directed to the full-length protein.44 However, hemophiliac mice treated with an adenoviral vector encoding human BDD FVIII generated little or no antibody response to human BDD FVIII. The induction of a cytotoxic T-lymphocyte (CTL) response was not investigated. Furthermore, the low antibody levels detected in either strain of hemophiliac mice indicate that the lack of FVIII immunogenicity was not dependent on the specific FVIII mutation. These observations represent preliminary evidence to suggest that constant level, endogenous expression of human FVIII may be less immunogenic than intermittent, intravenous protein administration. Verification of this hypothesis will require a controlled study in which the immunogenicity of BDD FVIII protein is compared directly with vector-mediated expression of BDD FVIII. Notably, vector-mediated expression of BDD FVIII can result in the generation of antibodies against the human protein, as was observed in vector-treated hemophiliac dogs.21 Because the murine immune system is well studied and easily manipulated, hemophiliac mice represent an excellent model for characterizing the immune response to FVIII. Furthermore, this model can be used for evaluating gene therapy strategies to treat FVIII inhibitory antibodies, a significant clinical problem in hemophilia.
Two human BDD FVIII-encoding vectors were evaluated in this study. The first generation Av1 vector is composed of an adenoviral backbone from which the E1 and E3 regions were removed. The third generation Av3 vector contains, in addition, the deletion of the E2a region. Av3 vectors are completely replication defective in vitro and do not express detectable levels of hexon capsid protein.29 In some animal models, attenuation of E2a gene expression results in prolonged transgene expression and a reduction in the host immune response to the vector.39-41 However, in other mouse strains and hemophilia B dogs, the inclusion of a temperature sensitive mutation or removal of the E2a gene had no effect on vector persistence.45,46 In this work, treatment of hemophiliac mice with either the Av1 or the Av3 FVIII vectors resulted in similar liver transduction efficiency, transgene transcriptional activity, and FVIII expression levels. Both Av1 and Av3 vectors achieved sustained expression with attenuation in the first 20 weeks followed by stable FVIII plasma levels in some animals for over 9 months. The finding that Av3 vectors did not provide for greater efficacy in the hemophiliac mice is consistent with the hypothesis that the immune system did not play a major role in limiting the duration of expression in this model. Furthermore, the attenuation of FVIII expression in the first 20 weeks was probably due to direct vector-mediated liver toxicity,20,46 which is similar with both Av1 and Av3 vectors (T.A.G. Smith, personal communication, August 1997) and may be related to the E4 gene,47-50which remains in both backbones. The removal of additional backbone genes may decrease toxicity, minimize the attenuation of expression in the first 20 weeks, and enable more sustained expression.
The data presented in this work support the use of recombinant adenoviruses for the treatment of hemophilia A. Prolonged curative therapy was achieved with a single vector administration in a hemophiliac animal model. However, clinical benefit in humans will require therapy that extends for years, necessitating repeated treatments or more sustained expression. Current studies to allow vector readministration51-53 together with the development of improved, more attenuated adenoviral vectors54-59 may soon provide long-term correction of hemophilia A.
ACKNOWLEDGMENT
We thank Drs Martin Woodle and Theodore A.G. Smith for critical review of the manuscript, Dr Theodore A.G. Smith for communication of data before publication, Dr Russette Lyons and Christoph Wey for assistance with the animal procedures, and Adam Shoemaker for vector production and quality control.
Address reprint requests to Michael Kaleko, MD, PhD, Genetic Therapy, Inc, 938 Clopper Rd, Gaithersburg, MD 20878.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal