Abstract
We have recently made the paradoxical observation that a single injection of recombinant murine interleukin-12 (IL-12) on the day of bone marrow transplantation (BMT) inhibits graft-versus-host disease (GVHD) in lethally irradiated mice receiving fully major histocompatability complex (MHC)-mismatched bone marrow and spleen cells. We have now examined the mechanism of this effect of IL-12 on acute GVHD. By day 4 post-BMT, IL-12–treated mice showed marked reductions in splenic donor CD4+ and CD8+ T cells compared with GVHD controls. Expression of the early activation markers IL-2R alpha chain (CD25) and CD69 on splenic donor CD4+ cells was considerably higher at early time points (36 and 72 hours post-BMT) in IL-12–treated mice compared with GVHD controls. However, the later, GVHD-associated increase in CD25 and very late antigen-4 (VLA-4) expression on donor T cells was greatly depressed in IL-12–protected mice compared with GVHD controls. The marked GVHD-associated expansion of host-reactive T helper cells by day 4 was also completely inhibited in the IL-12–treated group. Expression of Fas was increased on donor CD4 cells of IL-12–treated mice compared with those of controls on days 3 through 7 post-BMT. Furthermore, the ability of IL-12 to protect against GVHD was at least partially dependent on the ability of donor cells to express functional Fas molecules. We conclude that IL-12 treatment at the time of BMT markedly perturbs the activation of alloreactive donor CD4+ T cells that play a critical role in the pathogenesis of acute GVHD. We hypothesize that these perturbations culminate in Fas-dependent apoptosis of donor T cells, thus impeding their expansion and their GVHD-promoting activity.
DESPITE MAJOR ADVANCES in chemotherapeutics, immunosuppressive therapy, and supportive care, the full therapeutic potential of bone marrow transplantation (BMT) for the treatment of leukemia has not yet been realized. Graft-versus-host disease (GVHD), which arises from an attack of donor T cells against alloantigens of the recipient,1 is a continued threat to the successful outcome of allogeneic BMT, especially with the increasing use of unrelated donors. With modern immunosuppressive drugs for GVHD prophylaxis, significant GVHD still occurs in 30% to 40% of HLA-matched sibling transplants.2 The problems of GVHD and failure of engraftment are magnified in the presence of HLA mismatches,3,4 so that extensively HLA-mismatched BMT cannot be routinely performed. Removing T lymphocytes from the marrow graft (T-cell depletion [TCD]) effectively prevents GVHD, but at the expense of an increase in marrow graft rejection and leukemic relapse.5,6 Immunosuppressive drugs used for GVHD prophylaxis may also increase the rates of leukemic relapse by suppressing this T-cell reactivity.2 Therefore, an ability to separate the beneficial engraftment-promoting and antileukemic effects of donor T cells while diminishing their ability to induce GVHD might allow HLA-mismatched BMT to be performed and lead to reduced leukemic relapse rates.
We have recently made the paradoxical observation that a single injection of recombinant interleukin-12 (IL-12) inhibits acute GVHD in a fully major histocompatability complex (MHC) plus multiple minor antigen-mismatched murine BMT model.7 Administration of a single injection of IL-12 on the day of BMT led to marked inhibition of acute GVHD mortality in lethally irradiated H-2b mice receiving fully MHC plus multiple minor antigen-mismatched allogeneic bone marrow and spleen cells. A small additional protective effect was observed when T-cell–depleted host-type bone marrow cells (BMC) were added to the inoculum, even though those host-type BMC were rapidly eliminated, so that full donor chimerism was observed by about 7 days post-BMT.7 The protective effect of IL-12 against GVHD was surprising because this cytokine is known to induce Th1 differentiation and cytotoxic T-lymphocyte function, and both Th1 immune responses and cytotoxic T lymphocytes (CTL) have been implicated in the pathogenesis of acute GVHD, including the fully mismatched model in which the protective effect of IL-12 was originally discovered.7 Moreover, the ability of IL-12 to inhibit GVHD is dependent on the Th1 cytokine IFN-γ.8 Initial studies addressing the mechanism of this effect suggested that the early expansion of donor T cells that occurs in the first week post-BMT9 was inhibited by IL-12.7 We have now attempted to further delineate the mechanisms of this paradoxical inhibitory effect of IL-12 on acute GVHD by examining the effect of IL-12 on the upregulation of donor T-cell activation markers and on the expansion of host-reactive T cells. The results suggest that IL-12 markedly perturbs the activation and early expansion of host-reactive donor T cells, at least in part through a Fas-dependent mechanism.
MATERIALS AND METHODS
Mice.
Specific pathogen-free female C57BL/6 (B6, H-2b, Kb Ib Db) and A/J (H-2a, Kk Ik Dd) mice were purchased from the Frederick Cancer Research Facility (NCI, Frederick, MD). Female MRL/MpJ+ +/+ control (designated MRL) and lpr/lpr MRL/MpJ-lpr (designated LPR) mice (both H-2k) were purchased from the Jackson Laboratory, Bar Harbor, ME. Animals were housed in sterilized microisolator cages and received autoclaved feed and autoclaved, acidified drinking water.
Preparation of BMC, spleen cells, and T-cell–depleted BMC.
BMC and spleen cell suspensions were prepared as described.10 Host-type BMC were depleted of T cells with anti-CD4 monoclonal antibody (MoAb) (GK1.5 in ascites, 1:5,000 dilution),11 and anti-CD8 MoAb (2.43 in ascites, 1:1,500 dilution)12 plus low toxicity rabbit complement (1:14 dilution), as described previously.13
Total body irradiation and BMT.
Recipient mice were lethally irradiated (9.75 to 10.25 Gy,137Cs source, 1.1 Gy/min) and reconstituted within 4 to 8 hours with a single 1-mL intravenous inoculum containing 15 to 17 ×106 A/J spleen cells plus 10 × 106A/J BMC with 5 × 106 TCD B6 BMC (allogeneic group), or with 5 × 106 TCD B6 BMC only (syngeneic group). In other experiments, irradiated B6 mice received TCD B6 BMC plus either MRL control or Fas-deficient LPR BMC (10 × 106) and spleen cells (10 × 106). To avoid bias from cage-related effects, animals were randomized before and after BMT as described.14 Survival was followed for 60 to 100 days. All experiments used for cell or cytokine analysis in this report included at least five control mice in each group that were followed for survival and in which the protective effect of IL-12 was documented and was similar in magnitude to that we have reported.7
IL-12 administration.
In earlier experiments, 4,900 IU of murine recombinant IL-12 (kindly provided by Genetics Institute, Cambridge, MA), with specific activity of 4.9 to 5.5 × 106 IU/mg, was injected intraperitoneally into recipient mice approximately 1 hour before BMT. In later experiments, the dose was reduced to 2,400 U, because other experiments to be reported elsewhere indicated that similar protection was afforded by either dose of IL-12 (M.S., unpublished data, January 1996).
MoAbs and flow cytometry.
Spleen cells or cell suspensions from inguinal and mesenteric lymph nodes were analyzed by two- or three-color flow cytometry (FCM) on a FACScan (Becton Dickinson, Mountain View, CA). To block nonspecific FcγR binding of labeled antibodies, 10 μL of undiluted culture supernatant of 2.4G2 (rat antimouse FcγR MoAb)15 was added to the first incubation. To determine the percentage of donor- and host-type T cells in spleens, cells were stained with fluorescein isothiocyanate (FITC)-conjugated rat antimouse CD4 (PharMingen, San Diego, CA) and anti-CD8 (Caltag, San Francisco, CA) MoAbs for 30 minutes at 4°C, washed, incubated with biotinylated anti-H–2Dd MoAb 34-2-1216 for 30 minutes at 4°C, washed again, incubated for 10 minutes with phycoerythrin-streptavidin (PEA), then washed again. To study the expression of activation markers on T cells, spleen cells were stained with FITC-conjugated anti-CD25 or anti–VLA-4 MoAbs for 30 minutes at 4°C, washed, incubated with biotin-conjugated anti-CD4 or anti-CD8 MoAbs for 30 minutes at 4°C, washed again, incubated for 10 minutes with PEA, then washed again. A control tube contained nonreactive mouse IgG2a MoAb HOPC-FITC plus 34-2-12-biotin-PEA. Dead cells were excluded during FCM analysis by gating out low forward scatter/high propidium iodide-retaining cells. For three-color determination of the percentage of donor CD4 cells expressing CD25, CD69, and VLA-4, spleen cells were stained with FITC-conjugated anti-CD25, anti-CD69, or anti–VLA-4 MoAbs, plus PE-conjugated anti-CD4 MoAb, washed, incubated with biotin-conjugated anti-H-2KbMoAb 5F1 (recipient class I antibody), washed again, and incubated with CyChrome-Streptavidin for 10 minutes and then washed twice. To determine the expression of Fas on donor CD4 T cells, cells were stained with 5F1-FITC plus PE-conjugated anti-CD4 MoAb, washed, incubated with biotinylated anti-Fas MoAb (PharMingen), washed, incubated with CyChrome-Streptavidin, then washed twice. Five to ten thousand 5F1-negative (ie, donor), CD4-positive cells were collected for analysis of Fas expression.
Limiting dilution analysis.
For measurement of activated helper cell (Th) frequencies, varying numbers of responder cells were incubated with 6 × 105, 30 Gy irradiated stimulator cells in 96-well round-bottomed plates. Twenty-four wells each containing 30,000, 10,000, 3,333, 1,111, 370, and 123 responder cells were prepared. After 24 hours of incubation, 100 μL of supernatant was obtained from each well and transferred to parallel plates containing 8,000 IL-2/IL-4–dependent CTLL cells per well. These were incubated in the supernatants for 24 hours, and 1 μCi of3H-thymidine was then added to each well. After an additional 18 hours, cells were harvested with a Tomtec harvester (Wallac, Gaithersburg, MD) and 3H-thymidine uptake was counted on a Betaplate β counter (Pharmacia LKB, Gaithersburg, MD). Wells were considered positive if3H-thymidine uptake was three standard deviations greater than the mean 3H-thymidine uptake in 24 wells containing supernatants from stimulator cells alone. The Poisson distribution was used to determine the frequency of antigen-responsive Th that recognized each stimulator strain, and statistical analysis was performed by χ2 minimization analysis as described by Taswell.17
Analysis of serum cytokine levels.
Animals were selected randomly from each group and were killed by exsanguination under anesthesia. Their blood was clotted on ice for 10 or 15 minutes, and serum was separated by centrifugation at 4°C and was stored at −80°C. Serum tumor necrosis factor (TNF)-α levels were measured in duplicate wells using the Cytoscreen Mouse TNF-α enzyme-linked immunosorbent assay (ELISA) kit (Biosource International, CA). The minimum detectable concentration of TNF-α was less than 3 pg/mL.
Histopathologic evaluation.
These evaluations were performed blindly on hematoxylin and eosin-stained liver and lung tissue sections by a hematopathologist.
Statistical analysis.
Two-tailed Student's t-tests for comparison of means were performed for evaluation of the effects of IL-12 on cell populations and cytokine levels between groups at individual time points. Survival data were analyzed using the Kaplan-Meier method of life table analysis, and statistical analysis was performed with the Mantel-Haenzsen test or the log rank test. A P value of less than .05 was considered to be significant.
RESULTS
Effect of IL-12 on donor T-cell numbers.
We have previously shown marked reductions in donor CD4+and CD8+ T cells in the spleens of IL-12–protected compared with GVHD control mice on day 4 post-BMT, with a reversal of this difference by day 7.7 We confirmed these results in three additional experiments and extended the analysis to include day 5. Overall, T-cell numbers in the spleens of GVHD control mice increased to a peak level on day 5, then declined between days 5 and 7. In spleens of the IL-12–treated group, donor CD4+ and CD8+ cells were markedly decreased compared with GVHD controls on day 4 (P = .04), increased by day 5 to a peak that was lower than that in GVHD controls, but remained relatively constant between days 5 and 7 (Fig 1A). Because mortality in GVHD controls was considerable after day 7, no further comparisons could be made at later time points.
Effect of IL-12 treatment on donor T-cell expansion and activation. Lethally irradiated B6 mice received A/J BMC and spleen cells plus TCD host-type (B6) BMC with or without IL-12, 4,900 IU, administered on day 0. (A) Mean number of CD4+ and CD8+ T cells in spleens of GVHD control and IL-12–protected mice according to donor (▨;) versus host (▪) origin, as determined by separate staining with anti-CD4 versus anti-Dd and anti-CD8 versus anti-Dd. Mean values obtained from six mice per group at each time point are shown. (B) Altered expression of CD25 on donor T cells in IL-12–protected mice. Spleen cells were analyzed by two-color FCM to determine percentages of CD4+ and CD8+ T cells expressing CD25 on days 4, 5, and 7 after BMT. The mean of the products of the percentages of CD25+ CD4 or CD8 cells and the spleen cell yield for GVHD control (□) and IL-12–protected mice (▦) are shown (n = 3 mice per group per time point). Black bars (▪) represent splenic T cells from normal A/J mice. Similar results were obtained in two additional experiments. Because additional stains (anti-Ddv anti-CD25) showed that all CD25-expressing spleen cells were of donor origin in these animals, it can be inferred that all CD25+ T cells shown in this figure are of donor origin. (C) Mean number of VLA-4+ CD4 and CD8 cells in spleens of GVHD control (□) and IL-12–protected mice (▪) on days 4, 5, and 7 post-BMT. The average of the product of the percentage of VLA-4+ CD4 or CD8 cells and the spleen cell yield (n = 3 mice per group per time point) is shown.
Effect of IL-12 treatment on donor T-cell expansion and activation. Lethally irradiated B6 mice received A/J BMC and spleen cells plus TCD host-type (B6) BMC with or without IL-12, 4,900 IU, administered on day 0. (A) Mean number of CD4+ and CD8+ T cells in spleens of GVHD control and IL-12–protected mice according to donor (▨;) versus host (▪) origin, as determined by separate staining with anti-CD4 versus anti-Dd and anti-CD8 versus anti-Dd. Mean values obtained from six mice per group at each time point are shown. (B) Altered expression of CD25 on donor T cells in IL-12–protected mice. Spleen cells were analyzed by two-color FCM to determine percentages of CD4+ and CD8+ T cells expressing CD25 on days 4, 5, and 7 after BMT. The mean of the products of the percentages of CD25+ CD4 or CD8 cells and the spleen cell yield for GVHD control (□) and IL-12–protected mice (▦) are shown (n = 3 mice per group per time point). Black bars (▪) represent splenic T cells from normal A/J mice. Similar results were obtained in two additional experiments. Because additional stains (anti-Ddv anti-CD25) showed that all CD25-expressing spleen cells were of donor origin in these animals, it can be inferred that all CD25+ T cells shown in this figure are of donor origin. (C) Mean number of VLA-4+ CD4 and CD8 cells in spleens of GVHD control (□) and IL-12–protected mice (▪) on days 4, 5, and 7 post-BMT. The average of the product of the percentage of VLA-4+ CD4 or CD8 cells and the spleen cell yield (n = 3 mice per group per time point) is shown.
We hypothesized that the markedly reduced numbers of donor T cells in the spleens of IL-12–treated mice compared with GVHD controls on day 4 post-BMT might be due either to (1) redistribution of allogeneic T cells into GVHD target organs such as liver and lung, or (2) a significant inhibitory effect of IL-12 on the activation and expansion of donor T cells. To address the possibility of redistribution, histopathologic findings (tissue sections stained with hematoxylin-eosin) were compared for lungs and livers of GVHD control and IL-12–treated mice on day 4 post-BMT (n = 2 from each group). Livers and lungs did not show significant cellular infiltrations in either group on day 4 (data not shown), making tissue redistribution an unlikely explanation for the marked reductions in donor T cells in spleens of IL-12–treated mice at this time.
Effect of IL-12 on donor T-cell activation markers.
To address the hypothesis that IL-12 inhibits activation of donor T cells, we compared the expression of activation-associated markers on donor T cells in spleens of GVHD control and IL-12–protected mice. Results of two-color analyses are summarized in Fig 1. The absolute numbers of CD4+ and CD8+ cells expressing CD25 on day 4 after BMT were markedly decreased in IL-12–treated mice compared with GVHD controls (Fig 1B). These striking differences in CD25 expression on donor CD4 T cells on day 4 were confirmed directly using three-color FCM in three of three experiments (total, n = 9 per group). Although by day 5 the number of CD25+, CD4+, CD25+, and CD8+ T cells had increased in the spleens of IL-12–protected mice, their numbers remained lower than in GVHD controls. The number of CD25+donor T cells in both groups declined to baseline by day 7. The CD25+ T cells shown in Fig 1B were all of donor origin because there were no CD25-expressing host cells detected by FCM in either group (not shown).
The distribution and kinetics of VLA-4 expression were quite distinct from those of CD25. Very few VLA-4–expressing cells were detected in spleens of IL-12–protected mice on day 4, and half of these were of host origin, whereas GVHD controls contained significant numbers of VLA-4+ cells that were almost all of donor origin (not shown). These included substantial numbers of both CD4+ and CD8+ donor T cells (Fig 1C). By day 5, essentially all CD4+ and CD8+ cells and VLA-4+cells in spleens of both groups were entirely of donor origin (Fig 1A and data not shown). Therefore, the VLA-4+ CD4+and VLA-4+ CD8+ cells shown in Fig 1C are mainly of donor origin, except for the IL-12–treated group on day 4, when some of the very few VLA-4+ CD4+ cells present (Fig 1C, left half) might be of host origin. A marked reduction in VLA-4 expression on donor T cells was observed on day 4 post-BMT in IL-12–protected mice. Direct confirmation of this result for donor CD4 cells on day 4 was obtained using three-color FCM (total, n = 9 per group; data not shown). VLA-4 expression among donor T cells later increased in IL-12–protected mice to become similar to that of GVHD controls on days 5 and 7 (Fig 1C).
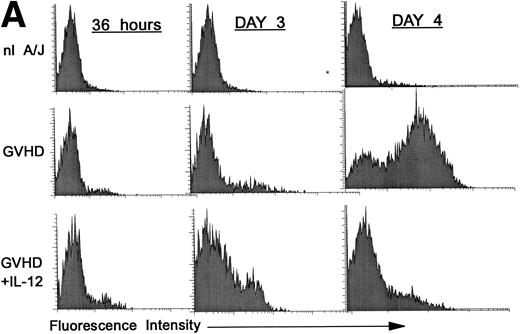
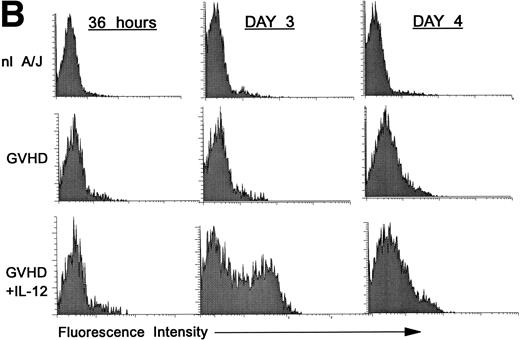
Premature activation of donor CD4 cells by IL-12.
We hypothesized that IL-12 might activate donor T cells earlier than in uninhibited GVHD, in which marked donor T-cell activation is not evident in the spleen before day 4. To address this hypothesis, we performed three-color FCM on spleen cells to examine CD25 and CD69 expression on gated donor CD4+ spleen cells at 36 hours and on days 3 and 4 post-BMT. As is shown in Fig 2A, CD25 expression on donor CD4+ cells was considerably higher (bottom row, left and middle) at 36 hours and on day 3 post-BMT in IL-12–treated mice compared with GVHD controls (middle row, left and middle). However, on day 4 the pattern reversed, and CD25 expression was markedly increased on donor CD4+ cells of GVHD controls (middle row, right), but not in IL-12–protected animals (bottom right), consistent with results presented above.
Increased expression of CD25 and CD69 on donor CD4+ T cells at 36 hours and on day 3 in spleens of IL-12–protected mice. Lethally irradiated B6 mice received A/J BMC and spleen cells plus TCD B6 BMC with or without IL-12 on day 0. Spleen cells were analyzed by three-color FCM to determine percentages of CD4+ Dd+ donor cells expressing CD25 (A) and CD69 (B). Similar results were obtained from six mice per group at each time point in two independent experiments.
Increased expression of CD25 and CD69 on donor CD4+ T cells at 36 hours and on day 3 in spleens of IL-12–protected mice. Lethally irradiated B6 mice received A/J BMC and spleen cells plus TCD B6 BMC with or without IL-12 on day 0. Spleen cells were analyzed by three-color FCM to determine percentages of CD4+ Dd+ donor cells expressing CD25 (A) and CD69 (B). Similar results were obtained from six mice per group at each time point in two independent experiments.
Expression of the early activation marker CD69 on donor CD4+ cells was also markedly higher in IL-12–treated mice (Fig 2B, bottom row, left and middle) compared with GVHD controls (middle row, left and middle) at 3 days and was also slightly higher at 36 hours post-BMT. Similar results were observed in a repeat experiment. Therefore, expression of CD69 and CD25 on donor CD4+ cells increased in IL-12–protected mice between 36 and 72 hours, then decreased by day 4, when CD25 expression increased markedly in GVHD controls.
Effect of IL-12 on donor Th activity.
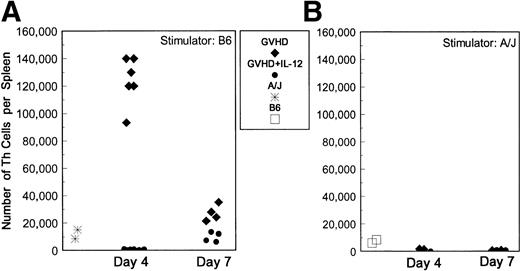
To investigate the possibility that IL-12 inhibits the function of host-reactive donor Th cells, we used limiting dilution analysis (LDA) to quantify these cells in spleens of GVHD control and IL-12–protected mice. This approach is quantitative and allows dilution of cells with suppressive activity,18 which we have found to inhibit T-cell responses in bulk culture at early time points (data not shown). To obtain the best estimate of activated Th present at the time of animal sacrifice, spleen cells from BMT mice were cultured with stimulator cells for only 24 hours before supernatants were obtained, when their ability to induce proliferation of the IL-2– and IL-4–responsive cell line CTLL was assessed. Results of Th LDA are presented in Fig 3, which shows that the total numbers of host-reactive (B6-reactive) Th per spleen were markedly higher in spleens of GVHD control mice than in unprimed A/J mice at day 4. However, the host-reactive Th frequencies and total number of host-reactive Th cells were markedly diminished in IL-12–treated mice compared with GVHD controls (P = .006), and were even lower than those detected for naive A/J mice (Fig 3A). Donor and host T cells were quantified by two-color FCM so that the frequency of host-reactive cells could be expressed as a fraction of the donor T cells present in spleens. Among the reduced numbers of donor CD4+ cells in spleens of IL-12–treated animals on day 4 post-BMT, a markedly lower fraction responded to host antigens compared with CD4+ cells in GVHD control mice (greater than 1 in 25 CD4 cells were host-reactive in GVHD controls v approximately 1 in 1,000 CD4 cells in IL-12–protected mice). Spleens of recipients of TCD B6 (syngeneic) marrow alone (with or without IL-12 treatment) contained undetectably low numbers of host-reactive Th, similar to those of normal B6 mice (data not shown).
Reduced host-reactive Th frequencies in spleens of IL-12–protected mice. Lethally irradiated B6 mice received A/J BMC and spleen cells plus TCD B6 BMC with or without IL-12 on day 0. B6-reactive and A/J-reactive (specificity control) Th frequencies per spleen were determined by limiting dilution analysis on days 4 and 7 after BMT. The total numbers of B6 (host)-reactive (A) and A/J (donor)-reactive (B) Th per spleen, as determined by the product of the Th frequency and the total number of spleen cells obtained from each individual animal, are shown. (⧫) GVHD control mice; (•) IL-12–protected mice; (*) normal A/J mice; (□) normal B6 mice. Results of three experiments are combined.
Reduced host-reactive Th frequencies in spleens of IL-12–protected mice. Lethally irradiated B6 mice received A/J BMC and spleen cells plus TCD B6 BMC with or without IL-12 on day 0. B6-reactive and A/J-reactive (specificity control) Th frequencies per spleen were determined by limiting dilution analysis on days 4 and 7 after BMT. The total numbers of B6 (host)-reactive (A) and A/J (donor)-reactive (B) Th per spleen, as determined by the product of the Th frequency and the total number of spleen cells obtained from each individual animal, are shown. (⧫) GVHD control mice; (•) IL-12–protected mice; (*) normal A/J mice; (□) normal B6 mice. Results of three experiments are combined.
By day 7, total numbers of host-reactive Th and the fraction of donor CD4+ cells reacting to host antigens had increased compared with day 4 in IL-12–treated mice. In the same period, total numbers of host-reactive Th (Fig 3A) and the fraction of donor CD4+cells responding to host antigens decreased markedly in spleens of GVHD control mice. However, both the absolute number of Th per spleen (Fig3A) and the fraction of CD4+ cells reacting to host antigens (approximately 1 in 125 CD4 cells for GVHD controls v1 in 250 to 500 in IL-12–protected mice) remained slightly higher in the GVHD control group than in the IL-12–protected group on day 7.
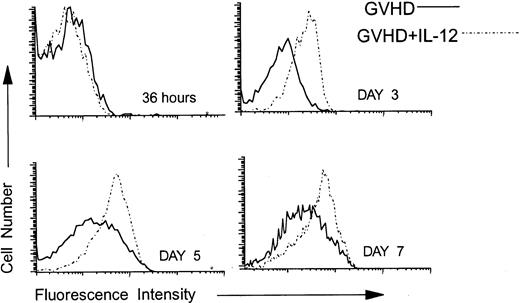
Fas is upregulated on the surface of splenic donor CD4 cells in IL-12–treated mice.
Using three-color FCM analysis, we compared the expression of Fas on gated splenic donor CD4 cells at 36 hours and on days 3, 4, 5, and 7 post-BMT from GVHD control and IL-12–treated mice. As is shown in Fig 4, at 36 hours after BMT, Fas was expressed at low, but detectable, levels on donor CD4 cells in both groups. By contrast, on days 3 (Fig 4), 4 (not shown), and 5 (Fig 4), Fas was upregulated on donor CD4 cells in IL-12–protected mice to a greater extent than in the GVHD control group. By day 7, Fas expression was increased above normal levels (not shown) on donor CD4 cells of both the GVHD control and the IL-12–treated group, but remained highest on those of IL-12–treated mice (Fig 4).
Upregulation of Fas on donor CD4 cells in IL-12–protected mice. Lethally irradiated B6 mice received A/J BMC and spleen cells plus TCD B6 BMC without or with IL-12 on day 0. At 36 hours and on days 3, 5, and 7 post-BMT, spleen cells were analyzed by three-color FCM to examine the expression of Fas on 5F1-negative, ie, donor CD4+ cells. Solid lines represent the Fas density on donor CD4 cells recovered from spleens of GVHD mice and dotted lines represent Fas expression on splenic donor CD4 cells recovered from IL-12–protected mice. Similar results were obtained from six mice per group at each time point in three independent experiments.
Upregulation of Fas on donor CD4 cells in IL-12–protected mice. Lethally irradiated B6 mice received A/J BMC and spleen cells plus TCD B6 BMC without or with IL-12 on day 0. At 36 hours and on days 3, 5, and 7 post-BMT, spleen cells were analyzed by three-color FCM to examine the expression of Fas on 5F1-negative, ie, donor CD4+ cells. Solid lines represent the Fas density on donor CD4 cells recovered from spleens of GVHD mice and dotted lines represent Fas expression on splenic donor CD4 cells recovered from IL-12–protected mice. Similar results were obtained from six mice per group at each time point in three independent experiments.
Role of donor Fas expression in IL-12–mediated GVHD protection.
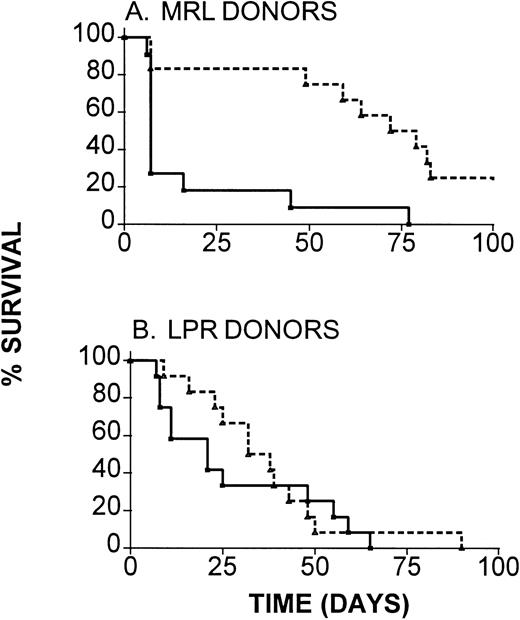
To determine the functional significance of the increase in expression of Fas by donor CD4 T cells in IL-12–protected compared with GVHD control mice, we evaluated the ability of IL-12 to inhibit GVHD when Fas-deficient H-2k LPR donors were used. For comparison, we used Fas-competent H-2k MRL mice as donors to B6 recipients. As is shown in Fig 5A, marked and highly significant (P < .001) prolongation of survival was induced by IL-12 treatment in recipients of wild-type MRL BMC and spleen cells. Although IL-12 treatment led to slight prolongation of survival in recipients of Fas-mutant LPR marrow and spleen cells in the same experiments (Fig 5B), this prolongation did not achieve statistical significance (P = .6 comparing IL-12–treated and control recipients of LPR marrow). A somewhat greater degree of IL-12 protection was detectable when a higher dose of LPR spleen cells was administered (median survival time [MST], 7 days in controlsv 37.5 days in IL-12–treated recipients), but this protection did not achieve statistical significance (P = .2) or approach the level of protection observed with wild-type donors in the same experiment. Thus, IL-12–mediated GVHD protection is at least partially dependent on the ability of donor cells to express functional Fas molecules, suggesting a role for Fas-mediated apoptosis of donor T cells in this phenomenon.
Requirement for donor Fas expression for maximal IL-12–induced GVHD protection. B6 mice received lethal irradiation followed by reconstitution with TCD B6 marrow, along with either (A) wild-type MRL or (B) with Fas-mutant LPR BMC and spleen cells (107). Results of two experiments, both of which produced similar results, are combined (n = 11 to 12 mice per group). (—), GVHD controls; (---), IL-12–treated.
Requirement for donor Fas expression for maximal IL-12–induced GVHD protection. B6 mice received lethal irradiation followed by reconstitution with TCD B6 marrow, along with either (A) wild-type MRL or (B) with Fas-mutant LPR BMC and spleen cells (107). Results of two experiments, both of which produced similar results, are combined (n = 11 to 12 mice per group). (—), GVHD controls; (---), IL-12–treated.
DISCUSSION
A single injection of IL-12 can significantly inhibit acute GVHD in a fully MHC and multiple minor antigen-mismatched, A/J→B10 murine BMT model.7 IL-12 significantly alters the kinetics of donor T-cell activation and expansion in the first week after BMT. By 4 days after BMT, the numbers of allogeneic CD4 and CD8 cells in the spleens of GVHD control mice are approximately two to three times greater than the number administered, increase further on day 5, then decline to approximately one third to one fourth of this level by day 7 post-BMT. This decline in splenic donor T cells could be due to their emigration to GVHD target tissues in this period. Consistent with this possibility, we have observed marked lymphocytic infiltration of the livers and lungs of GVHD control mice on day 7 post-BMT (M.S., P. Nguyen, unpublished data, March 1996). The decline may also reflect apoptotic cell death that can follow T-cell activation,19-22 ie, activation-inducted cell death (AICD).
The expression of the activation-associated markers CD25 and VLA-4 on donor T cells follows a generally similar kinetic pattern as the expansion of donor CD4 cells (Fig 1), as does the level of IFN-γ in the serum.7 All of these parameters peak at 4 to 5 days post-BMT, suggesting that this is the time at which the full extent of T-cell activation has occurred. These patterns are altered by IL-12 treatment in parallel with the observed protection from GVHD. A marked reduction in the number of donor T cells is detected in spleens of IL-12–treated mice compared with GVHD controls on day 47; by day 5, the number of donor T cells increases several-fold, but still remains significantly lower than that in GVHD controls (Fig 1). Between day 5 and day 7 post-BMT, the total numbers of donor T cells remain relatively constant in spleens of IL-12–protected mice, whereas they decrease markedly in spleens of GVHD controls. Most strikingly, IL-12 markedly attenuates the powerful host-specific Th response that is evident in spleens of GVHD mice on day 4. Although total donor T-cell numbers are reduced in IL-12–treated mice at this time point, the fraction of these donor CD4 cells that respond to host antigens is specifically and markedly diminished. In contrast, the frequencies of host-reactive CTL precursors, which are less markedly expanded than host-reactive Th in spleens of GVHD mice, are unaffected by IL-12 treatment (data not shown). Thus, IL-12–induced inhibition of activation, expansion, and cytokine production by donor CD4 cells by day 4 post-BMT may play a critical role in IL-12–induced protection against acute GVHD. This significant inhibitory effect of IL-12 on donor Th activity has also been observed in a haploidentical, haplotype-mismatched strain combination (CBD2F1→B6D2F1), in which even more potent GVHD protection is induced by IL-12 than in the A/J to B6 strain combination used here.23 The increasing numbers of donor CD4 and CD8 cells in the spleens of IL-12–treated mice between days 4 and 7, along with increasing numbers of host-reactive donor Th in the same period and the delayed increase in VLA-4 expression in this group, suggest that a delayed pattern of expansion of allogeneic T cells can result in a delay in the onset of GVHD.
Rather than reduced expansion, an alternative explanation for the early reduction in numbers of donor splenic T cells in IL-12–protected mice is that they redistribute to other tissues. However, histopathologic evaluation of livers and lungs and flow cytometric analysis of lymph node cells on day 4 post-BMT (data not shown) did not support this hypothesis. Thus, we favor the possibility that IL-12 blunts and delays donor T-cell proliferative responses to host antigens.
Although IL-12 can enhance T-cell proliferation,24,25recent studies showed that high doses26,27 of IL-12 administered to mice can lead to depletion of splenic CD4 and CD8 cells and can significantly inhibit virus-induced CD8+ T-cell expansion and CTL activation. These changes were accompanied by induction of TNF-α production.27 Our analyses of sera have not shown differences between GVHD control and IL-12–protected mice in TNF-α levels (data not shown), suggesting that different mechanisms may prevail in each model. Furthermore, in our studies, the reduction in donor CD4 cells in spleens of IL-12–treated animals was much more striking than that reported in the above studies.26 27
In parallel with the markedly reduced level of host-reactive Th expansion, IL-12 treatment in our GVHD model leads to inhibition of GVHD-associated increases in serum IFN-γ levels, and in T-cell expression of the activation markers CD25 and VLA-4. The inhibition of IFN-γ levels probably reflects the ability of IL-12 to inhibit early donor CD4+ T-cell expansion and activation, because the majority of IFN-γ appears to be produced by donor CD4+ T cells in GVHD controls on day 49 (M.S. et al, unpublished data, August 1995). Dallman et al28 have shown decreased expression of IL-2Rα (CD25) and β chain by graft-infiltrating leukocytes from animals rendered tolerant to an allogeneic kidney graft. These cells showed a very poor proliferative response to IL-2, and this altered regulation of the IL-2 pathway may have resulted in tolerance.28 Together, our results show that IL-12 alters the pattern of donor T-cell activation, but does not completely abrogate this process, thus permitting delayed VLA-4 upregulation (Fig 1).
We observed a significant increase in Fas expression on donor CD4 cells in IL-12–protected compared with control mice on days 3 through 7. Most importantly, the data presented in Fig 5 indicate that IL-12–induced GVHD protection is at least partially dependent on the expression of functional Fas molecules by the donor. The role played by IFN-γ in this early Fas-mediated apoptosis is currently under investigation. IL-12 is a potent inducer of IFN-γ production by both natural killer (NK) cells and T cells.29-33In our GVHD model, IL-12 treatment has a biphasic effect on serum IFN-γ levels, causing an early increase on day 2 and 3, followed by the later inhibition described above.7 IFN-γ has been reported to induce T-cell apoptosis by upregulating Fas expression.34 Furthermore, IFN-γ can upregulate Fas expression on tumor cells and increase their sensitivity to FasL-dependent killing.35 Despite the fact that IFN-γ has been implicated in GVHD pathophysiology, exogenous IFN-γ has recently been shown to be capable of inhibiting GVHD.36 Importantly, the early increase in serum IFN-γ production induced by IL-12 treatment plays a critical role in GVHD protection in our model.8 Together, our results lead to the hypothesis that early IFN-γ production induced by IL-12 treatment upregulates donor CD4 cell Fas expression and sensitivity to FasL-mediated killing, that these cells undergo apoptosis by a Fas-dependent pathway, and that the graft-versus-host response, which is CD4-dependent in this strain combination,37 is largely aborted. Consistent with this possibility, preliminary studies have shown that IL-12 increases the number of donor T cells undergoing apoptosis in the first few days after BMT. The source of Fas ligand responsible for this early Fas-mediated apoptosis of donor T cells is unknown. Fas ligand can be expressed by activated CD8 CTL and Th1 CD4 clones,38,39 and it has recently been reported that in vivo IL-12 treatment induces a CD3+CD4−CD8−B220+T-cell population that is capable of Fas ligand-mediated cytolysis of tumor cells.35
In addition to Fas, CD25 and CD69 expression was also increased on donor CD4 cells at 36 and 72 hours post-BMT in spleens of IL-12–protected mice compared with GVHD controls. In vitro studies have shown that IL-12 provides a second signal to Th1 clones to induce IL-2Rα chain expression and proliferation.40 It is possible that the early exposure to IL-12 leads to premature activation of donor CD4+ T cells, as is suggested by their higher expression of these activation markers at early time points. Such premature activation might make these cells susceptible to AICD before they have the opportunity to expand. Indeed, IL-2 can promote T-cell death in response to activation,41 and this AICD is dependent on the expression of the high-affinity IL-2 receptor that requires the α-chain, CD25.41-44 Thus, we hypothesize that IL-12–induced premature activation of donor T cells makes host-reactive donor T cells susceptible to AICD around day 2 to 3 post-BMT. Whether this AICD is mediated directly through the upregulated CD25 molecules is currently unclear. However, signaling through CD25 has been shown to be critical for Fas-mediated apoptosis of T cells activated in vitro.45
Importantly, graft-versus-leukemia effects are preserved in IL-12–protected mice, and, like IL-12–mediated GVHD protection, this GVL effect is largely IFN-γ–dependent.8 Thus, the particular effects of IL-12 on donor T-cell activation and expansion appear to be highly beneficial in that they inhibit GVHD, while preserving the graft-versus-leukemia effects of donor T cells, both of which are mediated, at least in part, by IFN-γ.
In conclusion, IL-12–induced protection against acute GVHD in mice is associated with evidence of premature activation of donor T cells, followed by inhibition of the GVHD-associated activation and expansion of donor T-helper cells that recognize host antigens. These changes may reflect early induction by IL-12 of Fas-mediated apoptosis of host-reactive donor T cells through an IFN-γ–dependent mechanism. Experiments now in progress should help to elucidate the pathway(s) by which this occurs.
ACKNOWLEDGMENT
We thank Drs Henry J. Winn and Michael Seiden for helpful review of the manuscript, Guiling Zhao for outstanding animal husbandry and technical assistance, and Diane Plemenos for expert assistance with the manuscript.
Supported by National Institutes of Health (Bethesda, MD) Grant No. CA64912 and American Cancer Society (Atlanta, GA) Grant No. RPG-95-071-03-CIM.
Address reprint requests to Megan Sykes, MD, Bone Marrow Transplantation Section, Transplantation Biology Research Center, Massachusetts General Hospital, MGH East, Bldg 149-5102, 13th St, Boston, MA 02129.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal