Abstract
T-cell large granular lymphocyte (T-LGL) leukemia is clinically indolent, but is associated with severe neutropenia in approximately 50% of cases. The pathogenesis of the neutropenia is unclear. We report reversal of severe neutropenia associated with T-LGL leukemia in five patients treated with cyclosporine (CSA). All five had persistent neutrophil counts below 0.5 × 109/L, two had agranulocytosis, and four had recurrent infections. Increased populations of LGL were present in blood and marrow, with a T-LGL immunophenotype (CD3+CD8+CD16±CD56±CD57+) shown by multiparameter flow cytometry, and clonal T-cell receptor (TCR) gene rearrangements in two of two pretreatment blood samples studied. CSA was initiated at doses of 1 to 1.5 mg/kg orally every 12 hours, with subsequent dose adjustments based on trough serum levels. Four patients attained normal neutrophil counts with CSA alone; one required addition of low-dose granulocyte-macrophage colony-stimulating factor. Time to attainment of 1.5 × 109/L neutrophils ranged from 21 to 75 days. Attempts to taper and withdraw CSA resulted in recurrent neutropenia. Three patients have maintained normal neutrophil counts on continued CSA therapy for 2, 8, and 8.5 years. Two patients died 1.7 and 4.6 years after initiation of CSA despite normal neutrophil counts—one of metastatic melanoma and one of complications after aortofemoral bypass surgery. Despite resolution of neutropenia, increased populations of T-LGL cells have persisted in all patients during CSA therapy, as shown by morphology and flow cytometry and by the presence of clonal TCR gene rearrangements in four patients' posttreatment blood samples. We conclude that CSA is an effective therapy for neutropenia associated with T-LGL leukemia, and that resolution of neutropenia despite persistence of abnormal cells implies that CSA may inhibit T-LGL secretion of yet unidentified mediators of neutropenia.
LARGE GRANULAR lymphocytes (LGL) are a morphologically distinct subset of lymphocytes which constitute 10% to 15% of normal peripheral blood mononuclear cells.1 LGL include two phenotypically distinct populations of cells, T-cell LGL (T-LGL), which express the T-cell antigen CD3, and natural killer cell LGL (NK-LGL), which lack CD3 expression.2 LGL leukemias are rare but well characterized.1 NK-LGL leukemia presents as an acute systemic illness which pursues a fulminant course. In contrast, T-LGL leukemia is generally clinically indolent. Nevertheless, approximately 50% of patients with T-LGL leukemia have severe neutropenia, which renders them susceptible to potentially lethal infectious complications.1
The pathogenesis of severe neutropenia associated with T-LGL leukemia is unclear, and its treatment has generally been unsatisfactory.1 Therapy with corticosteroids and cytotoxic agents is usually ineffective,3-5 splenectomy does not generally produce sustained increases in neutrophil counts,6 and colony-stimulating factor (CSF) therapy has not yielded consistent results.7-10 Weekly oral low-dose methotrexate was reported to be effective in reversing neutropenia in 6 of 10 patients; LGL counts normalized in 5 of the 6, and abnormal clones identified by T-cell receptor (TCR) gene rearrangements disappeared in 3.11 A response of T-LGL leukemia with severe neutropenia to 2-chlorodeoxyadenosine has also been reported.12
We report the use of cyclosporine (CSA) to successfully treat severe neutropenia associated with T-LGL leukemia in a series of five patients. Our patients have had long-term responses to CSA, but maintenance CSA therapy has been required to sustain responses. Neutrophil counts have normalized despite persistence of increased populations of T-LGL, suggesting that CSA may inhibit T-LGL secretion of yet unidentified mediators of neutropenia. CSA appears to represent effective therapy for neutropenia associated with T-LGL leukemia.
MATERIALS AND METHODS
Patients.
Six patients with T-LGL leukemia were seen at Roswell Park Cancer Institute (Buffalo, NY) between 1989 and 1995. The diagnosis of T-LGL leukemia was established by the presence of increased populations of LGL in the peripheral blood with T-LGL immunophenotypes (see below). One patient had maintained absolute neutrophil counts (ANC) between 0.8 and 1.2 × 109/L for 20 years without any therapy, and has continued to be observed with stable neutrophil counts without therapeutic intervention. The other five patients had persistent ANCs below 0.5 × 109/L. These five patients were treated with CSA.
CSA therapy.
Patients were treated with daily oral CSA (Sandimmune, Sandoz, East Hanover, NJ). CSA was initiated at a dose of 1 to 1.5 mg/kg orally every 12 hours. Doses were gradually increased until ANCs rose above 1.5 × 109/L, while maintaining trough cyclosporine levels in therapeutic range (250 to 400 ng/mL). One patient did not respond to CSA alone, and recombinant human granulocyte-macrophage CSF (GM-CSF; Schering-Plough, Kenilworth, NJ) was added at a dose of 1.5 μg/kg subcutaneously daily. After resolution of neutropenia, CSA doses were tapered to the lowest doses at which therapeutic responses were maintained.
Multiparameter flow cytometry.
Peripheral blood mononuclear cell expression of the CD3, CD8, CD16, CD57, and CD56 antigens was studied by multiparameter flow cytometry13 using the Leu4, Leu2, Leu11, Leu7 (Becton Dickinson, San Jose, CA), and NKH1 (Coulter, Hialeah, FL) monoclonal antibodies. A mononuclear gate was created by gating out granulocytes in the forward versus side scatter display. Populations of T-LGL, defined by coexpression of CD3 and CD57, were measured in pretreatment and posttreatment blood samples. For Patient 4, in whom CD57 was not studied pretherapy and whose T-LGL cells coexpressed CD3 and CD56, CD3+56+ counts were compared in pretherapy and posttherapy samples. To determine a normal range for CD3+CD57+ cells, cells coexpressing these two antigens were measured by multiparameter flow cytometry in peripheral blood samples from 10 normal donors. A normal range of 0.128 ± 0.118 × 109/L (mean ± SD) was established. The normal range for CD3+CD56+ cells in the same 10 donors was 0.098 ± 0.098 × 109/L.
Southern blot analysis.
Rearrangement of the TCR-β subunit gene was studied by Southern blot analysis using a probe for the constant region. Ten-microgram aliquots of genomic DNA extracted from peripheral blood mononuclear cells were digested with Hind III, EcoRI, and BamHI (New England Biolabs, Beverly, MA). Digested DNA was size-separated by electrophoresis in 0.8% agarose gels (Seakem GTG; FMC Bioproducts, Rockland, ME), transferred to Zetabind (Cuno, Meridien, CT), and hybridized with a radiolabeled 400-bp Bgl II/Pst I CTβ genomic probe.14Autoradiograms were interpreted according to Cossman et al.14
Polymerase chain reaction (PCR).
PCR was performed using a panel of 24 TCR-β chain variable region (TCR-Vβ) and generic TCR-β chain joining region (TCR-Jβ) primers.17 TCR-Vβ primers were fluorescently labeled. Amplification reagents included 300 ng of DNA template; 1.5 mmol/L MgCl2; 20 mmol/L Tris-HCl, pH 8.4; 50 mmol/L KCl; 0.2 mmol/L dNTPs; 7% dimethyl sulfoxide; and 0.1 μmol/L of each primer in a total volume of 100 μL, to which 1 U of Taq polymerase (GIBCO, Grand Island, NY) was added. Thermal cycling was performed in a DNA thermal cycler (Perkin-Elmer Cetus, Norwalk, CT) as follows: an initial denaturation for 5 minutes at 94°C, then 1 minute at 94°C, 1 minute at 60°C, and 2 minutes at 72°C for 35 cycles. One microliter of each PCR product was loaded on a 6% denaturant polyacrylamide gel, electrophoresed, and scanned on a Genescanner 373 (Applied Biosystems, Foster City, CA).
RESULTS
Pretreatment clinical data for the five patients with severe neutropenia associated with T-LGL leukemia are shown in Table1. Age at presentation ranged from 45 to 76 years (median, 62 years). Three patients were men and two were women. All five patients had persistent neutrophil counts below 0.5 × 109/L; two of the five had agranulocytosis. Four patients had had recurrent infections, including pneumonia, sinusitis, cutaneous abscesses, and urinary tract infections. One patient (Patient 3) was also anemic, with a hemoglobin level of 8.8 g/dL, a mean corpuscular volume of 101 μ, a low reticulocyte count, and absence of red blood cell antibodies. The other four patients had normal hemoglobin values. All five patients had normal platelet counts. Two patients had splenomegaly at presentation. Two patients had rheumatoid factor; one of the two (Patient 4) had a polyarthritis that was consistent with rheumatoid arthritis, but the other (Patient 5) had minimal symptoms and signs of arthritis.
Pretreatment Clinical Characteristics of Patients With T-LGL Leukemia
| Patient . | Age/Sex . | Neutropenia . | Recurrent Infections . | Anemia . | Splenomegaly . | Arthritis . |
|---|---|---|---|---|---|---|
| 1 | 62/F | + | + | — | + | — |
| 2 | 76/M | + | + | — | — | — |
| 3 | 45/M | + | + | + | — | — |
| 4 | 72/M | + | — | — | + | + |
| 5 | 50/F | + | + | — | — | + |
| Patient . | Age/Sex . | Neutropenia . | Recurrent Infections . | Anemia . | Splenomegaly . | Arthritis . |
|---|---|---|---|---|---|---|
| 1 | 62/F | + | + | — | + | — |
| 2 | 76/M | + | + | — | — | — |
| 3 | 45/M | + | + | + | — | — |
| 4 | 72/M | + | — | — | + | + |
| 5 | 50/F | + | + | — | — | + |
Abbreviations: F, female; M, male.
Pretreatment bone marrow biopsies were hypocellular in three patients, normocellular in one, and hypercellular in one. Granulocytic hypoplasia was present in all cases, with myeloid to erythroid ratios ranging between 1:1 and 1:16. Myeloid maturation was normal, without dysplasia or maturation arrest. Erythroid maturation was also normal in all patients. Patient 3, the patient with anemia, had 63% erythroblasts in his pretreatment marrow, with normal maturation. LGL were seen in marrow aspirates in all cases. Lymphoid nodules were present in four patients' pretreatment bone marrow biopsies.
All five patients had increased populations of LGL shown in peripheral blood smears. Immunophenotyping performed by multiparameter flow cytometry showed T-LGL immunophenotypes in all five cases (Table2). CD3, CD8, and CD57 were coexpressed on T-LGL cells in all five. CD16 was also expressed on the abnormal population in two cases, and CD56 in one.
T-LGL Immunophenotypes
| Patient . | Immunophenotype . |
|---|---|
| 1 | CD3+CD8+CD16−CD56−CD57+ |
| 2 | CD3+CD8+CD16−CD56−CD57+ |
| 3 | CD3+CD8+CD16+CD56−CD57+ |
| 4 | CD3+CD8+CD16−CD56+CD57+ |
| 5 | CD3+CD8+CD16+CD56−CD57+ |
| Patient . | Immunophenotype . |
|---|---|
| 1 | CD3+CD8+CD16−CD56−CD57+ |
| 2 | CD3+CD8+CD16−CD56−CD57+ |
| 3 | CD3+CD8+CD16+CD56−CD57+ |
| 4 | CD3+CD8+CD16−CD56+CD57+ |
| 5 | CD3+CD8+CD16+CD56−CD57+ |
Southern blot analyses of T-cell receptor genes performed on pretreatment blood samples from Patients 3 and 5 showed TCR-β gene rearrangements. PCR analysis showed clonal expansion of the TCR-Vβ24 and TCR-Vβ4 families in Patients 3 and 5, respectively. The other three patients did not have pretreatment material available for study.
Only one patient (Patient 1) had received previous therapy for severe neutropenia associated with T-LGL leukemia before treatment with CSA. Her initial treatment had consisted of splenectomy 6 years previously, with a partial response for 3 years. She had then been treated with prednisone, cyclophosphamide, antithymocyte globulin, plasmapheresis, lithium, and interferon-α, all without response. The other four patients received CSA as their initial therapy.
Responses to CSA therapy are summarized in Table3. Neutrophil counts normalized in all five patients. The time to attainment of ANC ≥ 1.5 × 109/L ranged between 21 and 75 days, with a median of 60 days. CSA doses required to achieve a therapeutic response ranged between 100 and 300 mg every twelve hours. Four patients responded to CSA alone, and one (Patient 3) to the combination of CSA and low-dose GM-CSF. All five patients tolerated cyclosporine therapy well. The only toxicities were mild hypertension in one patient and reversible renal dysfunction in another.
Clinical Response to CSA Therapy
| Patient . | CSA Dose Used to Induce Response (mg every 12 h) . | Days to ANC > 1.5 × 109/L . | Maintenance CSA Dose (mg every 12 h) . | Follow-Up (yr) . | Status . |
|---|---|---|---|---|---|
| 1 | 180 | 75 | 50 | 8.5+ | Alive, nonneutropenic |
| 2 | 100 | 21 | 130 (every other day) | 8.0+ | Alive, nonneutropenic |
| 3 | 300 | 60 | 275 | 1.7 | Died, metastatic melanoma |
| 4 | 100 | 60 | 200/100 (every other day) | 4.6 | Died, postoperative complications |
| 5 | 200 | 30 | 100 | 2.0+ | Alive, nonneutropenic |
| Patient . | CSA Dose Used to Induce Response (mg every 12 h) . | Days to ANC > 1.5 × 109/L . | Maintenance CSA Dose (mg every 12 h) . | Follow-Up (yr) . | Status . |
|---|---|---|---|---|---|
| 1 | 180 | 75 | 50 | 8.5+ | Alive, nonneutropenic |
| 2 | 100 | 21 | 130 (every other day) | 8.0+ | Alive, nonneutropenic |
| 3 | 300 | 60 | 275 | 1.7 | Died, metastatic melanoma |
| 4 | 100 | 60 | 200/100 (every other day) | 4.6 | Died, postoperative complications |
| 5 | 200 | 30 | 100 | 2.0+ | Alive, nonneutropenic |
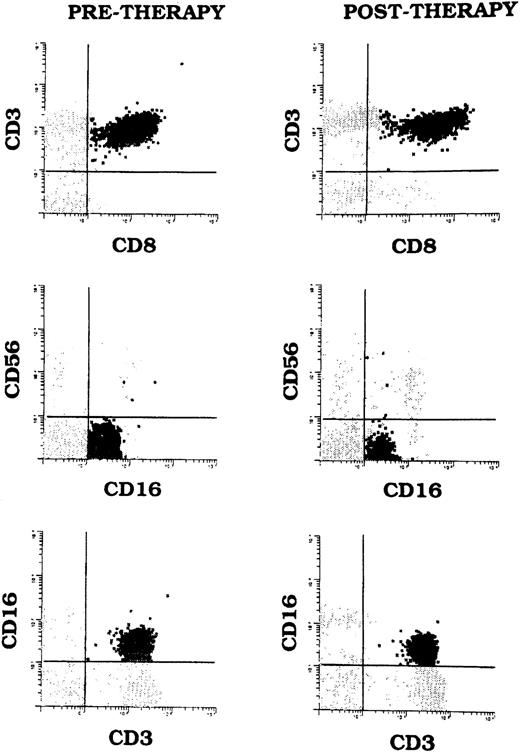
Despite normalization of neutrophil counts, increased populations of T-LGL persisted in all five patients' blood during CSA therapy. Posttreatment peripheral blood smears continued to show increased populations of LGL. Pretreatment and posttreatment T-LGL counts determined by multiparameter flow cytometry are compared in Table4. Post-CSA T-LGL counts were lower than pretreatment counts in two patients (Patients 1 and 5), but were similar to pretreatment counts in the other three. Figure1 shows populations of T-LGL cells demonstrated by multiparameter flow cytometry in Patient 5's blood before initiation of CSA therapy and after response to CSA.
White Blood Cell, Absolute Neutrophil, and LGL Counts Before and After CSA Therapy
| Patient . | WBC Count (×109/L) . | ANC (×109/L) . | LGL Count (×109/L) . | |||
|---|---|---|---|---|---|---|
| Pre . | Post . | Pre . | Post . | Pre . | Post . | |
| 1 | 7.6 | 8.4 | 0 | 2.8 | 2.0 | 0.6 |
| 2 | 2.3 | 3.0 | 0 | 1.3 | 0.6 | 0.5 |
| 3 | 5.2 | 4.8 | 0.1 | 1.7 | 2.1 | 2.4 |
| 4 | 1.4 | 3.2 | 0.1 | 2.2 | 0.3 | 0.5 |
| 5 | 7.2 | 9.3 | 0.4 | 3.3 | 4.7 | 1.7 |
| Patient . | WBC Count (×109/L) . | ANC (×109/L) . | LGL Count (×109/L) . | |||
|---|---|---|---|---|---|---|
| Pre . | Post . | Pre . | Post . | Pre . | Post . | |
| 1 | 7.6 | 8.4 | 0 | 2.8 | 2.0 | 0.6 |
| 2 | 2.3 | 3.0 | 0 | 1.3 | 0.6 | 0.5 |
| 3 | 5.2 | 4.8 | 0.1 | 1.7 | 2.1 | 2.4 |
| 4 | 1.4 | 3.2 | 0.1 | 2.2 | 0.3 | 0.5 |
| 5 | 7.2 | 9.3 | 0.4 | 3.3 | 4.7 | 1.7 |
Abbreviation: WBC, white blood cell.
T-LGL cells (CD3+CD8+CD16+CD56−) shown by multiparameter flow cytometry in Patient 5's peripheral blood before and after CSA therapy. The cells also expressed CD57 (not shown).
T-LGL cells (CD3+CD8+CD16+CD56−) shown by multiparameter flow cytometry in Patient 5's peripheral blood before and after CSA therapy. The cells also expressed CD57 (not shown).
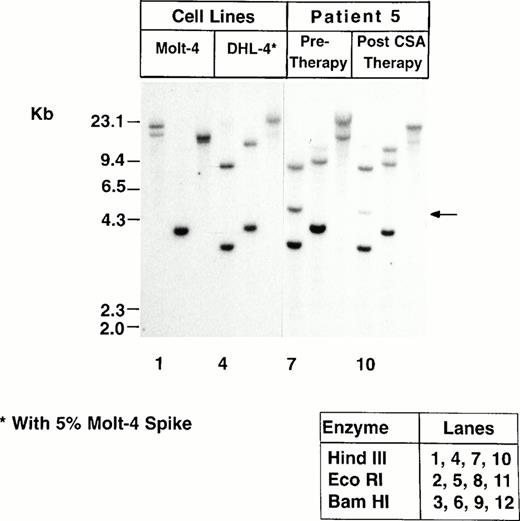
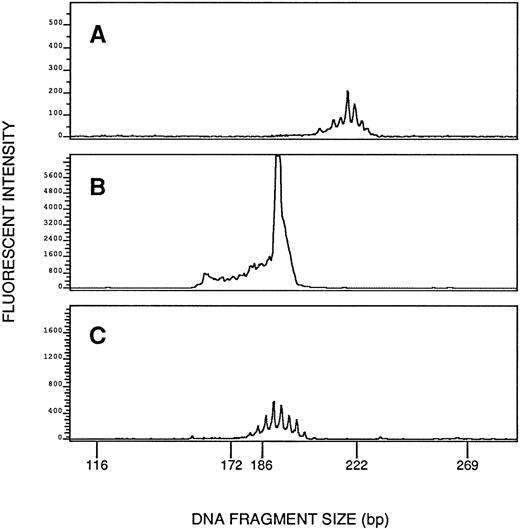
TCR gene rearrangement studies were performed on posttreatment blood samples from all five patients both by Southern blot analysis and by PCR. Clonal TCR-β gene rearrangements were shown in posttreatment samples from four patients both by Southern blot analysis and by PCR. Figure 2 shows TCR-β gene rearrangements demonstrated by Southern blot analysis in Patient 5's peripheral blood cells before and after CSA therapy, and Fig3 shows clonal rearrangement of the TCR-Vβ4 demonstrated by PCR in Patient 5's blood cells after CSA therapy. Patient 1's posttreatment blood cells did not show convincing evidence of a TCR-β gene rearrangement by Southern blot analysis or by PCR, and neither TCR-γ nor TCR-δ gene rearrangement was shown by Southern blot analysis. A pretreatment sample from Patient 1 was not available for study.
TCR-β gene rearrangements shown by Southern blot analysis in Patient 5's peripheral blood cells before and after CSA therapy. The abnormal band is indicated by an arrow. Molt-4 and DHL-4 cells are shown as positive and negative controls, respectively.
TCR-β gene rearrangements shown by Southern blot analysis in Patient 5's peripheral blood cells before and after CSA therapy. The abnormal band is indicated by an arrow. Molt-4 and DHL-4 cells are shown as positive and negative controls, respectively.
Clonal rearrangement of TCR-Vβ4 shown by the PCR in Patient 5's blood cells after cyclosporine therapy (B). Shown as negative controls are Patient 5's blood cells studied with Vβ1 primers (A) and blood cells from a normal donor studied with Vβ4 primers (C). The image in (A) is magnified 60-fold in relation to the images in (B) and (C). (A) and (C) show multiple small peaks produced by polyclonal rearrangements, whereas (B) shows a discrete tall peak produced by a clonal rearrangement and superimposed on a polyclonal background.
Clonal rearrangement of TCR-Vβ4 shown by the PCR in Patient 5's blood cells after cyclosporine therapy (B). Shown as negative controls are Patient 5's blood cells studied with Vβ1 primers (A) and blood cells from a normal donor studied with Vβ4 primers (C). The image in (A) is magnified 60-fold in relation to the images in (B) and (C). (A) and (C) show multiple small peaks produced by polyclonal rearrangements, whereas (B) shows a discrete tall peak produced by a clonal rearrangement and superimposed on a polyclonal background.
Pre-CSA and post-CSA bone marrow aspirate smears and biopsy sections were compared in three patients (Table 5).Although the lymphocyte mass (the percentage of lymphocytes in the bone marrow aspirate smear multiplied by the average cellularity of the bone marrow biopsy) decreased after treatment with CSA, the percentage of lymphocytes remained elevated in all three patients, as did the proportion of lymphocytes with LGL morphology (20% to 60%). All three patients' bone marrow samples showed marked absolute granulocytic hypoplasia before CSA therapy. There was a modest increase in granulocyte mass (the percentage of granulocytes in the bone marrow aspirate smear multiplied by the average cellularity of the bone marrow biopsy) in posttherapy samples, but granulocytic hypoplasia persisted in all three patients. Thus, despite normalization of ANCs, bone marrow samples, like peripheral blood samples, showed evidence of persistent involvement by LGL leukemia.
Bone Marrow Findings Before and After CSA Therapy
| . | Patient 1 . | Patient 2 . | Patient 3 . | |||
|---|---|---|---|---|---|---|
| Pre . | Post . | Pre . | Post . | Pre . | Post . | |
| Cellularity (%) | 80 | 50 | 20 | 70 | 20 | 20 |
| Lymphocytes (%) | 77 | 67 | 29 | 17 | 50 | 24 |
| Lymphocyte mass4-150 | 62 | 34 | 12 | 6 | 10 | 5 |
| Granulocytes (%) | 6 | 14 | 6 | 12 | 4 | 6 |
| Granulocyte mass4-150 | 5 | 7 | 1 | 4 | 1 | 2 |
| Erythroid cells (%) | 10 | 17 | 63 | 25 | 25 | 54 |
| M:E ratio | 1:2 | 1:1 | 1:16 | 1:4 | 1:5 | 1:5 |
| Megakaryocytes4-151 | 1 | 2 | 5 | 10 | 1 | 1 |
| . | Patient 1 . | Patient 2 . | Patient 3 . | |||
|---|---|---|---|---|---|---|
| Pre . | Post . | Pre . | Post . | Pre . | Post . | |
| Cellularity (%) | 80 | 50 | 20 | 70 | 20 | 20 |
| Lymphocytes (%) | 77 | 67 | 29 | 17 | 50 | 24 |
| Lymphocyte mass4-150 | 62 | 34 | 12 | 6 | 10 | 5 |
| Granulocytes (%) | 6 | 14 | 6 | 12 | 4 | 6 |
| Granulocyte mass4-150 | 5 | 7 | 1 | 4 | 1 | 2 |
| Erythroid cells (%) | 10 | 17 | 63 | 25 | 25 | 54 |
| M:E ratio | 1:2 | 1:1 | 1:16 | 1:4 | 1:5 | 1:5 |
| Megakaryocytes4-151 | 1 | 2 | 5 | 10 | 1 | 1 |
Abbreviations: M, myeloid; E, erythroid.
Derived by multiplying the average cellularity of the bone marrow biopsy section by the corresponding cell percentage determined on marrow aspirate smear.
Average per high-power field.
After attainment of responses in all five patients, attempts were made to taper CSA doses to the lowest levels at which therapeutic responses were maintained. The maintenance dose of CSA was lower than the induction dose in all but one patient (Table 3). Although CSA doses could be decreased, attempts to progressively taper and withdraw CSA resulted in recurrent neutropenia. Of note, neutropenia recurred when CSA therapy was tapered and withdrawn in Patient 3, the patient who had required the addition of low-dose GM-CSF to CSA to achieve a response. Recurrence of neutropenia when CSA was withdrawn showed that this patient had in fact responded to CSA as well as GM-CSF.
The duration of follow-up ranges from 1.7 to 8.5 years (median, 4.6 years). Three of the five patients are alive, with normal neutrophil counts. Patient 3 died of metastatic melanoma 1.7 years after initiation of CSA. Patient 4 died of postoperative complications following aorto-femoral bypass surgery 4.6 years after initiation of CSA, despite a normal neutrophil count.
DISCUSSION
We report sustained reversal of neutropenia associated with T-LGL leukemia in five patients treated with CSA. Our report represents the largest series of patients with LGL leukemia treated with CSA and has the longest follow-up. This report highlights the relatively low doses of CSA required to treat this disorder, the favorable toxicity profile of CSA therapy, and the rapid onset of clinical response. We have also shown the persistence of T-LGL cells despite resolution of neutropenia, and the need for ongoing maintenance CSA therapy to sustain responses.
The diagnosis of T-LGL leukemia was established in our patients by morphological demonstration of increased populations of LGL in peripheral blood and by demonstration of the CD3+CD8+CD57+ immunophenotype by multiparameter flow cytometry. LGL counts in normal peripheral blood have been reported as 0.198 ± 0.112 × 109/L,2 0.210 + 0.020 × 109/L,18 and 0.223 + 0.099 × 109/L,19 although higher values of 0.630 ± 0.261 × 109/L in men and 0.350 ± 0.176 × 109/L in women were found in one study.5 Using coexpression of CD3 and CD57 to identify T-LGL, we established a normal range of 0.128 ± 0.118 × 109/L. An LGL count of 2 × 109/L was used as the criterion for diagnosing LGL leukemia in some earlier studies.4,5 It has subsequently been recognized that otherwise typical LGL leukemia patients may have LGL counts below 2 × 109/L.20 The updated criterion for the diagnosis of LGL leukemia is the demonstration of clonal expansion of a population of granular lymphocytes.20Three of our patients had LGL counts of 2 × 109/L or greater. The other two had LGL counts below 2 × 109/L, but had flow cytometric evidence of T-LGL expansion, and also exhibited TCR-β gene rearrangements, albeit in posttreatment samples. Patient 1 in our series had an LGL count of 2 × 109/L and had expansion of CD3+CD8+CD57+cells shown by multiparameter flow cytometry, but did not have convincing evidence of a TCR gene rearrangement in a posttreatment blood sample, despite persistence of increased numbers of T-LGL shown by morphology and flow cytometry. A pretreatment sample was not available for study. The apparent absence of a TCR gene rearrangement in this patient's cells was surprising, but rare cases of otherwise typical T-LGL leukemia without TCR gene rearrangements have been reported.1 Of particular note is a report of a patient with T-LGL leukemia who had a clonal cytogenetic abnormality, but did not have a clonal TCR gene rearrangement.21
Clonal disorders of T-LGL have an indolent clinical course. Neutropenia is generally the most significant clinical problem in these patients. Neutropenia has been reported in approximately 85% of patients, and severe neutropenia (<0.5 × 109/L) is present in approximately 50%.1 Recurrent bacterial infections resulting from severe neutropenia are the presenting feature in the majority of cases. Anemia and thrombocytopenia are less common (approximately 50% and 20% of patients, respectively), and, when present, are generally mild. Rheumatoid arthritis is diagnosed in approximately 30% of patients with LGL leukemia, but its manifestations are generally mild.22 Thus, neutropenia represents a life-threatening complication of what is otherwise an indolent disease, and is the major indication for therapy.3
The mechanism by which severe neutropenia develops in patients with T-LGL leukemia is not well understood.1 Neutropenia does not appear to be caused by marrow infiltration, as the extent of bone marrow infiltration by LGL cells is usually not sufficient to explain the severity of the neutropenia, and neutropenia is more common and more severe than anemia and thrombocytopenia.1 Moreover, neutropenia does not appear to be caused by direct immune suppression. Normal LGL have been shown to suppress GM colony formation,23-25 but this phenomenon has not been observed when LGL cells from patients with LGL leukemia have been cocultured with bone marrow from normal donors,26,27 nor with autologous marrow.28
Immune destruction mediated by granulocyte antibodies may play a role in the neutropenia associated with T-LGL leukemia because anti-granulocyte antibodies are common,27,29 shortened neutrophil survival has been shown,30 and both complement fixation by the IgG fraction29 and antibody-dependent cell-mediated cytotoxicity26 have been observed. Nevertheless, peripheral destruction of granulocytes cannot be the only operative mechanism because granulocytic hypoplasia, as was noted in our patients, is a common finding and anti–neutrophil-reactive IgG persists when neutrophil counts normalize in response to methotrexate therapy.11
LGL from patients with T-LGL leukemia produce a variety of lymphokines which may play a role in the genesis of neutropenia. Production of interferon-γ has been shown, as has inhibition of myeloid colony growth by interferon-γ.31 Interleukin-2 (IL-2) synthesis has been demonstrated in T-LGL cells from patients with T-LGL leukemia, and IL-2–mediated autocrine proliferation has been suggested.32 Finally, tumor necrosis factor-α (TNF-α) synthesis has been shown, and TNF-α synthesis was stimulated by incubation with IL-2.33
Neutropenia may arise by more than one mechanism in patients with T-LGL leukemia. Baker et al34 showed both humoral and cellular suppression of granulopoiesis in a patient with neutropenia associated with a CD3+CD8+CD57+ population. Marrow colony forming unit-GM (CFU-GM) growth was markedly reduced, but normalized after T-cell depletion in the absence of autologous plasma. Addition of either autologous T cells or autologous plasma to cultures caused marked growth inhibition. Humoral suppression of CFU-GM was shown to be mediated by the IgG fraction and seemed to be complement-independent. The patient did not respond to plasmapheresis, aimed at reversing humoral suppression of granulopoiesis, nor to prednisone, cyclophosphamide, or vinblastine therapy, aimed at treating cellular suppression. CSA therapy was initiated based on the idea that it might be effective in inhibiting both cellular immune mechanisms and synthesis of immunoglobulins (see below). Single-agent CSA therapy produced rapid normalization of the neutrophil count, and in vitro studies showed reversal of both humoral and cellular suppression, but only when CSA was present in the culture medium. This in vitro observation suggested the need for ongoing CSA therapy.
CSA is an immunosuppressive agent which inhibits activation of CD4+ lymphocytes, thereby suppressing both cellular and humoral immunity.35 CSA also inhibits expression of the genes coding for IL-2 and the IL-2 receptor, as well as other cytokines. Our use of CSA to treat neutropenia associated with T-LGL leukemia was predicated on these properties, as well as on earlier reports of the successful use of CSA to treat other immunologically mediated cytopenias, including severe aplastic anemia,36pure red cell aplasia,37 and amegakaryocytic thrombocytopenia.38
There are five previous reports of successful CSA therapy of neutropenia associated with LGL leukemia,39-43 including our own 1989 abstract reporting the early results of treatment of Patients 1 and 2 in the present report.38 Three other patients successfully treated with CSA alone have been reported.40-42 Two had previously been unsuccessfully treated with other agents, including prednisone, cyclophosphamide, vincristine, and lithium. All three patients received ongoing CSA therapy after resolution of neutropenia. Numbers of T-LGL were unchanged in one patient, but decreased markedly in the other two. Jakubowski et al43 reported a patient with T-LGL leukemia whose neutropenia, previously unresponsive to prednisone and cyclophosphamide, did not respond to G-CSF alone but responded to the combination of G-CSF and CSA, and who was then able to be receive maintenance therapy consisting of G-CSF alone. T-LGL cells were not detectable in this patient during maintenance therapy. CSA has also been used to successfully treat adult-onset cyclic neutropenia,44 an entity associated with clonal T-LGL proliferation45; increased numbers of T-LGL persisted during maintenance CSA therapy. Of interest also is a report of successful CSA therapy of severe anemia in two patients with T-LGL leukemia.46 As with CSA therapy of neutropenia, expanded populations of T-LGL persisted despite successful therapy of anemia, and maintenance CSA therapy was needed to sustain responses.
The mechanism of response of T-LGL leukemia to CSA is poorly understood. We postulate that CSA reverses neutropenia associated with T-LGL leukemia by inhibiting T-LGL secretion of inhibitory cytokines as well as synthesis of anti-granulocyte antibodies. Based on our experience with unsuccessful withdrawal of CSA therapy and based on in vitro observations,34 it seems that maintenance of normal neutrophil counts is dependent on ongoing CSA therapy, albeit in reduced doses. Of note, increased populations of T-LGL persisted in all of our patients despite resolution of neutropenia. Of interest are the cases in the literature in which T-LGL decreased in number or became undetectable coincident with CSA-induced resolution of neutropenia. CSA likely interrupted an autocrine loop in these cases by inhibiting synthesis of IL-2 and possibly other cytokines. It is unclear whether ongoing CSA therapy is needed in cases in which T-LGL cells become undetectable.
Successful CSA therapy of neutropenia associated with T-LGL leukemia in a series of five patients, reported here, suggests that the response rate to CSA is high. Nevertheless, additional, larger prospective trials are needed to define the true rate of response to CSA in this rare disorder. Additionally, mechanisms of response remain to be defined.
ACKNOWLEDGMENT
The authors thank Dr William Lawrence (Buffalo, NY), Dr Alan Baer (Buffalo, NY), and Dr Loren Rosenbach (Pittsburgh, PA) for referring patients for this study.
Supported in part by National Institutes of Health Grant No. CA73773. P.D.A. is a scholar of the Leukemia Society of America.
Address reprint requests to Maria R. Baer, MD, Division of Medicine, Roswell Park Cancer Institute, Elm and Carlton Sts, Buffalo, NY 14263.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal