Abstract
Ex vivo cytokine production by circulating lymphocytes and monocytes is reduced in patients with infectious or noninfectious systemic inflammatory response syndrome. Very few studies have addressed the reactivity of polymorphonuclear cells (PMN). To analyze further the relative contribution of systemic inflammatory response syndrome alone or in combination with infection we studied the interleukin-8 (IL-8) production by PMN isolated from patients who had undergone cardiac surgery with cardiopulmonary bypass (CPB) and patients with sepsis. Cells were activated with either lipopolysaccharide (LPS) or heat-killed streptococci. Compared with healthy controls, the release of IL-8 by PMN in both groups of patients was significantly reduced whether activated by LPS, independently of its concentration and origin, or by heat-killed streptococci. These observations suggest that stressful conditions related to inflammation, independently of infection, rapidly dampened the reactivity of circulating PMN. We investigated whether the observed diminished reactivity of PMN might reflect an endotoxin tolerance phenomenon. Our in vitro experiments with PMN from healthy controls indicated that PMN could not be rendered tolerant stricto sensu. However, our data suggested that LPS-induced mediators such as IL-10 may be responsible for the observed anergy in patients.
POLYMORPHONUCLEAR neutrophils (PMN) are key cells in inflammatory processes and during sepsis syndrome. Their activation is associated with the release of many inflammatory mediators such as eicosanoids, free radicals, and proteolytic enzymes. Activated neutrophils display an upregulated expression of CD11b and CD351 and bind to endothelial cells, contributing to vascular endothelium damage.2,3 They migrate toward tissues where they maintain inflammation and favor organ dysfunction,4-6 which may lead to lethality.7The reactivity of neutrophils is modulated by many cytokines. Pro-inflammatory cytokines such as interleukin-1 (IL-1), IL-8, and tumor necrosis factor (TNF) are potent activators of PMN functions. The fact that IL-8 is one of the main cytokines produced by PMN8-10 allows these cells to perpetuate their own activation within an autocrine loop. Anti-inflammatory cytokines play a major role in dampening PMN functions, including IL-8 production. We and others have shown that IL-10 and, to a lesser degree, IL-4, IL-13, and transforming growth factor-β (TGF-β), can repress IL-8 release by activated PMN.11-14 However, the nature of the triggering signal influences the capacity of anti-inflammatory cytokines to downmodulate cytokine release.14
During the course of inflammation, both pro- and anti-inflammatory cytokines, rapidly released with excessive production, can be detected in the blood compartment15 where they modulate the reactivity of circulating leukocytes. Indeed, we have previously shown that monocytes isolated from septic patients have a reduced capacity to produce IL-1α, IL-1β, IL-6, and TNF-α upon activation by endotoxins (lipopolysaccharide [LPS])16 or heat-killed Gram-positive bacteria.17 This observation has been confirmed by others with whole blood assays of the above cytokines18,19 and extended to IL-10,20 IL-12, and interferon-γ (IFN-γ),21 but not to IL-1 receptor antagonist (IL-1ra) production.22 These observations probably follow the events occurring in the blood compartment, and experiments with whole blood take into account the plasma environment, which has been shown to play a major role in deactivating circulating leukocytes.23 However, the source of cytokines cannot be analyzed in whole blood assays. So far, McCall et al24 have been the only group to our knowledge to investigate the effect of sepsis on PMN behavior in terms of cytokine production. They reported a decreased capacity of neutrophils to produce IL-1β upon stimulation in ex vivo experiments. The investigators suggested that the observation was linked to the well-known endotoxin tolerance phenomenon. However, endotoxin tolerance is not initiated only by LPS nor is it restricted to the LPS-responsiveness.25
Because IL-8 is the most abundant cytokine produced by neutrophils, we were interested in analyzing the capacity of PMN from septic patients to produce this specific chemokine. It was of interest to compare the observable modifications in septic patients consecutive to the infectious process itself and those associated with the inflammatory reaction, independently of any microbial stimulus. Indeed, we had previously shown that inflammation after surgery was sufficient to reduce the capacity of monocytes to produce TNF-α, IL-1α, and IL-1β.26 For that purpose we investigated a group of patients who had undergone cardiac surgery associated with cardiopulmonary bypass (CPB). It is well known that, although surgery itself is an inflammatory process, the extracellular circulation is associated with an enhanced inflammatory reaction as a consequence of the interaction of blood with bio-materials.27 Finally, we attempted to mimic some of the events occurring in vivo to find out whether endotoxin and/or IL-10 could be responsible for the modifications we observed in both groups of patients.
MATERIALS AND METHODS
Patients.
Eleven patients with sepsis syndrome, as defined by Bone et al,28 were studied upon admission to intensive care units (ICU) or at initiation of their sepsis syndrome. The study included 9 men and 2 women, the average age being 49 ± 5 years (range, 21 to 69 years) and the mean simplified acute physiology score II (SAPS II) being 49 ± 7. There were 8 cases of pneumonia, 1 of pelvic abscess, and 2 of peritonitis, including 5 in septic shock. The outcome was survival for 4 patients. The patients' characteristics are given in Table 1.
Patients' Characteristics
| Patients . | Age . | Sex . | Etiology . | Bacteria . | Shock . | SAPS II . | Outcome . |
|---|---|---|---|---|---|---|---|
| DIA | 55 | M | Pneumopathy | E coli | + | 50 | Nonsurvival |
| LEG | 69 | M | Peritonitis | E coli + E faecalis | + | 74 | Nonsurvival |
| PIC | 21 | M | Peritonitis | E coli + E faecalis | 0 | 21 | Survival |
| COU | 67 | M | Pneumopathy | Aspergillus | + | 81 | Nonsurvival |
| TRU | 65 | F | Pneumopathy | E cloacae | 0 | 43 | Nonsurvival |
| ELK | 59 | F | Pelvis abscess | Streptococcus | 0 | 21 | Survival |
| AST | 34 | M | Pneumopathy | Staph aureus | + | 48 | Survival |
| MEB | 50 | M | Pneumopathy | Staph aureus | 0 | 50 | Nonsurvival |
| KHE | 60 | M | Pneumopathy | Not found | 0 | 87 | Nonsurvival |
| BEN | 24 | M | Pneumopathy | Legionellae | + | 22 | Nonsurvival |
| DUB | 31 | M | Pneumopathy | H influenzae + S pneumoniae | 0 | 38 | Survival |
| Patients . | Age . | Sex . | Etiology . | Bacteria . | Shock . | SAPS II . | Outcome . |
|---|---|---|---|---|---|---|---|
| DIA | 55 | M | Pneumopathy | E coli | + | 50 | Nonsurvival |
| LEG | 69 | M | Peritonitis | E coli + E faecalis | + | 74 | Nonsurvival |
| PIC | 21 | M | Peritonitis | E coli + E faecalis | 0 | 21 | Survival |
| COU | 67 | M | Pneumopathy | Aspergillus | + | 81 | Nonsurvival |
| TRU | 65 | F | Pneumopathy | E cloacae | 0 | 43 | Nonsurvival |
| ELK | 59 | F | Pelvis abscess | Streptococcus | 0 | 21 | Survival |
| AST | 34 | M | Pneumopathy | Staph aureus | + | 48 | Survival |
| MEB | 50 | M | Pneumopathy | Staph aureus | 0 | 50 | Nonsurvival |
| KHE | 60 | M | Pneumopathy | Not found | 0 | 87 | Nonsurvival |
| BEN | 24 | M | Pneumopathy | Legionellae | + | 22 | Nonsurvival |
| DUB | 31 | M | Pneumopathy | H influenzae + S pneumoniae | 0 | 38 | Survival |
Nine patients undergoing cardiopulmonary bypass for selective artery bypass grafting were studied. The study included 8 men and 1 woman, the average age being 68 ± 2 years (range, 63 to 74 years). Anesthesia induction and maintenance was achieved using flunitrazepam, sufentanyl, pancuronium bromide, and isoflurane. Administration of heparin before canulation and subsequent neutralization after bypass with protamine sulfate were performed in a standard fashion. Normothermic CPB was performed (32.5 ± 0.4°C). The mean timing of aortic clamping was 55 ± 2 minutes. Blood sampling was performed 1½ hours after CPB beginning and always before protamine administration.
Blood of healthy controls were obtained from 18 subjects either from the blood bank (Fondation Nationale de Transfusion Sanguine) or from the laboratory. The study included 7 men and 11 women, the average age being 32 ± 2 years (range, 24 to 51 years).
Isolation of human polymorphonuclear cells.
Blood was drawn onto heparin (20 IU/mL) from healthy volunteers or from patients. Ten volumes of blood was mixed with 2 vol of glucose dextran (3% glucose; 3% dextran T250; Pharmacia, Uppsala, Sweden) and the leukocytes were recovered after a 40-minute sedimentation at room temperature.29 The leukocytes were then diluted 1:2 in RPMI-1640 medium and layered on Ficoll-Hypaque (Milieu de Séparation des Lymphocytes [MSL]; Eurobio, Les Ulis, France). The ratio was 2 vol of leukocytes to 1 vol of MSL. After centrifugation for 25 minutes at 15°C and 500g, the cell pellet was washed and centrifuged once for 5 minutes at 300g. Contaminating erythrocytes were lysed after a 5-minute incubation of the cell pellet at 4°C by resuspension in 5 mL of lysis buffer (NH4Cl = 8.32 g/L; NaHCO3 = 0.84 g/L; Na4EDTA = 43.2 mg/L). Lysis was stopped by adding a large excess of RPMI-1640 medium (Glutamax; GIBCO Life Technologies, Paisley, UK) and the cells were washed and centrifuged for 10 minutes at 200g. The viability of polymorphonuclear cells (PMN) was assessed by counting the cells in 0.1% eosine. A nonspecific esterase staining was performed to evaluate the monocyte contamination, which never exceeded 0.5%.
In vitro culture.
PMN cells were cultured in RPMI-1640 medium (Glutamax; GIBCO Life Technologies) supplemented with antibiotics (penicillin, 100 IU/mL; streptomycin, 100 μg/mL) and 5% heat-inactivated normal human serum (a pool of sera from healthy volunteers). 0.5-mL aliquots of PMN suspension per well were incubated in a 5% CO2 incubator in 24-well multidish plates (Costar, Cambridge, MA) for 24 hours at 37°C. Stimuli in volumes ≤10 μL were added at the beginning of the culture. At the end of the culture, the supernatants were obtained, centrifuged for 10 minutes at 300g and 15°C, and kept at −20°C before cytokine assessments.
Reagents.
Escherichia coli (0111:B4) lipopolysaccharide was purchased from Sigma (St Louis, MO), TNF-α was obtained from Rhône Poulenc (Vitry/Seine, France), and Neisseria meningitidis LPS was the generous gift of Dr Martine Caroff (Institut de Biochimie, Orsay, France). Heat-killed Streptococcus pyogenes group A (A78) was a kind gift of Dr H. Müller-Alouf (Institut Pasteur de Lille). Recombinant human IL-10 was a generous gift of Dr J. Abrams (DNAX, Palo Alto, CA).
Two-step experiments.
PMN (2 × 106 cells/mL) were cultured in RPMI-1640 medium (Glutamax; GIBCO Life Technologies) supplemented with antibiotics and 5% heat-inactivated normal human serum (complete medium). A 0.5-mL aliquot of PMN suspension per well in 24-well multidish plates was incubated for 24 hours at 37°C in a 5% CO2 incubator in the presence of different reagents (“pretraitment period”). At the end of this preculture period, the supernatants were obtained and 0.25 mL of fresh complete medium was added to each well to preserve adherent neutrophils. Supernatants were centrifuged for 10 minutes at 200g and 15°C and kept at −20°C before IL-8 assessment. The cell pellets were resuspended in 0.25 mL of fresh complete medium and plated in the same well as during the first 24 hours. Then, multidish plates were incubated at 37°C for an additional 24 hours of culture in the presence of various activators (“activation period”). At the end of the activation period, the supernatants were obtained, centrifuged 10 minutes at 300g and 15°C, and kept at −20°C before IL-8 assessment.
Assessement of cell-associated forms of IL-8 in whole-blood samples.
One milliliter of blood was centrifuged for 10 minutes at 500g.Plasma was obtained and kept at −20°C before cytokine assessments. The cell pellet was lysed in 100 μL of lysis buffer (TRAx buffer; T-Cell Sciences, Inc, Needham, MA) before addition of RPMI-1640 medium to achieve a 1-mL total volume.
Assessement of cell-associated forms of IL-8 in PMN.
At the end of the culture period (2 × 106 PMN/mL), the PMN pellets were lysed by adding 100 μL of TRAx lysis buffer before adding 100 μL of diluent buffer and 300 μL of RPMI medium.
Cytokine enzyme-linked immunosorbent assay (ELISA).
IL-8 ELISA was performed as previously described30 using a monoclonal anti-human IL-8 antibody obtained by Dr J-C. Mazié(Hybridolab, Institut Pasteur) and a rabbit polyclonal anti–IL-8 antibody graciously provided by Dr N. Vita (Sanofi Recherche, Labège, France). The sensitivity of the ELISA was 3 pg/mL. In collaboration with Dr S. Berthold (DPC Biermann, Bad Nauheim, Germany) the values obtained with our ELISA were compared with those obtained for the same samples using the chemiluminescent quantification of IL-8 (IMMULITE; DPC Biermann). A correlation of r = .98 (P = .0001) was achieved. We ensured that the TRAx buffer did not interfere with the accuracy of the ELISA.
Apoptosis assay.
PMN apoptosis was assessed as the percent of cells with hypodiploid DNA by using the technique described by Nicoletti et al.31After 24 or 48 hours of culture of PMN with or without activators, cells were centrifuged at 200g for 10 minutes and washed in phosphate-buffered saline (PBS). The cell pellets were gently resuspended in hypotonic fluorochrome solution (50 μg/mL propidium iodide [PI], 0.1% sodium citrate, 0.1% Triton X-100) and stored at 4°C in the dark overnight before the flow-cytometric analysis using a FACScan flow cytometer (Becton Dickinson Immunocytometry System, San Jose, CA). The red fluorescence of PI in individual nuclei and the forward and side scatter were simultaneously measured. Cell debris were excluded from acquisition by raising the forward scatter threshold. Apoptotic nuclei were easily distinguishable from residual debris by the high size side scatter value due to the condensation of nuclear chromatin. Ten thousand events were collected and analyzed using the software Lysis II program. Apoptotic PMN nuclei were distinguished by their hypodiploid DNA content from the diploid DNA content of normal PMN nuclei.
Statistical analysis.
Statistical analyses were performed using the nonparametric Mann-Whitney U-test for comparing data between healthy controls and patients and the Wilcoxon signed-rank test for the two-step experiments performed with the PMN from healthy controls.
RESULTS
Circulating and cell-associated IL-8.
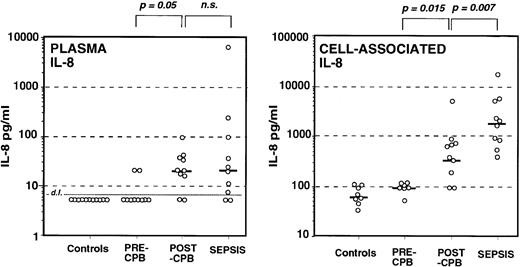
Levels of circulating IL-8 were under the detection limit in controls and in all but two patients undergoing cardiac surgery before cardiopulmonary bypass (Fig 1), whereas after cardiopulmonary bypass all patients but two had detectable levels of plasma IL-8. Similarly, IL-8 was measurable in all but two septic patients. The highest values were found among septic patients but the mean levels between post-CPB and sepsis were not significantly different.
Measurement of circulating IL-8 in plasma (left) of healthy controls, patients undergoing cardiac surgery before (pre-CPB), and after (post-CPB) cardiopulmonary bypass and patients with sepsis. Cell-associated IL-8 was assessed in whole-blood cell pellet of the same groups of donors (right). Each symbol represents an individual subject and the thick short lines represent the median value for each group.
Measurement of circulating IL-8 in plasma (left) of healthy controls, patients undergoing cardiac surgery before (pre-CPB), and after (post-CPB) cardiopulmonary bypass and patients with sepsis. Cell-associated IL-8 was assessed in whole-blood cell pellet of the same groups of donors (right). Each symbol represents an individual subject and the thick short lines represent the median value for each group.
The pattern was quite different for cell-associated IL-8. We have previously shown that cell-associated IL-8 assessed in whole blood is essentially associated with leukocytes whereas the contribution of red blood cells is limited.32 Naturally occurring cell-associated IL-8 was detected in healthy controls, and levels were similar in pre-CPB patients. Post-CPB patients had significantly higher levels than those measured before CPB (P = .015). The levels of cell-associated IL-8 in septic patients were also higher than in controls as well as higher than in post-CPB patients (P = .007). Although circulating levels of IL-8 were similar in infectious and noninfectious inflammation, the levels of cell-associated IL-8 were not.
IL-8 production by isolated PMN.
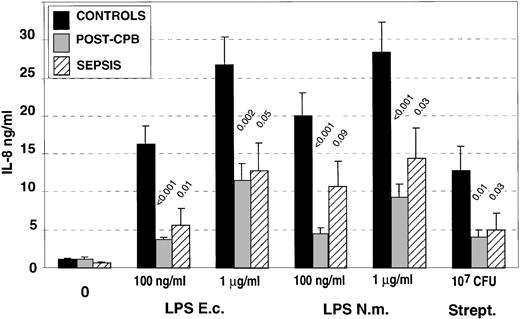
We studied the capacity of isolated circulating PMN to release IL-8 in response to either 0.1 or 1 μg/mL of LPS of two different bacterial origins or heat-killed streptococci (Fig2). A significant reduction in the levels of IL-8 produced in response to the different concentrations of LPS from different origins and to heat-killed streptococci was observed in both groups of patients. Sepsis or infectious systemic inflammatory response syndrome as well as noninfectious inflammation both led to a reduced capacity of PMN to release IL-8 upon in vitro stimulation. IL-8 production in patients undergoing cardiac surgery was also tested in a few patients before the initiation of the cardiopulmonary bypass, and the levels of released IL-8 were similar to those obtained in healthy controls (data not shown).
IL-8 production by isolated polymorphonuclear cells (PMN) after in vitro activation with LPS from different origins (Escherichia coli: E.c.; Neisseria meningitidis: N.m.) or with heat-killed, group A streptococcus. The data are the mean of experiments performed with isolated PMN from healthy controls (n = 13), post-CPB patients (n = 9), and patients with sepsis (n = 11). P values correspond to statistical analysis between post-CPB or sepsis groups versus control one.
IL-8 production by isolated polymorphonuclear cells (PMN) after in vitro activation with LPS from different origins (Escherichia coli: E.c.; Neisseria meningitidis: N.m.) or with heat-killed, group A streptococcus. The data are the mean of experiments performed with isolated PMN from healthy controls (n = 13), post-CPB patients (n = 9), and patients with sepsis (n = 11). P values correspond to statistical analysis between post-CPB or sepsis groups versus control one.
After cell activation, the levels of IL-8 were also analyzed at the cellular levels, ie, following cell lysis of PMN (Table 2). There was no difference between the levels of cell-associated IL-8 in septic and control groups whereas in most cases there was a significant reduction of the levels found in post-CPB patients.
Levels of PMN-Associated IL-8 (pg/mL) After Ex Vivo Activation
| Activators . | Controls (n = 13) . | Sepsis (n = 10) . | Post-CPB (n = 9) . |
|---|---|---|---|
| None | 3,621 ± 1,125 | 3,056 ± 1,121 | 2,534 ± 464 |
| LPS E.c. 0.1 μg/mL | 12,133 ± 1,681 | 12,225 ± 4,657 | 6,298 ± 740* |
| 1 μg/mL | 16,501 ± 2,861 | 18,164 ± 5,884 | 11,263 ± 1,181 |
| LPS N.m. 0.1 μg/mL | 13,154 ± 2,074 | 14,252 ± 5,195 | 6,222 ± 830* |
| 1 μg/mL | 19,559 ± 3,822 | 17,589 ± 5,852 | 9,605 ± 801* |
| Streptococcus 107 CFU | 11,743 ± 1,770 | 8,231 ± 2,252 | 6,024 ± 990* |
| Activators . | Controls (n = 13) . | Sepsis (n = 10) . | Post-CPB (n = 9) . |
|---|---|---|---|
| None | 3,621 ± 1,125 | 3,056 ± 1,121 | 2,534 ± 464 |
| LPS E.c. 0.1 μg/mL | 12,133 ± 1,681 | 12,225 ± 4,657 | 6,298 ± 740* |
| 1 μg/mL | 16,501 ± 2,861 | 18,164 ± 5,884 | 11,263 ± 1,181 |
| LPS N.m. 0.1 μg/mL | 13,154 ± 2,074 | 14,252 ± 5,195 | 6,222 ± 830* |
| 1 μg/mL | 19,559 ± 3,822 | 17,589 ± 5,852 | 9,605 ± 801* |
| Streptococcus 107 CFU | 11,743 ± 1,770 | 8,231 ± 2,252 | 6,024 ± 990* |
*P < .05 v control.
In vitro modulation of IL-8 production by PMN from healthy donors.
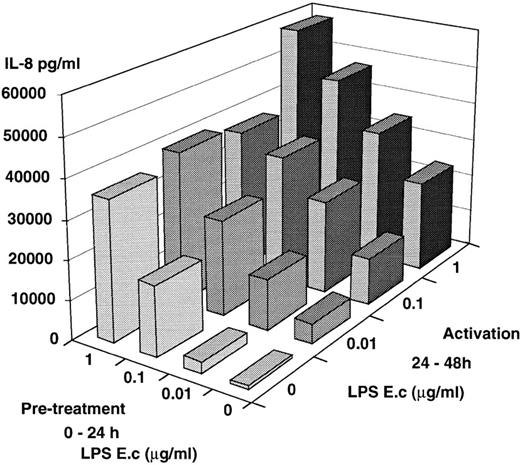
The reduced levels of IL-8 release by PMN isolated from septic patients or patients who underwent cardiopulmonary bypass could be the consequence of in vivo exposure of PMN to endotoxin and/or to IL-10. We attempted to mimic this situation by preculturing PMN from healthy donors in the presence of LPS, IL-10, or both, before further activation by LPS. Numerous investigators have reported a high percent of apoptotic PMN after a 24-hour culture period.33,34However, our PMN preparations (dextran + glucose sedimentation and a Ficoll-Hypaque [MSL] step) and our culture conditions in the presence of normal human serum led to a low percentage of apoptotic cells after a 24-hour culture period. Recovery of live cells after an overnight culture period was 89% ± 14% with 15% ± 2% and 9% ± 2% apoptotic cells in the absence or in the presence of LPS, respectively. After 48 hours the recovery was 46% ± 15% live cells and 35% ± 11% apoptotic cells. Thus, it was possible to perform the two-step experiments over 48 hours. The mean values of four experiments are shown in Fig 3: PMN maintained for 24 hours in culture medium alone were fully responsive to LPS during an additional 24-hour culture period and release significant amounts of IL-8 in a dose-dependent fashion, illustrating the good viability of the cells. Such IL-8 production (24 to 48 hours) also occurred when the cells had been precultured in the presence of increasing amounts of LPS and then left in cultured medium alone. LPS-pretreated cells responded in an additional fashion when reexposed to LPS. Similarly, LPS-pretreated PMN had an enhanced IL-8 production upon a further challenge by recombinant human TNF (10 ng/mL) compared with cells maintained for 24 hours in culture medium alone (8,631 ± 3,880v 3,293 ± 925, P = .04; mean of five experiments). Thus, in contrast to monocytes which upon preexposure to LPS are “tolerized” and have a lower capacity to release cytokines,35 PMN were not rendered tolerant to LPS by prior encounter with LPS. To see whether this effect was specific for LPS or could be obtained with other activating signals, PMN were first cultured in the presence of TNF, and further cultured in the presence of LPS (Table 3). TNF pretreated PMN continue to release IL-8 once TNF has been withdrawn. Addition of LPS acted synergistically and led to an enhanced release of IL-8, while a second TNF stimulation had an insignificant enhancing effect.
In vitro model of tolerization. PMN from healthy donors were first cultured over 24 hours in the presence or absence of increasing amounts of E coli LPS. Supernatants were obtained and fresh medium was added to the cells. PMN were further cultured for an additional 24 hours (24-48 h) in the presence or absence of increasing amounts of E coli. IL-8 was measured in the supernatants. The results are the mean of four different experiments; the mean of SEM was ±30%.
In vitro model of tolerization. PMN from healthy donors were first cultured over 24 hours in the presence or absence of increasing amounts of E coli LPS. Supernatants were obtained and fresh medium was added to the cells. PMN were further cultured for an additional 24 hours (24-48 h) in the presence or absence of increasing amounts of E coli. IL-8 was measured in the supernatants. The results are the mean of four different experiments; the mean of SEM was ±30%.
Priming Effect of TNF on IL-8 Production (pg/mL) by Human PMN
| 0-24 h Culture . | 24-48 h Culture . | ||
|---|---|---|---|
| None . | LPS E.c. (1 μg/mL) . | TNF (10 ng/mL) . | |
| None | 566 ± 83 | 7,773 ± 4,147 | 3,293 ± 925 |
| P = .04 | NS | ||
| TNF (10 ng/mL) | 4,237 ± 1,954 | 26,316 ± 11,576 | 5,682 ± 3,011 |
| 0-24 h Culture . | 24-48 h Culture . | ||
|---|---|---|---|
| None . | LPS E.c. (1 μg/mL) . | TNF (10 ng/mL) . | |
| None | 566 ± 83 | 7,773 ± 4,147 | 3,293 ± 925 |
| P = .04 | NS | ||
| TNF (10 ng/mL) | 4,237 ± 1,954 | 26,316 ± 11,576 | 5,682 ± 3,011 |
Mean ± SEM of five different experiments.
Abbreviation: NS, not significant.
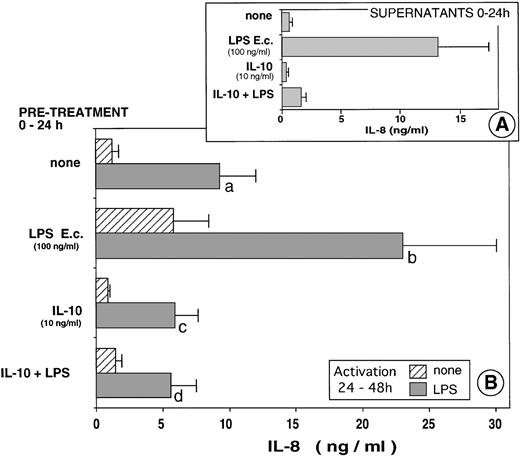
To further understand the mechanism leading to the PMN anergy we observed in the patients, we analyzed the effects of IL-10, known to circulate in these patients. As shown in Fig 4A, simultaneous addition of IL-10 and LPS led to an 88% inhibition of IL-8 production by PMN, confirming numerous previous studies, including ours.11 14 Pretreatment of PMN with IL-10 render the cells less reactive to a subsequent stimulation with LPS (P = .02) (Fig 4B). Furthermore, pretreatment of PMN with IL-10 abolished the priming effect of LPS to a further activation by LPS (P = .01).
In vitro model of tolerization. PMN from healthy donors were first cultured over 24 hours in the presence or absence of eitherE coli LPS (100 ng/mL) or recombinant human IL-10 (10 ng/mL) or both. Supernatants were obtained and IL-8 was measured (A). Fresh medium was added to the cells and the PMN were further cultured for an additional 24-hour period (24-48 h) in the presence or absence of E coli LPS (1 μg/mL). IL-8 was measured in the supernatants (B). The results are the mean of eight different experiments. (b va: P = .01; c v a: P = .02; d va: P = .01; d v b: P = .01.)
In vitro model of tolerization. PMN from healthy donors were first cultured over 24 hours in the presence or absence of eitherE coli LPS (100 ng/mL) or recombinant human IL-10 (10 ng/mL) or both. Supernatants were obtained and IL-8 was measured (A). Fresh medium was added to the cells and the PMN were further cultured for an additional 24-hour period (24-48 h) in the presence or absence of E coli LPS (1 μg/mL). IL-8 was measured in the supernatants (B). The results are the mean of eight different experiments. (b va: P = .01; c v a: P = .02; d va: P = .01; d v b: P = .01.)
DISCUSSION
Circulating and cell-associated IL-8.
Systemic inflammatory response syndrome of infectious (eg, sepsis syndrome) or noninfectious (eg, cardiopulmonary bypass surgery) origin can both be associated with the detection of circulating cytokines. Although the plasma levels of IL-8 could not discriminate between the two types of inflammation, significantly higher levels of cell-associated IL-8 were measured in septic patients (Fig 1). An enhanced cell-associated IL-8 was found in patients after cardiac surgery involving CPB as compared to pre-CPB in agreement with a previous report.36 We have previously shown that a high proportion of IL-8 in the blood compartment was associated with erythrocytes and mainly with mononuclear and polymorphonuclear cells.32 A major proportion of the cell-associated IL-8 found with leukocytes was the result of internalization of surrounding IL-8. Because post-CPB and septic patients had similar levels of circulating PMN (data not shown), the relative number of circulating cells may have had little influence on the lower level of cell-associated IL-8 observed in the CPB group. These results may rather reflect lower IL-8 receptor expression and IL-8 internalization process in this group of patients. The analysis of cell-associated IL-8 after in vitro activation which led to a diminished level of PMN-associated IL-8 among CPB patients as compared with healthy controls (Table 2) further argues for a lower IL-8 receptor expression. Indeed, C5a generated during extracorporal circulation and IL-8 itself known to modulate IL-8 receptor expression37 38 may be the causative agents which led to low IL-8 receptor expression. Further experiments will test it.
Among the septic patients, the reduced levels of IL-8 in PMN supernatants was not associated with reduced levels of the cell-associated form as compared with healthy donors. We can speculate that the receptor-mediated trapping of the IL-8 released by PMN upon activation and its internalization was achieved with a certain amount of IL-8, a level of which was reached in both healthy and septic patients. The PMN of healthy donors do produce more IL-8 than those from septic patients and the difference could only be found in the PMN supernatants.
Sepsis and reduced cytokine production/PMN hyporeactivity.
Whole-blood assays have been widely used these last years to investigate the capacity of circulating leukocytes to produce cytokines within their local environment. A reduced capacity was observed when IL-1, TNF-α, IL-6, IL-10, IL-12, and IFN-γ were investigated.18-22,39 40 We also studied the capacity of whole-blood samples to generate IL-8 upon in vitro activation. Low concentrations of endotoxins, independently of their origin, allow the detection of a diminished capacity to produce IL-8 in septic patients compared with healthy controls. On the contrary, when higher amounts of LPS or heat-killed streptococci were used, no significant changes were observed. In CPB patients the capacity to produce IL-8 was unchanged as compared to controls (data not shown).
Experiments performed with whole-blood assays give no information about the specific reactivity of a given cell lineage. We have previously investigated the capacity of monocytes from septic patients to be activated.16,17 Because IL-8 is mainly produced by both monocytes and neutrophils within the blood compartment we decided to analyze the PMN reactivity. Indeed, very few studies have investigated the production of cytokines by neutrophils from septic patients and we showed that, as previously reported for IL-1β,24circulating PMN have a significantly reduced capacity to release IL-8. In contrast to the data reported on IL-1β, our observation was made independently of the nature of the triggering signal. Endotoxins from various origins used at different concentrations as well as heat-killed streptococci led to a reduced production of IL-8 compared with healthy subjects.
Although PMN have been claimed to exist in one of three states (quiescent, primed, or activated),41 it is clear that a fourth state, ie, deactivated, exists. Indeed, other PMN functions have been reported to be reduced in infected patients such as the protein kinase C–dependent stimulation of superoxide production.42In rabbits, in vitro complement (C5)-dependent degranulation of PMN was reduced after intravenous injection of LPS,43 and in human volunteers administration of endotoxin leads to a markedly reduced ex vivo neutrophil chemotactic activity.44 Accordingly, in in vivo experiments, LPS-pretreated animals showed a diminished recruitment of PMN into tissues after local challenge.43,45Deactivation of PMN has also been reported in noninfectious pathologies. For example, Parsons et al46 reported that PMN from patients with adult respiratory distress syndrome (ARDS) produced less superoxide than did cells from normal subjects when primed with LPS and stimulated with formyl-methionyl-leucine-phenylalanine. However, not all PMN functions may be altered during inflammation and/or infection because H2O2generation was shown to be enhanced in septic patients47and in a PMN subpopulation from ARDS patients.48
Inflammatory stress and ex vivo cytokine production.
To see whether the reduced IL-8 production by PMN we observed in septic patients was specific to an infectious process or related to the systemic inflammatory response, we included another group of patients who underwent cardiac surgery associated with cardiopulmonary bypass. Inflammatory stress that occurs during CPB is linked to the surgical procedure itself as well as to the interaction of circulating leukocytes with biomaterials during extracorporal circulation.27 To our knowledge, very few investigators have tested the ex vivo capacity of leukocytes of CPB patients to release cytokine upon activation. Naldini et al49 have reported that phytohemagglutinin-induced production of IFN-γ, IL-2, and TNF by peripheral blood mononuclear cells were significantly diminished while IL-1β, IL-6, and IL-8 were not affected. In addition, we have previously shown that surgery itself leads to a reduced capacity of monocytes to release IL-1α, IL-1β, and TNF-α, but not IL-6.26 Other stressful conditions such as trauma,21,50,51 thermal injury,52 and hemorrhage53 are also associated with a reduced capacity of leukocytes to produce cytokine upon cell stimulation. We observed that IL-8 production by PMN as well as IL-10 production by peripheral blood mononuclear cells (manuscript submitted) were significantly reduced in CPB patients. In addition to the inflammatory stress, other parameters such as some drugs may affect cytokine production by circulating leukocytes. Among the drugs used in the patients, aprotinin, a serine protease inhibitor, has been shown to reduce IL-8 levels in broncho-alveolar lavages of patients after CPB.54 We have performed experiments by adding aprotinin to whole blood of healthy controls at concentrations similar to those generated in the patients' blood (500 KIU/mL). Blood was maintained at 37°C for 3 hours before performing the PMN preparation, similarly to the in vivo situation before sampling the blood from the post-CPB patients. After PMN activation by either LPS or streptoccoci, an enhanced release of IL-8 was observed (49% ± 9%) (data not shown), just at the opposite of the ex vivo observation. This observation indicates that aprotinin is not the causative agent of the decreased IL-8 production observed in CPB patients. This does not mean that other drugs such as those used for anesthesia do not interfere in the ability of PMN to produce IL-8. However, in a preliminary study on four patients undergoing prolonged anesthesia with a light surgical stress (arterial embolization for aneurysm), we did not observe any significant modification of the IL-8 production by isolated PMN upon activation.
The presence of circulating pro- and anti-inflammatory cytokines such as IL-6,55 IL-8,55,56 TNF,57IL-10,58 TGF-β,59 as well as the induction of cell-associated IL-160 in patients undergoing CPB, confirms that a systemic inflammatory process does occur. Thus, a rapid deactivation of circulating PMN in terms of IL-8 production was observed in CPB patients, suggesting that the inflammatory component of sepsis syndrome may be sufficient to alter the IL-8 synthesis machinery in PMN. Although CPB is not associated with infectious processes, circulating LPS has been detected in these patients61 and, as in infected patients, its role in affecting PMN reactivity could be considered as endotoxin tolerance.25
In vitro tolerization of PMN from healthy donors.
To determine whether an LPS encounter in vivo in patients could alter PMN reactivity to a later in vitro LPS stimulation, we performed two-step experiments with PMN prepared from healthy controls. PMN were first stimulated with LPS overnight and resuspended in fresh medium in the presence of activating LPS for another 24-hour culture. IL-8 production was then assessed in the cell supernatants. Although a high percentage of PMN has been reported to become rapidly apoptotic in cultures,33,34 our culture conditions had a low level of apoptotic cell after 24 hours and allowed us to perform these two-step analyses. Although a similar protocol led to tolerized monocytes,35 62 neutrophils that were first exposed to LPS before a secondary stimulation with LPS had an enhanced cytokine production.
In monocytes, IL-10 could mimic in vitro LPS tolerization,35 and septic63 as well as CPB58 patients have circulating IL-10. So, it was worthwhile to see whether pretreatment of PMN by IL-10 led to a reduction of IL-8 production. As previously reported,11,14IL-10 is a potent inhibitor of IL-8 production by PMN when added simultaneously with LPS. The effect of pretreatment of PMN by IL-10 led to a significant reduction of subsequent LPS-induced IL-8 production. Furthermore, when IL-10 and LPS were added simultaneously during the pretreatment period, IL-10 completely obliterated the “priming” effects of LPS. Thus, while PMN can be rendered less reactive to a secondary stimulation by LPS after pretreatment with IL-10, they cannot be rendered tolerant to endotoxin stricto sensu by LPS itself. McCall et al24 claimed that PMN from septic patients were endotoxin tolerant. However, this statement should be reconsidered very carefully. Although there are many earlier reports that PMN could be primed by a short preexposure to LPS for enhanced release of oxygen metabolites64 and for the synthesis of 5-lipoxygenase products65 there was no long exposure of PMN and, to our knowledge, this is the first demonstration of a priming effect of LPS on IL-8 production. A similar priming effect was noticed after a first encounter of PMN with TNF. These results obtained after a first activation of long duration are in contrast with a previous report66 in which a 2-hour pretreatment with low amounts of TNF led to deactivated PMN with reduced transcobalamine release and burst activity in response to TNF. In this report, in contrast to most other reports, a 2-hour pretreatment with LPS also led to a deactivation of PMN when further challenged with TNF.
We have focused our attention on the responsiveness of isolated neutrophils from patients with systemic inflammatory response syndrome of infectious or noninfectious origin. In both cases the release of IL-8 upon activation by endotoxins or heat-killed streptococci was significantly reduced, suggesting that stressful conditions rapidly dampen the reactivity of circulating PMN. As suggested by in vitro experiments performed with PMN from healthy controls, the observed phenomenon does not reflect an endotoxin tolerance phenomenon stricto sensu because LPS was unable to desensitize the cells to a second challenge by LPS as reported for monocytes. Our in vitro results suggest that LPS-induced mediators such as IL-10 may be responsible for the observed anergy in patients.
ACKNOWLEDGMENT
We thank the Cardiopulmonary Unit of the Hôpital Lariboisière for its support and help, and Dr Dorris Grossmann for linguistic advice.
Address reprint requests to Jean-Marc Cavaillon, DrSc, Unitéd'Immuno-Allergie, Institut Pasteur, 28 rue Dr Roux, 75015 Paris, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal