Umbilical cord blood (CB) transplantation is thought to be associated with a reduced risk of severe graft-versus-host-disease (GVHD) compared with bone marrow transplantation (BMT). The cytokine cascade is known to be important in the pathogenesis of GVHD; however, previous studies investigating the cytokine secretion pattern of CB cells have been contradictory because of variations in experimental techniques. In this study, the cytokine profile of cord and adult blood lymphocytes and lymphocyte subsets has been assessed at the single-cell level by flow cytometry, using CD4/CD8 and CD45RA/CD45RO markers. Cord and adult blood mononuclear cells were stimulated with phorbol 12-myristate 13-acetate (PMA) and ionomycin in the presence of monensin. After 4 to 24 hours of incubation, interleukin-2 (IL-2), IL-4, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) production was measured by three-color flow cytometry. The results show that cord blood lymphocytes (CBL) produce less IL-2, IL-4, IFN-γ, and TNF-α than adult peripheral blood lymphocytes (ABL). Further subset analysis showed that in CBL the majority of cytokine producing cells were CD4+CD45RA+, whereas in ABL the cytokine-producing cells were both CD4+CD45RO+ and CD8+CD45RO+. These results suggest that the reduced incidence of GVHD in CB transplantation may partly due to the altered cytokine profile seen in CBL.
ALLOGENEIC BONE MARROW transplantation (BMT) is an accepted form of therapy for hematological malignancies, BM failure syndromes, immunodeficiency states, and metabolic disorders.1 However, its success is dependent on the prompt identification of a suitable donor and on the avoidance of opportunistic infections and severe graft-versus-host disease (GVHD). To overcome some of these complications and to expand the available donor pool, cord blood (CB) has been used as an alternative source of hematopoietic stem cells.
CB is a rich source of primitive hematopoietic stem cells and progenitor cells,3 with extensive proliferative and self-renewal capacity ex vivo4,5 and possibly in vivo.6 The first successful CB transplant was performed in 1988 on a patient with Fanconi anemia.6 Subsequently, umbilical CB has been used in both related7 and unrelated BMT,8 and in both settings there appears to be a reduced incidence and severity of GVHD when compared with results obtained using BM.9 Because GVHD is a major cause of morbidity and mortality in allogeneic BMT, this feature of CB transplantation could prove to be crucial for its use in stem cell transplantation.
The development of GVHD involves recognition of alloantigen(s) present in the patient by donor T cells.10 However, the clinical manifestations largely result from cytokine dysregulation.11 Therefore, it is important to investigate whether altered cytokine production of CB could account for the reduced incidence of GVHD observed in CB transplantation.
A variety of different experimental techniques, including enzyme-linked immunosorbent assay (ELISA) or bioassays, have been used to measure cytokine secretion by CB cells, leading to limited and inconclusive results.12 13 In this study interleukin-2 (IL-2), IL-4, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) production by phorbol 12-myristate 13-acetate (PMA)-and-ionomycin–activated cord and adult lymphocytes has been assessed at single-cell level by flow cytometry. CB contains primarily unprimed T cells as documented by the expression of CD45RA marker on the vast majority of both CD4 and CD8 subsets of T cells. Therefore, a comprehensive phenotypic analysis of cytokine producing cells has been performed using CD4/CD8 and CD45RA/CD45RO markers.
MATERIALS AND METHODS
Reagents.
PMA, ionomycin, and monensin were purchased from Sigma (Poole, UK) and Calbiochem/Novachem (Nottingham, UK), respectively.
Monoclonal antibodies (MoAbs) used were CD4-PerCP (Becton Dickinson, Oxford, UK), CD8-PerCp (Becton Dickinson), CD45RA-FITC (Becton Dickinson), CD45RO-FITC (Dako, High Wycombe, UK), IL-2-PE (Pharmingen, Oxford, UK), IL-4-PE (Pharmingen), IFN-γ-PE (Pharmingen), and TNF-α-PE (Pharmingen). Isotype-specific anti-mouse IgG1 or IgG2a were purchased from Becton Dickinson, while anti-mouse IgG1 and anti-rat IgG2a were obtained from Pharmingen. The cells were fixed and permeabilized with Fix and Perm solution (Calbiochem).
Cells.
Human CB was obtained from the placentas of normal full-term deliveries at a local hospital, following ethical committee approval. Adult peripheral blood (AB) mononuclear cells were isolated from buffy coats obtained from local routine blood donations.
Mononuclear cells were obtained from all samples by centrifugation over Ficoll-Hypaque gradients (Nycomed, Birmingham, UK). Before separation CB was incubated with a glycophorin MoAb (Nycomed) at a concentration of 100 μL/10 mL of blood for 5 minutes at room temperature to aid removal of nucleated red blood cells and to improve separation. All cells were frozen in 10% dimethyl sulfoxide (DMSO) using a controlled rate freezer, stored in liquid nitrogen, and then thawed for use.
Intracellular cytokine analysis.
Cells were cultured in 24-well plates (1 × 106/mL) for 4 to 24 hours at 37°C and 5% CO2 in RPMI 1640 supplemented with 10% AB serum, 25 mmol/L HEPES, 100 nmol/L sodium pyruvate, 2 mmol/L L-glutamine, and penicillin/streptomycin. Cells were stimulated with PMA (5 ng/mL) and ionomycin (1 μmol/L). Monensin (3 μmol/L) was added 4 to 10 hours before the end of culture as described by Jung et al.14 Cultured cells were washed twice in phosphate-buffered saline (PBS)/1% bovine serum albumin (BSA) and then stained with MoAb to the following cell-surface markers: CD4, CD8, CD45RA, CD45RO. The cells were then fixed and permeabilized with Fix and Perm solution (Calbiochem) according to manufacturer's instructions. Finally, the cells were incubated with MoAb to the following cytokines: IL-2, IL-4, IFN-γ, and TNF-α.
Flow cytometry.
Phenotypic analyses of mononuclear preparations in CB and AB samples were performed by two- and three-color flow cytometry using a Becton Dickinson FACScan flow cytometer. A minimum of 10,000 lymphocyte-gated events were acquired in list mode and analyzed with Lysis II (Becton Dickinson), Cellnet (Becton Dickinson), and Flowmate (Dako) software.
Statistical analysis.
Student's t-tests were used to compare CB lymphocyte (CBL) versus adult peripheral blood lymphocyte (ABL) cytokine production.P values <.05 were considered to be significant.
RESULTS
Kinetics of cytokine synthesis.
The kinetics of IL-2, IL-4, IFN-γ, and TNF-α synthesis are shown in Fig 1. After PMA and ionomycin stimulation there is a rapid increase in IL-2 production in both CBL and ABL, reaching a peak at 12 hours. In ABL there is a similar rapid increase in TNF-α and IFN-γ, maximal at 8 and 12 hours, respectively. Although in CBL there was a much lower production of both TNF-α and IFN-γ, the maximal production was also at 8 and 12 hours, respectively. Both cord and adult mononuclear cells (MNC) produce low levels of IL-4, the peak production being at 8 hours.
Kinetics of cytokine synthesis in cord and adult blood. Cord and adult mononuclear cells were activated with PMA/ionomycin and IL-2, IL-4, IFN-γ, and TNF-α expression was measured every 4 hours for 24 hours as described in Materials and Methods. The results are expressed as a percentage of the lymphocyte gated cells (see Fig2).
Kinetics of cytokine synthesis in cord and adult blood. Cord and adult mononuclear cells were activated with PMA/ionomycin and IL-2, IL-4, IFN-γ, and TNF-α expression was measured every 4 hours for 24 hours as described in Materials and Methods. The results are expressed as a percentage of the lymphocyte gated cells (see Fig2).
Cytokine expression on cord and adult blood.
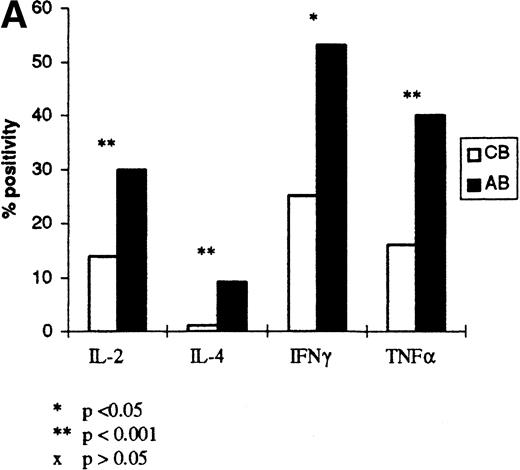
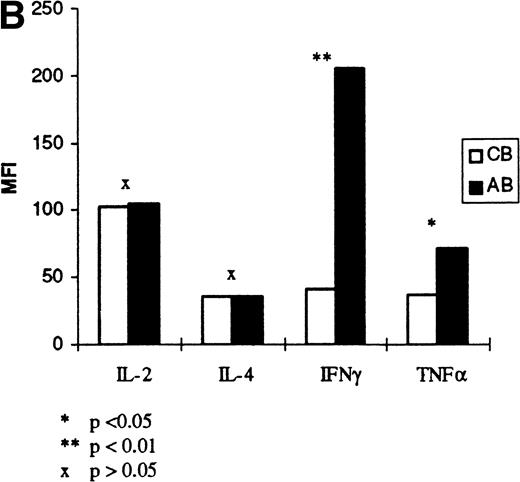
Figure 2 shows the expression of IL-2, IFN-γ, TNF-α, and IL-4 on individual samples of cord and adult lymphocytes, at the time of optimal production as determined by the results in Fig 1 (12, 12, 8, and 8 hours, respectively). The forward and side scatter (fsc/ssc) characteristics for cord and adult cells, and the gates used for analysis of lymphocytes, are also shown in Fig2. These results show that there is reduced production of IL-2, IFN-γ, TNF-α, and IL-4 in CBL compared with ABL. Further experiments were performed on six additional samples of cord and adult blood to confirm these findings (Fig 3). In these experiments, the mean percentage of cells secreting each cytokine and the level of expression, indicated by the mean fluorescence intensity (MFI), was analyzed (Fig 3A and B, respectively). These results show that the percentage of positive cells for each cytokine is significantly lower in CBL compared with ABL. Likewise the level of expression of IFN-γ and TNF-α was significantly lower in CBL than in ABL. However, there was a similar level of expression of IL-2 and IL-4, in both CBL and ABL positive cells.
Flow cytometric cytokine expression on cord and adult lymphocytes. The method for cytokine analysis is described in Materials and Methods. The top two traces show the forward scatter/side scatter distribution and the gate used to select lymphocytes for analysis. The quadrants were set using isotype controls for each of the antibodies. Percentages indicated in the right upper quadrant indicate the percentage of the total lymphoid population (ie, the cells within the region [R1] outlined in the dual scatter plot). For each plot cord blood (left column) is compared with the adult blood (right column).
Flow cytometric cytokine expression on cord and adult lymphocytes. The method for cytokine analysis is described in Materials and Methods. The top two traces show the forward scatter/side scatter distribution and the gate used to select lymphocytes for analysis. The quadrants were set using isotype controls for each of the antibodies. Percentages indicated in the right upper quadrant indicate the percentage of the total lymphoid population (ie, the cells within the region [R1] outlined in the dual scatter plot). For each plot cord blood (left column) is compared with the adult blood (right column).
(A) Cytokine expression on cord and adult blood. The percentage positivity of six samples of cord and adult blood is shown following PMA/ionomycin activation. Results are expressed as a percentage of R1 (see Fig 2). (B) Cytokine expression on cord and adult blood. The MFI for the cord and adult blood samples in (A) are shown. The results are expressed as a mean of six samples of cord and adult blood.
(A) Cytokine expression on cord and adult blood. The percentage positivity of six samples of cord and adult blood is shown following PMA/ionomycin activation. Results are expressed as a percentage of R1 (see Fig 2). (B) Cytokine expression on cord and adult blood. The MFI for the cord and adult blood samples in (A) are shown. The results are expressed as a mean of six samples of cord and adult blood.
CD45RA/CD45RO subset analysis.
Cytokine producing cells in CBL and ABL were further characterized, at the time of peak production, according to expression of CD45RA/CD45RO. The results are shown in Fig4A. In CBL, 70% of IL-2–producing cells were CD45RA+ and 30% were CD45RO+. Cells producing IFN-γ were 75% CD45RA+ and 25% CD45RO+, while TNF-α–producing cells were 90% CD45RA+ and 10% CD45RO+. IL-4–producing cells were 75% CD45RA+ and 25% CD45RO+. In contrast, in ABL, the predominant IL-2–, IFN-γ–, TNF-α–, and IL-4–producing cells were CD45RO+, rather than CD45RA+ (83%v 17%, 98% v 2%, 83% v 17%, and 94%v 6%, respectively).
(A) T-cell subset analysis. The cytokine producing cells in cord and adult blood were characterized according to expression of CD45RA and CD45RO. The results given were calculated using the ratio of CD45RA to CD45RO, when these values were expressed as a percentage of the total population analyzed. In each case the mean percentage positivity as a percentage of the total lymphoid population is noted as well as the number of samples tested (n). (B) T-cell subset analysis. The cytokine-producing cells in cord and adult blood were further characterized according to expression of CD4 and CD8. The values given were calculated using the results expressed as a percentage of the total population analyzed, as in (A).
(A) T-cell subset analysis. The cytokine producing cells in cord and adult blood were characterized according to expression of CD45RA and CD45RO. The results given were calculated using the ratio of CD45RA to CD45RO, when these values were expressed as a percentage of the total population analyzed. In each case the mean percentage positivity as a percentage of the total lymphoid population is noted as well as the number of samples tested (n). (B) T-cell subset analysis. The cytokine-producing cells in cord and adult blood were further characterized according to expression of CD4 and CD8. The values given were calculated using the results expressed as a percentage of the total population analyzed, as in (A).
CD4/8 subset analysis.
Further phenotypic analysis of the cytokine-producing cells was also performed according to CD4 and CD8 expression, and the results are shown in Fig 4B. In CBL, IL-2 is produced predominantly by CD4+ cells compared with CD8+ cells (80%v 20%). Similarly, IFN-γ, TNF-α, and IL-4 are produced mainly by CD4+ cells compared with CD8+ cells (83% v 17%, 86% v 14%, and 61% v 39%, respectively). In ABL, IL-2 is also produced predominantly by CD4+ cells compared with CD8+ cells (75%v 25%). However, IFN-γ, TNF-α, and IL-4 are produced by both CD4+ and CD8+ cells (49% v 51%, 44% v 56% and 42% v 58%).
The percentage and level of cytokine expression in different T-cell subsets.
A comparison of the percentage of CD4+CD45RA+, CD4+CD45RO+, CD8+CD45RA+, and CD8+CD45RO+ CBL and ABL producing IL-2, IFN-γ, TNF-α, and IFN-γ is shown in Fig5. The level of expression as determined by (MFI) of IL-2, IFN-γ, TNF-α, and IL-4 in CD4+CD45RA+, CD4+CD45RO+, CD8+CD45RA+, and CD8+CD45RO+ subsets in CBL and ABL is also shown in Fig 5.
The percentage and level of expression of each cytokine in different T-cell subsets. The percentage of IL-2–, IFN-γ–, TNF-α–, and IL-4–producing cells in CD4+CD45RA+, CD4+CD45RO+, CD8+CD45RA+, and CD8+CD45RO+ CBL and ABL is shown on the left. The level of expression (MFI) of IL-2, IFN-γ, TNF-α, and IL-4 on CD4+CD45RA+, CD4+CD45RO+, CD8+CD45RA+, and CD8+CD45RO+ CBL and ABL is shown on the right. The results shown are the mean values of the samples shown in Fig 4.
The percentage and level of expression of each cytokine in different T-cell subsets. The percentage of IL-2–, IFN-γ–, TNF-α–, and IL-4–producing cells in CD4+CD45RA+, CD4+CD45RO+, CD8+CD45RA+, and CD8+CD45RO+ CBL and ABL is shown on the left. The level of expression (MFI) of IL-2, IFN-γ, TNF-α, and IL-4 on CD4+CD45RA+, CD4+CD45RO+, CD8+CD45RA+, and CD8+CD45RO+ CBL and ABL is shown on the right. The results shown are the mean values of the samples shown in Fig 4.
There is a similar percentage of CD4CD45RA+ and CD4+CD45RO+ CBL and ABL producing IL-2. However, a lower number of CD8+CD45RA+ and CD8+CD45RO+ CBL express IL-2 than ABL with a similar phenotype. In CB, there is a reduced percentage of cells producing IFN-γ and TNF-α in all four T-cell subsets, compared with AB. Although, a similar percentage of CD4+CD45RA+ and CD8+CD45RA+ CBL and ABL produce IL-4, there is a lower percentage of CD4+CD45RO+ and CD8+CD45RO+ CBL producing IL-4 compared to ABL with the same phenotype.
In CBL and ABL there is a similar level of expression of IL-2 in all four T-cell subsets. There is a similar level of expression of IL-4 in CD4+CD45RA+, CD8+CD45RA+, and CD8+CD45RO+ CBL compared with ABL of similar phenotype; however, there is a significant decrease in the level of IL-4 in CD4+CD45RO CBL compared with CD4+CD45RO ABL. Furthermore, there is a significant reduction in the level of expression of IFN-γ and TNF-α in all CB T-cell subsets, compared with AB.
T-cell subsets in CBL and ABL.
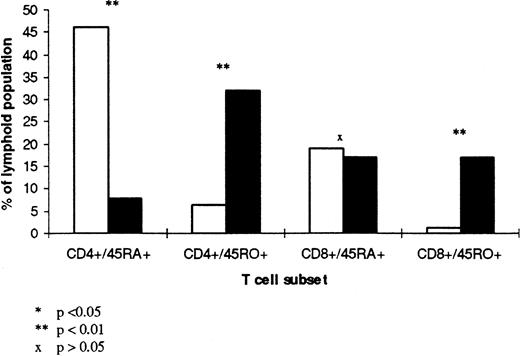
To understand the differences in cytokine secretion described above, it is important to consider the differences in T-cell subsets between CBL and ABL. Figure 6 shows the mean percentage of CD4+CD45RA+, CD4+CD45RO+, CD8+CD45RA+, and CD8+CD45RO+ subsets in CBL and ABL, expressed as a percentage of the total lymphoid population. In CB, the majority of T lymphocytes are either CD4+CD45RA+(46% ± 6%) or CD8+CD45RA+(19% ± 5%) with very few CD4+CD45RO+ and CD8+CD45RO+ (6% ± 2% and 1% ± 2%, respectively). In contrast, in AB there is a substantial proportion of CD4+CD45RO+, CD8+CD45RA+, CD8+CD45RO+ with fewer CD4+CD45RA+ (30% ± 12%, 21% ± 7%, 16% ± 7%, and 8% ± 4%, respectively).
T-cell subsets in CBL (□) and ABI (▧). The mean percentage of CD4+CD45RA+, CD4+CD45RO+, CD8+CD45RA+, and CD8+CD45RO+ subsets in six samples of CBL and six samples of ABL is shown. The results are expressed as a percentage of the total lymphoid population.
T-cell subsets in CBL (□) and ABI (▧). The mean percentage of CD4+CD45RA+, CD4+CD45RO+, CD8+CD45RA+, and CD8+CD45RO+ subsets in six samples of CBL and six samples of ABL is shown. The results are expressed as a percentage of the total lymphoid population.
DISCUSSION
Cytokine production has been extensively investigated in the past using ELISA or bioassay techniques which measure cytokine secretion in cell-culture supernatants. However, results have been variable and inconclusive, largely because of the different experimental conditions used.
In this study, the cytokine profile of CBL is reported, using a modification of the technique described by Jung et al.14This technique enables phenotypic characterization of cytokine-producing cells, and therefore allows a more accurate and detailed comparison of the cytokine profile of cord and adult lymphocytes, at single-cell level.
Previous studies have shown that in stimulated CB, IL-2 secretion into the supernatant is lower than15 or similar12 16to activated AB. The results of this study show that although the proportion of cells expressing IL-2 was lower in CBL, the level of expression of IL-2 and the kinetics of IL-2 production are similar in both CBL and ABL. Further subset analysis showed that the reduced proportion of IL-2–producing cells in CB was predominantly caused by the reduced percentage of IL-2–producing CD8+CD45RA+ and CD8+CD45RO+ CBL compared to ABL with the same phenotype.
These results show that not only is there a lower proportion of IFN-γ– and TNF-α–producing cells in CB, but there is also lower expression of IFN-γ and TNF-α on positive cells in each. Furthermore, in CBL the reduced percentage and level of expression of these cytokines is seen in all four T-cell subsets. Some investigators have suggested that there may be a discrepancy between intracellular stores and secretion of TNF-α in some clinical settings.17 In our study we have not been able to compare the intracellular cytokine production with secretion into the supernatant, but previous data confirm that CBMNC secrete less TNF-α and IFN-γ into the supernatant than adult blood.18-20
With regard to IL-4, this study shows that both CBL and ABL display similar kinetics and a similar level of expression of IL-4 on positive cells. However, the proportion of IL-4–producing cells was consistently lower in CBL compared with ABL, in keeping with previous data.12 21 Subset analysis has shown that in CBL the reduced proportion of IL-4–producing cells is related to the reduced percentage of IL-4–producing-CD4+CD45RO+ and CD8+CD45RO+ CBL. Overall, these results indicate that although there is a reduction in intracellular IL-2 and IL-4 production in CBL compared with ABL, the difference is less marked than that seen in IFN-γ and TNF-α production.
CB is known to contain primarily ‘unprimed’ T cells expressing the CD45RA phenotype, and this has been confirmed in this study.22 Therefore, it has been suggested that the reason for the altered cytokine profile seen in CBL is the low proportion of CD45RO+ ‘memory’ T cells present.23 24 However, the results presented here show that in CBL IL-2, IL-4, IFN-γ, and TNF-α were produced predominantly by CD45RA+ cells, in contrast to ABL where these cytokines are predominantly produced by CD45RO+ cells. Furthermore, there was a reduced level of expression of IFN-γ and TNF-α in both CD45RA+ and CD45RO+ CBL compared to ABL with the same phenotype. The latter observation suggests that CD45RA+ and CD45RO+. CBL are different to CD45RA+ and CD45RO+ ABL. Thus, the reduction in cytokine secretion by CBL may either be due to the reduced number of CD45RO+ cells in CB and/or the reduced capacity for CB CD45RA+ and CD45RO+ cells to produce cytokines other than IL-2.
Further CD4/CD8 subset analysis showed that in CBL IL-2, IL-4, IFN-γ, and TNF-α are all predominantly produced by CD4+CD45RA+ cells. On the other hand, in ABL IL-2 is predominantly produced by CD4+CD45RO+cells, while IL-4, IFN-γ, and TNF-α are produced by both CD8+CD45RO+ and CD4+CD45RO+ cells. In keeping with previous studies, these results have shown that there is a reduced proportion of CD8+CD45RO+ cells in CBL, which again could in part account for the marked reduction in IL-4, IFN-γ, and TNF-α secretion in CBL.25 26
PMA is a phorbol ester that acts on protein kinase C, while ionomycin is a calcium ionophore that elevates intracellular calcium. These agents act together to stimulate the T3-Ti–mediated and IL-2–mediated signal transduction pathways.27,28 In CB, reduced cytokine production seen after PMA/ionomycin activation may therefore be due to immaturity of the intracellular signaling mechanism. The precise mechanism has yet to be defined. Kilpinen et al29 have studied the production of NF-κB, which has a central role in the transcriptional activation of genes important in production of cytokines, particularly IL-2. In their study, activation with phorbol dibutyrate/calcium ionophore resulted in an increased activation of NF-κB in CB compared with AB. Therefore, it seems unlikely that the defect in CB involves NF-κB.29 On the other hand, Pino-Otin et al30 have shown that CB T cells have reduced expression of CD50 (ICAM-3), which has been shown to be important in intracellular signaling.31 32 Therefore, reduced CD50 expression may in part account for the altered cytokine profile of CBL.
It has been suggested that the production of IFN-γ and IL-2 by donor T cells and that of IL-1 and TNF-α by donor mononuclear cells play a central role in the pathogenesis of GVHD.33 The involvement of TNF-α and IFN-γ has also been described in the skin explant model.34 Other investigators have found raised levels of IFN-γ and TNF-α in the serum of patients with GVHD.35,36 Previous studies have supported the early role of IL-2 in GVHD, probably through stimulation of the production of other cytokines such as IL-1 and TNF-α.37 However, further studies have shown that IL-2 mRNA levels in peripheral blood were not found to be increased in patients with GVHD.38
In considering the reduced incidence of GVHD in CB transplantation it may be important to investigate the cytokine profile of CB T cells posttransplant, because the cytokine profile of CB T cells may be modified in vivo. Indeed, recent studies by Sornasse et al39 have shown that the cytokine profile of naı̈ve CB CD4+ T cells can be modified by the addition of differentiation-inducing cytokines.
Nevertheless, the results presented here show that CBL have an altered intracellular cytokine profile compared with ABL, particularly with respect to IFN-γ and TNF-α, which might account for the reduced capacity of transplanted CB T cells to induce severe GVHD. Furthermore, the reduction of cytokine production seen in CBL may be due to the predominance of CD4+CD45RA+ cells. Defining the underlying mechanisms of reduced cytokine production is of crucial importance in understanding the apparent reduced incidence and severity of GVHD in CB transplantation.
Address reprint requests to C. Navarrete, PhD, Department of Histocompatibility and Immunogenetics, North London Centre (NBS), Colindale Ave, London NW9 5BG, UK.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


![Fig. 2. Flow cytometric cytokine expression on cord and adult lymphocytes. The method for cytokine analysis is described in Materials and Methods. The top two traces show the forward scatter/side scatter distribution and the gate used to select lymphocytes for analysis. The quadrants were set using isotype controls for each of the antibodies. Percentages indicated in the right upper quadrant indicate the percentage of the total lymphoid population (ie, the cells within the region [R1] outlined in the dual scatter plot). For each plot cord blood (left column) is compared with the adult blood (right column).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/1/10.1182_blood.v92.1.11.413a39_11_18/5/m_blod41339002x.jpeg?Expires=1767717271&Signature=FVn7xli2~NRSDNfr2hAh9HJ-vP9yU1vhJxJRN-FjWNRbEBu8kHvkhF2Qdv0NoVYIw-6LXt4vgZH8SGFs8I8fdrsQaPm3r1ux9HzLt7MUBRh7aa4mZwP1xwPuYFnjuQT4n~gA8IDffszNRl30C5VG~oD-tlkhZ5-gtVANP0VXUx32GBjK6An05S-zCfvTmdbvzDb1o6ugoEkgp1YSIr1nnUSL~N9vuzx5va858zWI9~ziundhYiNAdlwRbj6rO9JAb-myApDNaMzpfJCesDy1yEEbaIbKzRRx94-aP~5Gnm-mlKnPpFc~LVEryAMlYZG0~KpyRLqsD0A7OIXHTKTbpg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal