Regulatory peptides, such as vasoactive intestinal peptide (VIP), somatostatin (SS), or substance P (SP), are considered to play a role in immune regulation. To localize the targets of these peptides in the human immune system, their receptors have been evaluated with in vitro receptor autoradiography in lymph nodes, tonsils, appendix, Peyer's patches, spleen, and thymus. The three peptide receptors were detected in all lymphoid tissues tested, but, unexpectedly, usually in distinct compartments. In lymph nodes, palatine tonsils, vermiform appendix, and Peyer's patches, VIP receptors were found in the CD3 positive zone around lymphoid follicles; SS receptors in the germinal centers of secondary follicles; and SP receptors mainly in interfollicular blood vessels. In the spleen, VIP receptors were detected in periarterial lymphatic sheaths, SS receptors in the red pulp, and SP receptors in the central arteries. In the thymus, VIP receptors were present in cortex and medulla, SS receptors in the medulla, and SP receptors in blood vessels. For comparison, cholecystokinin (CCK)-A and -B receptors were not demonstrated in any of these tissues. These results suggest a strong compartmentalization of the three peptide receptors in human lymphoid tissues and represent the molecular basis for the understanding of a very complex and interactive mode of action of these peptides.
REGULATORY PEPTIDES, in particular gut and brain peptides, have been shown to be involved in the regulation of immune processes in numerous animal and human studies.1-3First, receptors for various regulatory peptides, ie, somatostatin (SS), vasoactive intestinal peptide (VIP), or substance P (SP) are expressed by rat, mice, or human immunocytes, in particular lymphocytes.1,4-12 Second, various effects of these regulatory peptides have been reported in vivo or in vitro on several immunologic parameters: SS has been shown to inhibit murine lymphocyte proliferation,13-15 Ig synthesis,13,16,17 or the release of colony-stimulating factor18 and to enhance the formation of leukocyte-migration inhibiting factor in activated lymphocytes.13 VIP regulates immunocompetence in vitro including antibody production, natural killer activity, histamine production by mast cells, and lymphocyte proliferation.2,3Effects of SP on immunocompetence have also been documented19 and are probably exerted through the carboxy-terminal domain of the 11-amino acid neuropeptide.9
Moreover, several investigations have described interactions between several regulatory peptides involved in immune functions, suggesting close topographical links between the various peptide systems.2,20-25 For instance, in murine Peyer's patch cells, mesenteric lymph node cells and splenocytes, SS and VIP reduce cell proliferation driven by a T-cell lectin, whereas SP increases it. SS can antagonize the SP- or VIP-induced stimulation of immunoglobulin production.1,25 SS and VIP can increase the cholecystokinin (CCK)-induced inhibition of Molt-4 lymphoblast proliferation.21
Much progress has been made recently in the molecular characterization of the receptors for SS, VIP, SP, and CCK at the protein and mRNA levels, in particular with the identification of the existence of several subtypes for each of these receptors.26-29
Despite these data, little is known about the cellular localization of receptors for peptides and about regulatory interactions of peptides in human lymphoid tissues. Therefore, we have investigated the tissue localization of various regulatory peptide receptors, including SS receptors, VIP receptors, SP receptors, CCK-A receptors and CCK-B receptors, using in vitro receptor autoradiography in human thymus, lymph nodes, spleen, tonsils, appendix, and Peyer's patches.
MATERIALS AND METHODS
Aliquots of surgically resected tissues or of biopsies submitted for diagnostic histopathology were frozen immediately after surgical resection and stored at −70°C. The specimens originated primarily from the Institute of Pathology, University of Berne, Berne, Switzerland. The various nondiseased lymphoid tissue samples investigated included thymus, lymph nodes, spleen, tonsils, appendix, and Peyer's patches. Each tissue was cut in 10- or 20-μm thick, successive sections on a cryostat. Alternate sections were used for in vitro receptor autoradiography (20 μm) for VIP receptors, SS receptors, SP receptors, or CCK-A and CCK-B receptors, and for immunohistochemical staining (10 μm) for various antigens (CD3, MIB-1, DRC-1, F-VIII) known to be present in lymphoid tissues.
VIP receptor autoradiography.
[125I]-labeled VIP (2,000 Ci/mmol; Anawa, Wangen, Switzerland) was used as the radioligand. Only the mono125Iodo-[Tyr10]-VIP, eluted as single peak from the high-performance liquid chromatography (HPLC) and analyzed by mass spectrometry, was used. The slide-mounted tissue sections were incubated for 90 minutes in a solution of 50 mmol/L Tris-HCl (pH 7.4) containing 2% bovine serum albumin (BSA), 2 mmol/L EGTA, 0.1 mmol/L bacitracin, and 5 mmol/L MgCl2 to inhibit endogeneous proteases in the presence of 30 pmol/L [125I]-labeled VIP at room temperature as described previously.30 To estimate nonspecific binding, paired serial sections were incubated as described above, except that 1 μmol/L VIP (Bachem, Bubendorf, Switzerland) was added to the incubation medium. After this incubation, the slides were washed twice in ice-cold 50 mmol/L Tris-HCl (pH 7.4) containing 0.25% BSA, then in buffer alone, and quickly dried under a stream of cold air. The sections were subsequently exposed to a 3H-Hyperfilm (Amersham, Little Chalfont, UK) for 1 week.
Selected VIP receptor-positive lymphoid tissues were evaluated with125I-[Ac-His1]pituitary adenylate cyclase activating peptide (PACAP) 1-27 for their receptor subtype specificity: displacement experiments under the same conditions as for VIP receptor autoradiography using increasing concentrations of unlabeled VIP and PACAP 1-27 were performed to differentiate PACAP-I and PACAP-II receptors.31
Type and Number of Human Lymphoid Tissues Investigated and Their Peptide Receptor Status
| Tissue . | Presence of Regulatory Peptide Receptors . | ||||
|---|---|---|---|---|---|
| VIP-R . | SS-R . | SP-R . | CCK-A-R . | CCK-B-R . | |
| Lymph nodes | 27/27 | 27/27 | 9/9 | 0/7 | 0/7 |
| Tonsils | 8/8 | 8/8 | 8/8 | 0/3 | 0/3 |
| Appendix | 11/11 | 11/11 | 5/5 | 0/4 | 0/4 |
| Peyer's patches | 9/9 | 9/9 | 9/9 | 0/2 | 0/2 |
| Spleen | 6/6 | 6/6 | 6/6 | 0/4 | 0/4 |
| Thymus | 9/9 | 9/9 | 9/9 | 0/4 | 0/4 |
| Tissue . | Presence of Regulatory Peptide Receptors . | ||||
|---|---|---|---|---|---|
| VIP-R . | SS-R . | SP-R . | CCK-A-R . | CCK-B-R . | |
| Lymph nodes | 27/27 | 27/27 | 9/9 | 0/7 | 0/7 |
| Tonsils | 8/8 | 8/8 | 8/8 | 0/3 | 0/3 |
| Appendix | 11/11 | 11/11 | 5/5 | 0/4 | 0/4 |
| Peyer's patches | 9/9 | 9/9 | 9/9 | 0/2 | 0/2 |
| Spleen | 6/6 | 6/6 | 6/6 | 0/4 | 0/4 |
| Thymus | 9/9 | 9/9 | 9/9 | 0/4 | 0/4 |
Localization of VIP receptors, SS receptors, and SP receptors in the various compartments of a human appendix. Successive sections were used. (A) Autoradiogram showing total binding of125I-VIP. Predominant VIP receptor-location is in the zone surrounding lymphoid follicles. (B) CD3 immunohistochemical staining. (C) Hematoxylin-eosin stained section. Bar = 1 mm. (D) Blood vessels stained for F-VIII related antigen. (E) Autoradiogram showing total binding of 125I-Tyr3-octreotide. Predominant SS receptor location is in the germinal centers. (F) DRC-1 immunohistochemical staining for reticulum cells. (G) MIB-1 immunohistochemical staining for proliferating cells. (H) Autoradiogram showing total binding of 125I-BH-SP. Predominant SP receptor location is in the blood vessels, but not in lymphoid tissue.
Localization of VIP receptors, SS receptors, and SP receptors in the various compartments of a human appendix. Successive sections were used. (A) Autoradiogram showing total binding of125I-VIP. Predominant VIP receptor-location is in the zone surrounding lymphoid follicles. (B) CD3 immunohistochemical staining. (C) Hematoxylin-eosin stained section. Bar = 1 mm. (D) Blood vessels stained for F-VIII related antigen. (E) Autoradiogram showing total binding of 125I-Tyr3-octreotide. Predominant SS receptor location is in the germinal centers. (F) DRC-1 immunohistochemical staining for reticulum cells. (G) MIB-1 immunohistochemical staining for proliferating cells. (H) Autoradiogram showing total binding of 125I-BH-SP. Predominant SP receptor location is in the blood vessels, but not in lymphoid tissue.
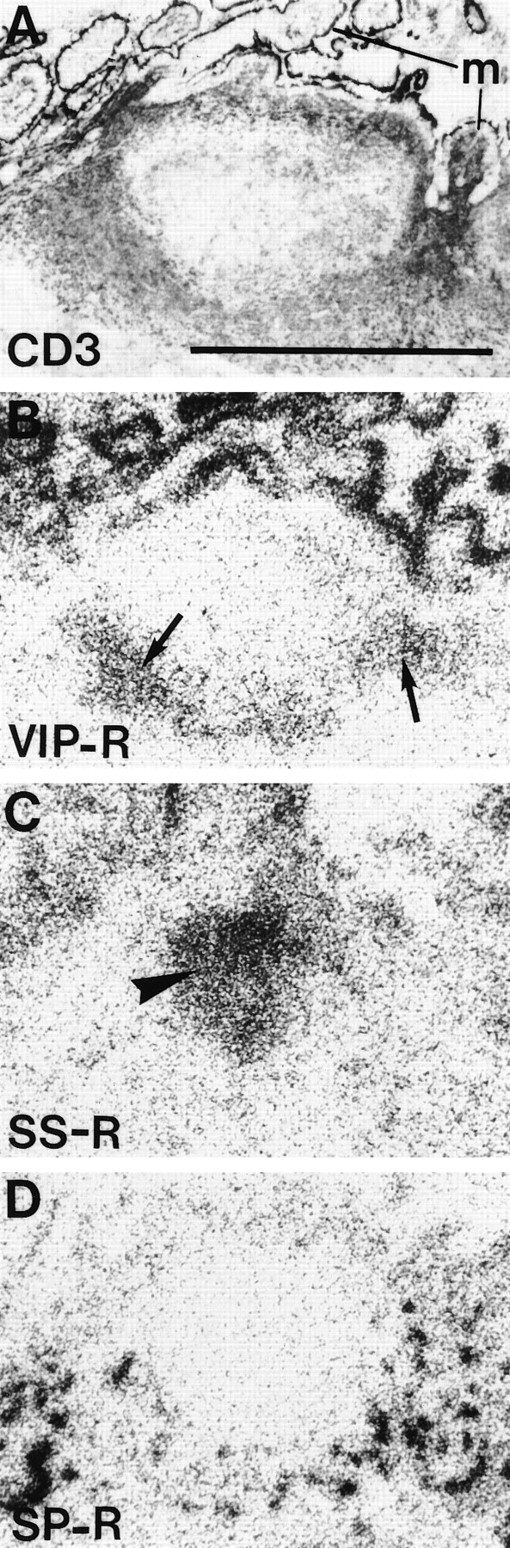
Localization of VIP receptors, SS receptors, and SP receptors in successive sections of a human Peyer's patch. (A) CD3 immunohistochemical staining. Bar = 1 mm. (B) Autoradiogram showing total binding of 125I-VIP. Arrows point towards VIP receptors located in the peripheral zone of the lymphoid follicle. (C) Autoradiogram showing total binding of125I-Tyr3-octreotide. Arrowhead shows SS receptors in the germinal center. (D) Autoradiogram showing total binding of 125I-BH-SP. SP receptors are located in the blood vessels.
Localization of VIP receptors, SS receptors, and SP receptors in successive sections of a human Peyer's patch. (A) CD3 immunohistochemical staining. Bar = 1 mm. (B) Autoradiogram showing total binding of 125I-VIP. Arrows point towards VIP receptors located in the peripheral zone of the lymphoid follicle. (C) Autoradiogram showing total binding of125I-Tyr3-octreotide. Arrowhead shows SS receptors in the germinal center. (D) Autoradiogram showing total binding of 125I-BH-SP. SP receptors are located in the blood vessels.
SS receptor autoradiography.
The radioligands used were 125I-labeled [Tyr3]-octreotide and125I-[Leu8,D-Trp22,Tyr25]-SS-28 (125I-[LTT]-SS-28) known to specifically label SS receptors. Both ligands were iodinated, purified by high pressure liquid chromatography column, and characterized in standard binding assays as described previously.4 32 Frozen sections were then incubated for 2 hours at ambient temperature in the presence of 65 pmol/L 125I-[Tyr3]-octreotide. The incubation solution was 170 mmol/L Tris-HCl buffer (pH 8.2) containing 1% BSA, bacitracin (40 μg/mL), and MgCl2 (10 mmol/L) to inhibit endogenous proteases. Nonspecific binding was determined by adding 1 μmol/L solution of unlabeled [Tyr3]-octreotide or SS-28. Incubated sections were washed twice for 5 minutes in cold incubation buffer containing 0.25% BSA and then in buffer alone and dried quickly. Finally, the sections were apposed to3H-Hyperfilms (Amersham, Little Chalfont, UK) and exposed for 1 week in x-ray cassettes.
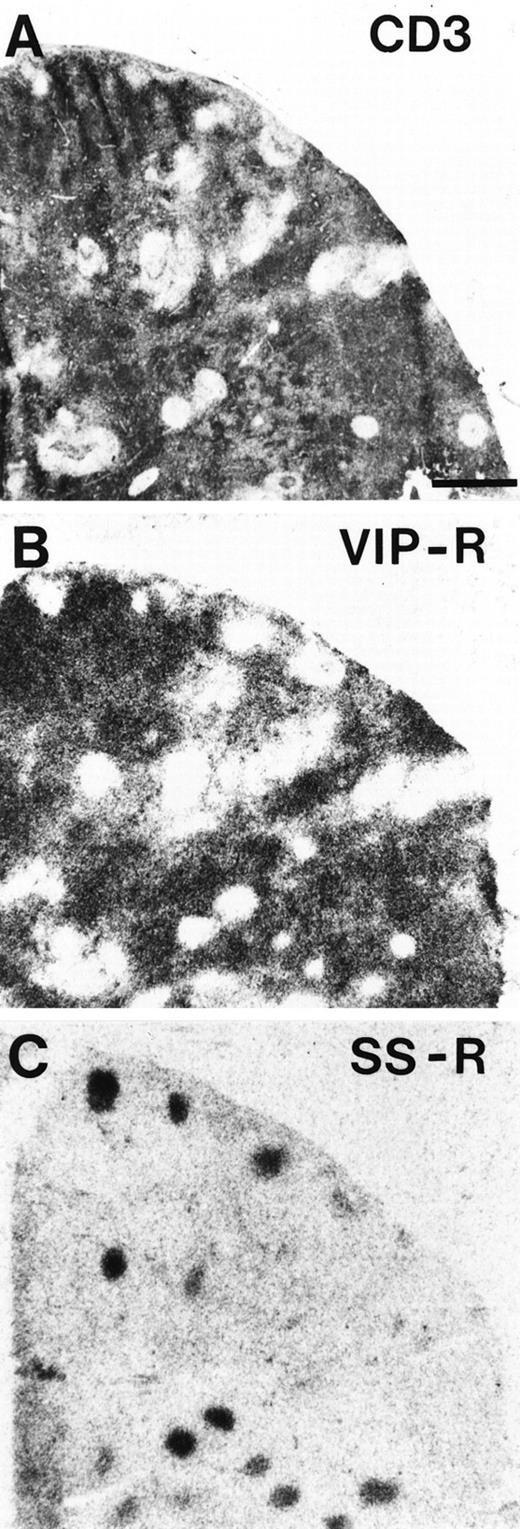
Complementary localization of VIP receptors and SS receptors in a human lymph node. (A) CD3 immunohistochemical staining. Bar = 1 mm. (B) Autoradiogram showing total binding of125I-VIP. VIP receptors are in the whole lymph node with exception of the germinal centers. (C) Autoradiogram showing total binding of 125I-Tyr3-octreotide. SS receptors are predominantly located in germinal centers.
Complementary localization of VIP receptors and SS receptors in a human lymph node. (A) CD3 immunohistochemical staining. Bar = 1 mm. (B) Autoradiogram showing total binding of125I-VIP. VIP receptors are in the whole lymph node with exception of the germinal centers. (C) Autoradiogram showing total binding of 125I-Tyr3-octreotide. SS receptors are predominantly located in germinal centers.
Adjacent sections from some of the tissues tested with125I-labeled [Tyr3]-octreotide were also examined for specific binding with the SS-28 radioligand125I-[LTT]-SS-28 to evaluate whether the same tissue elements were identified by both ligands.
SP receptor autoradiography.
The radioligand used was 125I-Bolton-Hunter–SP (125I-BHSP) (2,000 Ci/mmol; Anawa) as described.33 The sections were incubated for 2 hours in a solution of 50 mmol/L Tris-HCl (pH 7.4) containing BSA (0.02%), chymostatin (2 mg/L), leupeptin (4 mg/L), bacitracin (40 mg/L), MnCl2 (5 mmol/L), and 50 pmol/L 125I-BHSP at room temperature. To estimate nonspecific binding, paired serial sections were incubated as described above, except that 1 μmol/L SP (Bachem) was added to the incubation medium. After this incubation, the slides were rinsed with four washes of 30 seconds each in ice-cold 50 mmol/L Tris-HCl (pH 7.4), dipped in ice-cold distilled water, and then quickly dried in a refrigerator under a stream of cold air. The sections were subsequently exposed to a 3H-Hyperfilm (Amersham) for 1 week.
CCK receptor autoradiography.
Immunohistochemistry.
Immunostaining for CD3 (clone UCHT1; Dako, Glostrup, Denmark), follicular dendritic cells (clone R4/23; Dako), factor VIII-related antigen (clone F8/86; Dako), and proliferation antigen Ki-67 (clone MIB-1; Immunotech, Marseille, France) was performed on sections adjacent to those used for receptor autoradiography. Cryostat sections (5 μm) were fixed in acetone, air-dried, rehydrated in 5% rabbit serum, and then sequentially incubated with a primary antibody, a biotinylated rabbit antimouse immunoglobulin antibody (Dako), and a complex of avidin and biotinylated alkaline phosphatase (Dako), according to the avidin-biotin-complex (ABC)-technique. Finally, slides were developed in new fuchsin and mounted without counterstaining. In negative controls, the primary antibody was replaced with an irrelevant mouse monoclonal antibody.
RESULTS
As shown in Table 1, three peptide receptors were found to be consistently expressed in human lymphoid tissues, namely VIP receptors, SS receptors, and SP receptors, whereas CCK-A and CCK-B receptors were not identified. The most interesting finding is that in the lymph nodes, appendix, and Peyer's patches, VIP receptors, SS receptors, and SP receptors were localized in different, complementary tissue compartments: whereas SS receptors were found in the germinal centers of lymphoid follicles, VIP receptors were predominantly localized in the zone around the follicles and SP receptors in blood vessels located in this zone (Figs 1-3). In the thymus, a low density of VIP receptors was distributed in medulla and cortex, whereas SS receptors were mainly expressed in the epithelial cells of the medulla and SP receptors were found in the thymic vessels (Fig 4). In the spleen, the VIP receptors were located in the periarterial lymphatic sheets (Fig 5), whereas SS receptors were found diffusely in the red pulp and SP receptors in the central arteries of the white pulp (Fig 5).
Localization of VIP receptors, SS receptors, and SP receptors in a human thymus. (A) Hematoxylin-eosin stained section. Bar = 1 mm. (B) Autoradiogram showing total binding of125I-VIP. VIP receptors are diffusely distributed in cortex and medulla. (C) Autoradiogram showing total binding of125I-Tyr3-octreotide. SS receptors are located in the medulla. (D) Autoradiogram showing total binding of125I-BH-SP. SP receptors are located in blood vessels.
Localization of VIP receptors, SS receptors, and SP receptors in a human thymus. (A) Hematoxylin-eosin stained section. Bar = 1 mm. (B) Autoradiogram showing total binding of125I-VIP. VIP receptors are diffusely distributed in cortex and medulla. (C) Autoradiogram showing total binding of125I-Tyr3-octreotide. SS receptors are located in the medulla. (D) Autoradiogram showing total binding of125I-BH-SP. SP receptors are located in blood vessels.
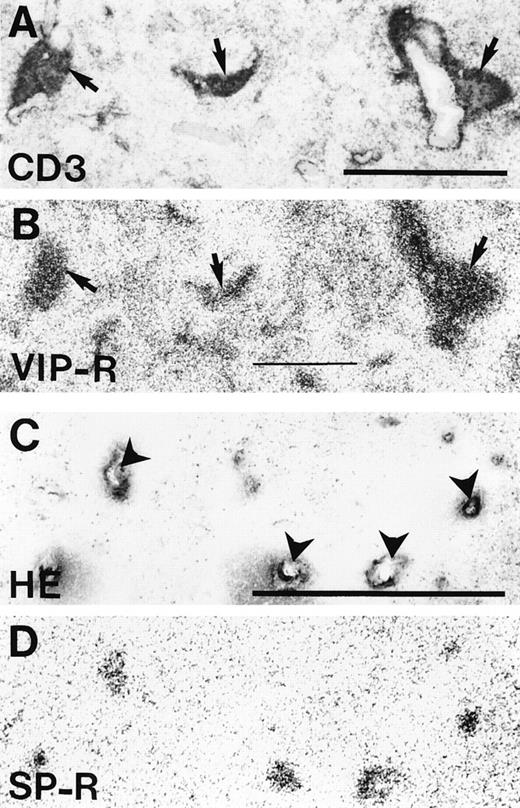
Localization of VIP receptors and SP receptors in the human spleen. (A) CD3 immunohistochemical staining of the periarterial lymphatic sheaths (arrows). Bar = 1 mm. (B) Autoradiogram showing total binding of 125I-VIP. Preferential labeling is seen in the periarterial lymphatic sheets (arrows). Nonspecific binding is negligible. (C) Hematoxylin-eosin staining showing several central arteries (arrowheads) Bar = 1 mm. (D) Autoradiogram showing total binding of 125I-BH-SP. Only the central arteries are labeled. Nonspecific binding is negligible.
Localization of VIP receptors and SP receptors in the human spleen. (A) CD3 immunohistochemical staining of the periarterial lymphatic sheaths (arrows). Bar = 1 mm. (B) Autoradiogram showing total binding of 125I-VIP. Preferential labeling is seen in the periarterial lymphatic sheets (arrows). Nonspecific binding is negligible. (C) Hematoxylin-eosin staining showing several central arteries (arrowheads) Bar = 1 mm. (D) Autoradiogram showing total binding of 125I-BH-SP. Only the central arteries are labeled. Nonspecific binding is negligible.
The tissue localization of the receptors was compared with that of lymphocyte subpopulations in defined tissue areas in lymphoid organs: in the lymph nodes, appendix, Peyer's patches, tonsils, and thymus, the VIP receptor localization correlated best with that of CD3 immunoreactivity (Figs 1-4), that is, it was located in T-lymphocyte areas. Conversely, SS receptor localization correlated best with that of germinal centers of lymphatic tissue, as indicated by the reactivity of the latter for DRC-1 and MIB-1, eg, in the appendix in Fig 1. SP receptors were primarily located in vessels (Fig 1), in particular, high endothelial venules and in vascular sinuses of the spleen, as shown by the endothelial reactivity for F-VIII related antigen.
The receptor subtypes expressed by the lymphoid tissues are likely to be the following: sst2 is the main somatostatin receptor subtype found in the germinal centers, as in all cases the sst2-preferring radioligand 125I-Tyr3-octreotide was the ligand of choice compared with the universal ligand125I-LTT-SS-28, which gave a slightly less intense labeling of the same tissue elements (data not shown). The VIP receptors in T-cell areas are considered to belong to the PACAP-II receptor subtypes, as in displacement experiments, VIP and PACAP displaced125I-VIP with high affinity; moreover, when125I-[Ac-His-1]-PACAP 1-27 was used as ligand, both PACAP and VIP displaced the ligand with high affinity (Fig 6). NK1 appears to be the SP receptor subtype expressed in the vessels of human lymphoid tissue, as NK1 selective analogs, but not NK2 or NK3 selective analogs, displaced 125I-BH-SP with high affinity (data not shown).
Displacement curves of125I-[Ac-His-1]-PACAP(1-27) to tissue sections of a human thymus (A) and a human lymph node (B). Tissue sections are incubated with 15,000 cpm/100 μL radioligand and increasing concentrations of PACAP(1-27) (▴) and VIP (▪), or somatostatin (SS-14) (•) at a concentration of 100 nmol/L. Nonspecific binding was subtracted from all values. In all cases, complete displacement of the ligand is achieved by VIP and PACAP. SS-14 was inactive in the nanomolar range.
Displacement curves of125I-[Ac-His-1]-PACAP(1-27) to tissue sections of a human thymus (A) and a human lymph node (B). Tissue sections are incubated with 15,000 cpm/100 μL radioligand and increasing concentrations of PACAP(1-27) (▴) and VIP (▪), or somatostatin (SS-14) (•) at a concentration of 100 nmol/L. Nonspecific binding was subtracted from all values. In all cases, complete displacement of the ligand is achieved by VIP and PACAP. SS-14 was inactive in the nanomolar range.
DISCUSSION
Foreign antigens are transported to and concentrated in peripheral (secondary) lymphoid organs and tissues, including the spleen, lymph nodes, and mucosa-associated tissues. The peripheral organs provide an environment in which mature T and B lymphocytes can interact with each other, with accessory cells, and with antigens to generate cellular and humoral immune responses, whereas central lymphoid organs are major sites of lymphopoiesis. The structural organization of the various cell populations in the lymph nodes is considered to be critical for the generation of immune responses.36 This study represents the first evidence for a striking and unexpected compartmentalization for three peptide receptors present in lymphoid organs. The results indicate and confirm that several regulatory peptide receptors are strongly expressed in central and peripheral human lymphoid organs and thus may participate in immunoregulatory functions.2,3 25It is the first time that the precise tissue distribution of VIP receptors is reported in human lymphoid organs, as they were consistently and predominantly expressed in regions rich in CD3 immunostained cells, ie, rich in T lymphocytes. These VIP receptor-expressing cells are quite different from the SS receptor-expressing cells in secondary follicles, immunoreactive for MIB-1 and DRC-1. Further, the SP receptor-expressing structures were identified as blood vessels. Thus, all three receptors, VIP receptors, SS receptors, and SP receptors, are expressed by human lymphoid organs in different, topographically distinct structures. The CCK receptors do not appear to play a major role in the tissues tested in this study.
In lymph nodes, the localization of SS receptors correlated well with that of germinal centers, defined by the presence of dendritic and proliferating cells, and suggests their functional involvement in the regulation of humoral antibody responses. The adjacent paracortex, containing mostly T lymphocytes of the helper phenotype, displayed a marked expression for VIP receptors. Dendritic cells located in T-cell–rich areas are known to present antigens circulating in the lymph to CD4+ helper T cells. Further, B lymphocytes from the blood must traverse helper T-cell–rich and VIP receptor-rich zones on their way to the follicles, hence maximizing the chance for cellular interactions. Because lymphocytes enter and egress via lymphatics and high endothelial venules, the presence of SP receptors in blood vessels may play a regulatory role for lymphocyte traffic and recirculation. Thus, the anatomic organization of lymph nodes provides multiple sites for cellular interactions. The compartmentalized distribution of receptors for small peptide hormones in the lymph nodes reflects, to a certain extent, the structural, and probably functional, organization of the various cell populations in the lymph nodes.
In the tonsils, Peyer's patches and vermiform appendix, which are part of the mucosa-associated lymphoid system, the distribution of receptors for peptides was comparable to that noted in lymph nodes. The secondary follicles displayed SS receptors, perifollicular areas VIP receptors, and vessels, SP receptors. The epithelium overlying mucosal lymphoid tissues are known to allow the transport of antigens. Mucosa-associated cells mainly recirculate within the mucosal lymphoid system.
In the spleen, which responds mainly to blood-borne antigens, the bulk of the lymphoid tissue, arranged around a central SP receptor-positive arteriole in the white pulp, constitutes the periarteriolar lymphoid sheath that contains mainly T lymphocytes and that also displayed VIP receptors. The primary or secondary follicles with a germinal center, attached to the periarteriolar sheath, have the same anatomic features and functions as in lymph nodes, and also display SS receptors. The red pulp contains resident leukocytes and diffusely distributed SS receptors. The venous sinuses in the red pulp bear SP receptors; blood cells can reenter the circulation by crossing the highly discontinuous walls of these sinuses.
In the human thymus, cortex and medulla display a low density of VIP receptors that seems to be associated with T cells. A network of epithelial cells in the lobules, expressing SS receptors in the medulla, plays a role in the thymocytic differentiation process. Cell traffic occurs via high endothelial, SP receptor-positive venules near the corticomedullary junction.
The observed distribution pattern of the respective peptide receptors is compatible with data reported in previous studies obtained by other methods, eg, by the use of isolated cells. For instance, the present findings of a predominant VIP receptor expression in T lymphocytes is compatible with several reports showing that VIP receptors are present in lymphocyte-rich fractions; these T cells have a much higher binding capacity for VIP than non-T cells, and the proportion of these cells in lymph nodes, spleen, and Peyer's patches is higher than in thymus.6,7,37 SS receptors were shown earlier by us to be predominantly expressed by the germinal centers of lymph nodes, tonsils, and Peyer's patches, as well as by the red pulp of the spleen and the medulla of the thymus.4,38 Further, SP receptors were identified previously by us33 and by Tang et al39 in the vascular compartments of lymphoid organs, ie, high endothelial venules and vascular sinuses, rather than in lymphoid cells. Our present results are in agreement with these observations and show, in addition, the presence of SP receptors in the splenic central arteries.
Endogenously synthesized regulatory peptides are likely to be present in sufficient amounts in the immediate vicinity of the reported receptor-expressing targets. Among all the potential sources of VIP, it appears that VIP originating from peripheral nerve endings is the most likely to act on VIP receptor-containing lymphoid cells, as dense VIP-containing fibers were identified in the immediate vicinity of lymphoid organs.40 The localization of these VIP fibers correlate with that of VIP receptors detected in the present study. These findings probably represent the basis for the proposed neuroimmune interactions.2,3 The peripheral nervous system is also likely to be the source for SS acting on germinal centers40 or SP acting on blood vessels.
It is presumably not yet possible, on the basis of these receptor data, to present a unifying concept of the specific actions of these peptides in human lymphoid tissues, as well as their specific interactions, and to bring them together with those proposed from in vivo experiments in animals and humans. Indeed, when interpreting the numerous in vivo studies involving regulatory peptides, we have to be aware of the fact that this class of substances is characterized by multiple actions in man: in addition to immunomodulatory actions, several endocrine, neural, gastrointestinal, vascular, and other actions have been described. In vivo effects of these peptides on immune function may therefore result from indirect effects on secretory cells, nerve cells, or cells of the vascular apparatus. Influences of peptides in vivo on immune functions may also vary with the presence of other signal molecules (ie, other peptides) with the resident cell population in the lymphoid tissue and with the actual microenvironment. The present study has primarily pointed towards a number of specific structures of the lymphoid system where these regulatory peptides may play a predominant role.
Address reprint requests to Jean Claude Reubi, MD, Professor of Pathology, Division of Cell Biology and Experimental Cancer Research, Institute of Pathology, University of Berne, Murtenstrasse 31, CH-3010 Berne, Switzerland.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.



![Fig. 6. Displacement curves of125I-[Ac-His-1]-PACAP(1-27) to tissue sections of a human thymus (A) and a human lymph node (B). Tissue sections are incubated with 15,000 cpm/100 μL radioligand and increasing concentrations of PACAP(1-27) (▴) and VIP (▪), or somatostatin (SS-14) (•) at a concentration of 100 nmol/L. Nonspecific binding was subtracted from all values. In all cases, complete displacement of the ligand is achieved by VIP and PACAP. SS-14 was inactive in the nanomolar range.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/1/10.1182_blood.v92.1.191.413k12_191_197/5/m_blod41312006x.jpeg?Expires=1769148891&Signature=eiMrT2lD2oKAobT~0jVxWW5TSgNoNCE3~7NJyx3rQDVUltjJ6Z7hAH52vU7Gqy41FXksyrx4IFHCMAoBSQLutJva0PwOaekB1DqUu0RtoGp5BshAyWPzHhCoruE3d28ncxnPWyPf5Dqzhb0HgqCefI12cC3GDlUlmnlwTnqy9FU7J6xm4t0f4AVC1yJqjmy~a9CXYBMZFju4lGJow7vDNGHoekIJZ8rMrlIZHCtMlNU-yAO06V5sr8d0QKdWdfZne1F0SbgdPgqon0O4Ma9K1~C6GGp-8TbeUo96hv~q46yEjYSqSZtkdUDcdT0YkNr~-2I05jk~m5P0~7CAhnT-WA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal