As an important determinant of response to chemotherapy, accurate measurement of cellular drug resistance may provide clinically relevant information. Our objectives in this study were to determine the relationship between in vitro resistance to prednisolone (PRD) measured with the colorimetric methyl-thiazol-tetrazolium (MTT) assay, and (1) short-term clinical response to systemic PRD monotherapy, (2) long-term clinical outcome after combination chemotherapy within all patients and within the subgroups of clinical good and poor responders to PRD, and (3) in vitro resistance to 12 other drugs in 166 children with newly diagnosed acute lymphoblastic leukemia (ALL). The 12 clinical poor PRD responders had ALL cells that were median 88-fold more in vitro resistant to PRD than 131 good responders (P = .013). Within all patients, increased in vitro resistance to PRD predicted a significantly worse long-term clinical outcome, at analyses with and without stratification for clinical PRD response, and at multivariate analysis (P ≤ .001). Within both the clinical good and poor responder subgroups, increased in vitro resistance to PRD was associated with a worse outcome, which was significant within the group of clinical good responders (P < .001). LC50 values, ie, lethal concentrations to 50% of ALL cells, for PRD and each other drug correlated significantly with those of all other 12 drugs, with an average correlation coefficient of 0.44 (standard deviation 0.05). The highest correlations were found between structurally related drugs. In conclusion, in vitro resistance to PRD was significantly related to the short-term and long-term clinical response to chemotherapy, the latter also within the subgroup of clinical good responders to PRD. There was a more general in vitro cross-resistance between anticancer drugs in childhood ALL, although drug-specific activities were recognized.
THEORETICALLY, CELLULAR drug resistance together with pharmacokinetics and the regrowth potential of minimal residual leukemic cells determines the clinical outcome after chemotherapy in leukemia. We have therefore adapted the short-term total cell kill methyl-thiazol-tetrazolium (MTT) assay for accurate measurements of cellular drug resistance in childhood and adult acute lymphoblastic leukemia (ALL) and nonlymphoblastic (ANLL) leukemia samples.1-4 Current combination chemotherapy regimens generally do not allow for single agent vitro-vivo comparisons. However, the German Berlin-Frankfurt-Münster (BFM)-ALL Group introduced a therapeutic window in its protocols, with 1 week of systemic predniso(lo)ne (PRD) monotherapy as an initial treatment of all patients.5 It appeared that the short-term clinical response to PRD was related to the long-term clinical outcome after combination chemotherapy, and in current BFM-based protocols, initial clinical response to PRD is one of the factors for risk-group stratification.6
The therapeutic window enabled a study on the relation between the in vitro antileukemic activity of PRD and the clinical response to an initial 1 week PRD therapy in children with newly diagnosed ALL. We also studied the relationship between in vitro PRD resistance and clinical outcome after combination chemotherapy, especially within the subgroups of clinically good and poor PRD responders. Finally, we studied the in vitro cross-resistance patterns between PRD and 12 other drugs. The relationship between resistance to each of the single drugs and a drug resistance profile and long-term clinical outcome after combination chemotherapy has been described separately.7
MATERIALS AND METHODS
Patients and patient samples.
Children (age 0 to 18 years) with non-B ALL, newly diagnosed between 1989 and 1994 were eligible. Bone marrow (BM) and peripheral blood (PB) samples, and smears of 306 patients were sent by local institutions to the Dutch Childhood Leukemia Study Group (DCLSG) laboratory for confirmation of the diagnosis ALL, French-American-British (FAB) classification8 and immunophenotyping as described previously.9 The DCLSG also calculated the BFM risk factor (based on peripheral leukemic cell count and liver and spleen size.10 Fresh samples with sufficient cells of 190 children were sent by the DCLSG laboratory to the research laboratory for pediatric hematology/oncology of the University Hospital Vrije Universiteit in Amsterdam for in vitro drug resistance testing, successfully performed in 166 of 190 (87%) children. We have recently described this process and consequences of selection of cases in a previous study.7 The selected group of patients tested for in vitro drug resistance has a significantly higher leukemic cell burden, more often concerns T-cell immunophenotype, and has a worse long-term clinical outcome than the total group of ALL patients. Moreover, assay failures more often concern DNA hyperdiploid cases.7 Characteristics of the 166 patients are shown in Table 1. Results for PRD of 93 of these 166 samples have been used in a previous preliminary meeting report limited to the short-term response to PRD only,11 while 152 of the 166 samples have also been used in our study on drug resistance versus long-term clinical outcome after combination chemotherapy (excluding in that study the 14 patients who were not treated according to protocol after the systemic PRD monotherapy).7 Hyperdiploidy was defined as a DNA index of 1.16 to 1.35, nonhyperdiploidy as a DNA index of <1.16 or >1.35, as determined by flow cytometry.12
Characteristics of 166 Children With Newly Diagnosed ALL in Whom In Vitro Drug Resistance Was Successfully Assessed
| Age in months, median (range) | 62 (2-190) |
| Female/male ratio | 71:95 |
| WBC × 109/L, median (range) | 24.1 (0.5-900) |
| BFM risk factor-150, median (range) | 1.05 (0.31-2.03) |
| FAB type | |
| L1 | 133 |
| L2 | 30 |
| AUL | 3 |
| Immunophenotype | |
| Pro-B | 6 |
| Common | 86 |
| Pre-B | 38 |
| T | 36 |
| DNA ploidy | |
| Nonhyperdiploid | 130 |
| Hyperdiploid | 20 |
| Unknown | 16 |
| Follow-up in months, median (range) | 46 (17-81) |
| Clinical response to PRD monotherapy | |
| Good | 144 |
| Poor | 12 |
| Unknown | 10 |
| Events | |
| No complete remission | 1 |
| Relapse | 43 |
| Early death | 2 |
| Toxic death | 3 |
| No event | 103 |
| Lost to follow-up or otherwise not evaluable | 14 |
| Age in months, median (range) | 62 (2-190) |
| Female/male ratio | 71:95 |
| WBC × 109/L, median (range) | 24.1 (0.5-900) |
| BFM risk factor-150, median (range) | 1.05 (0.31-2.03) |
| FAB type | |
| L1 | 133 |
| L2 | 30 |
| AUL | 3 |
| Immunophenotype | |
| Pro-B | 6 |
| Common | 86 |
| Pre-B | 38 |
| T | 36 |
| DNA ploidy | |
| Nonhyperdiploid | 130 |
| Hyperdiploid | 20 |
| Unknown | 16 |
| Follow-up in months, median (range) | 46 (17-81) |
| Clinical response to PRD monotherapy | |
| Good | 144 |
| Poor | 12 |
| Unknown | 10 |
| Events | |
| No complete remission | 1 |
| Relapse | 43 |
| Early death | 2 |
| Toxic death | 3 |
| No event | 103 |
| Lost to follow-up or otherwise not evaluable | 14 |
Abbreviation: AUL, acute undifferentiated leukemia.
A measure for the leukemic cell burden, based on peripheral leukemic cell count and liver and spleen size, as developed by the German Berlin-Frankfurt-Münster Group.10
In vitro drug resistance.
This was measured with the cell culture MTT assay.2 BM and PB ALL samples were evaluated together because they do not differ in drug resistance.13 Fresh (noncryopreserved) leukemic cells were cultured in RPMI 1640 (Dutch modification; GIBCO, Uxbridge, UK) containing fetal calf serum and other supplements.2
Thirteen drugs were tested, each at six concentrations, as described previously.13 Methotrexate was not included in the panel because this drug is not cytotoxic to human leukemia samples in nonclonogenic assays.2,13 Leukemic cells were incubated with each drug at each concentration in duplicate in wells of microculture plates at 37°C in humidified air with 5% CO2. Six wells contained leukemic cells in drug-free medium to determine the control cell survival and the percentage of leukemic cells after culture. Six wells contained medium only to blank the spectrophotometer. After 4 days, 10 μL (5 mg/mL) MTT salt (Sigma Chemical Corp, St Louis, MO) was added for 6 hours. MTT is reduced to colored formazan crystals by living cells only. The crystals were dissolved with 100 μL acidified isopropanol, and formazan production was quantitated using a spectrophotometer at 562 nm. The optical density (OD) is linearily related to the cell number.14Leukemic cell survival (LCS) was calculated at each drug concentration by the equation LCS = (OD treated well/mean OD control wells) × 100%. The drug concentration lethal to 50% of the ALL cells, the LC50, was used as measure of resistance. Samples were considered evaluable if the drug-free control wells contained ≥80% leukemic cells before and ≥70% leukemic cells after 4 days of culture (determined by morphology and occasionally in case of doubt, by immunology) and if the control OD at day 4 exceeded 0.050. The MTT assay gives reliable results under these conditions.2,15The coefficient of variation of the OD of the control wells in the successful assays is median 5.2% (range, 0.9% to 15.3%). The intraassay variation (duplicates) and interassay variation (repeated testing of frozen sample) in LC50 values for all drugs are well within one dilution step.16
Treatment.
Patients were treated according to DCLSG protocols (ALL-VII and ALL-VIII). All patients first received a 1-week systemic monotherapy with PRD (60 mg/m2/day), and one injection with methotrexate (the dose being age-dependent) intrathecally at day 1 of the PRD window. Patients were then stratified into one of three risk groups, according to several factors including BFM risk factor, immunophenotype, extramedullary disease, karyotype, clinical response to PRD (if poor, high risk), and achievement of complete remission (CR).
Treatment outcome.
The results of treatment were evaluated at the DCLSG operations office. Routine BM, PB, and cerebrospinal fluid examinations were performed at the central laboratory of the DCLSG during chemotherapy and up to 3 years after cessation of therapy. Patients were divided into clinical PRD poor responders (≥1,000 leukemic cells/μL PB at day 8) and good responders (<1,000 leukemic cells/μL PB at day 8) by protocol definition, independent of the number of leukemic cells/μL PB at the start of this treatment. CR was defined as less than 5% leukemic blasts in representative BM containing megakaryocytes and granulocytic precursors with some degree of maturation, and no manifestation of leukemia elsewhere. Failure to achieve CR after induction chemotherapy (induction failure) was considered an event at day 0. Early death was defined as death before completion of induction therapy. Disease-free survival (DFS) was defined as the time from diagnosis to induction failure or relapse (leukemia-related events). For estimation of DFS, toxic deaths in remission were censored at the time of occurrence and early deaths at day 0, and only patients eligible and treated according to one of the two DCLSG protocols were included. Patients who were disease-free were censored at the time of latest follow-up as evaluated at this planned analysis. The relation between in vitro PRD resistance or clinical PRD response and long-term clinical outcome was studied only within the 152 patients without protocol violation after the systemic PRD monotherapy.
Statistics.
Differences in distribution of variables were tested with the Mann-Whitney U (MWU) test or the χ2 test. Estimates of the probability (p) of DFS (with standard errors [SE]) were calculated according to the Kaplan-Meier product limit analysis.17 Because toxic and early deaths presumably are unrelated to cellular drug resistance, in contrast to induction failures and relapses, results of DFS analysis are shown. Univariate and multivariate statistical comparisons of outcome were conducted by proportional hazard Cox regression analysis, after stratification for risk group or clinical PRD response where appropriate.18The model for multivariate analysis included the conventional prognostic factors age, BFM risk factor, immunophenotype, DNA ploidy, clinical PRD response, and in vitro drug resistance. Information on karyotype was not available for the majority of patients and therefore was not included in this analysis. Cross-resistance patterns were studied by correlating the LC50 values for different drugs, using the Spearman test. Means were calculated with standard deviations (SD) for the correlation coefficients. The analyses were two-tailed at a significance level of 5%.
RESULTS
In vitro resistance to PRD versus short-term clinical response to PRD.
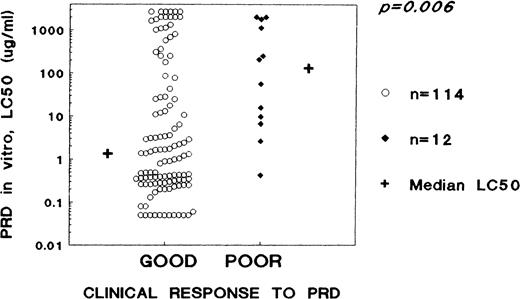
The clinical response to the window therapy with PRD was known in 156 patients. Differences between the good and poor responders are outlined in Table 2. Poor responders more often were male (although not significantly), had a higher leukemic cell burden (P = .024) and more often an unfavorable (pro-B or T-cell) immunophenotype (P < .001). The 12 (8%) clinical poor responders to PRD had significantly (P = .013) higher LC50 values for PRD (median, 130.9; range, 0.43 to >2,000 μg/mL) than the 131 good responders (median, 1.49; range, <0.06 to >2,000) tested successfully for PRD resistance. However, 17 (13%) of the 131 good responders had less than 1,000 leukemic cells/mm3 PB at day 0, but were by definition classified as clinical good responders. After omitting these 17 patients from the analysis to allow a more meaningful comparison, the 12 in vivo poor responders (10%) had a 97-fold higher median LC50 for PRD than the 114 good responders (P = .006, Fig 1 and Table 2). Relapses occurred in five of these 17 patients, and all five were highly in vitro PRD-resistant with LC50 values ≥250 μg/mL, compared with a median LC50 value of 2.1 μg/mL for the remaining 12 patients. Overall, events did not occur significantly more frequent in the group of clinical poor PRD responders (P = .08, Table2), and the far majority of relapses were seen within the group of clinical good PRD responders.
Characteristics and Clinical Outcome of 156 Children With ALL
| . | Clinical PRD Response . | P Value . | |
|---|---|---|---|
| Good . | Poor . | ||
| No. of patients | 144 | 12 | |
| Age in months, median (range) | 61 (3-190) | 86 (2-163) | .57 |
| Female/male ratio | 64/80 | 3/9 | .19 |
| WBC × 109/L, median (range) | 19.2 (0.5-900) | 129.7 (43-746) | <.001 |
| BFM risk factor,* median (range) | 1.0 (0.3-2.0) | 1.3 (0.9-1.9) | .024 |
| FAB type | .60† | ||
| L1 | 117 | 9 | |
| L2 | 27 | 2 | |
| AUL‡ | 0 | 1 | |
| Immunophenotype | <.001 | ||
| Pro-B | 4 | 2 | |
| Common | 81 | 0 | |
| Pre-B | 38 | 0 | |
| T | 21 | 10 | |
| DNA ploidy | .45 | ||
| Nonhyperdiploid | 112 | 11 | |
| Hyperdiploid | 18 | 1 | |
| Not yet available | 14 | 0 | |
| LC50 values PRD, median (range) (μg/mL) | 1.35 (<0.06->2,000) | 130.9 (0.43->2,000) | .0061-153 |
| Follow-up, median (range) (in months) | 46 (17-81) | 65 (40-79) | .15 |
| Events | .081-155 | ||
| No complete remission | 0 | 1 | |
| Relapse | 38 | 5 | |
| Early death | 0 | 0 | |
| Toxic death | 2 | 1 | |
| No event | 97 | 5 | |
| Lost to follow-up or otherwise not evaluable | 7 | 0 | |
| . | Clinical PRD Response . | P Value . | |
|---|---|---|---|
| Good . | Poor . | ||
| No. of patients | 144 | 12 | |
| Age in months, median (range) | 61 (3-190) | 86 (2-163) | .57 |
| Female/male ratio | 64/80 | 3/9 | .19 |
| WBC × 109/L, median (range) | 19.2 (0.5-900) | 129.7 (43-746) | <.001 |
| BFM risk factor,* median (range) | 1.0 (0.3-2.0) | 1.3 (0.9-1.9) | .024 |
| FAB type | .60† | ||
| L1 | 117 | 9 | |
| L2 | 27 | 2 | |
| AUL‡ | 0 | 1 | |
| Immunophenotype | <.001 | ||
| Pro-B | 4 | 2 | |
| Common | 81 | 0 | |
| Pre-B | 38 | 0 | |
| T | 21 | 10 | |
| DNA ploidy | .45 | ||
| Nonhyperdiploid | 112 | 11 | |
| Hyperdiploid | 18 | 1 | |
| Not yet available | 14 | 0 | |
| LC50 values PRD, median (range) (μg/mL) | 1.35 (<0.06->2,000) | 130.9 (0.43->2,000) | .0061-153 |
| Follow-up, median (range) (in months) | 46 (17-81) | 65 (40-79) | .15 |
| Events | .081-155 | ||
| No complete remission | 0 | 1 | |
| Relapse | 38 | 5 | |
| Early death | 0 | 0 | |
| Toxic death | 2 | 1 | |
| No event | 97 | 5 | |
| Lost to follow-up or otherwise not evaluable | 7 | 0 | |
Divided into two groups according to their clinical response to a 1-week systemic PRD monotherapy: good responders (<1,000 blasts/μL PB at day 8) and poor responders (≥1,000 blasts/μL PB at day 8 of treatment).
A measure for the leukemic cell burden, based on peripheral leukemic cell count, and liver and spleen size.10
Comparison of FAB L1 versus no L1.
Acute undifferentiated leukemia.
The 17 patients with <1,000 blasts/μL blood at the start of treatment are excluded from this analysis to perform the most meaningful vitro-vivo correlation. However, including these patients still results in a significant 88-fold difference at P = .013.
Comparison of yes versus no complete remission or relapse.
Distribution of LC50 values as a measure of in vitro resistance to PRD within potentially clinically poor PRD responders (blasts at day 0 ≥1,000/μL of peripheral blood) in clinical good (blasts day 8 of <1,000/μL, n = 114) and clinical poor (blasts day 8 of ≥1,000/μL, n = 12) responders to a 1-week systemic PRD monotherapy (plus one intrathecal injection with methotrexate at day 1) in childhood ALL.
Distribution of LC50 values as a measure of in vitro resistance to PRD within potentially clinically poor PRD responders (blasts at day 0 ≥1,000/μL of peripheral blood) in clinical good (blasts day 8 of <1,000/μL, n = 114) and clinical poor (blasts day 8 of ≥1,000/μL, n = 12) responders to a 1-week systemic PRD monotherapy (plus one intrathecal injection with methotrexate at day 1) in childhood ALL.
In vitro resistance to PRD versus long-term clinical outcome after combination chemotherapy.
The extent of in vitro resistance to PRD was known in 153 of the 166 patients. Table 3 outlines the differences between in vitro PRD sensitive (LC50 < 150 μg/mL) and in vitro PRD-resistant (LC50 ≥ 150 μg/mL) patients. This definition of sensitivity versus resistance is based on our previous retrospective study.19 The in vitro resistant group more often had pro-B or T-cell ALL (P = .007), and less often DNA hyperdiploidy (P = .03). In vitro PRD-resistant patients more often had a clinical poor response to PRD then in vitro PRD sensitive patients (P = .04). Finally, the frequency of no CR and relapses was higher in the in vitro PRD-resistant subgroup (P = .002).
Characteristics and Clinical Outcome of 153 Children With ALL
| . | In Vitro PRD Resistance Assay . | P Value . | |
|---|---|---|---|
| Sensitive . | Resistant . | ||
| No. of patients | 108 | 45 | |
| Age in months, median (range) | 61 (4-190) | 70 (2-184) | .71 |
| Female/male ratio | 44/64 | 21/24 | .50 |
| WBC × 109/L, median (range) | 21.9 (1.7-519) | 37.2 (2.7-900) | .17 |
| BFM risk factor,* median (range) | 1.0 (0.3-1.9) | 1.1 (0.4-2.0) | .19 |
| FAB type | .19 | ||
| L1 | 84 | 38 | |
| L2 | 23 | 5 | |
| AUL† | 1 | 2 | |
| Immunophenotype | .007 | ||
| Pro-B | 3 | 3 | |
| Common | 55 | 22 | |
| Pre-B | 32 | 4 | |
| T | 18 | 16 | |
| DNA ploidy | .03‡ | ||
| Nonhyperdiploid | 85 | 36 | |
| Hyperdiploid | 17 | 1 | |
| Unknown | 6 | 8 | |
| Clinical response to PRD2-153 | .042-155 | ||
| Good | 97 | 34 | |
| *Blasts day 0 ≥ 1,000/μL | 88 | 26 | |
| *Blasts day 0 < 1,000/μL | 9 | 8 | |
| Poor | 6 | 6 | |
| Unknown | 5 | 5 | |
| Events | .002# | ||
| No complete remission | 0 | 1 | |
| Relapse | 24 | 18 | |
| Early death | 1 | 1 | |
| Toxic death | 3 | 0 | |
| None | 74 | 17 | |
| Lost to follow-up or otherwise not evaluable | 6 | 8 | |
| . | In Vitro PRD Resistance Assay . | P Value . | |
|---|---|---|---|
| Sensitive . | Resistant . | ||
| No. of patients | 108 | 45 | |
| Age in months, median (range) | 61 (4-190) | 70 (2-184) | .71 |
| Female/male ratio | 44/64 | 21/24 | .50 |
| WBC × 109/L, median (range) | 21.9 (1.7-519) | 37.2 (2.7-900) | .17 |
| BFM risk factor,* median (range) | 1.0 (0.3-1.9) | 1.1 (0.4-2.0) | .19 |
| FAB type | .19 | ||
| L1 | 84 | 38 | |
| L2 | 23 | 5 | |
| AUL† | 1 | 2 | |
| Immunophenotype | .007 | ||
| Pro-B | 3 | 3 | |
| Common | 55 | 22 | |
| Pre-B | 32 | 4 | |
| T | 18 | 16 | |
| DNA ploidy | .03‡ | ||
| Nonhyperdiploid | 85 | 36 | |
| Hyperdiploid | 17 | 1 | |
| Unknown | 6 | 8 | |
| Clinical response to PRD2-153 | .042-155 | ||
| Good | 97 | 34 | |
| *Blasts day 0 ≥ 1,000/μL | 88 | 26 | |
| *Blasts day 0 < 1,000/μL | 9 | 8 | |
| Poor | 6 | 6 | |
| Unknown | 5 | 5 | |
| Events | .002# | ||
| No complete remission | 0 | 1 | |
| Relapse | 24 | 18 | |
| Early death | 1 | 1 | |
| Toxic death | 3 | 0 | |
| None | 74 | 17 | |
| Lost to follow-up or otherwise not evaluable | 6 | 8 | |
Divided into two groups according to the extent of in vitro resistance to PRD: highly and intermediately sensitive (LC50 < 150 μg/mL) and resistant (LC50 ≥ 150 μg/mL).
A measure for the leukemic cell burden, based on peripheral leukemic cell count, and liver and spleen size.10
Acute undifferentiated leukemia.
Analysis excluding cases with unknown ploidy.
Determined after 1 week systemic PRD monotherapy (plus one intrathecal injection with methotrexate) by the number of blasts/μL peripheral blood (if < 1,000 = > good responder, if ≥ 1,000 = > poor responder).
Comparison of the good versus poor responders within potentially poor responders only (>1,000 blasts/μL peripheral blood at start of systemic PRD monotherapy).
#Comparison of yes versus no complete remission or relapse.
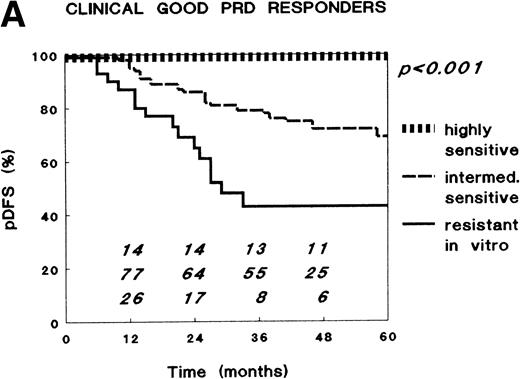
Patients were further classified as in vitro highly sensitive (LC50 <0.1 μg/mL), intermediately sensitive (LC50 0.1 to 150 μg/mL), or in vitro resistant (LC50 ≥150 μg/mL) to PRD according to the definition in our retrospective study.19 In the total patient-group, in vitro PRD resistance was related to the long-term clinical outcome after combination chemotherapy at a median follow-up of 46 months (range, 17 to 79) for patients at risk, at univariate analysis both without any stratification and with stratification for risk-group or clinical response to the PRD monotherapy (P < .001), and at multivariate analysis (P = .001, Table 4). The pDFS progressively decreased from highest (100%) in those patients with highly sensitive ALL-cells to lowest (42%, SE 10%) in those with resistant ALL-cells. This has been described by us in detail previously.7 However, within the subgroup of clinical good PRD responders, again, the extent of in vitro PRD resistance was related to the clinical outcome (Fig 2). Within this subgroup, the pDFS at 3 years was 100% for the in vitro PRD highly sensitive patients, 79% (SE, 5%) for the intermediately sensitive patients, and 43% (SE, 11%) for the in vitro PRD-resistant patients (P < .001 at univariate, risk-group stratified analysis, and P = .002 at multivariate analysis, Table 4). Apparently, relapses that had not been predicted by the clinical response to PRD were predicted by the in vitro antileukemic activity of PRD. Within the small subgroup of clinical poor PRD responders, none of the patients had ALL cells that were highly sensitive to PRD in vitro. At 3 years, the pDFS was 60% (SE 22%) for in vitro intermediately sensitive patients (n = 6) and 33% (SE 19%) for the remaining group of in vitro resistant patients (P = .29). Similarly, LC50 values for PRD were median sevenfold lower in the six patients who did achieve CR and did not suffer a relapse (median, 32.7 μg/mL) than in the remaining six clinical poor responders who did not achieve CR (n = 1) or relapsed (median, 229 μg/mL), although this again was not a statistically significant difference.
Multivariate Analysis of the Relationship Between Several Potential Prognostic Factors and the Probability of Leukemia-Related Events in the Total Group of Children With ALL and Within the Subgroup of Clinical Good PRD Responders
| Factor . | P Values . | |
|---|---|---|
| Total Group . | Only Clinical Good PRD Responders . | |
| Age (<2 or ≥10 yr v 2 to 9 yr) | .49 | .38 |
| BFM risk factor* (≥1.20 v <1.20) | .36 | .21 |
| Immunophenotype (pro-B/T-cell v common/pre-B) | .83 | .70 |
| DNA ploidy (nonhyperdiploid v hyperdiploid) | .63 | .97 |
| Clinical PRD response (poor v good) | .23 | — |
| In vitro PRD resistance (resistant vintermediately sensitive v highly sensitive) | .001 | .002 |
| Factor . | P Values . | |
|---|---|---|
| Total Group . | Only Clinical Good PRD Responders . | |
| Age (<2 or ≥10 yr v 2 to 9 yr) | .49 | .38 |
| BFM risk factor* (≥1.20 v <1.20) | .36 | .21 |
| Immunophenotype (pro-B/T-cell v common/pre-B) | .83 | .70 |
| DNA ploidy (nonhyperdiploid v hyperdiploid) | .63 | .97 |
| Clinical PRD response (poor v good) | .23 | — |
| In vitro PRD resistance (resistant vintermediately sensitive v highly sensitive) | .001 | .002 |
*Based on peripheral leukemic cell count and liver and spleen size.10
Relationship between in vitro PRD resistance (cut-off values for sensitivity and resistance 0.1 and 150 μg/mL, respectively) and long-term clinical outcome after combination chemotherapy in childhood ALL, within (A) the total group of clinical good PRD responders (15 highly sensitive, 79 intermediately sensitive, and 30 resistant to PRD in vitro), and (B) the group of clinical poor PRD responders (none highly sensitive, six intermediately sensitive, and six resistant to PRD in vitro). The numbers in the figures along the x-axis indicate the patients at risk for a relapse at the different time points.
Relationship between in vitro PRD resistance (cut-off values for sensitivity and resistance 0.1 and 150 μg/mL, respectively) and long-term clinical outcome after combination chemotherapy in childhood ALL, within (A) the total group of clinical good PRD responders (15 highly sensitive, 79 intermediately sensitive, and 30 resistant to PRD in vitro), and (B) the group of clinical poor PRD responders (none highly sensitive, six intermediately sensitive, and six resistant to PRD in vitro). The numbers in the figures along the x-axis indicate the patients at risk for a relapse at the different time points.
In vitro cross-resistance patterns.
LC50 values of the different drugs could be correlated for 104 up to 142 samples, except for ifosfamide. For the latter drug, being included in the drug panel later on in the study, this was possible for 48 to 60 samples. There was a significant cross-resistance between all drugs (Table 5). However, cross-resistance between structurally related drugs was more pronounced, eg, for the glucocorticoids, the vinca-alkaloids, the anthracyclines, and the thiopurines. There was a relatively high degree of cross-resistance between the epipodophyllotoxin teniposide (VM-26) and the three anthracyclines tested, and between the vinca-alkaloids and the anthracyclines. For prednisolone, the correlation coefficients ranged from 0.30 (vindesine and ifosfamide) to 0.84 (dexamethasone), with an average of 0.44 (SD 0.14). For the other drugs, the mean correlation coefficient ranged from 0.38 (l-asparaginase) to 0.53 (doxorubicin), with an overall mean correlation coefficient of 0.44 (SD 0.05).
Correlation Between LC50 Values in 166 Childhood ALL Samples Expressed by Spearman's Rank Correlation Coefficients
| . | DXM . | ASP . | VCR . | VDS . | DNR . | DOX . | MIT . | 6MP . | 6TG . | ARA . | TEN . | IFM . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRD | 0.84 | 0.42 | 0.38 | 0.30 | 0.45 | 0.47 | 0.46 | 0.43 | 0.41 | 0.36 | 0.46 | 0.30 |
| DXM | — | 0.41 | 0.34 | 0.38 | 0.57 | 0.44 | 0.44 | 0.41 | 0.36 | 0.29 | 0.40 | 0.28 |
| ASP | — | 0.34 | 0.30 | 0.33 | 0.37 | 0.31 | 0.49 | 0.49 | 0.31 | 0.24 | 0.53 | |
| VCR | — | 0.72 | 0.45 | 0.53 | 0.59 | 0.32 | 0.38 | 0.49 | 0.49 | 0.41 | ||
| VDS | — | 0.42 | 0.62 | 0.62 | 0.29 | 0.35 | 0.40 | 0.62 | 0.42 | |||
| DNR | — | 0.62 | 0.59 | 0.22 | 0.36 | 0.35 | 0.57 | 0.41 | ||||
| DOX | — | 0.79 | 0.36 | 0.49 | 0.39 | 0.74 | 0.48 | |||||
| MIT | — | 0.31 | 0.38 | 0.38 | 0.72 | 0.47 | ||||||
| 6MP | — | 0.60 | 0.49 | 0.40 | 0.31 | |||||||
| 6TG | — | 0.42 | 0.46 | 0.43 | ||||||||
| ARA | — | 0.45 | 0.46 | |||||||||
| TEN | — | 0.49 |
| . | DXM . | ASP . | VCR . | VDS . | DNR . | DOX . | MIT . | 6MP . | 6TG . | ARA . | TEN . | IFM . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRD | 0.84 | 0.42 | 0.38 | 0.30 | 0.45 | 0.47 | 0.46 | 0.43 | 0.41 | 0.36 | 0.46 | 0.30 |
| DXM | — | 0.41 | 0.34 | 0.38 | 0.57 | 0.44 | 0.44 | 0.41 | 0.36 | 0.29 | 0.40 | 0.28 |
| ASP | — | 0.34 | 0.30 | 0.33 | 0.37 | 0.31 | 0.49 | 0.49 | 0.31 | 0.24 | 0.53 | |
| VCR | — | 0.72 | 0.45 | 0.53 | 0.59 | 0.32 | 0.38 | 0.49 | 0.49 | 0.41 | ||
| VDS | — | 0.42 | 0.62 | 0.62 | 0.29 | 0.35 | 0.40 | 0.62 | 0.42 | |||
| DNR | — | 0.62 | 0.59 | 0.22 | 0.36 | 0.35 | 0.57 | 0.41 | ||||
| DOX | — | 0.79 | 0.36 | 0.49 | 0.39 | 0.74 | 0.48 | |||||
| MIT | — | 0.31 | 0.38 | 0.38 | 0.72 | 0.47 | ||||||
| 6MP | — | 0.60 | 0.49 | 0.40 | 0.31 | |||||||
| 6TG | — | 0.42 | 0.46 | 0.43 | ||||||||
| ARA | — | 0.45 | 0.46 | |||||||||
| TEN | — | 0.49 |
All correlations were statistically significant, and printed bold if >.50.
Abbreviations: PRD, prednisolone; DXM, dexamethasone; ASP, l-asparaginase; VCR, vincristine; VDS, vindesine; DNR, daunorubicin; DOX, doxorubicin; MIT, mitoxantrone; 6MP, mercaptopurine; 6TG, thioguanine; ARA, cytarabine; TEN, teniposide; IFM, 4-hydroperoxy-ifosfamide.
DISCUSSION
Cellular drug resistance is an important cause of the ultimate failure of chemotherapy. Therefore, the accurate measurement of cellular drug resistance on primary clinical specimens is of clinical importance. For that purpose, we have adapted the short-term semiautomated colorimetric MTT assay for its use on clinical specimens of children and adults with ALL and ANLL and have shown the clinical relevance of the results obtained so far.3,4,7,16,19-23 With regard to glucocorticoids, we showed that unfavorable subgroups as defined by age (infants or >10 years at diagnosis), immunophenotype (pro-B, T-cell ALL), leukemia type (ANLL), or disease status (relapses) are relatively resistant to PRD in vitro, and that the extent of in vitro PRD resistance is related to the long-term clinical outcome after combination chemotherapy.24,25 In the present study, again pro-B and T-cell immunophenotype was associated with a relative PRD resistance, both clinically and in vitro. The reason for this is unknown, but it is not related to decreased numbers of glucocorticoid receptors.24 Rather, it seems that ALL cells of these immunophenotypes show a more general drug resistance, because we showed that pro-B and T-cell ALL samples were also more resistant to a large number of drugs other than glucocorticoids in comparison with common and pre-B ALL samples.20 23
In this study on 166 untreated childhood ALL samples, we have shown that in vitro PRD resistance is significantly related to the short-term response to a systemic 1 week PRD monotherapy (Fig 1) and to the clinical outcome after combination chemotherapy (Fig 2), and that these ALL cells have a general cross-resistance, with drug type-specific patterns (Table 5). The relationship between in vitro PRD resistance and clinical outcome was significant also within the subgroup of clinical good PRD responders, in which the far majority of relapses occurred (Fig 2). Apparently, in vitro PRD resistance predicts relapses not identified by the clinical response to the PRD window. Within the subgroup of 12 clinical poor PRD responders, in vitro PRD-resistant patients also had a worse outcome than the in vitro sensitive patients, but this was not significant in this low number of cases. Therefore, this study is inconclusive with respect to the prognostic significance of in vitro PRD resistance within the subgroup of clinical poor PRD responders.
We previously showed that in addition to PRD, the extent of in vitro resistance to asparaginase and vincristine was also significantly related to the long-term clinical outcome in childhood ALL. In that study, we also showed that combining the results for these three drugs provided a drug resistance profile, which was the single most important prognostic factor at multivariate analysis, and a (slightly) better prognostic indicator than in vitro resistance to each of the single drugs.7 A drawback of the previous and present study is that karyotype was unknown in most patients and could not be included in the multivariate analyses. Although we did show that combinations with two drugs can be tested in vitro in the MTT assay,26 it does not seem feasible to mimick clinical combination chemotherapy in in vitro cellular drug resistance testing.
Clinical poor PRD responders were nearly 100-fold more resistant to PRD in vitro than the good responders, but there were many clinical good PRD responders who were relatively resistant to PRD in vitro. There are several possible explanations for this. First, 17 patients were not actually tested clinically for their PRD responsiveness because they had less than 1,000 blasts/μL blood before the start of the systemic PRD monotherapy. Of interest, five of these 17 patients relapsed, and all five had ALL cells highly resistant to PRD in vitro. Second, the systemic PRD monotherapy was accompanied by one intrathecal injection with methotrexate at day 1. It is well-known that methotrexate delivered intrathecally has systemic antileukemic activity27,28 up to inducing a tumor-lysis syndrome.29 The Children's Leukemia Cooperative Group recently reported that the percentage of clinical good responders to PRD alone was 70% and increased to 90.4% if methotrexate had been delivered before day 2 of the therapeutic window.30Preliminary data of our laboratory (not shown) showed that clinically good responders to systemic PRD plus intrathecal methotrexate with in vitro PRD-resistant ALL cells were not resistant to methotrexate in vitro, and there was no significant in vitro cross-resistance between PRD and methotrexate. Therefore, methotrexate may indeed have contributed to the good clinical response in this group of patients. In addition to the systemic antileukemic activity of intrathecal methotrexate itself, a synergistic interaction with PRD may contribute to a good clinical response in the case of relatively PRD-resistant ALL cells in vitro. Third, while our in vitro assay tests for cell death, counting the absolute number of blasts in the peripheral blood may speculatively be influenced by a temporary redistribution of the blasts under the influence of PRD. This has been described to occur for normal lymphocytes.31 Finally, some of the assay results may have been unreliable, although we do apply strict rules for evaluability. Whatever the explanation, in vitro PRD resistance was the single most important prognostic factor at multivariate analysis with respect to long-term clinical outcome after combination chemotherapy within the total group and within the clinical good PRD responders.
Part of the clinical poor PRD responders were in vitro intermediately (never highly) sensitive to PRD. In these cases, unfavorable pharmacokinetics may have caused a relatively low exposure of the ALL cells to PRD, contributing to the poor clinical response (“pharmacokinetic resistance”). In addition, an antagonistic interaction between PRD and methotrexate in individual patients may have occurred. Finally, it should be noted that the applied arbitrary definition of in vitro PRD resistance versus sensitivity was based on our previous retrospective study on the relationship between in vitro PRD resistance and long-term clinical outcome after combination chemotherapy19 and not on a proper single agent vitro-vivo comparison of the antileukemic activity of PRD. However, it seems appropriate to consider patients with ALL cells that are highly or intermediately sensitive to PRD in vitro as relatively sensitive to PRD clinically, in view of the 70% clinical response rate to single agent PRD in the study of the Children's Leukemia Cooperative Group.30
ALL cells were cross-resistant to all types of drugs, although not strongly, with an average correlation coefficient for PRD of 0.44. The extent of cross-resistance was more pronounced between structurally related drugs. Specific drug targets may be more accessible in ALL cells of certain patients than in others, and mechanisms of resistance affecting specific types of drugs may be operative. Regarding the latter, P-glycoprotein is unlikely to be a major cause of resistance in untreated childhood ALL.25 The lung-resistance protein, which may alter the subcellular drug distribution, is a candidate.32 We speculate that the general cross-resistance pattern that we observed is in agreement with our current knowledge on apoptosis and a trigger-independent common pathway leading to cell death.
In conclusion, in vitro PRD resistance is related to the short-term response to systemic PRD monotherapy. This has been previously reported for children and adults,33-35 although some could not confirm this.36-38 In vitro PRD resistance is also related to the long-term clinical response to combination chemotherapy, even within the subgroup of clinical good PRD responders and was the single most important prognostic factor at multivariate analysis. This may indicate the essential contribution of glucocorticoids to the successful chemotherapy of ALL and may also be partly explained by the cross-resistance between PRD and other drugs. ALL cells show a general cross-resistance to all drugs, but drug-specific patterns can be recognized. Cross-resistance patterns of new drugs can be tested in vitro. The use of noncross-resistant drug combinations may offer therapeutic advantage.39 In vitro drug resistance can be used as a prognostic factor, and in vitro PRD resistance adds prognostic value to that of the clinical PRD response. Within the setting of the BFM-ALL protocols, it seems rational to include in vitro drug resistance testing as second stratification factor, after a first stratification based on the clinical response to PRD.
ACKNOWLEDGMENT
The authors thank F.R. Rosendaal (Departments of Clinical Epidemiology and Hematology, University Hospital Leiden, The Netherlands) for statistical assistance. The Dutch Childhood Leukemia Study Group (DCLSG) provided the patient samples. Board members of the DCLSG are H. Van Den Berg, M.V.A. Bruin, J.P.M. Bökkerink, P.J. Van Dijken, K. Hählen, W.A. Kamps, F.A.E. Nabben, A. Postma, J.A. Rammeloo, I.M. Risseeuw-Appel, A.Y.N. Schouten-Van Meeteren, G.A.M. De Vaan, E. Th Van't Veer-Korthof, A.J.P. Veerman, M. Van Weel-Sipman, and R.S. Weening.
Supported by the Dutch Cancer Society (IKA 89-06, Amsterdam, The Netherlands) and by the project VONK (VU Onderzoek Naar Kinderkanker, Amsterdam, The Netherlands).
Address reprint requests to G.J.L. Kaspers, MD, PhD, Department of Pediatric Hematology/Oncology, University Hospital Vrije Universiteit, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal