Recently, several lines of evidence have indicated an expanded role for thrombopoietin (TPO) and its receptor, c-mpl, in hematopoiesis. In addition to being the primary physiological regulator of platelet production, it is now apparent that TPO also acts during early hematopoiesis. To futher define the role of TPO in early hematopoiesis we have identified discrete murine and human stem cell populations with respect to c-mpl expression and evaluated their potential for hematopoietic engraftment. Fluorescence-activated cell sorter analysis of enriched stem cell populations showed the presence of c-mpl expressing subpopulations. Approximately 50% of the murine fetal liver stem cell–enriched population, AA4+Sca+c-kit+, expressed c-mpl. Analysis of the murine marrow stem cell population LinloSca+c-kit+ showed that 70% of this population expressed c-mpl. Expression of c-mpl was also detected within the human bone marrow CD34+CD38− stem cell progenitor pool and approximately 70% of that population expressed c-mpl. To rigorously evaluate the role of TPO/c-mpl in early hematopoiesis we compared the repopulation capacity of murine stem cell populations with respect to c-mpl expression in a competitive repopulation assay. When comparing the fetal liver progenitor populations, AA4+Sca+c-kit+c-mpl+and AA4+Sca+c-kit+c-mpl−, we found that stem cell activity segregates with c-mpl expression. This result is complemented by the observation that the LinloSca+ population of c-mplgene-deficient mice was sevenfold less potent than LinloSca+ cells from wild-type mice in repopulating activity. The engraftment potential of the human CD34+CD38−c-mpl+ population was evaluated in a severe combined immunodeficient-human bone model. In comparison to the CD34+CD38−c-mpl− population, the CD34+CD38−c-mpl+ cells showed significantly better engraftment. These results demonstrate a physiological role for TPO and its receptor, c-mpl, in regulating early hematopoiesis.
THROMBOPOIETIN (TPO) is the primary physiological regulator of platelet production and was initially thought to be a lineage dominant factor primarily affecting megakaryocytopoiesis.1,2 Like other hematopoietic growth factors, however, several lines of evidence indicate that TPO has a more pleiotropic range of activities. This was first realized when TPO was found to accelerate red blood cell recovery in myelosuppressed mice,3-5 as well as to synergize with erythropoietin (EPO) to stimulate erythroid proliferation in vitro.5,6 In addition, in vivo administration of TPO caused an expansion of colony-forming unit granulocyte-macrophage (CFU-GM) and burst-forming unit-erythroid (BFU-E) in normal mice3 and expanded CFU-mixed in rhesus monkeys.7 It was also shown that megakaryocytopoietic activities of TPO are initiated at the early progenitor cell level.8 Recently, several in vitro studies have shown that TPO alone or in combination with the early acting growth factors, c-kit ligand (KL), interleukin-3 (IL-3), or Flt-3 ligand (FL), stimulates the proliferation of early hematopoietic progenitors.9-19Significantly, progenitors expanded by TPO in combination with KL or FL retain a primitive phenotype and maintain the capacity for multilineage differentiation, even in the presence of TPO.9,12-14,16-18TPO also enhances the expansion and viability of CD34+CD38− progenitors in culture.19
The phenotypic alteration of c-mpl- and TPO-deficient mice also indicate a role for TPO in regulating hematopoietic progenitors. As expected, the primary phenotype of these mice is severe thrombocytopenia. However, detailed analysis of their marrow shows deficiencies in their progenitor pool as well.20-23 In addition to reduced megakaryocyte progenitors, both TPO- andc-mpl–deficient mice show significant reductions in neutrophil, GM, erythroid, and multilineage progenitors in the bone marrow (BM), spleen, and peripheral blood (PB).20,21 A reduction in blast cell–colony-forming cell (CFC) was also observed inc-mpl–deficient mice.21 TPO administration toTPO-deficient mice restores their progenitors to normal or above-normal levels.22 These latter observations further indicate that TPO may act to maintain and/or expand early progenitors.
To rigorously characterize the role of c-mpl and TPO in hematopoiesis we have identified discrete murine hemopoietic progenitor populations with respect to c-mpl, and evaluated their capacity to repopulate lethally irradiated mice. Similarly, the repopulation capacity of hematopoietic progenitors from c-mpl gene-deficient mice was also determined. To further expand our analysis we have characterized the expression of c-mpl on human stem cell populations and their functional capabilities using the severe combined immunodeficient-human (SCID-hu) bone model. Collectively, these data show that the expression of c-mpl is important in early hematopoiesis.
MATERIALS AND METHODS
Flow cytometric analysis of murine fetal liver and BM.
Murine fetal liver stem cell populations were isolated from AA4.1+ (called AA4+) cells derived by immune panning from mid-gestation fetal liver essentially as previously described.8 The AA4+ cells were subsequently stained using directly conjugated antibodies against murine c-kit (fluorescein isothiocyanate [FITC]), Sca-1 (R-PE) (Pharmingen, San Diego, CA), and biotinylated hamster anti-murine c-mpl.24 For the biotinylated antibodies, streptavidin Red-670 (GIBCO-BRL, Grand Island, NY) was used for detection.
BM populations were derived using femurs from C57/BL/6 mice. Briefly, the femurs were obtained and flushed with PBS/2% fetal calf serum (FCS) and a single cell suspension was made. Any remaining red blood cells were removed by lysis with 10 mmol/L NH4Cl. The mononuclear fraction was then incubated with monoclonal antibodies (MoAbs) specific for lineage markers consisting of rat anti-mouse CD4/L3T4, CD8/Ly 2,3, B-220/CD45R (Caltag, South San Francisco, CA), Gr-1, Mac-1, and Ter-119 (Pharmigen, San Diego, CA). Lineage marker positive cells were then removed by magnetic bead depletion with a sheep anti-rat antibody conjugated to magnetic beads (Dynal, Great Neck, NY). The lineage depleted cells were then incubated with MoAbs specific for: c-kit (FITC), Sca-1 (R-PE) (purchased from Pharmigen), goat anti-rat Lin (Cascade Blue; Molecular Probes, Eugene, OR), and biotinylated hamster anti-murine c-mpl.24 A second step using Streptavidin Red-670 (GIBCO-BRL) was included for the biotinylated samples. All fetal liver or BM samples were analyzed or sorted on a Coulter EPICS Elite (Hialeah, FL) dual-laser flow cytometer configured with a Coherent model 302 Krypton laser (407 nm) and argon laser (488 nm). The appropriate isotype controls were included for each experiment. All analysis excluded propidium iodide positive cells and all cells with high forward or right angle light scatter.
Competitive repopulation of fetal liver progenitors and c-mpl–deficient BM.
Timed pregnant C57/BL/6 mice were killed and the murine day 14 fetal liver cell populations were isolated as described above. The following enriched populations were then sorted: AA4+Sca+c-mpl+ and AA4+Sca+c-mpl−. For the competitive repopulation analysis of c-mpl–deficient mice, the BM population LinloSca+ was isolated from either homozygote mutant mice (c-mpl−/−) or their wild-type (c-mpl+/+) littermate controls. Young adult male C57/BL/6 Ly 5.2 mice were obtained from NCI and used as recipients. A minimum of five animals was used per experimental group. Whole-body irradiation (1,100 rads, 190 cGy/min) was administered as a single dose from a 137Cs source. One million nucleated BM cells from the Ly 5.2 mice were used as a source competitor, and mixed with 1,000 or 2,000 fetal liver donor cells or 10,000 BM-derived donor cells. Cells were administered via tail-vein injection and PB samples (50 to 100 μL) were obtained via the retro-orbital sinus 4, 12, and 24 weeks postreconstitution. The percentage of Ly 5.1 (A20.1) donor cells was determined indirectly by staining with biotin-conjugated Ly 5.2 MoAb (A20.1.7). Briefly, PB was collected in 10 U/mL heparin, 1 mmol/L EDTA in phosphate-buffered saline (PBS) and immediately placed on ice. Erythrocytes were removed by the addition of 5 vol of 4% Dextran T500 (Pharmacia) in PBS followed by incubation at room temperature for 20 minutes. The red blood cell–depleted fraction was then centrifuged for 5 minutes at approximately 200g. The remaining red blood cells were then lysed using 10 mmol/L NH4Cl. The mononuclear fraction was then resuspended in PBS/2% FCS. This procedure removed nearly 100% of the erythrocytes (the remaining red blood cells were excluded by size gating) while leaving the leukocytes 95% viable by propidium iodide exclusion. Fluorescence-activated cell sorter (FACS) analysis of Ly 5.2 PB cells was nearly identical to cells prepared by lysing. Controls for the specificity of Ly 5.2 staining included cells from Ly 5.1 or Ly 5.2 animals and cells from animals repopulated with Ly 5.2 competitor cells only. To confirm repopulation by the donor Ly 5.1 cells in all lineages, PB cells and the BM mononuclear fraction were stained in conjunction with Ly 5.1 for B220, CD4, CD8 (lymphoid lineages), and Gr-1/Mac-1 (myeloid lineages).
The relative repopulating ability of each putative stem cell population was compared by calculating repopulation units (RU) as described by Harrison et al.25 Each RU represents the same repopulating ability as 1 × 105 fresh marrow cells. It has been determined that the long-term repopulating stem cell content of fresh marrow is one stem cell per 105 total marrow cells.25 Therefore, the number of RU from each donor population is defined by the following equation:
Isolation of CD34+ cells from human BM.
Fresh human BM aspirates were obtained from healthy donors (Poietic Technologies, Gaithersburg, MD). The mononuclear cells were then enriched for CD34 by immunomagnetic positive selection (Miltenyi Biotech, Inc, Auburn, CA) according to the manufacturer's instruction. CD34 content was assessed by FACS and purity was routinely greater than 90%. The CD34+ fraction was then stained with murine MoAbs against human CD34 (FITC), CD38 (R-PE), (Becton Dickinson, San Jose, CA), and c-mpl (biotin).26 The biotinylated samples were then subsequently stained with Streptavidin Red-670 (GIBCO-BRL).
SCID-hu bone mice.
CB-17 scid/scid mice were implanted with fetal bone as previously described.27 Briefly, human fetal bone fragments from femurs, tibias, and humeri of 17- to 22-week gestational fetuses were implanted subcutaneously in 8-week-old CB-17 scid/scidmice. The BM fragments were allowed to engraft for 8 weeks. Mice were then bled via the retro-orbital sinus and engraftment of the bone fragments was determined by flow cytometric analysis of the blood samples with MoAbs specific for human HLA class 1 epitopes (One Lambda, Canoga Park, CA).
SCID-hu bone mice were administered whole-body irradiation of 200 rads from a 137Cs source and sorted human stem cells were then injected directly into a bone graft using a Hamilton syringe. Mice were then killed 8 weeks postinjection, HLA disparate grafts were removed, and marrow mononuclear cells were analyzed by FACS for donor HLA contribution as well as CD19, CD33, and CD34 (Becton Dickinson).
RESULTS
Expression of c-mpl on murine stem cell populations.
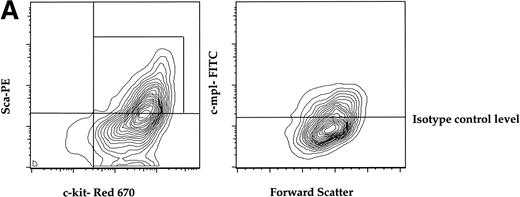
To determine if a murine primitive hematopoietic cell population enriched for stem cell activity expressed c-mpl, we isolated AA4.1+ (refered to as AA4+ here) cells from midgestational murine fetal liver by immunopanning and quantified the expression of c-mpl on the AA4+ fraction that is double-positive for the Sca-1 marker (called Sca here) and c-kit. The AA4+Sca+c-kit+ fraction has been shown to be highly enriched for stem cell activity.28 29Approximately 50% of the AA4+ cells that were positive for both Sca and c-kit (AA4+Sca+c-kit+) were c-mpl+. This c-mpl positive fraction (AA4+Sca+c-kit+ c-mpl+) constituted less than 0.1% of the total fetal liver (Fig 1A).
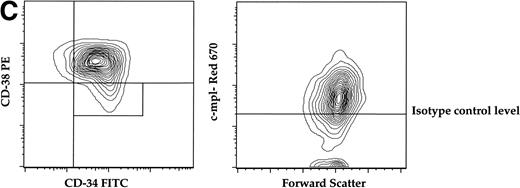
(A) Expression of c-mpl of murine fetal liver stem cell populations. Fetal liver stem cells were enriched for AA4 and stained with MoAbs to murine Sca, c-kit, and c-mpl as described in Materials and Methods. Fetal liver cells positive for both Sca and c-kit were gated and the resultant c-mpl positive cells are shown. Analysis was done a minimum of four times. (B) Expression of c-kit, Sca, and c-mpl on murine BM hematopoietic progenitors. The mononuclear fraction from BM was isolated by a density gradient and stained with a Lin cocktail of antibodies (see Materials and Methods). Lin stained cells were then removed by magnetic bead depletion. The lineage-depleted cells were then stained using directly conjugated MoAbs against Sca (R-PE), c-kit (FITC), c-mpl (biotin), and the Lin cocktail (Cascade Blue). The biotinylated c-mpl was detected using Streptavidin Red-670. Analysis was repeated three times. (C) Expression of CD34, CD38, and c-mpl on enriched human BM progenitor cells. CD34 cells were isolated as described. The enriched cells were then stained with MoAbs to CD34 (FITC), CD38 (R-PE), and c-mpl (Red -670). Cells were then analyzed on a Coulter Epics Elite flow cytometer as described. BM cells were enriched for CD34 using an immunomagnetic column. Analysis was repeated six times.
(A) Expression of c-mpl of murine fetal liver stem cell populations. Fetal liver stem cells were enriched for AA4 and stained with MoAbs to murine Sca, c-kit, and c-mpl as described in Materials and Methods. Fetal liver cells positive for both Sca and c-kit were gated and the resultant c-mpl positive cells are shown. Analysis was done a minimum of four times. (B) Expression of c-kit, Sca, and c-mpl on murine BM hematopoietic progenitors. The mononuclear fraction from BM was isolated by a density gradient and stained with a Lin cocktail of antibodies (see Materials and Methods). Lin stained cells were then removed by magnetic bead depletion. The lineage-depleted cells were then stained using directly conjugated MoAbs against Sca (R-PE), c-kit (FITC), c-mpl (biotin), and the Lin cocktail (Cascade Blue). The biotinylated c-mpl was detected using Streptavidin Red-670. Analysis was repeated three times. (C) Expression of CD34, CD38, and c-mpl on enriched human BM progenitor cells. CD34 cells were isolated as described. The enriched cells were then stained with MoAbs to CD34 (FITC), CD38 (R-PE), and c-mpl (Red -670). Cells were then analyzed on a Coulter Epics Elite flow cytometer as described. BM cells were enriched for CD34 using an immunomagnetic column. Analysis was repeated six times.
In adult murine marrow, we analyzed the expression of c-mpl on the LinloSca+c-kit+ population which is enriched for stem cell activity.30 In these experiments the mononuclear fraction of BM was depleted of lineage-committed progenitors with immunomagnetic beads (see Materials and Methods) and the Linlo mononuclear cells were then further fractionated into the LinloSca+c-kit+population. FACS analysis of the LinloSca+c-kit+ population showed that 70% of this population was c-mpl+ (Fig 1B). This population comprised less than 0.1% of total adult BM. As expected, we detected c-mpl on the Linhi cells (data not shown) that are presumably the lineage committed megakaryocyte progenitors.
Expression of c-mpl on human stem cell progenitor populations.
To evaluate the presence of c-mpl on human progenitors we analyzed the expression of c-mpl on the CD34+CD38−stem cell progenitor population derived from human marrow. The CD34+CD38− population comprises 10% of the total CD34+ population and is highly enriched for multiprogenitor and stem cell activity.31-33CD34+ cells were isolated from adult BM by immunomagnetic positive selection (>90% purity), then stained with MoAbs against human CD34, CD38, and c-mpl for FACS analysis. Similar to the expression pattern on murine progenitors, approximately 70% of the CD34+CD38− population expressed c-mpl (Fig 1C).
Long-term self-renewing stem cell activity segregates with c-mpl expression.
In this study, we used a competitive repopulation assay to compare the long-term functional abilities of murine hematopoietic stem cell populations with respect to c-mpl expression. Briefly, progenitor-enriched donor populations derived from either mid-gestational fetal liver or adult BM were mixed with 106competitor cells from BM of C57/BL6 Ly 5.2 animals and then injected into lethally irradiated C57/BL6 Ly 5.2 recipients. At the 4-, 12-, and 24-week time points, the relative contribution of donor cells to hematopoietic lineages was determined by FACS analysis of the Ly 5.1 and Ly 5.2 allotypes.
Using this model we directly compared the repopulating activity of AA4+Sca+c-mpl+ and AA4+Sca+c-mpl− fetal liver cells derived from wild-type mice (Table1). This direct comparison shows that all of the long-term repopulating activity of the AA4+Sca+ population segregated with c-mpl expression. At the 24-week time point more than 50% of the myeloid and lymphoid cells were derived from the AA4+Sca+c-mpl+ donor cells with equal contribution to each of these hematopoietic compartments. At 24 weeks the AA4+Sca+c-mpl+ population exhibited a reconstituting activity of 220 RU. In contrast, the AA4+Sca+c-mpl− cells exhibited no repopulating activity at the 24-week time point. The AA4+Sca+c-mpl− population did exhibit short-term reconstituting activity, indicating that this population consists primarily of committed progenitors. These data indicate that the enriched fetal liver AA4+Sca+population can be fractionated into functionally and phenotypically different populations based on differential expression of c-mpl, and that c-mpl expression in this subset is associated with pluripotent repopulating activity.
Competitive Repopulation Analysis of Murine Stem/Progenitor Cell Populations
| No. and Phenotype of Donor Cells . | Week 4 RU . | Week 12 RU . | Week 24 . | ||
|---|---|---|---|---|---|
| % Lymphoid . | % Myeloid . | RU . | |||
| 1K AA4+Sca+c-mpl+ | 112 | 142 | 58 ± 5 | 67 ± 2 | 220 |
| 2K AA4+Sca+c-mpl+ | 101 | 210 | 68 ± 3 | 73 ± 4 | 179 |
| 1K AA4+Sca+c-mpl− | 12 | 0 | 0 | 0 | 0 |
| 2K AA4+Sca+c-mpl− | 15 | 0 | 0 | 0 | 0 |
| 10K LinloSca+ (c-mpl−/−) | 2 | 1 | 3 ± 1 | 2 ± 0.5 | 5 ± 2-150 |
| 10K LinloSca+ (c-mpl+/+) | 12 | 39 | 57 ± 12 | 61 ± 10 | 37 ± 5-150 |
| No. and Phenotype of Donor Cells . | Week 4 RU . | Week 12 RU . | Week 24 . | ||
|---|---|---|---|---|---|
| % Lymphoid . | % Myeloid . | RU . | |||
| 1K AA4+Sca+c-mpl+ | 112 | 142 | 58 ± 5 | 67 ± 2 | 220 |
| 2K AA4+Sca+c-mpl+ | 101 | 210 | 68 ± 3 | 73 ± 4 | 179 |
| 1K AA4+Sca+c-mpl− | 12 | 0 | 0 | 0 | 0 |
| 2K AA4+Sca+c-mpl− | 15 | 0 | 0 | 0 | 0 |
| 10K LinloSca+ (c-mpl−/−) | 2 | 1 | 3 ± 1 | 2 ± 0.5 | 5 ± 2-150 |
| 10K LinloSca+ (c-mpl+/+) | 12 | 39 | 57 ± 12 | 61 ± 10 | 37 ± 5-150 |
Comparison of the competitive repopulation capacity of fetal liver AA4+Sca+c-mpl+ cells with AA4+Sca+c-mpl− cells, and LinloSca+ cells isolated from mice deficient in the c-mpl receptor (c-mpl−/−) with LinloSca+ cells isolated from their homozygous normal littermates (c-mpl+/+). Long-term repopulation analysis of genetically marked fetal liver or BM in C57BL/6 mice congenic at the Ly 5 locus, designated Ly 5.1 (A20.1) and Ly 5.2 (AL1-4A2). Fetal liver and BM stem cell populations were isolated as described.8 Five animals were used per stem cell population as described in Materials and Methods. Ly 5.1 expression in the Ly 5.2 mice was determined 4, 12, and 24 weeks postengraftment. RU are reported per 105 cells and were calculated as described in Materials and Methods. Contribution of the populations to all lineages was determined by staining both PB leukocytes and BM cells from the following antigens: lymphoid: B-220, CD4, CD8; and myeloid: Gr-1 and Mac-1.
P = .0001.
To further expand this analysis, we isolated LinloSca+ cells from the BM ofc-mpl–deficient mice and their wild-type littermate controls. The LinloSca+ cells from wild-type animals contained significantly more RU when compared with LinloSca+ cells from thec-mpl–deficient mice (37 v 5). Preferential engraftment of the lymphoid or myeloid compartments was not observed with the LinloSca+ donor populations isolated from either wild-type or c-mpl–deficient mice. In addition, for the mice injected with wild-type LinloSca+ cells, the majority of lymphoid and myeloid cells were derived from this donor population (Table 1).
To evaluate the in vivo functional characteristics of the human CD34+CD38−c-mpl+ population in comparison to the CD34+CD38−c-mpl−fraction, the multilineage engraftment potential of these populations was compared in the SCID-hu bone model.27 Thirty thousand cells of each phenotype were injected directly into the bone fragments of the SCID-hu animals and at 8 weeks postinjection, the mice were killed, and mononuclear cells from the grafts were analyzed by FACS. Cell suspensions were made of each graft and analyzed for overall donor contribution by FACS using HLA haplotype-specific antibodies. In addition, the contribution of donor cells to myeloid (CD33), lymphoid (CD19), and hematopoietic progenitors (CD34) was determined. As shown in Table 2, the CD34+CD38−c-mpl+ population engrafted better (donor HLA) than the other populations. The combined results of two separate experiments show that the CD34+CD38−c-mpl+ population engrafted in 90%(9 of 10) of the mice injected with this cell population. In contrast, of the mice receiving the CD34+CD38−c-mpl− cell population only 22% (2 of 9) showed donor derived reconstitution. In addition, the contribution of donor derived cells to the progenitor (CD34+), myeloid (CD33+), or lymphoid (CD19+) compartments was higher with the CD34+CD38−c-mpl+ than the CD34+CD38−c-mpl−population 8 weeks postreconstitution. This was especially true for the CD34+ progenitor population. In both experiments, with mice injected with the CD34+CD38−c-mpl+ cells, greater than 22% of CD34+ cells were donor derived. This compares to 2% to 4% donor-derived CD34+ cells for the two mice that engrafted with the CD34+CD38−c-mpl− cells.
Hematopoietic Engraftment Potential of Human Stem/Progenitor Cell Populations in the SCID-hu Bone Assay
| Cell Phenotype . | Study No. . | No. of Mice Engrafted . | % Donor HLA/CD34* . | % Donor HLA/CD33 . | % Donor HLA/CD19 . |
|---|---|---|---|---|---|
| CD34+CD38− | 1 | 3/5 | 8.4 ± 2.8 | 14.3 ± 5.3 | 64.2 ± 11.3 |
| CD34+CD38− c-mpl+ | 1 | 5/5 | 22.7 ± 5.7 | 18.9 ± 3.6 | 67.6 ± 2.5 |
| CD34+CD38− c-mpl− | 1 | 1/4 | 4.2 | 12.5 | 18.9 |
| CD34+CD38− | 2 | 3/5 | 7.9 ± 1.2 | 17.7 ± 4.2 | 48.5 ± 3.3 |
| CD34+CD38− c-mpl+ | 2 | 4/5 | 27.8 ± 3.5 | 12.9 ± 4.9 | 59.3 ± 9.6 |
| CD34+CD38− c-mpl− | 2 | 1/5 | 1.8 | 5.6 | 5.9 |
| Cell Phenotype . | Study No. . | No. of Mice Engrafted . | % Donor HLA/CD34* . | % Donor HLA/CD33 . | % Donor HLA/CD19 . |
|---|---|---|---|---|---|
| CD34+CD38− | 1 | 3/5 | 8.4 ± 2.8 | 14.3 ± 5.3 | 64.2 ± 11.3 |
| CD34+CD38− c-mpl+ | 1 | 5/5 | 22.7 ± 5.7 | 18.9 ± 3.6 | 67.6 ± 2.5 |
| CD34+CD38− c-mpl− | 1 | 1/4 | 4.2 | 12.5 | 18.9 |
| CD34+CD38− | 2 | 3/5 | 7.9 ± 1.2 | 17.7 ± 4.2 | 48.5 ± 3.3 |
| CD34+CD38− c-mpl+ | 2 | 4/5 | 27.8 ± 3.5 | 12.9 ± 4.9 | 59.3 ± 9.6 |
| CD34+CD38− c-mpl− | 2 | 1/5 | 1.8 | 5.6 | 5.9 |
CB17 (scid/scid) mice were transplanted with human fetal BM fragments and injected with 3 × 104 sorted progenitor cells derived from adult BM of the above phenotype. Each population was HLA mismatched to the fetal BM grafts. At 8 weeks posttransplantation, the mice were killed and the BM fragments removed and processed as described in Materials and Methods. FACS analysis of the grafts is described in Fig 1. In comparing the engraftment potential of the CD34+CD38−c-mpl+ and CD34+CD38−c-mpl− cell populations, statistical significance of the number of mice engrafted was determined by an unpaired two-tailed Student's t-test with a 95% confidence interval.
P < .0002.
DISCUSSION
Recent in vitro data clearly indicate that TPO may function not only to expand the megakaryocyte compartment, but also to play a role in the expansion and maintenance of early progenitors. TPO alone is capable of activating quiescent progenitors into cycle13,18 and acts in synergy with either IL-3, KL, or FL to expand the early progenitor pool.9-19 In support of these observations, we have recently shown that hematopoietic progenitors expanded in a stroma-free liquid culture using a cytokine cocktail consisting of FL, KL, and TPO maintain their long-term lymphohematopoietic repopulating activity in vivo.34 The hematopoietic deficiencies that occur inc-mpl and TPO-deficient mice, which exhibit a significant reduction of multipotent progenitors, further indicate a role for TPO/c-mpl in early hematopoiesis.20 21
At the molecular level, expression of c-mpl mRNA has been detected in human CD34+cells35 and noncycling progenitors.36 FACS analysis shows that c-mpl is expressed on the stem cell enriched murine fetal liver population AA4+Sca+.8 To further characterize stem cell populations that express c-mpl, we have identified discrete progenitor populations derived from murine and human sources that express c-mpl. FACS analysis of murine BM (LinloSca+c-kit+) or fetal liver (AA4+Sca+c-kit+) stem cell populations shows that c-mpl is expressed on at least 50% of each of these stem cell–enriched populations.
The differential expression of c-mpl within the AA4+Sca+ fetal liver progenitor population allowed evaluation of its functional characteristics with respect to c-mpl expression. Using a murine competitive repopulation model,25 we directly compared the in vivo hematopoietic reconstituting activity of AA4+Sca+c-mpl+ and AA4+Sca+c-mpl− murine fetal liver cell populations. These results show that all of the long-term repopulating activity of the fetal liver progenitor population (AA4+Sca+) segregates with c-mpl expression. Repopulating activity showed equal contribution to the myeloid and lymphoid compartments by the donor cell population (AA4+Sca+c-mpl+). The AA4+Sca+c-mpl− population exhibited a low level of short-term reconstituting activity, indicating that this population is devoid of true stem cell activity. Furthermore, marrow progenitors (LinloSca+) derived fromc-mpl gene-deficient mice showed a significant (sevenfold) reduction in repopulating activity when compared with the LinloSca+ progenitor population derived from wild-type littermates. The segregation of stem cell activity with c-mpl expression on the fetal liver AA4+Sca+population indicates that c-mpl is expressed on a pluripotent stem cell and that this subpopulation may further define a long-term self-renewing stem cell.
It is interesting to note that although the level of hematopoietic progenitors is diminished by 50% in adultc-mpl−/−mice, day 14 fetal liver progenitors are not reduced inc-mpl−/− mice,21suggesting that TPO does not play a critical role in early fetal hematopoiesis. However, the inability of the AA4+Sca+c-mpl− fetal liver cells to repopulate in the adult mouse indicates that c-mpl expression may be essential for fetal liver progenitors to exhibit repopulating activity in the adult marrow microenviroment. Because the LinloSca+ progenitor population from thec-mpl−/−mice had a low level of stem cell activity and that only platelets, and not other hematopoietic blood cells, are affected in these mice, suggests that for adult hematopoiesis, c-mpl may play a crucial role in the function of primitive stem/progenitors cells only when the homeostasis of the hematopoietic compartment is perturbed. Thus, expression of c-mpl on a subset of stem/progenitor cells may allow them to respond to feedback mechanisms that maintain homeostasis in the hematolymphoid compartment.
As was observed for the murine progenitor populations, c-mpl is also expressed on the stem cell–enriched progenitor population, CD34+CD38−, derived from human marrow. The expression pattern of c-mpl expression within this population is similar to that observed for the murine progenitors with greater than 50% of CD34+CD38− expressing c-mpl. These results are in contrast to those of Debili et al,37who suggest that the c-mpl+ cells present in the CD34+ fraction are primarily caused by late megakaryocyte progenitors and transitional cells. Expression of c-mpl on CD34+CD38− cells and the ability of TPO to activate noncycling progenitors13 confirm that c-mpl is indeed expressed on an early progenitor.
Until recently the study of long-term human hematopoiesis in vivo was difficult because of the lack of an appropriate in vivo model. However, using immunodeficient, nonobese diabetic (NOD)/SCID and scid/scid mice, models have been developed in recent years to assay for human hematopoietic stem cells and allow evaluation of their multilineage repopulating capacity.27, 32,38In this study we used the SCID-hu bone model27 to determine the effect of c-mpl expression on the in vivo reconstituting activity of the human progenitor CD34+CD38− cell population. A direct comparison of the CD34+CD38−c-mpl+ and CD34+CD38−c-mpl− cell populations shows that c-mpl expression correlates with significantly better donor-derived engraftment. This was apparent in both overall engraftment and donor contribution to various hematopoietic lineages. With equal numbers of cells injected, 90% of the mice receiving the CD34+CD38−c-mpl+ cells engrafted compared with 22% engraftment for the mice receiving the c-mpl− fraction. In addition, a higher level of donor contribution to the progenitor, myeloid, and lymphoid compartments was observed with the CD34+CD38−c-mpl+ donor cells. This was especially true for the CD34+ fraction in which greater than 20% were donor derived using CD34+CD38−c-mpl+ cells compared with only 4% for the CD34+CD38−c-mpl− donor cells or 8% for the c-mpl unenriched CD34+CD38− fraction. Taken together, these results suggest that the multilineage long-term reconstituting activity of the human CD34+CD38− cell population correlates with c-mpl expression.
The expanded role of TPO in hematopoiesis suggest that TPO may be useful clinically for more than just alleviating thrombocytopenia. Recent in vitro studies have shown that TPO in combination with other early growth factors can cause multilineage expansion of CD34+ cells in a long-term culture.17,33 Ex vivo expansion would be a great benefit for the banking of umbilical cord progenitors for future engraftment procedures. Additionally, recent clinical trials have reported that TPO treatment results in an increase in marrow hematopoietic progenitors and peripheral CD34+ cells.39 These preliminary clinical results again suggest that TPO may be useful not only as a thrombopoietic agent but also in peripheral stem cell harvest procedures.
ACKNOWLEDGMENT
We thank Jim Chin for excellent technical assistance and Daniel Tumas for critically reviewing the manusript.
Address reprint request to Dan L. Eaton, PhD, Genentech, 1 DNA Way, South San Francisco, CA 94080.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal