Inefficient retroviral-mediated gene transfer to human hematopoietic stem cells (HSC) and insufficient gene expression in progeny cells derived from transduced HSC are two major problems associated with HSC-based gene therapy. In this study we evaluated the ability of a murine stem cell virus (MSCV)-based retroviral vector carrying the low-affinity human nerve growth factor receptor (NGFR) gene as reporter to maintain gene expression in transduced human hematopoietic cells. CD34+ cells lacking lineage differentiation markers (CD34+Lin−) isolated from human bone marrow and mobilized peripheral blood were transduced using an optimized clinically applicable protocol. Under the conditions used, greater than 75% of the CD34+ cell population retained the Lin− phenotype after 4 days in culture and at least 30% of these expressed a high level of NGFR (NGFR+) as assessed by fluorescence-activated cell sorter analysis. When these CD34+Lin−NGFR+ cells sorted 2 days posttransduction were assayed in vitro in clonogenic and long-term stromal cultures, sustained reporter expression was observed in differentiated erythroid and myeloid cells derived from transduced progenitors, and in differentiated B-lineage cells after 6 weeks. Moreover, when these transduced CD34+Lin−NGFR+ cells were used to repopulate human bone grafts implanted in severe combined immunodeficient mice, MSCV-directed NGFR expression could be detected on 37% ± 6% (n = 5) of the donor-type human cells recovered 9 weeks postinjection. These findings suggest potential utility of the MSCV retroviral vector in the development of effective therapies involving gene-modified HSC.
HEMATOPOIETIC STEM CELLS (HSC) provide an attractive target for somatic cell-based gene therapy because they have the potential to continue producing progeny cells containing a therapeutic gene indefinitely. Hematological diseases that could benefit from HSC-based gene therapy approaches include hereditary diseases as well as other diseases such as acquired immunodeficiency syndrome and cancer.1 Retroviral vectors, which are being used in the majority of current clinical trials, are a primary choice as the vehicle for gene delivery. They are capable of integrating into cellular chromosomes, resulting in stable transmission to every progeny cell derived from transduced HSC.2-4 However, it has become clear that current protocols for transducing human HSC with retroviral vectors are inefficient.5,6 Although transgenes in engrafting cells have been detected using sensitive assays such as polymerase chain reaction (PCR), they have rarely been found in long-term repopulating cells.5-7 These results are in striking contrast to the efficient transduction of less primitive human progenitors, which are able to form colonies under in vitro culture conditions,7 and of mouse HSC.8Therefore, it is important to directly assess gene transduction into rare HSC capable of repopulating in vivo and of generating multiple (myeloid and lymphoid) lineages of differentiated hematopoietic cells.
Human HSC are included in a rare population of cells that bear the CD34 surface antigen (CD34+) and lack all lineage differentiation markers (Lin−). However, only a small fraction of CD34+Lin− cells have HSC activities, operationally defined by long-term in vivo marrow repopulating activity and the ability to give rise to both myeloid and lymphoid progeny.9,10 Several animal models have been developed in an attempt to detect HSC among the CD34+Lin− population. One of these is the severe combined immunodeficient-human (SCID-hu) bone system, in which a human fetal bone fragment is implanted in SCID mice as a supportive human hematopoietic microenvironment.11,12Using this assay, it was found that the in vivo marrow repopulating activities of CD34+Lin− cells mainly resided in a subset of cells expressing the Thy-1 antigen (Thy-1+CD34+Lin−).12,13In contrast, Thy-1−CD34+Lin− cells were found to lack in vivo SCID-hu repopulating activity, although they were enriched for cells with in vitro colony-forming potential.12,13 Analogous results have been obtained using other SCID mouse models and a human-fetal sheep model.14,15Thus, SCID repopulating cells (SRC) are considered to be more primitive than hematopoietic progenitor cells with in vitro activities, making the surrogate SCID-hu system a useful small animal assay to distinguish candidate human HSC from hematopoietic progenitors. In a recent report it was found that SRC within the CD34+ population from human bone marrow (BM) were rarely transduced (<1%) even though retroviral-mediated gene transfer to colony-forming progenitors was highly efficient (up to 95% gene marking) under the conditions used.14 The inability to efficiently transduce SRC in that study mirrors the low level of HSC transduction observed with existing protocols in various human gene therapy trials.1 5-7
The second potential problem associated with retroviral vector–based gene therapy is transcriptional silencing of the introduced transgene. Retroviral vectors derived from Moloney murine leukemia virus (MoMLV) are the most commonly used retroviral vectors in clinical trials.2-4 In a standard configuration, the gene of interest is placed under the transcriptional control of the viral long terminal repeat (LTR) because gene expression driven by the LTR is generally higher than when the exogenous gene is under the control of an internal promoter.16,17 However, it has been reported that MoMLV LTR-mediated gene expression is frequently downregulated during differentiation of HSC and inactive in several cell types.17-19 Because the LTR of the murine stem cell virus (MSCV) retroviral vector is permissive for expression in murine HSC,8 20 we were interested in examining the performance of the MSCV vector in candidate human HSC as well as in their differentiated progeny.
Recently, a number of groups have used various cell-surface molecules, including murine CD24 (HSA), murine CD8a (Lyt-2), and the low-affinity human nerve growth factor receptor (NGFR), to measure efficiency of gene transfer into hematopoietic precursors and to follow transgene expression in their marked progeny.17,21,22 Retroviral vectors encoding NGFR have been used successfully by others to transduce human T lymphocytes and hematopoietic cells, and no adverse effects on the transduced cells have been observed.22Therefore, we decided to use the human NGFR gene as a selectable marker and reporter in this study. Multiparameter flow cytometric analysis allowed cell-surface expression of NGFR to be easily monitored in various hematopoietic cell populations defined by cell-surface markers (either CD34+Lin− cell populations or differentiated cells belonging to a particular lineage). Similar efficiencies of gene transfer into colony-forming progenitors were obtained by a MSCV-based vector as for a MoMLV-based vector. However, we found that NGFR transgene expression mediated by MSCV LTR was substantially higher in differentiated erythroid, myeloid, and B-lymphoid progeny than that mediated by MoMLV LTR. Based on these findings we evaluated persistence of MSCV LTR-directed expression in progeny derived from transduced SRC. We show that our protocol for transducing SRC with the MSCV-based vector was efficient, and that a high percentage of transduced human cells continued to express NGFR after long-term reconstitution of SCID-hu bone mice.
MATERIALS AND METHODS
Construction and detection of retroviral expression vectors.
The LXSN type of MoMLV vector was used as the parental vector for LINGFR.23 After deleting the internal SV40 promoter and theneo gene in LXSN, an internal ribosome entry site (IRES) from the encephalomyocarditis virus was inserted.24 The human (p75) NGFR gene was then placed after the IRES.25 The resultant vector was named LINGFR to reflect the order of essential components (LTR-IRES-NGFR). The MINGFR vector was similarly constructed by replacing the neo gene driven by an internal promoter in MSCVneoEB with the IRES-NGFR cassette from LINGFR.20 Gene expression mediated by the MINGFR vector is directed by the MSCV LTR, whereas in LINGFR gene expression is driven by the MoMLV LTR. A third vector, MINT, was created by truncating the NGFR gene in MINGFR after the transmembrane domain (at the Nae I site). All the plasmids were purified and used in packaging cell transfections as described.26
The primers used to amplify transgene-specific (IRES) DNA sequences common to all three vectors were: upstream, 5′-CGT TAC TGG CCG AAG CCG CT-3′; and downstream, 5′-AAC CTC GAC TAA ACA CAT GT-3′. The primers used to amplify the endogenous human β-globin sequence of genomic DNA as a control for PCR assays were: upstream, 5′-ACA CAA CTG TGT TCA CTA GC-3′; and downstream, 5′-CAA CTT CAT CCA CGT TCA CC-3′. Forty-cycle PCR reactions for both target sequences were performed with an annealing temperature of 62°C in the presence of 1.5 mmol/L MgCl2. PCR products (a 485-bp IRES-specific fragment and a 220-bp β-globin–specific fragment) were separated by 4% agarose gel electrophoresis.
Specific antibodies for fluorescence-activated cell sorting (FACS) analyses.
A hybridoma producing mouse IgG1 monoclonal antibody (MoAb) against the human NGFR was obtained from the American Type Culture Collection (ATCC HB8737; Rockville, MD). Purified antibodies from mouse ascites were conjugated either directly with fluorescein isothiocyanate (FITC) or with R-phycoerythrin (PE) after deleting the Fc fragment. A CD34 antibody (Tuk3) conjugated with sulfo-rhodamine (SR), and a panel of FITC-conjugated mouse MoAbs against lineage differentiation markers were used to isolate and analyze transduced cells.27 This lineage panel (collectively called Lin) comprised CD2, CD4, CD14, CD15, CD16, CD19 (Becton Dickinson, San Jose, CA) and glycophorin A (Immunotech, Westbrook, ME). An FITC-conjugated antibody (MA2.1, ATCC HB54) against HLA-A2 was used to identify and monitor MA2.1+ donor cells in SCID-hu bone mice.12
Propidium iodide (0.5 μg/mL) was added to cell suspensions after antibody staining to exclude dead/dying cells from FACS analyses. FACStarPlus or FACS Vantage cell sorters (Becton Dickinson) equipped with a primary Agron laser and a dye-laser (required for detecting SR signals) were used for cell sorting. FACScan analyzers (Becton Dickinson) equipped only with an Argon laser were used to phenotype harvested cells after in vitro and in vivo assays.
Cytokines, media, and cell lines.
Recombinant human interleukin-3 (IL-3), IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), steel factor (SLF, also called stem cell factor), and leukemia inhibitory factor (LIF) were obtained from Sandoz Pharma (Basel, Switzerland), and erythropoietin (Epo) was purchased from Amgen (Thousand Oaks, CA). Dulbecco's modified Eagle's medium (DMEM), Iscove's modified Dulbecco's medium (IMDM), and RPMI 1640 culture media were purchased from GIBCO-BRL (Gaithersburg, MD) and fetal calf serum (FCS) from Hyclone (Logan, UT). TF1 cells (ATCC CRL-2003) were maintained in RPMI 1640 plus 10% FCS and 2 ng/mL GM-CSF.28
Production of retroviral supernatants and transduction protocol.
Amphotropic supernatants produced by the human 293 (embryonic kidney fibroblast) cell-based ProPak-A packaging line were made through transduction with VSV-G pseudotyped viral stocks as described.29 30 NGFR-expressing ProPak-A cells were enriched by flow cytometry sorting and expanded in culture. Amphotropic supernatants were then collected from stable ProPak-A producers, filtered, and stored at −80°C until use. For transduction, fresh or previously frozen vector supernatants were mixed at a 1:1 ratio with media containing target cells in the presence of 8 μg/mL polybrene (Sigma, St Louis, MO). The transduction mixture was then centrifuged at 1,800g at 32°C to 35°C for inoculation. After the 4-hour “spinoculation,” the cells were washed once and cultured in appropriate media.
Isolation and transduction of human hematopoietic progenitors.
Human BM aspirates and mobilized peripheral blood (mPB, collected at day 4 or 5 after G-CSF treatment) were obtained from healthy donors in compliance with regulations established by the federal and state governments. Low-density (<1.077 g/cm3) mononuclear BM cells after Ficoll-Hypaque gradient (Pharmacia, Piscataway, NJ) or apheresed mPB cells were stained with a CD34 antibody included in the Isolex kit (Baxter Biotech Immunotherapy Division, Irving, CA). CD34+ cells were magnetically isolated by Isolex using a modified protocol developed at Systemix.27 The purity of CD34+ cells from both BM and mPB was usually greater than 90% (n = 10 for BM and n = 20 for mPB). These isolated cells were then stained with CD34-SR and Lin-FITC, and CD34+Lin− cells were sorted by FACS and activated ex vivo for gene transduction. Cells (≈106/mL) were cultured overnight in IMDM/RPMI 1640 (1:1) medium plus 10% FCS supplemented with 10 ng/mL IL-3 and IL-6, and 100 ng/mL SLF. The next day cells were transduced with ProPak-A viral supernatants for 4 hours as described above. The transduction procedure was repeated the following day, and then the medium was changed and the cells were cultured for additional 2 days to allow gene expression.
In vitro progenitor assays.
Methylcellulose and reagents for clonogenic progenitor assays were obtained from StemCell Technologies (Vancouver, Canada). Cells (4 × 102) were added to 1 mL methylcellulose medium (MethoCult H4230) supplemented with IL-3, IL-6, GM-CSF (10 ng/mL each), SLF (100 ng/mL), and Epo (2 U/mL). Cell mixtures were plated in 35-mm suspension culture dishes (Nunc, Roskilde, Denmark), and incubated at 37°C. After 2 weeks, colonies (>100 cells) were enumerated in each of three triplicate plates. Subsequently some individual colonies were randomly picked. Cells were then lysed and an aliquot was used for a 40-cycle PCR analysis to detect specific DNA sequences. Colonies which yielded a positive signal for the human β-globin sequence were included in the calculations of colony-forming cell (CFC) gene transfer efficiency. Colonies which also yielded a transgene signal equal to or stronger than the β-globin signal in the same PCR reaction were considered to be positive for the transgene. The remaining cells were obtained in bulk at the end of 2-week CFC assays for FACS analysis of NGFR expression. Cells were washed three times with phosphate-buffered saline plus 0.5% human IgG (Gammimmune, Miles Inc, Elkhart, IN) before being stained with a PE-conjugated NGFR antibody and FITC-conjugated antibodies against glycophorin A, CD14, or CD15.
For stromal-dependent cobblestone area-forming cell (CAFC) assays,31 a stromal cell line (SyS-1) derived from murine BM was used.13 Cultures were maintained with IMDM/RPMI 1640 (1:1) medium plus 10% FCS supplemented with 10 ng/mL IL-6 and 50 ng/mL LIF. Up to 100 cells were cultured on sub-confluent monolayers of SyS-1 stromal cells in each well of 96-well plates. Half of the medium was replaced weekly, and the cultures were monitored for 6 weeks. CAFC frequencies were calculated based on the results obtained at week 5. Total cells were procured and pooled from wells which contained at least one cobblestone area at week 6. After being filtered through a 30-μm mesh filter, cells were stained as described before, with the PE-conjugated NGFR antibody and the FITC-conjugated CD19 antibody.
In vivo SCID-hu bone assays.
Immunodeficient C.B-17 scid/scid (SCID) mice were used as recipients of human fetal bone fragments to construct the SCID-hu bone mice.11,12 In each of two independent experiments, bone fragments were derived from the same human fetal tissue, which was negative for HLA-A2 and B17 and was not recognized by the corresponding MoAb MA2.1 (ATCC HB-54). Eight weeks after bone implantation, SCID-hu bone mice were used as recipients of transduced human hematopoietic precursors whose HLA-type was MA2.1+. The transduced donor cells were injected directly into the implanted bones (MA2.1−) residing in SCID mice according to the published protocol.12 Fifty thousand to 100,000 cells (based on CD34+Lin− cell counts) from the same cell population were injected into each of two bone fragments implanted in SCID mice. Nine weeks after injection, recipient mice were terminated, the implanted bone fragments were surgically removed, and total cells from BM were obtained. Then harvested cells were then stained with the PE-conjugated NGFR antibody and the FITC-conjugated MA2.1 antibody. Propidium iodide was added after antibody staining to cell suspensions to exclude dead/dying cells from FACS analyses. Using a FACScan analyzer, levels of engraftment (based on the presence of MA2.1+ donor cells) and transgene expression (based on the presence of NGFR on the surface of donor cells) were assessed by FACS.
RESULTS
Transduction of human TF1 hematopoietic progenitor cell line with MSCV- and MoMLV-based vectors encoding NGFR.
Several retroviral vectors containing the NGFR gene as a reporter were constructed to compare gene transfer and expression in human hematopoietic cells (Fig 1). NGFR expression is directed by the MSCV LTR in MINGFR and MINT, or directed by the MoMLV LTR in the LXSN-derived vector LINGFR.20,23 To achieve a higher efficiency of transduction of human hematopoietic precursors than has been routinely accomplished with recombinant retroviral stocks prepared using conventional murine packaging lines, amphotropic retroviral vector supernatants were produced using a new human 293 cell-based packaging line (ProPak-A).29Transduction efficiencies were first assessed on the growth factor–dependent human CD34+ progenitor line TF1.28 As shown in Fig 2A, ≈60% of TF1 cells were transduced by either MINGFR or LINGFR vector 4 days after the cells were exposed to 50% (vol/vol) of viral supernatants in a “spinoculation” protocol. Subsequent limiting dilution experiments with MINGFR and LINGFR vector preparations established that the two viral stocks had comparable titers (data not shown). Moreover, as indicated by the mean fluorescence intensities of the positive peaks, both vectors directed similar NGFR expression levels in TF1 cells (Fig 2A). The two vectors also performed equally well when tested on other cell lines such as murine NIH3T3 fibroblasts (data not shown).
Schematic representation of NGFR-encoding retroviral vectors. The LXSN type of MoMLV vector was used as the parental vector backbone in the construction of LINGFR and the MSCVneoEB vector was used to derive the MINGFR and MINT vectors. In all cases, a NGFR gene has been placed downstream of an IRES, replacing the previousneo genes driven by internal promoters. Inclusion of the IRES potentially allows for coexpression of an upstream gene on bicistronic transcripts which also encode the NGFR reporter. The LTR of MoMLV directs NGFR gene transcription in LINGFR whereas the MSCV LTR is used to express the NGFR gene in MINGFR and MINT. The NGFR gene in MINT was truncated after the transmembrane (TM) domain.
Schematic representation of NGFR-encoding retroviral vectors. The LXSN type of MoMLV vector was used as the parental vector backbone in the construction of LINGFR and the MSCVneoEB vector was used to derive the MINGFR and MINT vectors. In all cases, a NGFR gene has been placed downstream of an IRES, replacing the previousneo genes driven by internal promoters. Inclusion of the IRES potentially allows for coexpression of an upstream gene on bicistronic transcripts which also encode the NGFR reporter. The LTR of MoMLV directs NGFR gene transcription in LINGFR whereas the MSCV LTR is used to express the NGFR gene in MINGFR and MINT. The NGFR gene in MINT was truncated after the transmembrane (TM) domain.
FACS analysis of NGFR expression in retrovirally transduced TF1 cells. (A) Histograms of TF1 cells expressing the NGFR transgene after transduction with the LINGFR, MINGFR, or MINT vectors. TF1 cells were transduced with equal volumes of amphotropic viral supernatants for 4 hours (as described in Materials and Methods) and NGFR expression was analyzed by FACS 4 days later after staining for cell-surface NGFR with a FITC-conjugated anti-NGFR antibody. The profile of nontransduced TF1 cells (thin lines representing 0.5%) was overlaid to highlight transduced cell NGFR+ populations. Approximately 62%, 59%, and 40% of TF1 cells were transduced with the LINGFR, MINGFR, and MINT vectors, respectively. (B) Kinetics of NGFR expression in TF1 cells after transduction with the MINT vector. After 4-hour exposure to MINT vector supernatant, TF1 cells were either processed immediately (day 0) or cultured for 1, 2, 3, or 4 days and then stained for NGFR expression. Based on FACS histograms as shown in (A), the percentages of NGFR-expressing cells and relative levels of NGFR expression in transduced cells were plotted as a function of time in culture. The relative intensity of NGFR expression is defined by increased mean fluorescence intensity (MFI) normalized by the MFI of nontransduced cells.
FACS analysis of NGFR expression in retrovirally transduced TF1 cells. (A) Histograms of TF1 cells expressing the NGFR transgene after transduction with the LINGFR, MINGFR, or MINT vectors. TF1 cells were transduced with equal volumes of amphotropic viral supernatants for 4 hours (as described in Materials and Methods) and NGFR expression was analyzed by FACS 4 days later after staining for cell-surface NGFR with a FITC-conjugated anti-NGFR antibody. The profile of nontransduced TF1 cells (thin lines representing 0.5%) was overlaid to highlight transduced cell NGFR+ populations. Approximately 62%, 59%, and 40% of TF1 cells were transduced with the LINGFR, MINGFR, and MINT vectors, respectively. (B) Kinetics of NGFR expression in TF1 cells after transduction with the MINT vector. After 4-hour exposure to MINT vector supernatant, TF1 cells were either processed immediately (day 0) or cultured for 1, 2, 3, or 4 days and then stained for NGFR expression. Based on FACS histograms as shown in (A), the percentages of NGFR-expressing cells and relative levels of NGFR expression in transduced cells were plotted as a function of time in culture. The relative intensity of NGFR expression is defined by increased mean fluorescence intensity (MFI) normalized by the MFI of nontransduced cells.
A third vector, MINT, in which the NGFR gene in the MINGFR vector was C-terminally truncated after the transmembrane domain, was used to examine the kinetics of NGFR expression after viral transduction. The titer of MINT viral stock was somewhat lower and ≈40% TF1 cells were transduced and expressed NGFR on cell surface after 4 days (Fig 2A). To determine the kinetics of NGFR transgene expression after transduction, MINT-transduced TF1 cells were either stained for NGFR surface protein immediately posttransduction or cultured for various periods of time and then analyzed as described above. No NGFR signal could be detected on the cell surface immediately after the transduction protocol (Fig2B). Subsequently, the percentage of NGFR-expressing (NGFR+) cells as well as NGFR signal intensities progressively increased with time, reaching a maximum of 40% 4 days posttransduction (Fig 2A and B). Afterward the values of these two parameters remained constant for at least 2 months if transduced TF1 cells were maintained in a proliferating phase. The doubling time of TF1 cells is ≈20 hours; thus, it took a period equivalent to three cell divisions for continuously proliferating TF1 cells to reach the maximal and stable level of the NGFR surface signal. This finding is consistent with accumulated data that efficient retroviral-mediated expression (transcription and translation) occurs only after integration into cellular chromosomes which, in the case of C-type retroviruses, is dependent on cell division.
The MINT vector was initially constructed to minimize the possibility of NGFR functioning as a signal transducer in hematopoietic cells. However, no adverse effect was observed due to full-length NGFR expression on the growth of transduced TF1 cells (data not shown) or primary human hematopoietic cells assayed in vitro (see below). Because the titer of the MINT vector was somewhat lower than that of MINGFR or LINGFR vector (Fig 2A), we restricted our attention solely to the latter two vectors to compare directly the expression properties of MSCV- and MoMLV-based retroviral vector backbones after transduction of primary human hematopoietic precursors.
Transduction of human CD34+Lin− cells.
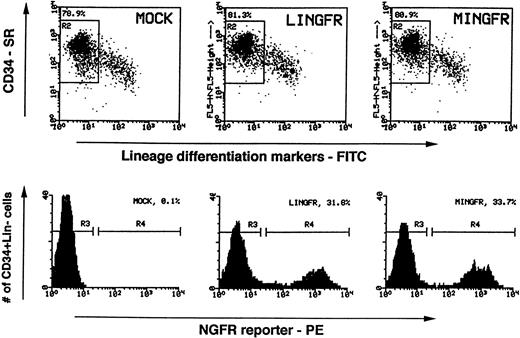
Enriched CD34+ cells from BM or mPB were further purified by FACS to reach greater than 95% purity with respect to CD34 expression and lack of expression of lineage-specific differentiation markers (CD2 and CD4 for T cells, CD16 for natural killer [NK] cells, CD19 for B cells, CD14 for monocytes, CD15 for granulocytes, and glycophorin A for erythroid cells). These highly purified cell populations (denoted as CD34+Lin−) were prestimulated in a cytokine cocktail (IL-3, IL-6 plus SLF) overnight and then exposed to retroviral supernatants in the “spinoculation” protocol. Viral supernatants were added on days 1 and 2, and then the cells were cultured for 2 more days to allow gene expression. Gene transfer efficiency and NGFR expression level were then investigated by FACS analysis. Under the conditions used, total cell numbers increased during this period by approximately fourfold or twofold for input CD34+Lin− cells isolated from BM (n = 3) or mPB (n = 6), respectively. Shown in Fig 3 as an example, ≈80% of the cells retained the CD34+Lin− phenotype after 4 days in culture while the remaining cells lost the CD34 antigen and gained one or more lineage differentiation markers (n = 3 for mPB). The CD34+ content of the cultures decreased rapidly thereafter, concomitant with the continued increase in total cell numbers. Among mPB cells which retained the CD34+Lin−phenotype (gated in the R2 region in Fig 3), greater than 30% expressed the NGFR transgene at day 4, irrespective of which retroviral vector backbone was used. The percentages of NGFR+ cells and the corresponding mean fluorescence intensities were, however, marginally but consistently higher for cells transduced by MINGFR than for those transduced by LINGFR (n = 3 for mPB). Similar results were obtained with transduced CD34+Lin-cells of BM origin (n = 2, data not shown).
FACS analyses of NGFR transgene expression in transduced CD34+Lin− cells. Sorted human mPB CD34+Lin− cells were activated and transduced with LINGFR or MINGFR vector supernatants. Two days after viral transduction (4 days in culture), expression of CD34 (stained with an SR-conjugated antibody) and the Lin markers (stained with FITC-conjugated antibodies) were analyzed (dot plots in upper panels). Live cells which retained the CD34+Lin−phenotype were gated (R2 regions) and percentages of gated cells among the total live cells were determined (≈80% in all cases). Efficiencies of gene transfer and expression levels in these CD34+Lin− cell populations were assessed by presence of NGFR (stained by a PE-conjugated antibody) and are plotted as histograms in the lower panels. The gates set up to sort cells expressing NGFR (NGFR+, R4) and cells lacking the NGFR surface reporter (NGFR−, R3) are indicated as are the percentages of NGFR+ cells.
FACS analyses of NGFR transgene expression in transduced CD34+Lin− cells. Sorted human mPB CD34+Lin− cells were activated and transduced with LINGFR or MINGFR vector supernatants. Two days after viral transduction (4 days in culture), expression of CD34 (stained with an SR-conjugated antibody) and the Lin markers (stained with FITC-conjugated antibodies) were analyzed (dot plots in upper panels). Live cells which retained the CD34+Lin−phenotype were gated (R2 regions) and percentages of gated cells among the total live cells were determined (≈80% in all cases). Efficiencies of gene transfer and expression levels in these CD34+Lin− cell populations were assessed by presence of NGFR (stained by a PE-conjugated antibody) and are plotted as histograms in the lower panels. The gates set up to sort cells expressing NGFR (NGFR+, R4) and cells lacking the NGFR surface reporter (NGFR−, R3) are indicated as are the percentages of NGFR+ cells.
Transduction of human CFC and CAFC progenitors.
At day 2 posttransduction (4 days in culture), cells were plated in methylcellulose to detect clonogenic myeloid and erythroid progenitors in a standard CFC assay. Total numbers of colonies were first enumerated by visual inspection and then the efficiency of gene transduction was assessed either by PCR to detect the presence of the vector sequences in individually picked colonies or by FACS to examine NGFR expression in populations of CFC-derived myeloid and erythroid cells. Approximately 25% of the input cells transduced by either MINGFR or LINGFR formed colonies, similar to nontransduced controls (Table 1). PCR analysis indicated that 28% (out of 64 picked CFC colonies) were transduced by MINGFR and 34% (out of 65 picked CFC colonies) were transduced by LINGFR, indicating that both vectors were capable of achieving similar efficiencies of gene transfer to CFC. When NGFR expression in differentiated cells derived from CFC was analyzed after 2 weeks in culture, we found that only 7.7% to 12.5% of the total cell populations expressed detectable cell surface NGFR (Table 1).
NGFR Transgene Expression in CFC-Derived Progeny
| Cells . | Vectors . | CFC Frequency (%) . | % of Individual Colonies That Show Transgene Presence (no.+/no. analyzed) . | % of Pooled Cells That Show Transgene Expression at Day 14 . |
|---|---|---|---|---|
| MINGFR | 26.3 ± 0.9 | 28 (16/64) | 12.5 | |
| (81% CD34+Lin−, 34% NGFR+) | ||||
| Unsorted | LINGFR | 25.1 ± 0.2 | 34 (22/65) | 7.7 |
| (81% CD34+Lin−, 32% NGFR+) | ||||
| None | 23.6 ± 1.2 | ND | 0.5% | |
| (81% CD34+Lin−) | (background) | |||
| Sorted | MINGFR | 22.9 ± 3.5 | ND | 78.0 |
| (NGFR+CD34+Lin−) | LINGFR | 19.9 ± 0.9 | ND | 52.6 |
| Cells . | Vectors . | CFC Frequency (%) . | % of Individual Colonies That Show Transgene Presence (no.+/no. analyzed) . | % of Pooled Cells That Show Transgene Expression at Day 14 . |
|---|---|---|---|---|
| MINGFR | 26.3 ± 0.9 | 28 (16/64) | 12.5 | |
| (81% CD34+Lin−, 34% NGFR+) | ||||
| Unsorted | LINGFR | 25.1 ± 0.2 | 34 (22/65) | 7.7 |
| (81% CD34+Lin−, 32% NGFR+) | ||||
| None | 23.6 ± 1.2 | ND | 0.5% | |
| (81% CD34+Lin−) | (background) | |||
| Sorted | MINGFR | 22.9 ± 3.5 | ND | 78.0 |
| (NGFR+CD34+Lin−) | LINGFR | 19.9 ± 0.9 | ND | 52.6 |
Two days after transduction using mPB CD34+Lin− cells, unsorted and the transgene-expressing subset of CD34+Lin−cells were each plated into methylcellulose culture. Colonies were enumerated at day 14. The presence of the retroviral DNA in individually picked colonies was determined by PCR, and transgene expression in CFC-derived cells was determined by flow cytometry of pooled colonies.
Abbreviation: ND, not done.
We next sorted transduced CD34+Lin− cells into two fractions on the basis of NGFR expression at day 2 posttransduction (Fig 3). The fact that a small percentage of NGFR− cells were capable of generating NGFR+ differentiated cells in the CFC assays (by FACS, data not shown) illustrated that not all of the retrovially transduced cells expressed sufficient levels of NGFR transgene at day 2 posttransduction to permit their identification and isolation. Thus, the frequencies of NGFR+ cells at this time point (day 2 posttransduction) were presumed to be underestimates of the actual values of cells containing the transgene. Nonetheless, since enrichment for cell-surface NGFR greatly simplified functional analyses of transduced cells and studies of LTR-mediated gene expression in functionally heterogeneous CD34+Lin− cells, we subsequently focused on those NGFR+ subpopulations that were clearly transduced and could be separated from untransduced cells. Approximately 20% of the CD34+Lin−NGFR+ cells generated by transduction with MINGFR or LINGFR formed CFC colonies (Table 1), a rate slightly less than that of unsorted cells (which is ≈25%). Based on these plating efficiencies (20% v 25%), this result would indicate that the majority of transduced CFC progenitors by both vectors also expressed the NGFR transgene 2 days posttransduction. Interestingly, however, NGFR transgene expression in CFC-derived cells at the end of the 2 week CFC assay period are different among NGFR+ cells transduced by the two vectors. Whereas 78% of CFC-derived progeny cells derived from MINGFR-transduced, sorted CD34+Lin−NGFR+ cells continued to express the NGFR transgene after 2 weeks, approximately one half of cells derived from LINGFR-transduced NGFR+ CFC had completely lost NGFR transgene expression while the remaining expressed NGFR at a reduced level (Table 1 and Fig 4). PCR analysis after cell sorting of LINGFR-transduced, CFC-derived cells which did not express NGFR after 2-week CFC assays confirmed that the transgene was still present in this NGFR− differentiated cell population (data not shown). Further multiparameter FACS analyses shown in Fig 4 indicated that downregulation of LINGFR-mediated NGFR expression occurred in differentiated erythroid (glycophorin A+) cells and granulocytes (CD15+), as well as in (CD14+cells) monocytes (data not shown). The downregulation of MoMLV LTR-mediated NGFR expression by the LINGFR vector in CD14+and CD15+ myeloid cells was also seen in a suspension culture assay (data not shown). These findings are consistent with those of a recent report in which the MoMLV LTR-mediated transcription is downregulated in differentiated erythroid/myeloid progeny derived from transduced CD34+ cells in CFC and suspension cultures, and the MSCV LTR-mediated transcription is substantially higher.19
LTR-mediated NGFR transgene expression in progeny of transduced CFC. Sorted NGFR-expressing, CD34+Lin− cells transduced with either the LINGFR (LINGFR+) or MINGFR (MINGFR+) vectors (see Fig 3) were assayed for CFC activity. After 14 days CFC numbers were enumerated (shown in Table 1), and total cells were obtained and stained for lineage markers and NGFR transgene expression. Fifty-two percent and 88% of erythroid cells (glycophorin A+), and 71% and 91% of granulocytes (CD15+) derived from LINGFR+ and MINGFR+ CFC, respectively, retained the NGFR reporter on cell surface.
LTR-mediated NGFR transgene expression in progeny of transduced CFC. Sorted NGFR-expressing, CD34+Lin− cells transduced with either the LINGFR (LINGFR+) or MINGFR (MINGFR+) vectors (see Fig 3) were assayed for CFC activity. After 14 days CFC numbers were enumerated (shown in Table 1), and total cells were obtained and stained for lineage markers and NGFR transgene expression. Fifty-two percent and 88% of erythroid cells (glycophorin A+), and 71% and 91% of granulocytes (CD15+) derived from LINGFR+ and MINGFR+ CFC, respectively, retained the NGFR reporter on cell surface.
Because silencing of MoMLV LTR-mediated expression had previously been observed in resting human T cells,17 and in differentiated erythroid/myeloid cells as shown above, we next examined whether this phenomenon also occurred in differentiated CD19+ B-lineage cells. The sorted NGFR+ and NGFR−fractions of CD34+Lin− cells (lacking CD19 expression) were plated at limiting dilution on stromal cell monolayers in a CAFC assay. There are two unique aspects of this long-term stromal-dependent CAFC assay compared with CFC assays: (1) CD19+ B-lineage cells as well as myeloid cells are generated from CD34+Lin− cells; and (2) late-appearing (after 5 weeks) cobblestone areas are considered to be derived from progenitors which are more primitive than CFC.13 31 The frequencies of CAFC in the various transduced cell populations were calculated based on Poisson distribution (Fig 5A). The frequencies of CAFC present in the NGFR+ subpopulations transduced by the MINGFR and LINGFR vectors were similar. However, both frequencies were more than 10-fold lower than the values calculated for the corresponding NGFR− subpopulations, indicating that the majority of CAFC were either not transduced at all or not expressing NGFR transgene 2 days posttransduction. An additional experiment using different preparations of viral supernatants confirmed this finding (data not shown).
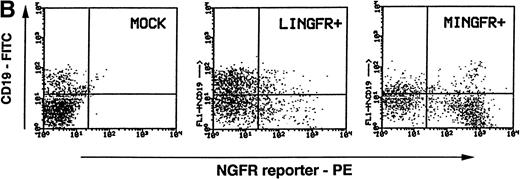
Maintenance of NGFR transgene expression in CAFC progeny. (A) Sorted NGFR-expressing, CD34+Lin− cells transduced with either the LINGFR (LIN, NGFR+) or MINGFR (MIN, GFR+) vectors, or the sorted CD34+Lin− cells lacking NGFR expression at day 2 posttransduction with LINGFR (LIN, NGFR−) or MINGFR (MIN, NGFR−) vectors (see Fig 3) were plated on SyS-1 stromal cell monolayers. Two-fold serial dilution of 100 CD34+Lin− input cells were seeded per well and examined for cobblestone area (CA) formation weekly up to 6 weeks. The number of wells lacking any CA at week 5 were plotted as a function of numbers of sorted cells, respectively. The frequencies of CAFC were estimated based on Poisson distribution and the results are indicated. (B) NGFR transgene expression in B cells formed at week 6 of CAFC assays. CD19+ B cells (in addition to CD14+and CD15+ myeloid cells) were generated from sorted CD34+Lin−NGFR+ cells in the presence of SyS-1 stromal cells as shown by the presence of the CD19 marker. Among CD19+ B cells, approximately 14% and 50% of the progeny cells derived from LINGFR-transduced (LINGFR+) and MINGFR-transduced (MINGFR+) CD34+Lin−NGFR+ cells, respectively, expressed the NGFR transgene at the end of CAFC assays.
Maintenance of NGFR transgene expression in CAFC progeny. (A) Sorted NGFR-expressing, CD34+Lin− cells transduced with either the LINGFR (LIN, NGFR+) or MINGFR (MIN, GFR+) vectors, or the sorted CD34+Lin− cells lacking NGFR expression at day 2 posttransduction with LINGFR (LIN, NGFR−) or MINGFR (MIN, NGFR−) vectors (see Fig 3) were plated on SyS-1 stromal cell monolayers. Two-fold serial dilution of 100 CD34+Lin− input cells were seeded per well and examined for cobblestone area (CA) formation weekly up to 6 weeks. The number of wells lacking any CA at week 5 were plotted as a function of numbers of sorted cells, respectively. The frequencies of CAFC were estimated based on Poisson distribution and the results are indicated. (B) NGFR transgene expression in B cells formed at week 6 of CAFC assays. CD19+ B cells (in addition to CD14+and CD15+ myeloid cells) were generated from sorted CD34+Lin−NGFR+ cells in the presence of SyS-1 stromal cells as shown by the presence of the CD19 marker. Among CD19+ B cells, approximately 14% and 50% of the progeny cells derived from LINGFR-transduced (LINGFR+) and MINGFR-transduced (MINGFR+) CD34+Lin−NGFR+ cells, respectively, expressed the NGFR transgene at the end of CAFC assays.
NGFR transgene expression was examined in differentiated B cells at week 6 of CAFC assays. Cells in cobblestone area–containing wells initially seeded with preselected NGFR+ transduced cells were obtained and pooled for simultaneous FACS analysis of the CD19 B-cell marker and NGFR transgene expression (Fig 5B). The majority of harvested hematopoietic cells were myeloid cells. A population of CD19+ cells was detected in pooled CAFC+ wells containing transduced cells by either LINGFR or MINGFR vector, or mock-transduced cells (Fig 5B). Although ≈50% of CD19+cells derived from MINGFR-transduced CD34+Lin−NGFR+ cells expressed a high level of NGFR reporter, a low to medium level of cell-surface NGFR could only be detected on ≈14% of CD19+ cells derived from LINGFR-transduced CD34+Lin−NGFR+ cells. Taken together, the results show that the MSCV LTR appears to be less susceptible to transcriptional silencing mechanisms than the MoMLV LTR in multiple lineages during in vitro differentiation of transduced human hematopoietic precursors.
Transduction of candidate human HSC assayed in SCID mice.
It has been shown that subsets of CD34+Lin− cells capable of long-term (>8 weeks) engraftment of fetal human bone implants in SCID mice (SCID-hu bone assay) exhibit B-lymphoid and myeloid potential as well as secondary repopulating capacity.12 13 To assess whether transduced CD34+Lin− cells expressing the NGFR reporter have candidate HSC activity, transduced mPB cells were tested in the SCID-hu bone assay for the presence of SCID repopulating cells (SRC). Because NGFR expression is higher in progeny derived from CD34+Lin− cells transduced with MINGFR than with LINGFR in the in vitro assays described above, for SRC assays we restricted our attention to the MINGFR-transduced cells. Nine weeks postinjection, the presence of donor (MA2.1+) cells and the NGFR transgene expression was examined by FACS analysis of total cells obtained from the human bone implants (Fig6). Cell-surface expression of NGFR could not be detected in nontransduced human hematopoietic cells (Fig 6A and B). The sorted CD34+Lin−NGFR+ cells that had been transduced by the MINGFR vector readily engrafted and maintained NGFR transgene expression in donor cells 9 weeks postinjection (Fig 6C and D).
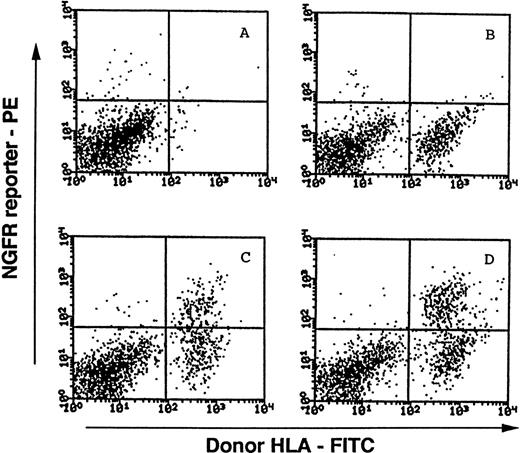
Representative FACS analyses of NGFR transgene expression in progeny of MINGFR-transduced, CD34+Lin−cells recovered from SCID-hu bone mice. Harvested cells from implanted human bone fragments were stained with the FITC-conjugated MA2.1 antibody recognizing the donor cell's HLA, and the PE-conjugated anti-NGFR antibody recognizing the transgene expression on cell surface. Viable cells were then collected and analyzed by FACS. Bone fragments in the absence of injected cells (A) and the presence of mock-transduced cells (B) were included as controls to distinguish donor cells (MA2.1+) from endogenous human cells and/or contaminating murine cells (MA2.1−). Cells recovered from two different bone implants injected with sorted CD34+Lin−NGFR+ cells transduced with MINGFR are shown in (C) and (D). Approximately 35.5% (C) and 44.7% (D) of donor-derived cells were expressing the NGFR reporter after 9 weeks in vivo. See Materials and Methods for experimental details and Table 2 for a summary.
Representative FACS analyses of NGFR transgene expression in progeny of MINGFR-transduced, CD34+Lin−cells recovered from SCID-hu bone mice. Harvested cells from implanted human bone fragments were stained with the FITC-conjugated MA2.1 antibody recognizing the donor cell's HLA, and the PE-conjugated anti-NGFR antibody recognizing the transgene expression on cell surface. Viable cells were then collected and analyzed by FACS. Bone fragments in the absence of injected cells (A) and the presence of mock-transduced cells (B) were included as controls to distinguish donor cells (MA2.1+) from endogenous human cells and/or contaminating murine cells (MA2.1−). Cells recovered from two different bone implants injected with sorted CD34+Lin−NGFR+ cells transduced with MINGFR are shown in (C) and (D). Approximately 35.5% (C) and 44.7% (D) of donor-derived cells were expressing the NGFR reporter after 9 weeks in vivo. See Materials and Methods for experimental details and Table 2 for a summary.
The results of our first two experiments are summarized in Table 2. Nine of 10 injections with mock-transduced cells showed donor cell engraftment. Because this frequency (90%) is similar to historic data obtained using freshly isolated human hematopoietic precursors,12 we believe that our protocol for ex vivo cell culture and transduction did not significantly alter the long-term SRC potential of CD34+Lin− cells. Both NGFR+and NGFR− subpopulations selected at day 2 posttransduction by MINGFR engrafted (5 of 5 and 2 of 2, respectively). All 5 bone grafts that successfully repopulated with MINGFR-transduced, preselected CD34+Lin−NGFR+cells showed reasonable levels of NGFR transgene expression (28% to 45% [37% ± 6%, n = 5] of the donor cells). In one experiment where sufficient numbers of donor-derived cells were available for an additional FACS analysis, both CD19+ (B-lineage) and CD19−NGFR+ (transduced donor) cells were found (data not shown). These findings showed that the MSCV LTR is functional in primitive human hematopoietic precursors with SRC potential and remains active for long periods in their transduced progeny after in vivo differentiation.
NGFR Transgene Expression in Transduced Human Cells Recovered From SCID-hu Bone Mice
| Cells Tested . | Engraftment Rate . | % Donor Cells per Graft . | % of Donor Cells That are NGFR+ . |
|---|---|---|---|
| Mock-transduced | 9/10 | 8.5-45.7 | N/A |
| NGFR+CD34+Lin− | 5/5 | 11.6-43.2 | 28.4-44.7 |
| NGFR−CD34+Lin− | 2/2 | 14.4-29.4 | 7.6-12.6 |
| Cells Tested . | Engraftment Rate . | % Donor Cells per Graft . | % of Donor Cells That are NGFR+ . |
|---|---|---|---|
| Mock-transduced | 9/10 | 8.5-45.7 | N/A |
| NGFR+CD34+Lin− | 5/5 | 11.6-43.2 | 28.4-44.7 |
| NGFR−CD34+Lin− | 2/2 | 14.4-29.4 | 7.6-12.6 |
Two days after transduction with the MINGFR retrovirus, CD34+Lin− cells were sorted into NGFR-expressing (NGFR+) and NGFR− subsets and injected into SCID-hu bone grafts. Nine weeks later grafts were analyzed by flow cytometry for donor-derived cells and NGFR transgene expression.
Abbreviation: N/A, not applicable.
DISCUSSION
In this report we evaluated the MSCV-based MINGFR vector carrying the NGFR reporter gene for its ability to efficiently transduce hematopoietic precursors purified from adult human BM or mPB. Based on FACS analyses and in vitro functional assays, similar efficiencies of gene transfer into CD34+Lin− cells as well as CAFC and CFC progenitors were observed as for the MoMLV-based LINGFR vector. Moreover, stable gene transfer into HSC (or SRC), based on the NGFR reporter expression in engrafted donor cells, was observed in 7 of 7 grafts of MINGFR-transduced cells. Because of the qualitative nature of the SCID-hu mouse model (in the absence of a limiting dilution analysis which requires large numbers of engineered animals), it is difficult to estimate the frequencies of HSC/SRC-based gene transfer by MINGFR, and to what extent the improved retroviral vector stocks and the “spinoculation” transduction protocol used in this study contributed to the success of these experiments. Because others reported recently that inclusion of certain cytokines (eg, Flk2/Flt3 ligand) and the presence of cell extra-cellular matrix molecules (eg, fibronectin fragments) can further preserve/activate candidate HSC and increase efficiencies of gene transfer into them, we expect that the transduction protocol reported here can be further optimized with these molecules.14,32 33
Although the level of NGFR expression directed by the LTR of either vector was comparable in several cell lines and in the total CD34+Lin− cell populations shortly after transduction, NGFR transgene expression mediated by MSCV LTR is substantially higher than that directed by MoMLV LTR in differentiated progeny derived from transduced CD34+Lin−cells (Figs 4 and 5B). We observed that the LTR-mediated gene expression from LINGFR (which is based on the LXSN-type of MoMLV vector) was downregulated in differentiated cells belonging to multiple-lineages including erythroid, myeloid, and B-lymphoid cells. Similar observations with MoMLV vectors have been made by other investigators in erythroid/myeloid lineages,19 in T cells,17 and in mouse hematopoietic cells after in vivo BM repopulation.18 Moreover, the phenomenon of the in vivo downregulation of gene expression of MoMLV LTR (typically caused by transcriptional silencing/inactivation) is not limited to the hematopoietic system, as it has also been observed in transduced primary fibroblasts, myoblasts, and hepatocytes in a variety of animal models.34 However, it should be noted that others have documented MoMLV LTR-directed gene expression in the T-cell or myeloid progeny of transduced CD34+ cells purified from cord blood or fetal liver after repopulation of SCID-hu thymus or SCID-hu bone grafts.35-37 Nonetheless, the possible extinction of MoMLV LTR-mediated expression needs to be taken into account when sustained expression in multiple myeloid and lymphoid lineages is deemed necessary for therapeutic benefits in HSC-based gene transfer applications.
Based on the outcome of this study, it would appear that MSCV-based vectors may offer advantages over conventional MoMLV-based vectors for gene delivery to the human hematopoietic system. The ability to direct sustained high-level expression of exogenous genes in differentiated cells derived from transduced HSC/progenitors is obviously a desirable goal of a number of human gene therapy protocols targeting congenital blood disorders.1 In addition, a vector that is permissive for expression in HSC may be of utility in those cancer gene therapy applications where the intent is to confer a drug-resistant phenotype to the patient's mature hematopoietic cells and their precusors to augment the therapeutic index of high-dose anti-tumor chemotherapy. In this regard, other types of retroviral vectors are currently being investigated for this purpose and are promising in human progenitor cells assayed in vitro.38 It remains to be determined whether these vectors are also functional in directing gene expression in HSC/SRC and whether they are more active than MSCV.
The sensitivity of the NGFR reporter system allowed facile monitoring of transgene expression during differentiation of transduced human hematopoietic precursors into progeny cells belonging to multiple (myeloid and lymphoid) lineages.22 Because the NGFR reporter gene is of human origin, it provides advantages in terms of increased specificity in SCID mouse models (compared with other mouse reporter genes in use) and, presumably, reduced immunogenicity in humans (compared with bacterial or mouse reporter genes). It was a concern at the outset that endogenous NGFR expression in human hematopoietic cells may complicate the SCID-hu bone assay. However, we have not been able to detect cell-surface NGFR expression in primary CD34+ cells, their progeny cells differentiated in vitro and in vivo, or in fetal hematopoietic cells residing in the recipient bone fragments in any of our experiments. In any case, although the presence of the full-length NGFR gene did not exert any noticeable adverse effects on the transduced hematopoietic precursors we evaluated, use of vectors like MINT expressing C-terminally truncated NGFR genes should alleviate any remaining misgivings. Therefore, the stable and nonimmunogenic NGFR reporter coexpressed with a therapeutic gene from the same vector may be useful in human gene therapy based on human HSC as well as T cells,39 if an easily detectable marker is desired to monitor and isolate transduced cells.
In summary, MSCV-based retroviral vectors encoding easily detectable and selectable markers should facilitate studies aimed at further characterizing the CD34+Lin− subset containing SRC. It is anticipated that the information gained will lead to improvements in HSC-based gene and cellular therapies.
ACKNOWLEDGMENT
We are grateful to Dr Richard Rigg for providing ProGag and ProPak-A packaging cells, and to Dr Ivan Plavec for providing a LXSN-based retroviral vector and an NGFR-containing plasmid. We also thank the SyStemix Cell Processing Group for isolation of CD34+ cells and the Comparative Medicine Group for production of SCID-hu mice.
R.G.H. is supported in part by a grant from the National Cancer Institute of Canada.
Address correspondence to Linzhao Cheng, PhD, Johns Hopkins University School of Medicine and Osiris Therapeutics, Inc, 2001 Aliceanna St, Baltimore, MD 21231; e-mail: LCheng@Osiristx.com.
Address reprint requests to Beth Hill, PhD, Systemix, Inc, 3155 Porter Dr, Palo Alto, CA 94304.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal