Abstract
Little is known about the mechanisms and the kinetics of the so-called graft-versus-leukemia (GVL) response induced by donor lymphocyte infusions (DLI) in patients with leukemic relapse after allogeneic bone marrow transplantation (BMT). We sought to elucidate this problem by sequentially studying three patients with relapsed chronic myeloid leukemia after sex-mismatched BMT from time before donor leukocyte infusion until achievement of complete molecular remission. Lineage-specific chimerism was assessed longitudinally by a combined fluorescent immunophenotyping and sex chromosome-specific in situ hybridization approach. Results were related to quantitative detection of bcr-abl transcripts by competitive differential reverse transcriptase-polymerase chain reaction (RT-PCR), qualitative bcr-abl RT-PCR, and multiplex PCR-based DNA donor/recipient chimerism. All patients had predominant donor lymphopoiesis at the time of DLI, suggesting a state of tolerance to recipient leukemic and/or normal cells. In contrast, granulopoiesis and erythropoiesis were mainly recipient derived in both patients with hematologic relapse and partly recipient derived in the patient with molecular relapse. Eighty percent, 90%, and 8% of CD34+cells, respectively, were found to be of recipient origin at relapse, and few donor stem cells predicted for cytopenia post-DLI. Responses were seen after a time lag of 5 to 13 weeks after DLI and resulted in reversal to full donor chimerism within a critical switch period of 4 to 5 weeks. A sudden decrease in recipient cells was paralleled by a sharp decrease in bcr-abl transcript numbers detectable several weeks before achievement of molecular remission and onset of clinical graft-versus-host disease (GVHD). This response pattern was confirmed by retrospective RT-PCR analysis in an additional five patients. Prospective monitoring of stem cell chimerism and response may enable us to individually tailor adoptive immunotherapy in the future.
DONOR LEUKOCYTE infusions are now a well-established treatment option for patients with chronic myeloid leukemia (CML) suffering from relapse of their disease after allogeneic bone marrow transplantation (BMT). Sustained remissions can be achieved in up to 80% of treated patients; however, they are complicated by severe marrow aplasia and/or graft-versus-host disease (GVHD) in a substantial number of cases.1 2 Although considerable evidence suggests that this so-called graft-versus-leukemia (GVL) effect is mediated by a direct cytotoxic activity of mature donor T cells, eventually leading to the eradication of the leukemic clone, still little is known about the targets, mechanisms, and kinetics of this response.
We sought to investigate the effects of donor lymphocyte infusions (DLI) in three consecutive patients with CML in relapse after allogeneic BMT by sequentially analyzing chimerism in different hematopoietic lineages before and after DLI until achievement of a complete response. Chromosome-specific fluorescent in situ hybridization (FISH) with simultaneous immunophenotyping of interphase cells (FICTION) was used as a primary tool of investigation. Results were related to those obtained by a quantitative competitive differential polymerase chain reaction (PCR) assay for the detection of bcr-abl fusion transcripts (CD-PCR), conventional nested bcr-abl reverse transcriptase-PCR (RT-PCR), and multiplex PCR-based donor/recipient DNA chimerism analysis. In addition, five patients were also studied using CD-PCR only.
PATIENTS AND METHODS
Patients
Three consecutive patients with relapsed CML after sex-mismatched allogeneic BMT were prospectively studied until achievement of hematologic and molecular remission (CR). Another five patients were analyzed retrospectively using CD-PCR only. All patients had been grafted with unmanipulated donor marrow. Donors were HLA-identical siblings with the exception of patient no. 2. Further patient characteristics are listed in Table 1.
Patient Characteristics and Treatment Response
| Patient No./UPN . | Age/ Sex . | Diagnosis at BMT . | Diagnosis at Relapse . | Tx Before DLI . | Cells/kg Infused at DLI . | Onset GVHD (wk) . | Start of Tx for GVHD (wk) . | Response . | |
|---|---|---|---|---|---|---|---|---|---|
| CD3 ×108 . | CD34 ×106 . | ||||||||
| 1/113 | 43/F | CML CP | CML CP | — | 3.7 | 2.3 | 13 | 14 | mol CR |
| 2/396 | 45/M | CML CP | CML CP | Hydroxyurea | 3.8 | — | 21 | 26 | mol CR |
| 3/338 | 23/M | CML CP | CML mol relapse | — | 1.8 | 2.4 | 12 | 15 | mol CR |
| 4/196 | 54/M | CML CP | CML CP | IFN | 1.6 | 18.2 | 9 | 14 | hem CR |
| 5/311 | 22/M | CML CP | CML CP | — | 1.7 | 1.1 | 7 | 15 | mol CR |
| 6/384 | 20/F | CML CP | CML CP | — | 1.2 | 2.4 | 7 | 10 | mol CR |
| 7/450 | 36/M | CML CP | CML mBC | Ida/AraC | 1.3 | 5.8 | 2 | — | hem CR |
| 8/468 | 39/M | CML CP | CML mBC | IFN, Ida/AraC | 0.8 | 1.6 | 8 | 22 | mol CR |
| Patient No./UPN . | Age/ Sex . | Diagnosis at BMT . | Diagnosis at Relapse . | Tx Before DLI . | Cells/kg Infused at DLI . | Onset GVHD (wk) . | Start of Tx for GVHD (wk) . | Response . | |
|---|---|---|---|---|---|---|---|---|---|
| CD3 ×108 . | CD34 ×106 . | ||||||||
| 1/113 | 43/F | CML CP | CML CP | — | 3.7 | 2.3 | 13 | 14 | mol CR |
| 2/396 | 45/M | CML CP | CML CP | Hydroxyurea | 3.8 | — | 21 | 26 | mol CR |
| 3/338 | 23/M | CML CP | CML mol relapse | — | 1.8 | 2.4 | 12 | 15 | mol CR |
| 4/196 | 54/M | CML CP | CML CP | IFN | 1.6 | 18.2 | 9 | 14 | hem CR |
| 5/311 | 22/M | CML CP | CML CP | — | 1.7 | 1.1 | 7 | 15 | mol CR |
| 6/384 | 20/F | CML CP | CML CP | — | 1.2 | 2.4 | 7 | 10 | mol CR |
| 7/450 | 36/M | CML CP | CML mBC | Ida/AraC | 1.3 | 5.8 | 2 | — | hem CR |
| 8/468 | 39/M | CML CP | CML mBC | IFN, Ida/AraC | 0.8 | 1.6 | 8 | 22 | mol CR |
Clinical data of patient no. 1 and patients no. 3 through 8 were reported previously.3
Abbreviations: CP, chronic phase; mBC, myeloid blast crisis; Tx, treatment; IFN, interferon α; Ida/AraC, induction course by idarubicin and araC; GVHD, graft-versus-host disease; DLI, donor lymphocyte (+/− peripheral stem cell) infusion; mol, molecular; hem, hematologic; CR, complete remission; UPN, unique patient number.
In June 1990, patient no. 1 received a BMT from her brother for Philadelphia chromosome-positive (Ph+) CML in chronic phase (CP) after conditioning with total body irradiation (TBI)/cyclophosphamide. She developed acute GVHD (aGVHD) grade II and limited chronic GVHD (cGVHD) of the skin and received a prolonged course of cyclosporine A. Since November 1993, a positive bcr-abl RT-PCR reaction was documented. At the time of hematologic relapse in November 1995, she was found to have 19 × 109/L peripheral blood (PB) leukocytes and a bone marrow (BM) with the morphologic characteristics of CP CML. Cytogenetics showed 15 of 15 female metaphases containing the Ph chromosome and additional complex abnormalities, including multiple deletions involving chromosomes 1, 2, and 15 as well as monosomy 15. In December 1995, she received one infusion of unmanipulated donor lymphocytes, followed by one infusion of peripheral donor stem cells 4 days later. The peripheral stem cell harvest of the donor was performed after a 4-day mobilization course with granulocyte colony-stimulating factor (G-CSF) at 10 μg/kg subcutaneously (SC). At week 11, the number of granulocytes of the patient decreased to a minimum of 0.78 × 109/L and a concomitant BM aspiration showed an excess of lymphocytes making up over 20% of BM cells. Molecular remission was documented at week 13. One week later, immunosuppressive treatment had to be introduced because of aGVHD of the skin and liver. The patient died 1 year after immunotherapy from complications of extensive cGVHD.
Patient no. 2 was grafted in March 1994 from an HLA A, B, DRB1-matched unrelated female donor for Ph+ CML CP. Conditioning consisted of busulfan/thiotepa/cyclophosphamide and antithymocyte globulin. He did not develop GVHD, and in March 1995 bcr-abl transcripts were documented by RT-PCR of PB. In October, overt hematologic relapse was evident, with 30 × 109/L peripheral leukocytes, a BM exam consistent with CP CML, and 25 of 25 BM metaphases showing the presence of a Ph chromosome. In January 1996, he received one infusion of unmanipulated buffy coat cells from his original donor. The patient was treated with hydroxyurea (HU) to control leukocytosis until week 4 after DLI, when transient leukopenia developed due to HU overdose. Because no response attributable to DLI was seen, interferon α (IFN) at 3 million units three times weekly was added from week 13 to 17. Cell counts rapidly decreased and cytopenia with a minimum of 0.5 × 109/L leukocytes and 10 × 109/L platelets developed at week 20. At the same time, an increase in liver enzymes was noted. At week 21, molecular remission was documented and cell counts returned to normal, but immunosuppressive treatment for extensive cGVHD of the oral mucosa and liver had to be instituted 5 weeks later.
In June 1993, patient no. 3 received a BMT from his sister for bcr-abl+ CML CP after conditioning with busulfan/cyclophosphamide. He did not develop GVHD. In December 1995, bcr-abl transcripts reappeared and were consistently present until DLI 7 months later. There were no signs of hematologic relapse; however, blood subgroup phenotyping for a known polymorphism in the Duffy system documented a growing proportion of recipient red blood cells. In July 1996, the patient received one infusion of peripheral stem cells from his sister harvested after a 4-day course of SC G-CSF at a dose of 10 μg/kg donor body weight. Molecular remission was documented after week 9 following immunotherapy without marrow depression. Acute and chronic GVHD of the buccal mucosa developed at week 12, requiring a 6-month course of prednisone.
Patients no. 4 through 8 were included into a pilot protocol evaluating the potential role of G-CSF–mobilized unmanipulated peripheral donor stem cell preparations as a mode of immunotherapy and are reported in more detail elsewhere.3
Methods
Before and after DLI, blood samples of the patient were obtained after informed consent at 2-week intervals for FICTION analysis. In parallel, bcr-abl fusion RNA copy numbers were determined by a competitive and differential PCR-based technique. Whenever possible, conventional qualitative RT-PCR for bcr-abl transcripts and donor/recipient DNA chimerism using a commercially available gene polymorphism detection kit (AmpliType PM; Perkin Elmer/Roche Molecular Systems Inc, Branchburg, NJ) were also performed.
FICTION.
FICTION was performed as published before with few modifications.4-6 Briefly, cytospin preparations of mononuclear cells were obtained after FICOLL density separation using standard protocols. Slides were fixed in acetone at room temperature for 10 minutes and incubated with an individually pretested dilution of monoclonal antibodies for 30 minutes. The following monoclonal antibodies were used: anti-CD34 (HPCA-1; Becton Dickinson [BD], San Jose, CA) as a marker of hematopoietic precursor cells; anti-CD15 (LeuM15 BD) for granulocytic cells; anti-glycophorin A (Immunotech, Marseille, France) for erythroblasts; anti-CD3 (Leu4; BD), anti-CD4 (IOT4; Immunotech), and anti-CD8 (IOT8; Immunotech) for T lymphocytes; and anti-CD22 (Pan-B; Dako, Hamburg, Germany) for B lymphocytes. Stained cells were visualized by sequential incubation with Cy3-conjugated goat antimouse, rabbit antigoat, and donkey antirabbit antibodies (Jackson/Dianova, Hamburg, Germany) at room temperature for 30 minutes. Incubations were separated by 2-minute washes in phosphate buffer, pH 8 (PN). All antibodies were diluted in PNM (5% nonfat dry milk, 0.02% Na-azide in PN). After immunophenotyping, slides were postfixed in cold methanol:acetic acid 3:1 for 10 minutes, followed by 1% paraformaldehyde/phosphate-buffered saline (PBS) for 2 minutes and dehydrated.
For in situ hybridization, commercially available biotinylated or fluorescein isothiocyanate (FITC)-conjugated centromeric probes specific for chromosomes X, Y, and 15 (DXZ1, DYZ1/DYZ3, and D15Z; Oncor/Appligene, Heidelberg, Germany) were used according to the following procedure: 2 μL of hybridization mixture (1 μg of [each] probe, 100 ng sonicated salmon sperm DNA, 10% Dextran, 60% formamide, and 1× SSC) were placed on the cytospin area, covered with a 10-mm coverslip, and sealed with rubber cement. After denaturation in a water bath for 5 minutes at 80°C, slides were incubated at 39°C for 4 hours. The slides were then washed three times for 3 minutes each in 50% formamide/2× SSC at 45°C and reequilibrated in PN. Biotinylated and FITC-conjugated probes were simultaneously detected by a mixture of AMCA-conjugated avidin (Jackson) and monoclonal anti-FITC (Dako), followed by a mixture of biotinylated goat antiavidin (Vector, Burlingame, CA) and FITC-conjugated donkey antimouse antibody (Jackson). For amplification, both steps were repeated as necessary. In case of single-color hybridization with a biotinylated probe, only FITC-conjugated avidin (Jackson) and biotinylated goat antiavidin were used.
Slides were mounted in antifade solution (Oncor) and examined with a Zeiss Axioplan fluorescence microscope (Zeiss, Jena, Germany) equipped with filters 00, 02, and 09. Results were documented using the digital image analysis system ISIS from Metasystems (Altlussheim, Germany).
Only slides with contiguous areas of intact cells with a hybridization efficiency of greater than 98% were analyzed. At least two slides per antibody and date were scored. We attempted to examine 200 antibody positive cells per slide or, in case of rare antibody positive cells, as many as possible. Differential hybridization results of slides stained with different antibodies within one series and date were used as internal controls. Percentages of male cells defined by the presence of only one X-chromosome were confirmed by at least one experiment using the two-color simultaneous XY-technique. Percentages of antibody stained cells were controlled by morphologic evaluation of cytospin preparations or, if necessary, by APAAP immunocytochemistry (results not shown).
Quantitative bcr-abl CD-PCR and conventional nested RT-PCR.
bcr-abl mRNA copy numbers of patient samples were quantified using a standardized, internally controlled, differential PCR-based technique described earlier.7 In brief, total RNA was extracted from PB samples and reverse transcribed using random hexamers. Subsequently, samples were subjected to PCR amplification with the addition of logarithmic dilutions of bispecific competitor fragments. bcr-abl and reference gene copy numbers were quantified by determination of the equivalence points with their specific competitors. Evaluated bcr-abl copy numbers were then divided through reference gene levels and expressed as the bcr-abl ratio.
Quantitative data were compared with results formerly obtained by a routinely performed qualitative PCR assay.8 In brief, randomly transcribed cDNA was subjected to PCR amplification using outer primers (sense: 5′-TTC AgA AgC TTC TCC CTg gCA TCC gT [b2a] and antisense 5′-ggT ACC Agg AgT gTT TCT CCA gAC Tg [abl3]) in a 480 DNA Thermal Cycler (Perkin Elmer, Rockville, MD) under the following conditions: cycle 1 of 94°C for 3 minutes, 60°C for 1 minute, and 72°C for 1 minute; cycles 2 through 31 of 94°C for 1 minute, 60°C for 1 minute, and 72°C for 1 minute; and a final cycle of 94°C for 1 minute and 60°C for 9 minutes. A second PCR was performed using 1 μL of first-round amplification mix and the inner primer pair (sense, 5′-gTC CAC AgC ATT CCg CTg ACC ATC AAT [bcr], and antisense, 5′-TgT TgA CTg gCg TgA TgT AgT TgC TTg g [ca3]), similar to first-round conditions with an annealing temperature of 62°C and a total of 25 cycles. The samples were judged positive by appearance of any signal in simple or nested amplification.
DNA donor/recipient chimerism.
Using the AmpliType Polymarker (PM) PCR Amplification and Typing Kit, the allelic polymorphism of 5 index genes plus an internal standard was simultaneously analyzed by a combination of multiplex PCR and reverse dot blot technique.9 10 DNA isolation from PB samples, PCR, and agarose gel electrophoresis were performed according to standard procedures. PCR primers were designed to yield six distinct PCR products sized from 133 to 242 bp. Amplified products were hybridized to nylon membrane strips containing immobilized sequence-specific oligonucleotide probes, as recommended by the manufacturer. Specifically bound DNA was visualized upon enzymatic conversion of a colorless substrate to a blue precipitate. Results were interpreted by reading the pattern of blue dots on the nylon strips and summarized as full recipient or donor versus mixed DNA chimerism.
RESULTS
FICTION
All patients analyzed were mixed chimeras at the time of relapse (Table 2).
Sequential Differential Count of Recipient and Donor Cells as a Percentage According to Cell Type
| Patient No. 1 CD . | BM wk −2 . | Blood wk 3 . | Blood wk 7 . | Blood wk 9 . | BM wk 11 . | Blood wk 11 . | Blood wk 13 . | Blood wk 16 . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XX . | X0 . | XX . | X0 . | XX . | X0 . | XX . | X0 . | XX . | X0 . | XX . | X0 . | XX . | X0 . | XX . | X0 . | |
| 15 | 99.3* | 0.7 | 100 | 0 | 98 | 2† | 15.4‡ | 84.6 | 46.0 | 54.0 | 0 | 100‡ | 0 | 100 | ||
| (6) | (33) | (62) | ||||||||||||||
| 3 | 13.3 | 86.6 | 4.3* | 95.7 | 11.5 | 88.5 | 1.4* | 98.6 | 0.5 | 99.5 | 0 | 100 | ||||
| (10) | (65) | |||||||||||||||
| 22 | 2.0 | 98.0 | 11.3* | 87.7 | 0 | 100 | 6.7* | 93.3 | 0.9 | 99.1 | ||||||
| (1) | (49) | (11)‡ | (85)‡ | (25)‡ | ||||||||||||
| 34 | 81.4‡ | 19.6 | 12.8‡ | 87.3 | ||||||||||||
| (11) | (82) | |||||||||||||||
| GlyA | 94.3‡ | 5.7 | 1.9* | 98.1 | ||||||||||||
| Patient No. 2 CD | BM wk −8 | Blood wk −8 | Blood wk 6 | Blood wk 8 | Blood wk 10 | Blood wk 13 | Blood wk 17 | Blood wk 21 | ||||||||
| X0 | XX | X0 | XX | X0 | XX | X0 | XX | X0 | XX | X0 | XX | X0 | XX | X0 | XX | |
| 15 | 100 | 0 | 98.0 | 2.0 | 91.8 | 8.2 | 96.5 | 3.5 | 96.8 | 3.2 | 0 | 100 | 0 | 100 | ||
| (14) | ||||||||||||||||
| 3 | 3.0 | 97.0 | 7.5 | 92.5 | 0 | 100 | 5.4 | 94.6 | 0.8 | 99.2 | 1.9 | 98.1 | 2.9 | 97.1 | ||
| 22 | 0 | 100 | 4.0 | 96.0 | 0 | 100 | 0 | 100 | 5.0 | 95.0 | 1.0 | 99.0 | ||||
| (19) | (61) | |||||||||||||||
| 34 | 90.0 | 10.0 | 88.3 | 11.7 | 88.5 | 12.5 | ||||||||||
| (5) | (2) | (56) | (8) | (2) | ||||||||||||
| Gly | 93.2 | 6.8 | ||||||||||||||
| (82) | (6) | |||||||||||||||
| Patient No. 3 CD | BM wk 0 | Blood wk 0 | Blood wk 5 | Blood wk 7 | Blood wk 9 | Blood wk 12 | ||||||||||
| XY | XX | XY | XX | XY | XX | XY | XX | XY | XX | XY | XX | |||||
| 15 | 61.0 | 39.0 | 67.6 | 30.9 | 69.7 | 25.4 | 6.0 | 94.0 | 0 | 100 | 0 | 100 | ||||
| (3) | (47) | (87) | ||||||||||||||
| 3 | 1.9 | 98.1 | 0.6 | 99.4 | 0 | 100 | ||||||||||
| 22 | 0.8 | 99.2 | 0 | 2.2 | 97.8 | 0 | 100 | |||||||||
| 34 | 8.0 | 92.0 | 0 | 1002-153 | 0.9 | 99.12-153 | ||||||||||
| (18) | ||||||||||||||||
| GlyA | 11.5 | 88.5 | 0 | 100 | ||||||||||||
| (18) | ||||||||||||||||
| Patient No. 1 CD . | BM wk −2 . | Blood wk 3 . | Blood wk 7 . | Blood wk 9 . | BM wk 11 . | Blood wk 11 . | Blood wk 13 . | Blood wk 16 . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XX . | X0 . | XX . | X0 . | XX . | X0 . | XX . | X0 . | XX . | X0 . | XX . | X0 . | XX . | X0 . | XX . | X0 . | |
| 15 | 99.3* | 0.7 | 100 | 0 | 98 | 2† | 15.4‡ | 84.6 | 46.0 | 54.0 | 0 | 100‡ | 0 | 100 | ||
| (6) | (33) | (62) | ||||||||||||||
| 3 | 13.3 | 86.6 | 4.3* | 95.7 | 11.5 | 88.5 | 1.4* | 98.6 | 0.5 | 99.5 | 0 | 100 | ||||
| (10) | (65) | |||||||||||||||
| 22 | 2.0 | 98.0 | 11.3* | 87.7 | 0 | 100 | 6.7* | 93.3 | 0.9 | 99.1 | ||||||
| (1) | (49) | (11)‡ | (85)‡ | (25)‡ | ||||||||||||
| 34 | 81.4‡ | 19.6 | 12.8‡ | 87.3 | ||||||||||||
| (11) | (82) | |||||||||||||||
| GlyA | 94.3‡ | 5.7 | 1.9* | 98.1 | ||||||||||||
| Patient No. 2 CD | BM wk −8 | Blood wk −8 | Blood wk 6 | Blood wk 8 | Blood wk 10 | Blood wk 13 | Blood wk 17 | Blood wk 21 | ||||||||
| X0 | XX | X0 | XX | X0 | XX | X0 | XX | X0 | XX | X0 | XX | X0 | XX | X0 | XX | |
| 15 | 100 | 0 | 98.0 | 2.0 | 91.8 | 8.2 | 96.5 | 3.5 | 96.8 | 3.2 | 0 | 100 | 0 | 100 | ||
| (14) | ||||||||||||||||
| 3 | 3.0 | 97.0 | 7.5 | 92.5 | 0 | 100 | 5.4 | 94.6 | 0.8 | 99.2 | 1.9 | 98.1 | 2.9 | 97.1 | ||
| 22 | 0 | 100 | 4.0 | 96.0 | 0 | 100 | 0 | 100 | 5.0 | 95.0 | 1.0 | 99.0 | ||||
| (19) | (61) | |||||||||||||||
| 34 | 90.0 | 10.0 | 88.3 | 11.7 | 88.5 | 12.5 | ||||||||||
| (5) | (2) | (56) | (8) | (2) | ||||||||||||
| Gly | 93.2 | 6.8 | ||||||||||||||
| (82) | (6) | |||||||||||||||
| Patient No. 3 CD | BM wk 0 | Blood wk 0 | Blood wk 5 | Blood wk 7 | Blood wk 9 | Blood wk 12 | ||||||||||
| XY | XX | XY | XX | XY | XX | XY | XX | XY | XX | XY | XX | |||||
| 15 | 61.0 | 39.0 | 67.6 | 30.9 | 69.7 | 25.4 | 6.0 | 94.0 | 0 | 100 | 0 | 100 | ||||
| (3) | (47) | (87) | ||||||||||||||
| 3 | 1.9 | 98.1 | 0.6 | 99.4 | 0 | 100 | ||||||||||
| 22 | 0.8 | 99.2 | 0 | 2.2 | 97.8 | 0 | 100 | |||||||||
| 34 | 8.0 | 92.0 | 0 | 1002-153 | 0.9 | 99.12-153 | ||||||||||
| (18) | ||||||||||||||||
| GlyA | 11.5 | 88.5 | 0 | 100 | ||||||||||||
| (18) | ||||||||||||||||
Unshaded columns correspond to recipient values and shaded columns correspond to donor values. Vertical rows show cell types defined by positivity for different clusters of differentiation (CD). Results are expressed as the percentage of antigen-positive cells counted. In parentheses are the absolute numbers of counted cells in as less than 100.
Abbreviations: X0/XX, male/female cells defined by FISH using an X-specific probe; XY/XX, male/female cells defined by FISH using an X- and a Y-specific probe simultaneously; GlyA, glycophorin A; BM, bone marrow.
All female cells are XX1515.
Confirmed by simultaneous hybridization of an X- and a Y-specific probe.
Week −2: 6% to 7% of female cells are XX15−; week 11: 1/11 CD34+, 1/6 CD15+ female cells are XX15−.
Partial overlap with GlyA+ cells, as morphologically circulating erythroblasts − confirmed by MGG count.
Lymphoid cells.
At relapse, the vast majority of T cells were still of donor origin. However, in all patients, a minority of 1.9% to 13.3% of CD3+ cells were recipient derived (Fig 1A). The same pattern was true for CD4+ and CD8+ cells (results not shown). At CR, the minor recipient T-cell fraction had further decreased, with 0% to less than 2% of host T cells detectable. Similarly, at relapse, the majority of B cells were derived from donor hematopoiesis (see Fig 3D). Only 0.8% to 13.3% of CD22+cells were demonstrated to be of host origin. Again, this percentage decreased to ≤1% at CR. In patient no. 1, neither CD3+nor CD22+ cells displayed the clonal abnormality XX15− demonstrated by karyotyping.
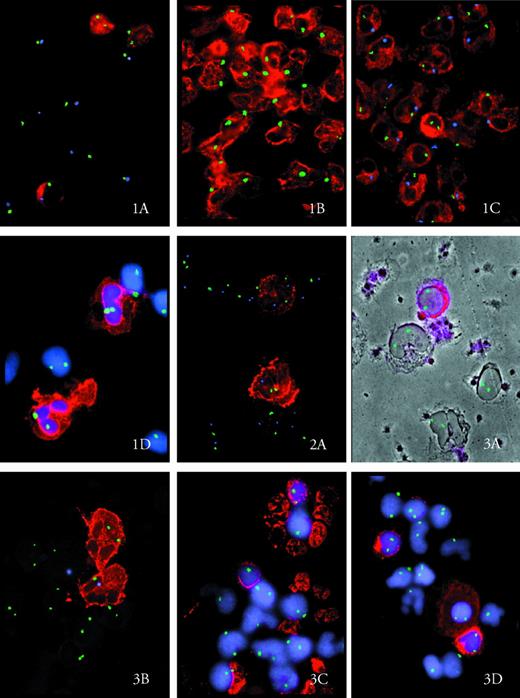
Original magnification × 420. Antigen-positive cells appear in red. In Figs 1D, 3C, and 3D, nuclei are counterstained in blue (DAPI; Oncor). Chromosome-specific hybridization signals are seen as green dots in simple and as green and blue dots in double hybridization experiments. (A) Mixed lymphoid chimerism in patient no. 2. Two CD3+ cells are XX, one is XY. CD3− cells are XY in relapse. X, green dot; Y, blue dot. (B) Male CD15+ cells at relapse in patient no. 2. X, green dot. (C) One female CD15+ cell (XX) within male CD15+ cells (XY) at week 6 after DLI in patient no. 2. X, green dot; Y, blue dot. (D) CD15+ and CD15−cells are all XX at week 17 after DLI in patient no. 2. X, green dot.
Original magnification × 420. Antigen-positive cells appear in red. In Figs 1D, 3C, and 3D, nuclei are counterstained in blue (DAPI; Oncor). Chromosome-specific hybridization signals are seen as green dots in simple and as green and blue dots in double hybridization experiments. (A) Mixed lymphoid chimerism in patient no. 2. Two CD3+ cells are XX, one is XY. CD3− cells are XY in relapse. X, green dot; Y, blue dot. (B) Male CD15+ cells at relapse in patient no. 2. X, green dot. (C) One female CD15+ cell (XX) within male CD15+ cells (XY) at week 6 after DLI in patient no. 2. X, green dot; Y, blue dot. (D) CD15+ and CD15−cells are all XX at week 17 after DLI in patient no. 2. X, green dot.
(A) Two weakly positive CD34 cells in patient no. 1 at relapse. The upper has two chromosomes 15 (XX1515); the lower belongs to a subclone with monosomy 15 (XX15). X, green dot; chromosome 15, blue dot.
(A) Two weakly positive CD34 cells in patient no. 1 at relapse. The upper has two chromosomes 15 (XX1515); the lower belongs to a subclone with monosomy 15 (XX15). X, green dot; chromosome 15, blue dot.
(A) Donor CD34+ cells (XX) circulating in the blood in patient no. 3 at week 9 after DLI. X, green dot. Outlines of CD34− cells are visualized by simultaneous phase contrast. (B) Mixed chimerism within the granulocytes in patient no. 3 at molecular relapse. One CD15+ cell is XX and one is XY. X, green dot; Y, blue dot. (C) Mixed chimerism within bone marrow erythroblasts in patient no. 3 at molecular relapse. One GlyA+ nucleated cell is XX and one is X. Most GlyA− BM cells are female. X, green dot. (D) Donor B cells, including one weakly CD22+ plasmacell in the BM of patient no. 3 at molecular relapse. X, green dot.
(A) Donor CD34+ cells (XX) circulating in the blood in patient no. 3 at week 9 after DLI. X, green dot. Outlines of CD34− cells are visualized by simultaneous phase contrast. (B) Mixed chimerism within the granulocytes in patient no. 3 at molecular relapse. One CD15+ cell is XX and one is XY. X, green dot; Y, blue dot. (C) Mixed chimerism within bone marrow erythroblasts in patient no. 3 at molecular relapse. One GlyA+ nucleated cell is XX and one is X. Most GlyA− BM cells are female. X, green dot. (D) Donor B cells, including one weakly CD22+ plasmacell in the BM of patient no. 3 at molecular relapse. X, green dot.
Granulocytic cells.
In CML, the predominant cell type in BM and PB is CD15+. These cells were 99% to 100% of recipient type in patients no. 1 and 2 at hematologic relapse and shortly after DLI (Fig 1B). In patient no. 3, who was treated while still in hematologic remission, two thirds of mature granulocytes were derived from the host, whereas one third were still of donor origin (see Fig 3B). In patients no. 1 and 2, a small number (2%) of donor CD15+ cells appeared as early as week 9 and week 6 after DLI in the PB, respectively. This minority in patient no. 2 remained stable between 2% and 8% for a period of 8 weeks (Fig 1C). In all three patients, after a variable length of time between week 5 (patient no. 3) and week 13 (patient no. 2), a sudden and sharp decrease in recipient granulocytes was documented and led to a complete reversal to full donor chimerism within a period of 4 to 5 weeks. For the purpose of this report, this time will be defined as critical switch period.
Erythroid cells.
All patients had mixed chimerism within the erythropoietic lineage at relapse. Whereas in patients no. 1 and 2 only 6% to 7% of BM Glycophorin A-positive cells were left from the donor, in patient no. 3, just 11.5% of erythroblasts had reverted to recipient type (see Fig3C).
Hematopoietic precursor cells.
In all three patients we were able to analyze enough precursor cells to demonstrate chimerism within the CD34+ compartment. In patients no. 1 and 2, the majority of BM CD34+ cells before DLI were recipient derived and therefore at least in part belonging to the malignant clone. However, in both patients there was a considerable percentage of CD34+ cells (10% to 20%) surviving from the donor, even during overt hematologic relapse. Donor CD34+cells were usually smaller in size and brighter positive for HPCA-1 antibody than their recipient, probably malignant counterparts. In patient no. 3, whose relapse was confined to a submicroscopic level, only a minority of CD34+ cells (8%) were of host origin. In all patients the same sudden reversal of recipient/donor ratio for CD34+ cells took place during the critical switch period, as seen for CD15 and glycophorin A-positive cells. Interestingly, also in PB, variable numbers of donor and not only host CD34+cells were demonstrable until CR.
In patient no. 1, an attempt was made to further characterize the malignant recipient cells by simultaneous hybridization with a chromosome X- and a chromosome 15-specific probe. However, uniformly, only 6% to 7% of CD34+, CD15+, and glycophorin A-positive female cells, not of lymphocytes, were found to have lost one chromosome 15, as suggested by classical cytogenetics at relapse (Fig2A). This minority of XX15− cells therefore seems to reflect the emergence of a subclone within the myeloid lineage, which, through its preferential growth, constituted the majority of dividing cells at karyotyping.
bcr-abl CD-PCR
A prospective analysis (patients no. 1, 2, and 3) was performed.
Time from relapse until the critical switch period.
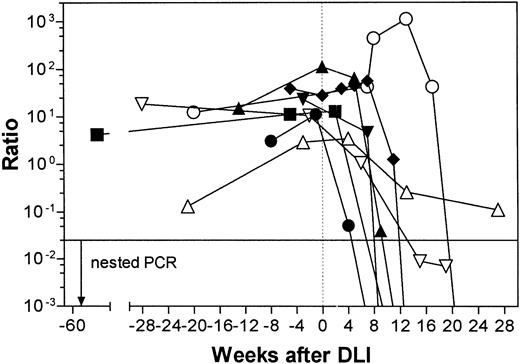
The slopes of these curves are depicted in Fig 4A, B, and C and are different for each patient. In patient no. 2, the bcr-abl ratio stayed on a relatively low level during the first weeks after DLI while the patient was receiving HU. At week 7, there was a sharp increase in the bcr-abl ratio up to a 10-fold maximum at week 13, when IFN was instituted. This value is by far the highest compared with the other two patients, possibly reflecting the higher tumor load of patient no. 2. Interestingly, the strong increase in the number of bcr-abl transcripts parallels the period of the appearance of a steady-state low percentage of peripheral donor granulocytes in patient no. 2.
DLI response in three patients (A, B, and C). Results of four different techniques are displayed in parallel relative to time. The shaded areas indicate the critical switch period. DNA-C: DNA chimerism by multiplex PCR ([▪] recipient DNA chimerism; [□] mixed recipient/donor DNA chimerism; [▨] donor DNA chimerism). PCR: qualitative RT-PCR results ([•] positive; [○] negative). QPCR: quantitative RT-PCR results (▴). FISH: fluorescent in situ hybridization ([⧫] absolute granulocyte counts, left Y-axis; [▾] donor granulocytes as a percentage as determined by percentage of CD15+ donor cells, right Y-axis); GVHD, onset of GVHD; HU, hydroxyurea treatment; IFN, IFN treatment.
DLI response in three patients (A, B, and C). Results of four different techniques are displayed in parallel relative to time. The shaded areas indicate the critical switch period. DNA-C: DNA chimerism by multiplex PCR ([▪] recipient DNA chimerism; [□] mixed recipient/donor DNA chimerism; [▨] donor DNA chimerism). PCR: qualitative RT-PCR results ([•] positive; [○] negative). QPCR: quantitative RT-PCR results (▴). FISH: fluorescent in situ hybridization ([⧫] absolute granulocyte counts, left Y-axis; [▾] donor granulocytes as a percentage as determined by percentage of CD15+ donor cells, right Y-axis); GVHD, onset of GVHD; HU, hydroxyurea treatment; IFN, IFN treatment.
Critical switch period.
In all three patients a critical decrease of bcr-abl transcript numbers to 0 can be seen within a period of 4 to 5 weeks after a varying time lag from DLI. This time lag was shortest for patient no. 3, with subclinical relapse (5 weeks), and longest for patient no. 2 (13 weeks); in the latter case, the lag time was coincidental with the introduction of IFN. In every case it paralleled the switch to donor cell type in the different hematopoietic lineages, with a steep increase in the percentage of donor granulocytes and a variable degree of depression in absolute granulocyte counts, as shown in Fig 4A, B, and C. This decrease was mildest in patient no. 3, lasted 1 week in patient no. 1, and led to a 4-week period of granulocytopenia in patient no. 2.
A retrospective analysis (patients no. 4 through 8) was performed.
A comparable pattern of abrupt disappearance of bcr-abl transcripts was seen in patients no. 5, 6, and 7, whereas patients no. 4 and 8 had a more prolonged and incomplete decrease in bcr-abl transcript numbers (Fig 5).
Time course plot of GVL response of all patients included in this study. Quantitative RT-PCR data are given as the bcr-abl ratio. Connecting lines crossing the X-axis indicate a PCR-negative follow-up sample. (⧫) Patient no. 1; (○) patient no. 2; (▴) patient no. 3; (▵) patient no. 4; (•) patient no. 5; (▾) patient no. 6; (▪) patient no. 7; (▿) patient no. 8.
Time course plot of GVL response of all patients included in this study. Quantitative RT-PCR data are given as the bcr-abl ratio. Connecting lines crossing the X-axis indicate a PCR-negative follow-up sample. (⧫) Patient no. 1; (○) patient no. 2; (▴) patient no. 3; (▵) patient no. 4; (•) patient no. 5; (▾) patient no. 6; (▪) patient no. 7; (▿) patient no. 8.
Qualitative bcr-abl RT-PCR and DNA Donor/Recipient Chimerism
The relation of the qualitative bcr-abl results, DNA chimerism analysis, and occurrence of clinical GVHD is plotted in Fig 4A, B, and C. The AmpliType PM system was informative only in host direction for donor/recipient pair 1, in both directions for donor/recipient pair 2, and not informative in donor/recipient pair 3, because patient no. 3 shared all alleles of the five index genes with his sister (results not shown). In patient no. 1, reversal to full donor chimerism was documented from week 11 onwards. In patient no. 2, before DLI, only recipient DNA could be demonstrated. As early as week 4, donor DNA was detectable in addition to recipient DNA. At week 20, reversal from mixed DNA chimerism to complete donor chimerism was documented.
DISCUSSION
The present study prospectively reports on three patients with relapsed CML after allogeneic non–T-cell–depleted BMT. At relapse, the patients were at different stages of their disease: Whereas patient no. 1 had stable hematologic relapse, patient no. 2 had actively evolving leukocytosis, necessitating the use of hydroxyurea. In contrast to the former two patients, patient no. 3 had not relapsed beyond the molecular level. All three received different forms of adoptive immunotherapy: patient no. 2 donor lymphocytes only and patients no. 1 and 3 donor lymphocytes and peripheral stem cells harvested according to different protocols in an attempt to abrogate post-DLI aplasia. Although the latter approach has not been shown to definitely prevent or shorten cytopenia, it seems to be as efficacious in inducing remission as donor lymphocytes alone.3,11 12 Bearing these obvious differences in mind, a rather consistent pretreatment pattern of chimerism and response could be demonstrated by the FICTION and quantitative bcr-abl PCR techniques used in this work. In addition, we were able to confirm our conclusions by a retrospective CD-PCR–based analysis of another five CML patients after donor cell infusion.
To monitor the DLI response and, in contrast to other investigators,13 we present our quantitative PCR data as the ratio between the amplification of the target gene and that of a reference gene and not as absolute bcr-abl copy numbers per cell. This difference is because we believe it difficult to predict an average bcr-abl mRNA content per cell for inhomogenous blood samples even when taking differentials into account. By standardizing and normalizing the detected bcr-abl copy numbers to a reference gene via the addition of a bispecific heterologous competitor fragment and parallel amplification in one reaction tube, we were able to minimize the problem of sample to sample variation and to detect even slight transcriptional changes. This strategy had already proven to be suitable in the context of ICSBP expression in myeloid leukemic cells, as reported previously.14
Chimerism studies after BMT have so far been hampered by the use of different tools to identify donor versus recipient lymphohematopoiesis, leading to conflicting results about the consequences of mixed chimerism on GVHD, GVL, and relapse.15-24 In addition, few investigators have addressed lineage specificity in the setting of mixed chimerism.15-17,19,25 26 Our FICTION data allow insights into the donor versus recipient nature of different lymphoid and hematopoietic lineages on a cell by cell level, therefore giving an accurate view of the changing donor/host ratio even within one cell type and at any stage of the treatment process.
All three patients reported here during relapse of their disease still had lymphoid cells predominantly of donor type, an observation that has been made by others before.16,17,19,27 If these donor T cells normally are thought to maintain remission by the so-called GVL effect, then their presence during relapse means that they do not mediate an efficient immune effect towards host leukemic and/or normal cells.28 However, the fact that a minority of T cells were of recipient origin at the time of relapse, a minority that disappeared at CR, argues against a leukemia-specific and towards a broader allo-specific target for the GVL response in this setting, because it is generally agreed that the majority of T cells in CP CML are normal.29,30 Our results are in line with the hypothesis of Mackinnon et al,19 31 who proposed that mixed T-cell chimerism post-BMT could favor relapse by allowing donor/host tolerance to establish, thereby abrogating the GVL effect. According to this line of thought, the infusion of mature donor lymphocytes grown up in the donor environment would break tolerance to recipient cells and therefore suppress growth of leukemic and normal host cells at the same time.
In contrast to lymphocytes, the majority of granulocytic and erythrocytic cells were clearly of recipient origin at the time of hematologic relapse. The percentage of host cells within one lineage correlated well with the clinical stage of relapse; in patient no. 3, only a few cells had reverted to recipient type at treatment. Although the small number of patients studied and the different treatment protocols preclude general conclusions, in our cases the percentage of host cells and the time to achieve CR were inversely correlated. Rapanotti et al,32 using PCR amplification of VNTR regions, have made a similar observation in two patients. It is easily conceivable that the time for clonal expansion of transfused donor cells varies according to host tumor burden and donor T-cell repertoire and that a critical effector/target ratio of T cells has to be reached before an overwhelming GVL effect can take place. However, a preliminary change in host/donor balance, possibly of different mechanism, could be seen in patients no. 1 and 2 as early as 6 weeks after DLI, with low but stable percentages of donor granulocytes appearing in PB. Also, in patient no. 2, donor DNA was detectable already at week 4 by multiplex PCR. At least in patient no. 2, who received donor lymphocytes only, these donor granulocytes cannot be interpreted as progeny of newly engrafted stem cells. Instead, they seem to have differentiated from those cells within the CD34+ compartment, which at relapse were shown to have survived from the donor. As expected, the number of these progenitor cells varied according to the stage of relapse before treatment. In patient no. 3, obviously donor CD34+ cells at molecular relapse were present in sufficient numbers to prevent a measurable decrease in peripheral cell counts after eradication of recipient cells. In contrast, patient no. 2 had only 10% of donor CD34+ cells left and experienced a 4-week period of spontaneously resolving cytopenia. Keil et al33 recently published a study with sorted cells and showed that a level of about 5% donor cells was sufficient to protect against critical aplasia post-DLI. Healthy recipient CD34+ cells do not protect against cytopenia, as shown by the same investigators, again arguing for an alloreactive mechanism of GVL. The FICTION technique used here accurately documented residual donor cells within the stem cell compartment at relapse and may therefore be of direct prognostic value for the patient if used before DLI.
The most original insight of this study, however, lies in the description of a critical switch period demonstrable in all complete responders of this study. After a variable length of time, possibly allowing expansion of reactive cells, a rather uniform phenomenon was seen in every case. A steep decrease in bcr-abl ratio was paralleled by a sudden increase in donor granulocytes and other cells of the myeloid series until molecular remission was achieved about 4 weeks later. This process in complete responders seemed to be self-governed and, once started, only limited by the disappearance of targets, including nonmalignant cells such as host lymphocytes. In patient no. 2, it appears that the introduction of IFN triggered this response, although a simple coincidence cannot be ruled out. After the eradication of host hematopoiesis, total granulocyte counts depended on the availability of donor stem cells spared by the violence of GVL reaction. Clinically evident GVHD in these cases occurred only after complete disappearance of leukemic and normal host cells. In contrast, patients no. 4 and 8, who did not reach a molecular but only a hematologic remission as defined by CD-PCR, had a more prolonged decrease in bcr-abl ratio. Clinical GVHD in these cases occurred before disappearance of bcr-abl transcripts, possibly arguing for a mechanism of immune escape of the malignant cells.
The qualitative techniques of bcr-abl RT-PCR and DNA chimerism used for comparison were able to demonstrate molecular CR of complete responders and reversal to full donor chimerism only in coincidence with clinical GVHD. However, by defining the turning point at the beginning of the critical switch period already several weeks before CR, the FICTION technique and quantitative bcr-abl CD-PCR used here were able to document an efficient GVL effect about 1 month earlier. Whether this time lag reflects a difference in mechanisms and/or effector cells or whether GVHD is simply the consequence of a broadening of the initial response remains to be determined. Mackinnon et al31 have demonstrated that, by choosing the lowest possible cell dose, the reaction can be confined to a GVL effect only, therefore avoiding GVHD in most patients. Sensitive detection of early relapse as well as systematic characterization of pretreatment chimerism and critical switch period with the help of the diagnostic tools described in this report may enable us to tailor future adoptive immunotherapy for the individual patient to achieve the most durable and safe response in relapsed CML after BMT.
ACKNOWLEDGMENT
The authors thank Barbara Oertel and Petra Glomp for preparing the cytospins and Jutta Laser for performing the qualitative bcr-abl RT-PCR. We are indebted to Dr Andreas Plesch from Metasystems for assistance in setting up the composite color plate. Dr Klaus Weber-Matthiesen helped with technical hints in the beginning of the FICTION project.
H.B. and S.N. contributed equally to this report.
Supported by grants from the Deutsche Forschungsgemeinschaft and the Deutsche José-Carreras-Stiftung (A.N.). The fluorescence microscope used for this work was financed with the help of the Berliner Krebsgesellschaft.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Herrad Baurmann, MD, BMT-Center, Deutsche Klinik für Diagnostik, Aukammallee 33, 65191 Wiesbaden, Germany; e-mail: Herrad.Baurmann@t-online.de.

![Fig. 4. DLI response in three patients (A, B, and C). Results of four different techniques are displayed in parallel relative to time. The shaded areas indicate the critical switch period. DNA-C: DNA chimerism by multiplex PCR ([▪] recipient DNA chimerism; [□] mixed recipient/donor DNA chimerism; [▨] donor DNA chimerism). PCR: qualitative RT-PCR results ([•] positive; [○] negative). QPCR: quantitative RT-PCR results (▴). FISH: fluorescent in situ hybridization ([⧫] absolute granulocyte counts, left Y-axis; [▾] donor granulocytes as a percentage as determined by percentage of CD15+ donor cells, right Y-axis); GVHD, onset of GVHD; HU, hydroxyurea treatment; IFN, IFN treatment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3582/4/m_blod42233004ax.jpeg?Expires=1769286227&Signature=AxGzUAB4e7fi5I5n3UDQRiLsDl61vFYbwd63PWCFu2TZFcPKXyOWR7GX~LtKpuxoXccBFEiwatWPZJWDhKAwFPSpnuGV~ea5ldMM~oQsvNSWTXnGjANu2cuB7C-Zvwy536aibAKyVwDvQ-htygDJYMg9AdccFR9VYzKiFe4JGdIVoAFQ3VdvnIEMjcz2odspJADkt5QBhaiEYm4WYi6MnozOOl~Tzzu2IBCoe8~iP2VFeCbE1vx9iEAPPB7vTK3W61DWX6rkF9FcwzhZtcnf~30OBeZy7UsullCFPXbHUxXHTj6J6O4cDLrMFChBH0bA~XtMKxzyqmDX8Tzu4qADrg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. DLI response in three patients (A, B, and C). Results of four different techniques are displayed in parallel relative to time. The shaded areas indicate the critical switch period. DNA-C: DNA chimerism by multiplex PCR ([▪] recipient DNA chimerism; [□] mixed recipient/donor DNA chimerism; [▨] donor DNA chimerism). PCR: qualitative RT-PCR results ([•] positive; [○] negative). QPCR: quantitative RT-PCR results (▴). FISH: fluorescent in situ hybridization ([⧫] absolute granulocyte counts, left Y-axis; [▾] donor granulocytes as a percentage as determined by percentage of CD15+ donor cells, right Y-axis); GVHD, onset of GVHD; HU, hydroxyurea treatment; IFN, IFN treatment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3582/4/m_blod42233004bx.jpeg?Expires=1769286227&Signature=ymUbJRyks~gMZuMhQEOvmzbxO4A75gwsTu7olEQazSOX7LX9DC9mkjcuwlXiYjTTU1OrmFagdo6mCpnJ~RucRnF6~AQAa9wcy1F4tM6QuIa3yoJNDXhAlBYddLm9pWX8XPLaGNlzWvHtWFklu9~mJ9dD5NlwvOFJmD9eQwo4tTML2Jpb7cT4akJZ9oXIo7ACMcRY0jKhPItZSETkOkKbgpEKTKCVoObqWwkVODjG70aU8YQ~58gSYEjothJBChxVopzph2me1CZ6tmkM4zGVCaMCqbRBbG6X5spKmJvcGVGm9QyRpwrD-b-kJm8TRwmlqlAPoBjcUW-1GqMryoq54g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. DLI response in three patients (A, B, and C). Results of four different techniques are displayed in parallel relative to time. The shaded areas indicate the critical switch period. DNA-C: DNA chimerism by multiplex PCR ([▪] recipient DNA chimerism; [□] mixed recipient/donor DNA chimerism; [▨] donor DNA chimerism). PCR: qualitative RT-PCR results ([•] positive; [○] negative). QPCR: quantitative RT-PCR results (▴). FISH: fluorescent in situ hybridization ([⧫] absolute granulocyte counts, left Y-axis; [▾] donor granulocytes as a percentage as determined by percentage of CD15+ donor cells, right Y-axis); GVHD, onset of GVHD; HU, hydroxyurea treatment; IFN, IFN treatment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3582/4/m_blod42233004cx.jpeg?Expires=1769286227&Signature=BgKqdm~y9~C0ebY6v7smZekxYM~bPUCvJrvkzTMvwbEmQEyShshf255M3GCx8lELLIyDY8Xt8bUH55obi-H276nhQA6jp3pSs7NDdaVk~rVNNyr7N9ktG2l69H9ZhHBCePEdb2itwq7aRT0I2qQcKjZNZ8AM8UT6qxrfDR7n5DSH3GnMqWnlYO9aX2bufisZdcGA~ij3DPuMYHaBlLsvT0Exyk--XM~ScGA8p3DmUAcBCjLFPZH17uA0J-jyUHgJp7nFwDe7a9nKEQxqAqzanjPfDRTTMfG6q6rFBRyR0oRehJD3nXF1qwOhvySKWefn7eUuaQ4DdeXx0U79YLXmyg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal