Abstract
We examined the mechanisms used by eosinophils to tether and accumulate on interleukin-4 (IL-4)–stimulated human umbilical vein endothelial cells (HUVECs) under flow conditions. As previously reported, HUVECs treated for 24 hours with 20 ng/mL IL-4 had increased expression of P-selectin and vascular cell adhesion molecule-1 (VCAM-1) but not E-selectin. We found that eosinophils tethered and rolled on IL-4–stimulated HUVECs at physiologic shear stresses. Eosinophil rolling was quickly followed by firm adhesion. Treatment with either an anti–P-selectin monoclonal antibody (MoAb) or an anti–VCAM-1 MoAb decreased both eosinophil tethering and accumulation at 2 dyn/cm2. VCAM-1 interacts with 4-integrins expressed on eosinophils. We found that an anti–4-integrin MoAb also blocked eosinophil tethering and accumulation at 2 dyn/cm2. None of these MoAbs alone had an impact on eosinophil accumulation at lower shear stresses, but when either an anti–VCAM-1 or an anti–4-integrin MoAb was used in combination with an anti–P-selectin MoAb, all eosinophil tethering and accumulation on IL-4–stimulated HUVECs were blocked. This was true at both high and low shear stresses. These data show that both P-selectin and VCAM-1 are required to tether eosinophils at high shear stresses, but at low shear stresses these adhesion proteins can act independently to recruit eosinophils to IL-4–stimulated HUVECs.
EOSINOPHILS REPRESENT a minor fraction of circulating leukocytes; however, they are a major component of the cellular infiltrate found in allergic diseases such as bronchial asthma,1,2 allergic rhinitis, and atopic dermatitis.3 Eosinophil cytotoxic mediators are believed to participate in epithelial cell shedding, mucous hypersecretion, microvascular leakage, and smooth muscle contraction associated with bronchiolar asthma.4 Because of the central role eosinophils play in these events, investigators are now trying to understand the molecular mechanisms responsible for selective recruitment of eosinophils into sites of allergic inflammation (reviewed by Wardlaw5). Because eosinophils must exit the vasculature and migrate into tissue to exert their effects, recent studies have focused on the adhesive interactions between vascular endothelial cells and eosinophils.
Leukocyte tissue infiltration is a multistep process that is initiated by leukocyte tethering and rolling along activated endothelium under flow conditions.6,7 Leukocyte tethering and rolling is mediated predominately through the selectin family of adhesion proteins.8 Selectin expression is restricted to the vasculature, with L-selectin expressed on most circulating leukocytes, E-selectin expressed on cytokine-activated endothelial cells, and P-selectin expressed on both activated endothelial cells and platelets.9 Eosinophils can use all of the selectins to form attachments under shear conditions10,11; however, P-selectin is much better at mediating eosinophil recruitment than E-selectin both in vivo12 and in vitro.10
The α4-integrins (α4β1 and/or α4β7) can also support leukocyte tethering and rolling by interacting with their ligands vascular cell adhesion molecule-1 (VCAM-1) and mucosal addressin cell adhesion molecule-1 (MadCAM-1).13,14 Although α4-integrins can participate in primary tethering to VCAM-1, several studies have demonstrated that leukocyte subclasses interact differently with VCAM-1. α4-integrins on T cells participate in both initial tethering and rolling on VCAM-1,13-15 whereas α4-integrins on monocytes do not participate in initial tethering but instead act to stabilize rolling adhesions and support firm adhesion.16 Cytokines can also alter leukocyte interactions with VCAM-1. VCAM-1 expressed on interleukin-1 (IL-1)– or IL-4–stimulated human umbilical vein endothelial cells (HUVECs) support T-cell rolling, but VCAM-1 expressed on HUVECs stimulated with both IL-1 and IL-4 does not support T-cell rolling.17 Thus, the role that VCAM-1 plays in selective recruitment of eosinophils will depend not only on expression and activation of α4-integrins on eosinophils, but also on the mechanism of endothelial cell activation. Finally, some studies suggest that shear forces will also govern leukocyte–VCAM-1 interactions.13,14 18
This study examines the interactions of eosinophils with HUVECs stimulated for 24 hours with IL-4, a cytokine purported to play a critical role in eosinophil recruitment in allergic inflammation.19 We show that eosinophils tether and adhere to IL-4–stimulated HUVECs under shear conditions. Surprisingly, we have found that eosinophil tethering and accumulation are dependent on both P-selectin and VCAM-1/α4-integrin interactions. Blocking any of these proteins decreases both eosinophil tethering and accumulation at higher shear stresses. As the shear stress is lowered, P-selectin and VCAM-1/α4-integrins act independently to accumulate eosinophils. This study clearly demonstrates both overlapping and cooperative roles for these adhesion proteins in eosinophil recruitment.
MATERIALS AND METHODS
Reagents and antibodies.
Human recombinant IL-4 and human recombinant tumor necrosis factor-α (TNF-α) were from R & D Systems, Inc (Minneapolis, MN). Hanks’ balanced salt solution with Ca2+ and Mg2+(HBSS), lymphoprep 1077, Dulbecco’s modified Eagle’s medium (DMEM), and Media 199 (M199) were from GIBCO BRL, Life Technologies (Grand Island, NY). Thirty-five–millimeter dishes were from Corning (Corning, NY) and all other plasticware was from Becton Dickinson (Franklin Lakes, NJ). Human serum albumin (HSA) was from Immuno US (Rochester, MI). Enhanced chemiluminescence reagents were from Amersham (Buckinghamshire, UK). Histamine was from Sigma (St Louis, MO). All other chemicals were from BDH, Inc (Toronto, Ontario, Canada).
The antihuman P-selectin monoclonal antibodies (MoAbs) S12 and G1 (both IgG1-κ) were prepared and characterized as described.20,21 G1, but not S12, blocks binding of P-selectin to leukocytes.22 The blocking antihuman E-selectin MoAbs ES1 (IgG1-κ) was prepared and characterized as described.23 The blocking antihuman VCAM-1 MoAb 1.G11B1 (IgG1), the antihuman α4-integrin MoAb H2/1 (IgG1), and the antihuman β2-integrin MoAb (IgG2b) were purchased from Serotec (Oxford, UK). Anti-CD16 MoAb conjugated to paramagnetic beads was purchased from Miltenyi (Auburn, CA).
Cell culture and isolation.
HUVECs were isolated from individual umbilical cords and grown in M199 with 20% human serum as primary cultures in either 35-mm dishes or in 48-well plates, as described.24 Only monolayers of primary cultures that were tightly confluent were used for these studies. Granulocytes were isolated from normal human donors by dextran sedimentation, hypotonic lysis, and density centrifugation on lymphoprep 1077, as described.24 Eosinophils were isolated from granulocytes by negative selection with CD16 microbeads using the Magnetic Cell Separation System (MACS), as described.10Eosinophils were greater than 94% pure as assessed by Kimura staining and/or Wright-Geimsa staining. L cells stably expressing human VCAM-1 were kindly provided by Dr John Elliott (University of Alberta, Edmonton, Alberta, Canada) and were maintained in DMEM with 10% fetal calf serum (FCS), as described.25
Adhesion under flow conditions.
Fluid shear stresses present in the microvasculature were simulated in a dual chamber parallel-plate flow chamber, as previously described.23,26 Confluent monolayers of primary HUVECs were treated with 20 ng/mL of IL-4 in M199 with 0.5% HSA (M199/A) for 24 hours and then washed once with HBSS. In other experiments, L cells stably transfected with human VCAM-1 were grown to confluence and used in the flow chamber. Eosinophils (5 × 105/mL) in HBSS/0.5% HSA (HBSS/A) were perfused through the chamber at the desired wall shear stresses. Eosinophil accumulation was determined after 4 minutes of rolling. These interactions were visualized with a ×10 objective using phase-contrast video microscopy. Interactions were quantified using a computer imaging system (NIH Image, Bethesda, MD). The number of adherent or rolling leukocytes was measured by digitizing six random image frames per condition. Tethering events were determined using frame-by-frame analysis during the first 60 seconds of perfusion, as described.10 Only cells that directly tethered to the surface (primary tethers) were counted. All experiments were performed at 37°C, unless otherwise indicated. In certain experiments, eosinophils or HUVECs were preincubated for 10 minutes with the specified MoAb and rolling was assayed in the continued presence of the MoAb.
Determination of adhesion protein expression.
Statistics.
All experiments were performed at least three times and the data are presented as the mean and SEM of those replicates. Statistical differences between experimental groups were evaluated using the unpaired Student’s t-test. P values ≤ .05 were considered significant.
RESULTS
Eosinophils accumulate on IL-4–stimulated HUVECs under shear conditions.
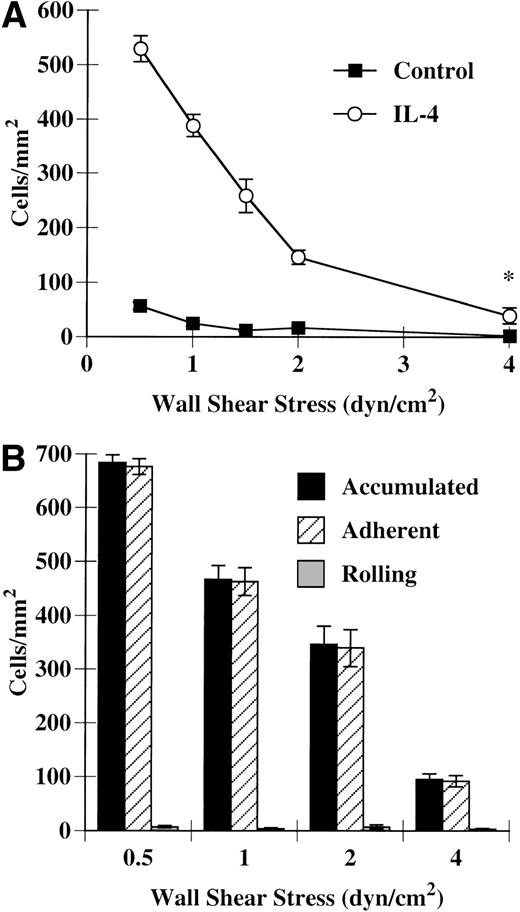
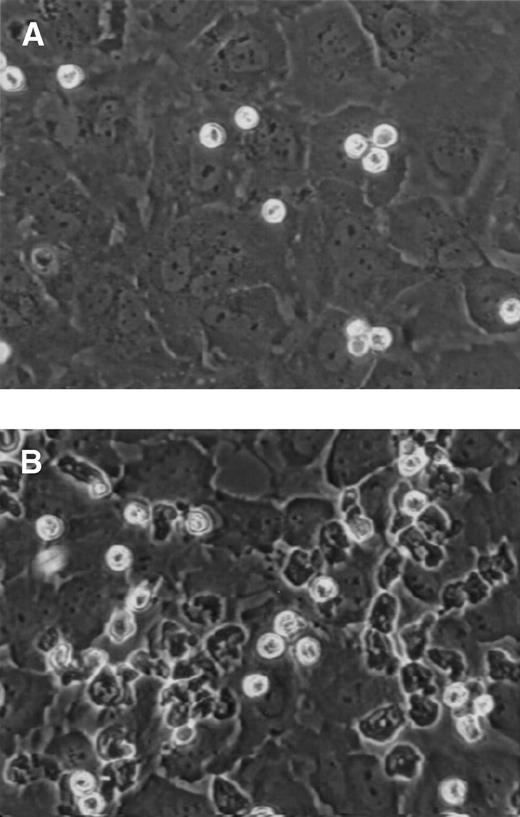
We found that eosinophils accumulated on IL-4–stimulated HUVECs at shear stresses as high as 4 dyn/cm2(Fig 1A); however, because there were many more interactions occurring at 2 dyn/cm2, this shear stress was the highest used in later experiments. Unlike eosinophil rolling on purified E-selectin or P-selectin,10 most eosinophils rolled for less than 1 cell diameter before becoming firmly adherent (Fig 1B). Thus, eosinophils do not participate in rolling interactions on IL-4–stimulated HUVECs, but instead become firmly adherent. Over a period of minutes, these eosinophils become activated, changing shape and spreading on the endothelial cell monolayer (Fig 2A and B). These data demonstrate that IL-4–stimulated HUVECs can tether, bind, and activate eosinophils under shear conditions.
Eosinophil accumulation on IL-4–stimulated HUVECs. Confluent monolayers of primary HUVECs were washed once with HBSS and then stimulated with either M199/A alone (control) or M199/A containing 20 ng/mL IL-4 (IL-4). After 24 hours at 37°C, the monolayers were washed with HBSS and assembled in the flow chamber. Purified eosinophils (5 × 105/mL) were perfused over the monolayers at the specified shear stresses and eosinophil accumulation (A), rolling, and adhesion (B) were determined. The data represent the mean and SEM of at least three independent experiments. *P ≤ .05.
Eosinophil accumulation on IL-4–stimulated HUVECs. Confluent monolayers of primary HUVECs were washed once with HBSS and then stimulated with either M199/A alone (control) or M199/A containing 20 ng/mL IL-4 (IL-4). After 24 hours at 37°C, the monolayers were washed with HBSS and assembled in the flow chamber. Purified eosinophils (5 × 105/mL) were perfused over the monolayers at the specified shear stresses and eosinophil accumulation (A), rolling, and adhesion (B) were determined. The data represent the mean and SEM of at least three independent experiments. *P ≤ .05.
Eosinophils bound to IL-4–stimulated HUVECs. Eosinophils were perfused over (A) control or (B) IL-4–stimulated HUVECs as described in Fig 1. After attachment under shear conditions, video images were obtained using a ×40 objective. These images were captured as described in Materials and Methods and photographs were made. Eosinophils bound to control HUVECs (A) remain phase-bright, whereas eosinophils bound to IL-4–stimulated HUVECs (B) change shape and spread on the monolayer.
Eosinophils bound to IL-4–stimulated HUVECs. Eosinophils were perfused over (A) control or (B) IL-4–stimulated HUVECs as described in Fig 1. After attachment under shear conditions, video images were obtained using a ×40 objective. These images were captured as described in Materials and Methods and photographs were made. Eosinophils bound to control HUVECs (A) remain phase-bright, whereas eosinophils bound to IL-4–stimulated HUVECs (B) change shape and spread on the monolayer.
P-selectin mediates eosinophil tethering to IL-4–stimulated HUVECs at high shear stresses.
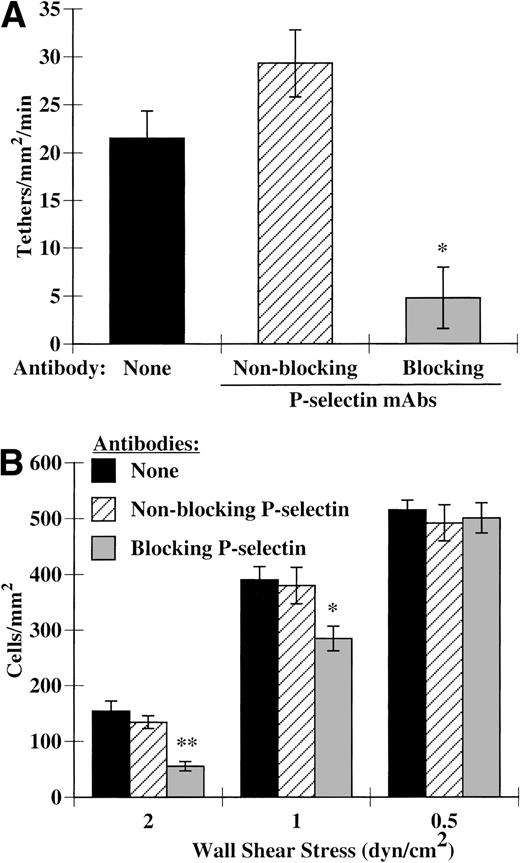
IL-4–stimulated HUVECs have increased expression of P-selectin, both intracellularly and on the cell surface (Yao et al28 and data not shown). Further treatment of these endothelial cells with histamine dramatically increases the surface expression of P-selectin and eosinophil attachment (Yao et al28 and data not shown); however, because eosinophils bind to P-selectin at very low site densities,29 we chose to explore the role of P-selectin on IL-4–activated endothelial cells in the absence of histamine in these experiments. We treated IL-4–stimulated HUVECs with a blocking MoAb directed against P-selectin to determine if the elevated levels of P-selectin present on the surface of IL-4–stimulated HUVECs were sufficient to participate in eosinophil recruitment. We found that blocking P-selectin significantly inhibited eosinophil tethering (Fig 3A). This inhibition of tethering translated to a 65% reduction in accumulation (Fig 3B). A nonblocking P-selectin MoAb had no effect on either tethering (Fig 3A) or accumulation (Fig 3B). As we decreased the shear stress below physiological levels, eosinophils began to accumulate on these HUVECs in a P-selectin–independent manner (Fig 3B). Thus, P-selectin participates in tethering and rolling of eosinophils at high shear stresses, but other mechanisms are sufficient to mediate these interactions at lower shear stresses.
P-selectin participates in eosinophil accumulation and tethering to IL-4–stimulated HUVECs. Monolayers of HUVECs were treated as described in Fig 1. Before assembly of the flow chamber, HUVECs were treated for 10 minutes at 37°C with HBSS/A alone or HBSS/A containing 5 μg/mL of either a nonblocking (S12) or blocking (G1) MoAb directed against P-selectin. The flow chamber was assembled and eosinophils also containing the specified MoAb were perfused through the chamber. (A) Tethering of eosinophils was determined at 2 dyn/cm2, as described in Materials and Methods. (B) Accumulation of eosinophils was determined at the specified wall shear stresses as described in Materials and Methods. The data represent the mean and SEM of at least three independent experiments. *P ≤ .05; **P ≤ .0001.
P-selectin participates in eosinophil accumulation and tethering to IL-4–stimulated HUVECs. Monolayers of HUVECs were treated as described in Fig 1. Before assembly of the flow chamber, HUVECs were treated for 10 minutes at 37°C with HBSS/A alone or HBSS/A containing 5 μg/mL of either a nonblocking (S12) or blocking (G1) MoAb directed against P-selectin. The flow chamber was assembled and eosinophils also containing the specified MoAb were perfused through the chamber. (A) Tethering of eosinophils was determined at 2 dyn/cm2, as described in Materials and Methods. (B) Accumulation of eosinophils was determined at the specified wall shear stresses as described in Materials and Methods. The data represent the mean and SEM of at least three independent experiments. *P ≤ .05; **P ≤ .0001.
VCAM-1 and α4-integrins participate in eosinophil tethering and accumulation on IL-4–stimulated HUVECs.
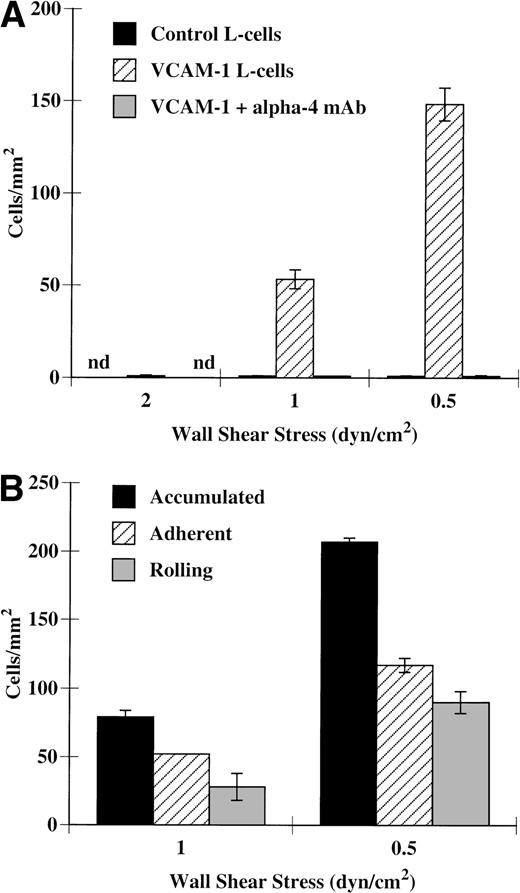
VCAM-1 expressed on L cells is capable of mediating tethering, rolling, and adhesion of eosinophils under shear conditions (Fig 4A and B). These interactions only occurred at shear stresses less than 2 dyn/cm2 (Fig 4A). Almost half of the eosinophils that tethered to VCAM-1 also participated in rolling interactions on this adhesion protein (Fig 4B). We found that an antibody directed against the α4-integrins was able to completely block these interactions (Fig 4A), confirming that α4-integrins on eosinophils interact with VCAM-1 under shear conditions.
Eosinophils tether and roll on VCAM-1–transfected L cells. Confluent monolayers of nontransfected and VCAM-1–transfected L cells were washed once with HBSS and then placed in flow assembly. Eosinophils (5 × 105/mL) were perfused over these cells at the specified wall shear stresses. (A) Accumulation, (B) rolling, and adherence were determined as described in Materials and Methods. In some experiments, eosinophils were pretreated for 10 minutes at 37°C with 2 μg/mL of H2/1, an anti–4-integrin MoAb, before perfusion over VCAM-1–transfected cells. The data represent the mean and SEM of at least three independent experiments.
Eosinophils tether and roll on VCAM-1–transfected L cells. Confluent monolayers of nontransfected and VCAM-1–transfected L cells were washed once with HBSS and then placed in flow assembly. Eosinophils (5 × 105/mL) were perfused over these cells at the specified wall shear stresses. (A) Accumulation, (B) rolling, and adherence were determined as described in Materials and Methods. In some experiments, eosinophils were pretreated for 10 minutes at 37°C with 2 μg/mL of H2/1, an anti–4-integrin MoAb, before perfusion over VCAM-1–transfected cells. The data represent the mean and SEM of at least three independent experiments.
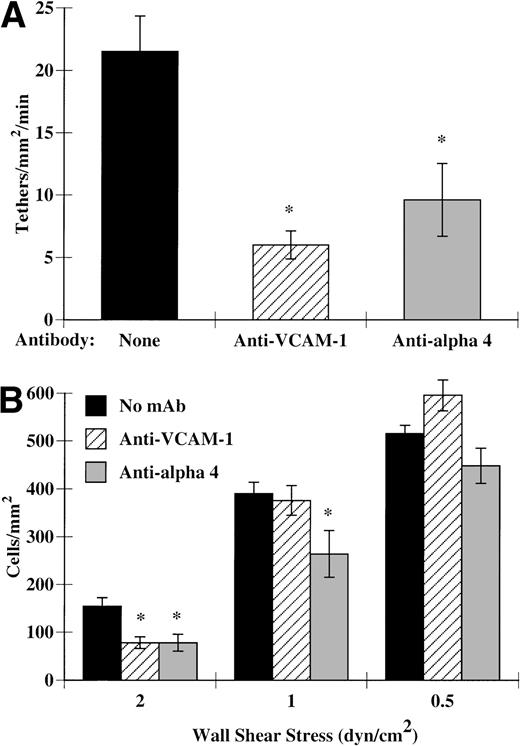
To determine if VCAM-1 or α4-integrins were participating in eosinophil tethering and accumulation on IL-4–stimulated HUVECs, we used MoAbs to block these adhesion proteins. Surprisingly, these MoAbs dramatically decreased eosinophil tethering at 2 dyn/cm2(Fig 5A). This inhibition of tethering led to decreased eosinophil accumulation on IL-4–stimulated HUVECs at 2 dyn/cm2 (Fig 5B). As with P-selectin, as the shear stress was decreased below physiological shear, the ability of these antibodies alone to block accumulation also diminished (Fig 5B). Taken together, these data show that VCAM-1/α4-integrins can mediate eosinophil tethering and accumulation under shear conditions both in a purified system and in the context of IL-4–stimulated HUVECs. Strikingly, VCAM-1 and α4-integrins can mediate significant tethering at 2 dyn/cm2, a value above any described for other leukocytes.
VCAM-1 and 4-integrins participate in eosinophil accumulation and tethering to IL-4–stimulated HUVECs. Monolayers of HUVECs were treated as described in Fig 1. Before assembly of the flow chamber, HUVECs were treated for 10 minutes at 37°C with HBSS/A alone or HBSS/A containing 5 μg/mL of 1.G11B1, an MoAb directed against VCAM-1. Alternatively, eosinophils were pretreated 10 minutes at 37°C with 2 μg/mL of H2/1, an anti–4-integrin MoAb, before perfusion over IL-4–stimulated HUVECs. The flow chamber was assembled and eosinophils also containing the specified MoAb were perfused through the chamber. (A) Tethering of eosinophils was determined at 2 dyn/cm2, as described in Materials and Methods. (B) Accumulation of eosinophils was determined at the specified wall shear stresses as described in Materials and Methods. The data represent the mean and SEM of at least three independent experiments. *P ≤ .05; **P ≤ .0001.
VCAM-1 and 4-integrins participate in eosinophil accumulation and tethering to IL-4–stimulated HUVECs. Monolayers of HUVECs were treated as described in Fig 1. Before assembly of the flow chamber, HUVECs were treated for 10 minutes at 37°C with HBSS/A alone or HBSS/A containing 5 μg/mL of 1.G11B1, an MoAb directed against VCAM-1. Alternatively, eosinophils were pretreated 10 minutes at 37°C with 2 μg/mL of H2/1, an anti–4-integrin MoAb, before perfusion over IL-4–stimulated HUVECs. The flow chamber was assembled and eosinophils also containing the specified MoAb were perfused through the chamber. (A) Tethering of eosinophils was determined at 2 dyn/cm2, as described in Materials and Methods. (B) Accumulation of eosinophils was determined at the specified wall shear stresses as described in Materials and Methods. The data represent the mean and SEM of at least three independent experiments. *P ≤ .05; **P ≤ .0001.
P-selectin, VCAM-1, and α4-integrins act together to recruit eosinophils to IL-4–stimulated HUVECs.
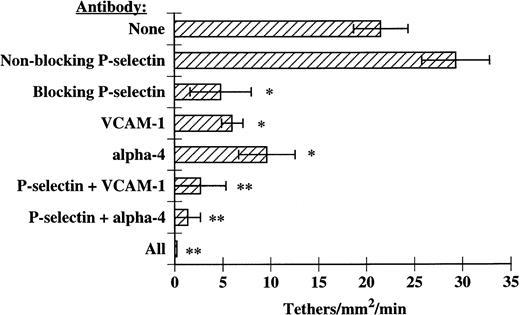
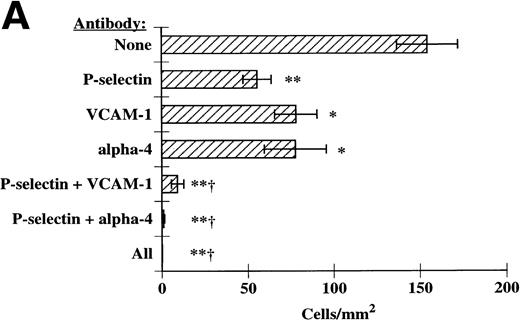
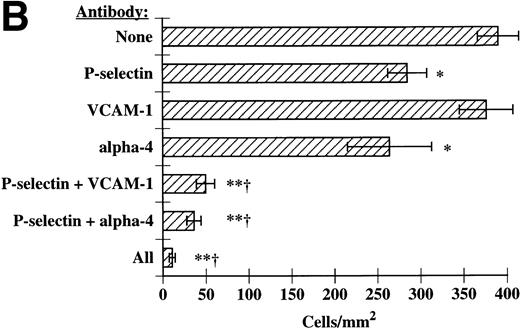
We next determined if P-selectin and VCAM-1/α4-integrins were acting together to optimally recruit eosinophils to IL-4–stimulated HUVECs. In these experiments, MoAbs directed against P-selectin, VCAM-1, and α4-integrins were used alone or in combination and eosinophil tethering and accumulation on IL-4–stimulated HUVECs were determined. We found that blockade of P-selectin and either VCAM-1 or α4-integrins completely blocked eosinophil tethering at 2 dyn/cm2 (Fig 6). Inhibition of tethering lead to ablation of accumulation at 2 dyn/cm2(Fig 7A). As the shear was lowered, either P-selectin or VCAM-1/α4-integrins could independently recruit eosinophils; however, inhibition of both of the adhesive pathways abolished accumulation at low shears as well (Fig 7B). Using a P-selectin MoAb in conjunction with either a VCAM-1 MoAb or an α4-integrin MoAb was nearly as effective as using all three MoAbs together (Figs 6 and 7A and B). Taken together, these data demonstrate that P-selectin and VCAM-1 expressed on the surface of IL-4–stimulated HUVECs act in concert to recruit eosinophils under shear conditions, in part through interactions with α4-integrins on eosinophils.
P-selectin, VCAM-1, and 4-integrins together mediate eosinophil tethering on IL-4–stimulated HUVECs. Monolayers of HUVECs were treated as described in Fig 1. Before assembly of the flow chamber, HUVECs were treated for 10 minutes at 37°C with HBSS/A alone; HBSS/A containing 5 μg/mL of 1.G11B1, an MoAb directed against VCAM-1; 5 μg/mL of either a nonblocking (S12) or blocking (G1) MoAb directed against P-selectin; or 5 μg/mL of both anti–VCAM-1 and G1 MoAbs. In some experiments, eosinophils were also pretreated for 10 minutes at 37°C with 2 μg/mL of H2/1, an anti–4-integrin MoAb, before perfusion over IL-4–stimulated HUVECs. The flow chamber was assembled and eosinophils also containing the specified MoAbs were perfused through the chamber. Tethering of eosinophils was determined at 2 dyn/cm2, as described in Materials and Methods. The data represent the mean and SEM of at least three independent experiments. *P ≤ .05; **P ≤ .01.
P-selectin, VCAM-1, and 4-integrins together mediate eosinophil tethering on IL-4–stimulated HUVECs. Monolayers of HUVECs were treated as described in Fig 1. Before assembly of the flow chamber, HUVECs were treated for 10 minutes at 37°C with HBSS/A alone; HBSS/A containing 5 μg/mL of 1.G11B1, an MoAb directed against VCAM-1; 5 μg/mL of either a nonblocking (S12) or blocking (G1) MoAb directed against P-selectin; or 5 μg/mL of both anti–VCAM-1 and G1 MoAbs. In some experiments, eosinophils were also pretreated for 10 minutes at 37°C with 2 μg/mL of H2/1, an anti–4-integrin MoAb, before perfusion over IL-4–stimulated HUVECs. The flow chamber was assembled and eosinophils also containing the specified MoAbs were perfused through the chamber. Tethering of eosinophils was determined at 2 dyn/cm2, as described in Materials and Methods. The data represent the mean and SEM of at least three independent experiments. *P ≤ .05; **P ≤ .01.
P-selectin, VCAM-1, and 4-integrins together mediate eosinophil accumulation on IL-4–stimulated HUVECs. These experiments were performed exactly as described in Fig 6. Accumulation of eosinophils was determined at (A) 2 dyn/cm2 and (B) 1 dyn/cm2, as described in Materials and methods. The data represent the mean and SEM of at least three independent experiments. *P ≤ .05; **P ≤ .0001; †P ≤ .001 with respect to P-selectin MoAb alone.
P-selectin, VCAM-1, and 4-integrins together mediate eosinophil accumulation on IL-4–stimulated HUVECs. These experiments were performed exactly as described in Fig 6. Accumulation of eosinophils was determined at (A) 2 dyn/cm2 and (B) 1 dyn/cm2, as described in Materials and methods. The data represent the mean and SEM of at least three independent experiments. *P ≤ .05; **P ≤ .0001; †P ≤ .001 with respect to P-selectin MoAb alone.
α-4-integrins and β2-integrins both participate in firm adhesion of eosinophils to IL-4–stimulated endothelial cells.
As stated earlier, eosinophils do not roll on IL-4–stimulated endothelial cells; instead, they immediately arrest and eventually activate and spread on the endothelial cell monolayer (Figs 1B and 2). In all of the experiments performed in this study using P-selectin, VCAM-1, and α4-integrin MoAbs either alone or in concert, all the cells remaining on the endothelial cell surface were firmly adherent, not participating in rolling adhesion (Table 1). This suggested that another adhesive mechanism was participating in the firm adhesion of eosinophils to IL-4–stimulated endothelial cells. Like other leukocytes, eosinophils have β2-integrins on their surface. When activated, β2-integrins can interact with ICAM-1 on the surface of endothelial cells supporting firm adhesion. We treated eosinophils with an anti–β2-integrin MoAb to evaluate the role of this adhesion molecule in eosinophil firm adhesion in this system. We found that an anti–β2-integrin MoAb alone had no effect on either accumulation (data not shown) or firm adhesion (Table 1). When we used this MoAb together with an anti–P-selectin MoAb, we found no further effect on accumulation (data not shown) or firm adhesion (Table 1). However, when an anti–β2-integrin MoAb was used with an anti–α4-integrin MoAb, the percentage of firmly adherent cells decreased dramatically (Table1). Instead, eosinophils were participating in rolling interactions similar to those observed during eosinophil interactions with purified P-selectin. These data suggest that both α4- and β2-integrins act together to firmly adhere eosinophils to IL-4–stimulated endothelial cells.
The Effect of MoAbs on Eosinophil Interactions With IL-4– Stimulated HUVECs
| Antibody . | % Adherent . |
|---|---|
| None | 97.8 ± 2.2 |
| P-selectin | 97.9 ± 1.4 |
| α-4-integrin | 96.2 ± 2.4 |
| β2-integrin | 98.1 ± 0.6 |
| P-selectin + α4-integrin | 97.6 ± 1.1 |
| P-selectin + β2 integrins | 97.6 ± 1.1 |
| α4-integrin + β2-integrin | 20.7 ± 15.9 |
| Antibody . | % Adherent . |
|---|---|
| None | 97.8 ± 2.2 |
| P-selectin | 97.9 ± 1.4 |
| α-4-integrin | 96.2 ± 2.4 |
| β2-integrin | 98.1 ± 0.6 |
| P-selectin + α4-integrin | 97.6 ± 1.1 |
| P-selectin + β2 integrins | 97.6 ± 1.1 |
| α4-integrin + β2-integrin | 20.7 ± 15.9 |
HUVECs were stimulated with IL-4 as described in Fig 1. Before assembly of the flow chamber, HUVECs were treated for 10 minutes at 37°C with HBSS/A alone or HBSS/A containing 5 μg/mL of G1, an MoAb directed against P-selectin. In some experiments, eosinophils were also pretreated 10 minutes at 37°C with either 2 μg/mL of H2/1, an anti–α4-integrin MoAb; 5 μg/mL of an anti–β2-integrin MoAb; or both antibodies before perfusion over IL-4–stimulated HUVECs. To determine the percentage of rolling and adherent cells, images were captured 5 seconds apart and the cells that were stationary during that time were considered adherent. Cells that moved at least 1 cell diameter were considered rolling. The data represent the mean ± SD of at least two experiments.
DISCUSSION
Eosinophils may represent a minor fraction of the circulating leukocytes; however, they are a major component of the cellular infiltrate found in diseases such as atopic dermatitis, allergic rhinitis, and bronchial asthma.1 30 Selective infiltration of these cells may be achieved at several levels, including the initial interaction between eosinophils and activated endothelium at sites of allergic inflammation.
We examined eosinophil accumulation on IL-4–stimulated HUVECs, because IL-4 has been shown to be an important cytokine in allergic inflammation.19 We found that HUVECs stimulated 24 hours with IL-4 tethered and accumulated eosinophils at shear stresses as high as 4 dyn/cm2 (Fig 1A). At 2 dyn/cm2, this response was quite robust. We initially hypothesized that tethering at 2 dyn/cm2 was due exclusively to the expression of P-selectin on IL-4–activated endothelial cells, because low site densities of P-selectin have been shown to support eosinophil rolling.29 Yet, when we used an antibody directed against P-selectin, there was still residual tethering of eosinophils at 2 dyn/cm2 (Fig 3A). As a result of this residual tethering, some eosinophils were still able to accumulate on IL-4–stimulated HUVECs in the presence of an anti–P-selectin MoAb. These data suggested that another adhesion protein was mediating eosinophil tethering to these endothelial cells.
Although the selectins mediate leukocyte recruitment under shear conditions both in vitro and in vivo,8 there are now several in vivo models of leukocyte recruitment in which selectin blockade does not prevent leukocyte recruitment and tissue infiltration. Sriramarao et al12,31 showed that inhibition of either L-selectin or α4-integrins blocked eosinophil infiltration into the rabbit mesentery after IL-1 stimulation, suggesting a role for the α4-integrins in eosinophil recruitment in this model. Johnston et al32 went on to show that α4-integrins could mediate both rolling and adhesion in chronically inflamed rat mesentery and that rolling could occur independent of selectins. These data suggest that, under some conditions, leukocyte recruitment can occur independent of selectins.
α4-integrins can mediate leukocyte tethering to purified VCAM-1.13-15 T cells13,15 and eosinophils18 tether to purified VCAM-1 at shear stresses between 0.5 and 1 dyn/cm2, but, as the shear stress is increased to 2 dyn/cm2, few cells tether and accumulate on VCAM-1. In contrast, monocytes expressing α4-integrins do not tether to VCAM-1 at either high or low shear stresses.33 To date, VCAM-1 expressed on cytokine-activated HUVECs has not been shown to participate in tethering of peripheral blood leukocytes,16,18,33,34 although data using lymphocyte cell lines suggest that the VCAM-1 expressed is capable of mediating attachment in the absence of a selectin.17 Instead, data suggest that VCAM-1 acts to stabilize the adhesion of both peripheral blood T cells and monocytes after these cells have been tethered by a selectin.
We first examined the ability of freshly isolated eosinophils to interact with VCAM-1 transfectants and found that eosinophils tethered and accumulated at low shear stresses in a manner similar to T cells. At increased site densities, VCAM-1 may be able to mediate eosinophil tethering at higher shear stresses; however, we only examined eosinophil interactions at a single site density. We then addressed the role of VCAM-1/α4-integrins interactions in eosinophil recruitment on IL-4–stimulated HUVECs. In contrast to other leukocytes, we found that eosinophils tethered to VCAM-1 expressed on IL-4–activated HUVECs, and this tethering was occurring at physiologic shear stresses.
P-selectin and VCAM-1/α4-integrins work cooperatively to optimize eosinophil interactions at physiologic shear stresses, because MoAbs directed against either VCAM-1 or α4-integrins used together with an anti–P-selectin MoAb blocked all eosinophil tethering and accumulation on IL-4–stimulated HUVECs. As the shear stress was lowered, P-selectin and VCAM-1 acted independently to recruit eosinophils. This difference in adhesion molecule use is likely due to the forces exerted on the flowing eosinophil at high versus low shear stresses. The loss of one receptor ligand pair at high shear stresses is sufficient to impair eosinophil tethering and accumulation, whereas the decreased forces exerted on the eosinophil at lower shear stresses allow for loss of some interactions without affecting eosinophil accumulation.
A striking observation was that eosinophils did not roll on IL-4–stimulated endothelial cells. Instead, eosinophils tethered and rapidly became firmly adherent. MoAbs directed against P-selectin, VCAM-1, and/or α4-integrins alone or in concert did not alter the nature of these eosinophil interactions, yet eosinophils do roll on purified surfaces expressing P-selectin10 or VCAM-1 (this study). When we explored a role for the β2-integrins in firm adhesion, we found that an anti–β2-integrin MoAb alone did not alter the percentage of adherent cells (Table 1). However, an anti–β2-integrin MoAb together with an anti-α4 MoAb not only decreased attachment, but also led to a dramatic increase in the percentage of rolling eosinophils (Table 1). Thus, just as P-selectin and VCAM-1/α4-integrins act together to tether eosinophils, β2-integrins and VCAM-1/α4-integrins act together to support firm adhesion.
Activation of β2-integrins on eosinophils along with the data in Fig2 showing the dramatic shape change and transmigration of eosinophils on IL-4–stimulated endothelial cells suggest that these endothelial cells express or release an eosinophil activator(s). Ligation of adhesion receptors may prime eosinophils to respond optimally to these activation signals. Ligation of P-selectin glycoprotein ligand-1 on neutrophils leads to increased tyrosine phosphorylation on several target proteins and activates members of the MAP kinase family.35 Similar activation occurs in eosinophils upon ligation of PSGL-1 (manuscript in preparation). Ligation of the α4-integrins has also been shown to activate MAP kinase family proteins in monocytic cells36 and may also play a role in eosinophil activation. Thus, both outside-in signaling through PSGL-1 or α4-integrins and release of eosinophil activators from IL-4–stimulated endothelial cells may be responsible for the abrupt adhesion of eosinophils in this system.
Our results show that eosinophils use both P-selectin and VCAM-1 to tether and accumulate on IL-4–stimulated HUVECs. Both of these molecules are critical at high shear stresses, but either can independently meditate accumulation at lower shear stresses. At all shear stresses, eosinophils rapidly become firmly adherent, an interaction that uses both α4- and β2-integrins. The ability of eosinophils to effectively use multiple adhesion molecules both for tethering and firm adhesion may be particularly important in understanding the mechanisms of eosinophil trafficking in vivo where vascular flow can regulate the efficacy of interactions. MoAbs directed against both the selectins and VCAM-1/α4-integrins may be required to prevent eosinophil recruitment at lower shear stresses, whereas in faster flowing vessels, blocking the selectins would be sufficient to inhibit recruitment. This is the first time that endothelial VCAM-1, through interactions with α4-integrins, has been shown to support significant tethering of eosinophils at shear stresses greater than 1 dyn/cm2. Thus, these experiments may shed some light on the mechanisms used for the selective accumulation of eosinophils at sites of allergic inflammation.
ACKNOWLEDGMENT
The author thanks Dr Rodger McEver and Dr John Elliot for their generous gifts of reagents; Dr Paul Kubes for critical reading of this manuscript; Evelyn Lailey for her technical assistance; and the Labor and Delivery unit at the Foothills Hospital in Calgary for their assistance in providing umbilical cords.
Supported by grants from the Medical Research Council of Canada (MT-14180) and the Alberta Heritage Foundation for Medical Research (970234).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Kamala D. Patel, PhD, Department of Physiology and Biophysics, University of Calgary, 3330 Hospital Dr NW, Calgary, Alberta, Canada T2N 4N1.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal