Abstract
Defective function of dendritic cells (DC) in cancer has been recently described and may represent one of the mechanisms of tumor evasion from immune system control. We have previously shown in vitro that vascular endothelial growth factor (VEGF), produced by almost all tumors, is one of the tumor-derived factors responsible for the defective function of these cells. In this study, we investigated whether in vivo infusion of recombinant VEGF could reproduce the observed DC dysfunction. Continuous VEGF infusion, at rates as low as 50 ng/h (resulting in serum VEGF concentrations of 120 to 160 pg/mL), resulted in a dramatic inhibition of dendritic cell development, associated with an increase in the production of B cells and immature Gr-1+ myeloid cells. Infusion of VEGF was associated with inhibition of the activity of the transcription factor NF-κB in bone marrow progenitor cells. Experiments in vitro showed that VEGF itself, and not factors released by VEGF-activated endothelial cells, affected polypotent stem cells resulting in the observed abnormal hematopoiesis. These data suggest that VEGF, at pathologically relevant concentrations in vivo, may exert effects on pluripotent stem cells that result in blocked DC development as well as affect many other hematopoietic lineages.
DENDRITIC CELLS (DC), as the most potent antigen-presenting cells, play a central role in antitumor immunity. Tumor cells appear to have developed mechanisms to avoid immune system recognition and control, and among these is the inhibition of antitumor immune responses. One of the targets for this inhibition is the professional antigen-presenting cell, particularly the dendritic cell.1 Defective function of DC in cancer has been reported recently by several groups.2-6 Ineffective or adverse antigen presentation may be an important mechanism of tumor evasion from immune system surveillance. Previously, we have shown that one of the possible mechanisms of DC dysfunction in cancer is an abnormal functional maturation of these cells from progenitors.7,8This was recently confirmed by other investigators.9-11Several soluble factors have been implicated in defective DC maturation in cancer, including vascular endothelial growth factor (VEGF).8 VEGF is produced in large amounts by most tumors and its production is closely associated with a poor prognosis.12,13 VEGF stimulates the proliferation of endothelial cells and plays an important role in the formation of tumor neovasculature.14 We have shown that anti-VEGF neutralizing antibodies block the negative effects of tumor cell supernatants on DC maturation in vitro.8 VEGF binds to hematopoietic progenitor CD34+ cells through one of the VEGF-specific receptors (Flt-1) and inhibits the activation of transcription factor NF-κB in these cells.15 However, recombinant VEGF has only limited effects on DC maturation from CD34+progenitors in vitro even when it was applied at relatively high concentrations. We ask in this study whether recombinant VEGF alone at relevant concentrations would cause significant alterations in DC development in vivo in otherwise healthy animals, which cell populations would be most affected, and what role other factors may play in the effects mediated by VEGF.
To answer these questions we studied the effects of long-term continuous infusion of recombinant VEGF in mice. We have found that VEGF not only had a dramatic effect on DC development, but also significantly affected the development of other cell lineages. We have also demonstrated that the most likely target for VEGF action is the population of pluripotent stem cells, rather than lineage-committed progenitor cells. The results of our experiments further suggest that VEGF may exert its effects by blocking NF-κB activation.
MATERIALS AND METHODS
Animals.
Six- to 8-week-old female BALB/c and CBA mice were purchased from Harlan Inc (Indianapolis, IN) and were housed in specific pathogen-free units of the Division of Animal Care at Vanderbilt University Medical Center.
Reagents and cell lines.
VEGF was a generous gift from Genentech Inc (South San Francisco, CA). The following antibody-producing hybridomas were obtained from American Type Culture Collection (ATCC; Rockville, MD) and used as culture supernatants: anti-CD4 (L3T4, TIB-207), anti-CD8 (Lyt-2.2, TIB-210), anti-B cells (HB-146), and anti-MHC class II (I-Ad,k, HB-120). Fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-labeled antibodies used in flow cytometry were purchased from Pharmingen (San Diego, CA) (Table 1). FITC- and PE-conjugated mouse IgG were used in control. Biotinylated I-Ad and I-Ak antibodies (Pharmingen) were used in the analysis of Langerhans cells (LC). FITC-dextran was obtained from Molecular Probes (Eugene, OR). The D459 tumor cell line is a poorly immunogenic fibrosarcoma described in detail elsewere.2,16 The polyoma virus middle-sized tumor antigen transformed mouse brain capillary endothelial cell line (bEND.3) was obtained from W.Risau (Max Plank Institute, Bad Nauheim, Germany).17 18
Antibodies Used in This Study
| CD Classification . | Specificity . |
|---|---|
| CD3 | T cells |
| CD4 (L3T4) | T-cell helper |
| CD8 (Ly-2) | T-cell suppressor/cytotoxic |
| CD11b (Mac-1) | Mostly macrophages |
| CD11c (N418) | Mostly dendritic cells |
| CD19 | B cells |
| CD34 | Endothelial cells, hematopoietic progenitor cells |
| CD40 | Dendritic cells, B cells |
| CD45 | Leukocyte common antigen |
| CD45R/B220 | B cells |
| CD80 (B7-1) | Mostly dendritic cells |
| CD86 (B7-2) | Mostly dendritic cells |
| Ly-6G (Gr-1) | Granulocytes |
| Sca-1 (Ly-6A/E) | Multipotent hematopoietic stem cells |
| TER-119 | Cells of erythroid lineage |
| I-Ad | MHC class II |
| CD Classification . | Specificity . |
|---|---|
| CD3 | T cells |
| CD4 (L3T4) | T-cell helper |
| CD8 (Ly-2) | T-cell suppressor/cytotoxic |
| CD11b (Mac-1) | Mostly macrophages |
| CD11c (N418) | Mostly dendritic cells |
| CD19 | B cells |
| CD34 | Endothelial cells, hematopoietic progenitor cells |
| CD40 | Dendritic cells, B cells |
| CD45 | Leukocyte common antigen |
| CD45R/B220 | B cells |
| CD80 (B7-1) | Mostly dendritic cells |
| CD86 (B7-2) | Mostly dendritic cells |
| Ly-6G (Gr-1) | Granulocytes |
| Sca-1 (Ly-6A/E) | Multipotent hematopoietic stem cells |
| TER-119 | Cells of erythroid lineage |
| I-Ad | MHC class II |
VEGF administration.
VEGF was delivered via implanted Alzet osmotic pumps (ALZA Corp, Palo Alto, CA) with an infusion rate of 0.25 μL/h. Two types of pumps (durations of infusion of 14 days and 28 days) were used. The pumps were inserted subcutaneously into the back of the mice through a small skin incision. Wound edges were reapproximated with surgical clips. All procedures were performed in aseptic conditions and were approved by the Vanderbilt University Animal Care Committee. In controls, pumps were filled with phosphate-buffered saline (PBS).
VEGF clearance from the circulation and distribution in tissues.
BALB/c nude mice were injected intravenously (IV) with 10 μg of125I-VEGF165 (specific activity [SA] > 106 cpm/μg protein). At different time points, blood samples were collected and aliquots of plasma were counted in a gamma counter. To determine the in vivo distribution of radiolabeled VEGF, mice were killed 25 minutes after injection with125I-VEGF. All major organs were harvested, weighed, and counted in a gamma counter. The total injected dose was calculated by counting a 2-μL sample of radioactive VEGF before administration. The accumulated radioactivity in each organ was expressed as the percentage of the total dose per gram of tissue. Radiolabeled VEGF165was proven to retain greater than 90% of functional capacity after iodination as measured by effectiveness of labeled and unlabeled VEGF to induce proliferation of human umbilical vein endothelial (HUVE) cells in vitro.
Cell preparation.
For analysis of cell-surface molecules, lymph node and spleen cells were used either directly (red blood cells removed by hypotonic shock) or cells were enriched for DC. For functional tests, only DC-enriched fractions were used. Cells were prepared from lymph nodes as described earlier.2 Briefly, a single cell suspension was prepared from inguinal, axillary, and brachial lymph nodes by pressing the tissues through a wire mesh. Cells were washed and then layered onto a metrizamide (Nygaard, Oslo, Norway) gradient (14.5 g plus 100 mL RPMI 1640 medium) and centrifuged for 10 minutes at 600g. Cells at the interface were washed once and resuspended in complete culture medium (CCM) (RPMI-1640; GIBCO-BRL, Gaithersburg, MD, with 100 IU/mL penicillin, 0.1 mg/mL streptomycin, 1 × 10−5mol/L 2-mercaptoethanol, and 10% fetal calf serum; HyClone, Logan, UT). DC were identified by their distinctive morphology and by labeling with N418 antibody. The final purity of DC in control mice was greater than 60%.
Pelleted cells from the lymph node suspension were passed through nylon wool columns to obtain greater than 90% pure T cells.
DC from spleen were prepared as described before.2 Briefly, spleen cells were incubated overnight, and nonadherent cells were collected and separated on metrizamide gradient. T and B cells were removed using a cocktail of anti-T and anti-B cell antibodies and Low-Tox-Guinea Pig Complement (Cedarline, Hornby, Ontario, Canada). The final purity of the DC fraction in control mice was greater than 60%.
Bone marrow (BM) was obtained from the femurs and tibias of BALB/c mice and used as a source of hematopoietic progenitor cells in NF-κB experiments. T and B cells, macrophages, and DC were depleted by incubation with the following cocktail of antibodies: TIB 207, TIB 210, HB 146, HB120, and complement.
Epidermal sheet preparation and Langerhans cell identification.
Epidermal sheets were prepared from abdominal skin using the EDTA separation procedure described previously.19-21 The ventral trunk skin was shaved and the mice were killed by cervical dislocation. The remaining hair was removed by chemical depilation with a thioglycollate-based commercial depilatory cream. The keratin layer was then removed by two or three applications of cellophane tape and the skin surgically excised while firmly attached to fresh cellophane tape. The skin was incubated for 2.5 hours at 37°C in PBS containing 20 mmol/L EDTA (pH 7.3) and 0.001% trypsin. The cellophane tape, with the epidermis attached, was separated from the dermis, washed, and incubated overnight at 4°C with biotinylated anti–I-Ador I-Ak antibodies. After that time, these sheets were washed and incubated with streptavidin-peroxidase (Pharmingen) for 2 hours at room temperature. I-A+ cells were visualized in 0.7% mg/mL, 3,3′-diaminobenzidine containing 2 mg/mL H2O2 (Sigma FAST DAB tablet set; Sigma, St Louis, MO) for 5 minutes at room temperature. The sheets were then washed, lightly dried, and mounted on slides. I-A+ cells were counted in 1 mm2 by counting 10 separate, randomly selected fields.
FITC-dextran uptake assay.
DC obtained from the spleen were incubated at 37°C for 60 minutes with 1 mg/mL FITC-dextran. To assess the background uptake, an equal number of cells was incubated at 4°C. After incubation, the cells were washed three times and analyzed by flow cytometry. For each sample the mean value of fluorescence of cells incubated at 37°C was divided by that value of cells incubated at 4°C. Increases of fluorescence intensity in test tubes over background were compared between tested groups.
Assessment of colony formation.
Colony formation by hematopoietic progenitor cells was measured using two different techniques. “Late” lineage restricted progenitor cells were evaluated on semisolid 1% methylcellulose medium supplemented with recombinant cytokines (erythropoietin [Epo], stem cell factor [SCF], interleukin-6 [IL-6], IL-3) supporting the optimal growth of burst-forming unit-erythroid (BFU-E), colony-forming unit granulocyte-macrophage (CFU-GM), CFU-M, CFU-G, and mixed CFU-GEMM colonies (Methocult M3434; Stemcell Technologies, Vancouver, Canada). BM cells were seeded at 15,000 cells per plate. BFU-E colonies were scored on day 8-9, all others on day 12-13. “Early” pluripotent progenitors were assessed using the spleen colony-formation technique.22Briefly, BALB/c mice were lethally irradiated with a split dose of 1,080 cGy (540 cGy with a 3-hour interval). BM cells from control or VEGF-treated mice were injected into the tail vein of irradiated mice (105 nucleated cells per mouse). Mice were killed on day 11. The number and type of colonies in spleens were analyzed after staining of paraffin-embedded sections with hematoxylin-eosin.
T-cell proliferation assay.
DC were obtained from lymph nodes and spleens of control and VEGF-treated mice as described above. Cells were irradiated (2,000 cGy) and the cell number was adjusted to 5 × 104/mL. DC were incubated in triplicates for 3 days with T cells obtained from CBA mice for allogeneic mixed leukocyte reaction. For the analysis of primary antigen-dependent T-cell proliferation, DC from spleens were incubated in triplicates in serum-free medium with influenza virus strain PR8 (A/Puerto Rico/8/34) for 2 hours, washed, and cultured for 3 days with T cells obtained from control syngeneic (BALB/c) mice. The cultures were pulsed with 1 μCi [3H]thymidine (Amersham, Arlington Heights, IL). [3H]Thymidine uptake was counted using a liquid scintillation counter. The level of T-cell proliferation observed after incubation of noninfected DC with T cells was subtracted.
Electrophoretic mobility shift assay (EMSA).
Double-stranded oligonucleotide probes were prepared by annealing the appropriate single-stranded oligonucleotides at 65°C for 10 minutes in 10 mmol/L Tris, 1 mmol/L EDTA, 10 mmol/L NaCl followed by slow cooling to room temperature. The probes were end-labeled with32P-labeled CTP by filling in 5′ overhangs with the Klenow fragment. We used the following murine intronic κ chain κB site23 24: normal: 5′-AGTTGAGGGGACTTTCCCAGG-3′; mutant: 5′-AGTTGAGGCGACTTTCCCAGG-3′.
Nuclear extracts were obtained from the cells as described.25 Ten micrograms of nuclear extract was incubated for 20 minutes with labeled probe (50,000 cpm) in the presence of 4 μg of poly(dI-dC) (Pharmacia) in binding buffer (20 mmol/L HEPES, 5% glycerol, 0.2 mmol/L EDTA, 1 mmol/L dithiothreitol, 5 mmol/L MgCl2). Competition assays were performed with a 200-fold excess of unlabeled probes. The DNA-protein complexes were separated on 4% polyacrylamide gels and visualized and analyzed on a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
RESULTS
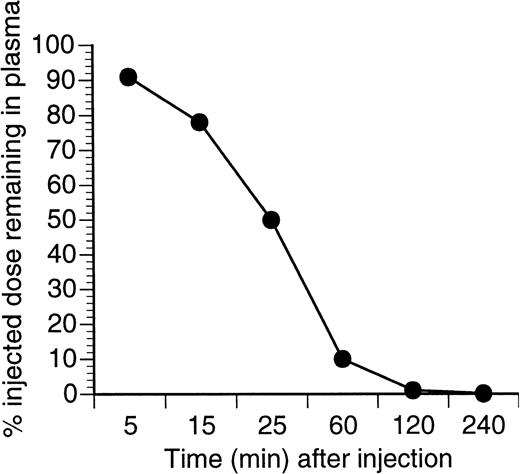
Experiments with 125I-labeled VEGF showed that it quickly disappeared from the circulation after iv injection with a t1/2 of about 25 minutes (Fig1). After 50 minutes, VEGF was undetectable in all organs, except kidney (0.35% injected dose per gram) and liver (0.5% injected dose per gram). To maintain a constant level of VEGF, we used a continuous-infusion osmotic pump. Several rates of infusion were tested (300 ng/h, 100 ng/h, 50 ng/h, and 10 ng/h). The level of VEGF in the serum was measured 7 and 28 days after the start of the infusion using an ELISA kit (R&D Systems, Minneapolis, MN). Four to 5 ng/mL VEGF was detected in the sera at the 300 ng/h rate of infusion and 120 to 160 pg/mL at the rate of 50 ng/mL (3 mice per group). No detectable level of VEGF was found at the 10 ng/h rate of infusion. These serum concentrations are within the range detected in tumor-bearing mice and in patients with cancer.26-29 Mice tolerated even the highest dose used, 300 ng/h for 28 days, without apparent difficulty. After 14 days of VEGF infusion at a rate of 50 ng/h or higher, new vessel formation around the pumps was observed. Blood vessel formation became much more pronounced by the end of the fourth week. No such effect was seen at a VEGF infusion rate of 10 ng/h. All rates of infusion were further studied.
Clearance of 121I-VEGF from the circulation. BALB/c nude mice were injected IV with 10 μg of125I-VEGF165. At different time points blood samples were collected, and aliquots of plasma were counted in a gamma counter. The total injected dose was calculated by counting a 2-μL sample of radioactive VEGF before administration.
Clearance of 121I-VEGF from the circulation. BALB/c nude mice were injected IV with 10 μg of125I-VEGF165. At different time points blood samples were collected, and aliquots of plasma were counted in a gamma counter. The total injected dose was calculated by counting a 2-μL sample of radioactive VEGF before administration.
Effect of VEGF on DC in vivo.
DC function was investigated on day 14 and on day 28 after the start of the infusion. The number of DC in spleen and lymph nodes was analyzed based on their morphology and expression of CD11c and B7-2 molecules. DC in skin (LC) were analyzed based on the expression of I-Ad molecule and morphology.
A 14-day infusion of VEGF, even at the highest tested dose of 300 ng/h (a total of 100 μg of VEGF), did not affect the number of DCs in lymph nodes, spleen, or skin (data not shown). The total number of lymph node cells and spleen cells was also normal. The ability of DCs to stimulate allogeneic and primary antigen-specific immune responses are the major functional characteristics of these cells. To test the ability of DCs to stimulate an allogeneic T-cell response, DCs isolated from lymph nodes and spleens were cultured with T cells obtained from allogeneic CBA mice and T-cell proliferation was measured using 3H-thymidine uptake. DCs isolated from lymph nodes of control and VEGF-treated mice were equally capable of stimulating allogeneic T cells (Fig 2A). However, splenic DCs isolated from VEGF-treated mice showed a statistically significantly decreased stimulation ability (Fig 2A). This was associated with a slight, but significant, decrease in the expression of MHC class II and B7-2 molecules on the DC surface (CD11c+ cells, data not shown). To assess the DC ability to stimulate antigen-specific primary T-cell responses, DCs obtained from spleens were infected with influenza virus and then incubated with naı̈ve syngeneic T cells. As shown in Fig 2B, DCs isolated from PBS-, but not VEGF-treated, mice were able to stimulate T-cell proliferation under these conditions. Another function of DCs is their ability to take up soluble antigens, which we assessed by FITC-dextran uptake. This capacity also may serve as an indirect indicator of the stage of DC maturation. DCs isolated from spleens were cultured with FITC-dextran at 37°C for 60 minutes and nonspecific binding, as determined by incubation of cells at 4°C, was subtracted. DCs isolated from mice treated with VEGF had significantly higher levels of FITC-dextran uptake than control mice (Fig 2C). The same effects were observed at a VEGF infusion rate of 50 ng/h (total of 16.8 μg VEGF), but not at 10 ng/h. Thus, a 14-day infusion of VEGF, even at the highest tested dose, did not affect the number or function of lymph node DC, but resulted in decreased function of splenic DCs.
Functional activity of splenic DC after 14 days of treatment with VEGF. VEGF was infused for 14 days at 50 ng/h. DC were obtained from spleens and lymph nodes as described in Materials and Methods. In all experiments, open bars represent data from mice treated with PBS, hatched bars represent mice treated with VEGF. (*)Statistically significant differences between control and VEGF groups. In all experiments each group included three mice. Two experiments with the same results were performed. (A) Stimulation of allogeneic T cells by DC. DC were incubated for 3 days with T cells from allogeneic CBA mice at different DC:T cells ratios.3H-thymidine uptake was measured in triplicate. (B) The ability of DC obtained from control and VEGF-treated mice to stimulate primary T-cell proliferation. DC were infected with influenza (FLU) virus as described in Materials and Methods and then cultured for 3 days with T cells obtained from control mice at a T-cell:DC ratio of 20:1. 3H-thymidine uptake is shown. The background T-cell proliferation (syngeneic non-infected DC cultured with T cells) was subtracted. Mean ± SE of 3H-thymidine uptake of three experiments is shown. (C) FITC-dextran uptake by DC isolated from VEGF-treated mice. DC were isolated from spleens and the FITC-dextran uptake assay were performed as described in Materials and Methods. Results (mean ± SE) of two experiments are shown.
Functional activity of splenic DC after 14 days of treatment with VEGF. VEGF was infused for 14 days at 50 ng/h. DC were obtained from spleens and lymph nodes as described in Materials and Methods. In all experiments, open bars represent data from mice treated with PBS, hatched bars represent mice treated with VEGF. (*)Statistically significant differences between control and VEGF groups. In all experiments each group included three mice. Two experiments with the same results were performed. (A) Stimulation of allogeneic T cells by DC. DC were incubated for 3 days with T cells from allogeneic CBA mice at different DC:T cells ratios.3H-thymidine uptake was measured in triplicate. (B) The ability of DC obtained from control and VEGF-treated mice to stimulate primary T-cell proliferation. DC were infected with influenza (FLU) virus as described in Materials and Methods and then cultured for 3 days with T cells obtained from control mice at a T-cell:DC ratio of 20:1. 3H-thymidine uptake is shown. The background T-cell proliferation (syngeneic non-infected DC cultured with T cells) was subtracted. Mean ± SE of 3H-thymidine uptake of three experiments is shown. (C) FITC-dextran uptake by DC isolated from VEGF-treated mice. DC were isolated from spleens and the FITC-dextran uptake assay were performed as described in Materials and Methods. Results (mean ± SE) of two experiments are shown.
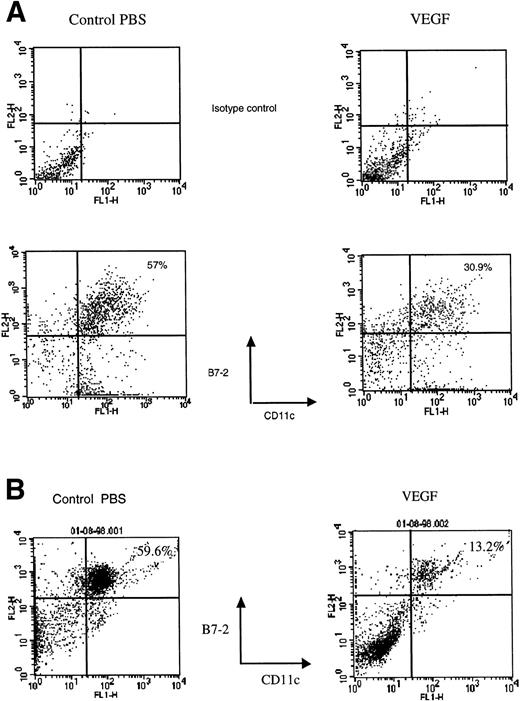
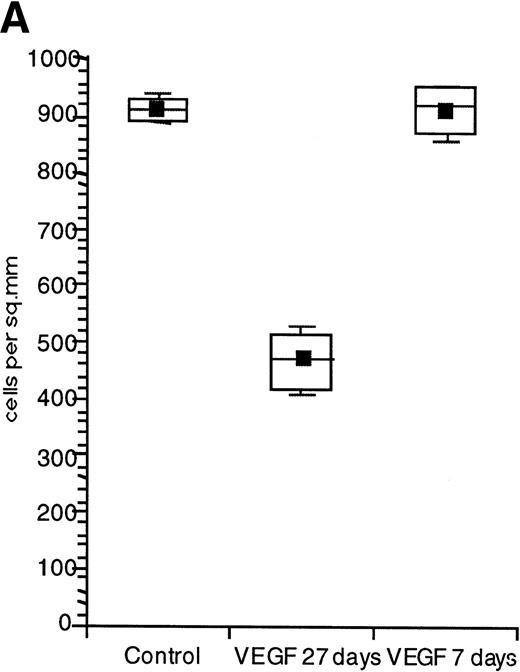
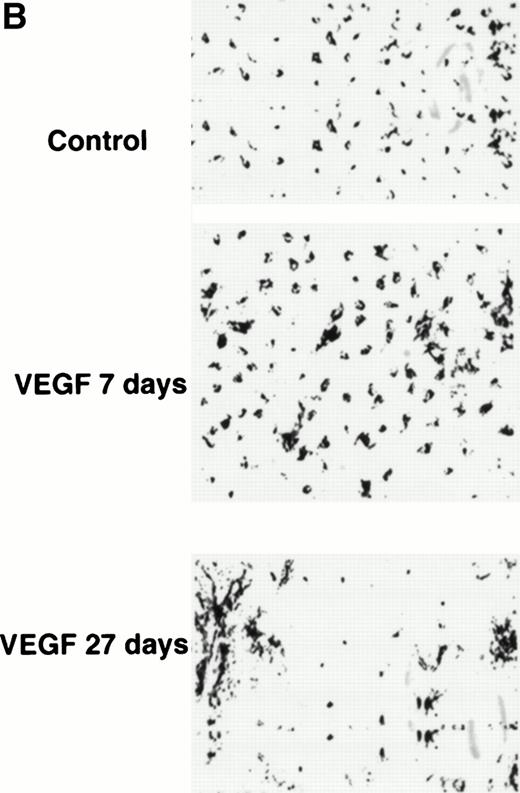
After 28 days of VEGF infusion, however, splenomegaly was observed in all mice treated at infusion rates of 50 ng/h or greater. The total number of spleen and lymph node cells was increased threefold to fourfold and almost twofold, respectively. Because DCs represent only about 1% to 2% of the total population of lymph node and spleen cells, it is difficult to directly detect decreases in their numbers. Therefore, DCs from lymph nodes were enriched using a metrizamide gradient. DCs from spleens were enriched using an overnight incubation, metrizamide gradient, and lymphocyte depletion as described in Materials and Methods. The remaining cells were then labeled with anti-CD11c and anti–B7-2 antibodies and assayed by fluorescence-activated cell sorting (FACS). In control mice, about 60% of cells expressed both of these markers of mature DCs. The proportion of mature DCs in lymph nodes from VEGF-treated mice was decreased twofold (30% double-positive cells) and in spleens more than fourfold (<15% double-positive cells, Fig 3A and B). The total number of DCs per one lymph node or spleen was calculated using the total number of cells in DC fractions. Mice treated with VEGF had 37.2 ± 3.4 (×103) DCs per lymph node, whereas control mice had 44.6 ± 3.1 (×103) (results from three experiments, P > .05). The number of DCs per spleen was decreased from 0.62 ± 0.05 (×106) in control mice to 0.5 ± 0.02 (×106) in VEGF-treated mice (five experiments,P = .048). The ability of cells from the lymph node DC fraction to stimulate allogeneic T cells was decreased almost twofold. Cells in the DC fraction from spleen were able to stimulate neither allogeneic nor FLU-specific T-cell proliferation (data not shown), similar to what was shown in Fig 2 after a 14-day infusion. To investigate the effect of VEGF treatment on the number of Langerhans cells in skin, epidermal sheets were stained with biotinylated anti–major histocompatibility complex (MHC) class II (anti-IAd) antibody and visualized with streptavidin-peroxidase followed by diaminobenzidine-H2O2. The number of LCs (IAd+ cells) was calculated per square millimeter of tissue. In the epidermis of control mice, LCs formed a network that was not affected after 7 days (Fig 4B) and 14 days of VEGF infusion (data not shown) at rates as high as 300 ng/h. However, 28-day infusions at a rate as low as 50 ng/h resulted in significant decreases in the number of LC in skin (Fig 4A and B). Thus, a prolonged 28-day infusion of recombinant VEGF resulted in a more pronounced decrease in the number and function of DC than short infusions. Moreover, the observed effects on DC function occurred at VEGF serum levels more than 100 times lower than that required in vitro.8 15 Because 28-day infusions of VEGF at a dose of 300 ng/h had the same effect as 50 ng/h, the latter dose (total dose of VEGF 33.6 μg) was used in all subsequent experiments.
Decreased presence of DC in spleen and lymph nodes after 28-day VEGF infusion. DCs were isolated from lymph nodes (A) and spleen (B) from control mice (left column) and mice treated with VEGF (right column) as described in Materials and Methods. Cells were labeled with FITC-conjugated anti-CD11c antibody and PE-conjugated anti–B7-2 antibody. The percentage of cells positive for both markers is indicated in the upper right corner of each panel. Typical results of one of three experiments is shown.
Decreased presence of DC in spleen and lymph nodes after 28-day VEGF infusion. DCs were isolated from lymph nodes (A) and spleen (B) from control mice (left column) and mice treated with VEGF (right column) as described in Materials and Methods. Cells were labeled with FITC-conjugated anti-CD11c antibody and PE-conjugated anti–B7-2 antibody. The percentage of cells positive for both markers is indicated in the upper right corner of each panel. Typical results of one of three experiments is shown.
Decreased numbers of LC in skin after 4 weeks of VEGF infusion. Mice were treated with either PBS or VEGF for 7 or 27 days (VEGF infusion rate of 50 ng/h). The number of LC in the ventral trunk epidermis was counted as described in Materials and Methods. (A) Results of three independent experiments are presented. (B) Microphotograph of one representative experiment (original magnification × 50).
Decreased numbers of LC in skin after 4 weeks of VEGF infusion. Mice were treated with either PBS or VEGF for 7 or 27 days (VEGF infusion rate of 50 ng/h). The number of LC in the ventral trunk epidermis was counted as described in Materials and Methods. (A) Results of three independent experiments are presented. (B) Microphotograph of one representative experiment (original magnification × 50).
The nature of cells generated during VEGF infusion.
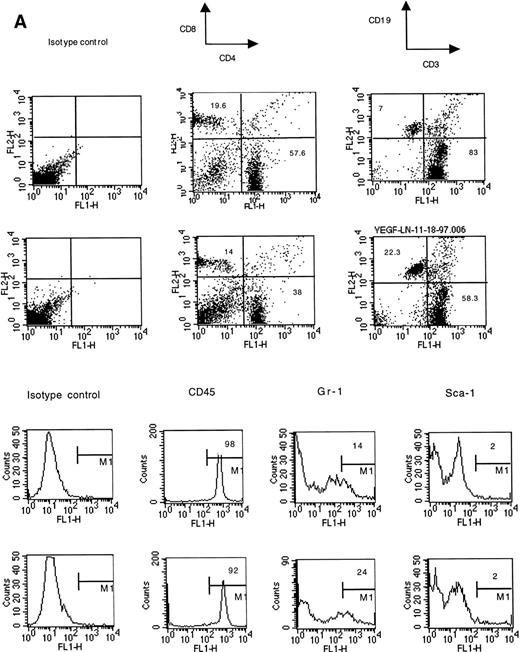
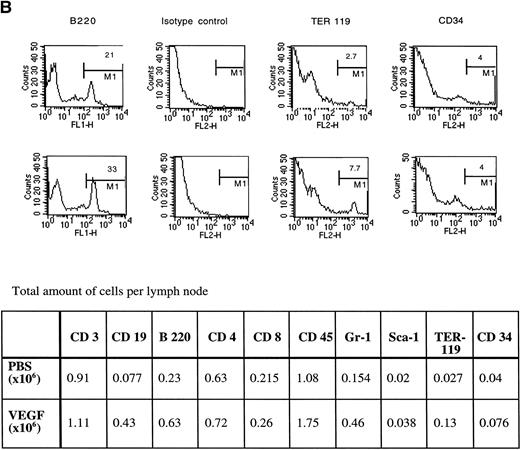
Cells for these experiments were isolated from lymph nodes and spleens and were not subjected to any other purification procedures. Red blood cells were removed from spleens by osmotic shock lysis. A 28-day infusion of VEGF did not significantly decrease the fraction of lymphocytes in lymph nodes (Fig 5). However, the balance between T cells and B cells was dramatically affected. The percentage of B cells was increased more than threefold, and the T-cell fraction was correspondingly decreased. The total number of T cells was not changed, but the total number of B cells increased almost six times (Fig 5). The CD4/CD8 cell ratio in lymph nodes was not affected. Infusion with VEGF led to a significant increase in the number of myeloid cells expressing the granulocyte-specific marker Gr-1 and in the number of erythroid cells (TER 119+ cells) (Fig5). No changes in the proportion of stem cells (Sca-1+, CD34+) were detected. Lack of increased proportion of CD34+ cells also suggests that there was no significant increase in the numbers of endothelial cells in lymph nodes. This was confirmed by positive staining of almost all of the cells with an anti-CD45 antibody (Fig 5) and a lack of increased Flk-positivity in the frozen sections (data not shown). Flk is one of the VEGF receptors abundantly expressed on the endothelial cells.
Phenotype of lymph node cells in mice treated with VEGF (50 ng/h). Representative results of one of three independent experiments are shown. Two groups of mice were compared. For each cell-surface marker, the top panel is from control mice (pumps contained PBS), the bottom panel shows VEGF-treated mice. Lymph nodes from three mice in each group were pooled together for this analysis.
Phenotype of lymph node cells in mice treated with VEGF (50 ng/h). Representative results of one of three independent experiments are shown. Two groups of mice were compared. For each cell-surface marker, the top panel is from control mice (pumps contained PBS), the bottom panel shows VEGF-treated mice. Lymph nodes from three mice in each group were pooled together for this analysis.
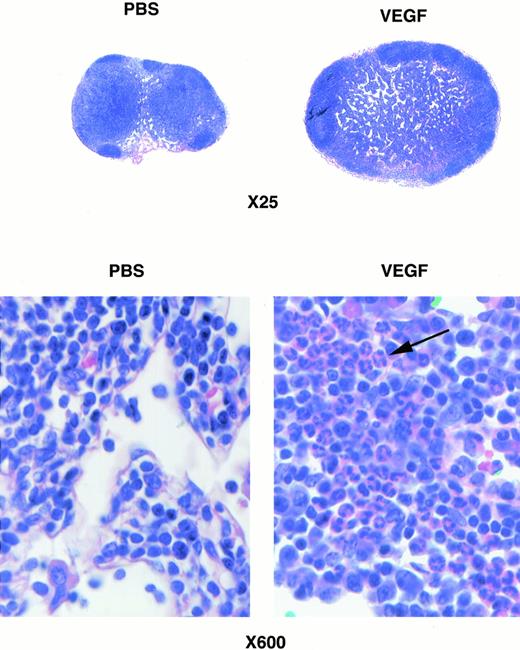
Histological analysis of lymph nodes from VEGF treated mice showed no appreciable changes in follicles (B-cell–rich areas), whereas the cellularity of the parafollicular cortex (T-cell zone) was decreased (Fig 6, top panel). Groups of granulocytes were seen in the parafollicular cortex of lymph nodes obtained from VEGF-treated mice (Fig 6, bottom panel).
Photomicrograph of lymph nodes stained with hematoxylin-eosin. (Bottom panel) Higher magnification of one of the fields in a parafollicular zone of lymph nodes. Arrow points to a group of granulocytes in the lymph node from a VEGF-treated mouse.
Photomicrograph of lymph nodes stained with hematoxylin-eosin. (Bottom panel) Higher magnification of one of the fields in a parafollicular zone of lymph nodes. Arrow points to a group of granulocytes in the lymph node from a VEGF-treated mouse.
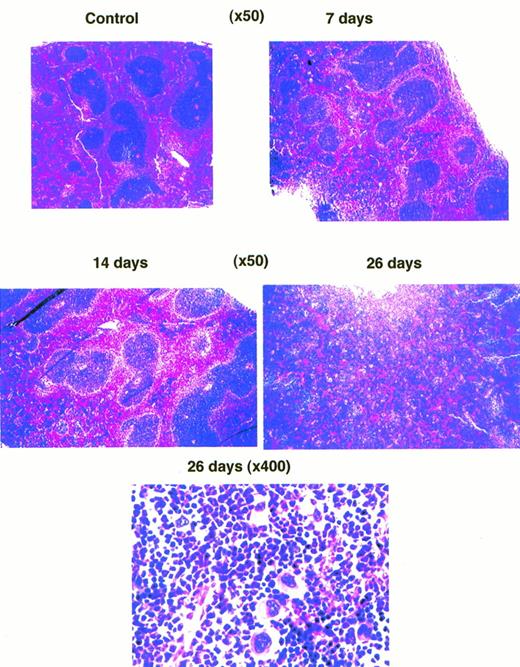
In the spleen, a 7-day VEGF infusion resulted in very little change in morphology, and a 14-day infusion led to slight increase in the size of the follicles (Fig 7). By the end of the fourth week of infusion, however, the splenic structure was effaced by expansion of the white pulp, associated with considerable increases in the number of megakaryocytes (Fig 7). The presence of megakaryocytes in spleens was confirmed by staining with cholinesterase61 (data not shown).
Photomicrograph of spleens stained with hematoxylin-eosin from mice treated with VEGF for different times. (Bottom panel) Higher magnification of one of the fields in the spleen from a mouse treated with VEGF for 28 days showing increased numbers of megakaryocytes.
Photomicrograph of spleens stained with hematoxylin-eosin from mice treated with VEGF for different times. (Bottom panel) Higher magnification of one of the fields in the spleen from a mouse treated with VEGF for 28 days showing increased numbers of megakaryocytes.
The proportion of lymphocytes in the spleen was dramatically decreased after 28 days of VEGF infusion. Despite a fourfold increase in the total amount of splenocytes in VEGF-treated mice, the number of T and B cells per spleen was reduced (Fig 8, CD3, CD19 markers). At the same time, the number of myeloid cells expressing the Gr-1 marker was markedly increased (Fig 8). Significantly increased numbers of erythroid cells (TER119+) were also detected. As was the case in lymph nodes, no increase in the proportion of cells expressing Sca-1 and CD34 markers was detected. Staining with anti-Flk antibodies did not show significant increases in the number of endothelial cells in spleens from these animals (data not shown). We detected no marked increase in the number of mature granulocytes in the spleens from VEGF-treated mice. To clarify whether these Gr-1+ cells were immature granulocytes, spleen cells were stained for myeloperoxidase, a cytochemical marker for granulocytes. No significant increase in the number of myeloperoxidase positive cells was found (data not shown). However, the increased proportion of Gr-1+ cells in the spleen was associated with significant increases in the percentage of granulocytes in peripheral blood from 28.6% ± 4.5% to 46.3% ± 5.2% (P < .05, six mice per group) after 28 days of VEGF infusion. Thus, the data presented so far indicate that continuous infusion with VEGF results in the inhibition of DC function, alterations in T- and B-cell numbers, and accumulation of immature myeloid cells and granulocytes in the spleen, lymph nodes, and peripheral blood.
Phenotype of the spleen cells in VEGF-treated mice. Three experiments with the same results were performed. For each cell-surface marker: (top), control mice (pumps contained PBS); (bottom), VEGF-treated (50 ng/h) mice.
Phenotype of the spleen cells in VEGF-treated mice. Three experiments with the same results were performed. For each cell-surface marker: (top), control mice (pumps contained PBS); (bottom), VEGF-treated (50 ng/h) mice.
The continuous presence of VEGF is required for the observed effects on hematopoietic cells.
VEGF induces the growth of endothelial cells, increases vascular permeability, and may affect the function of macrophages. Therefore, we asked whether the continuous presence of VEGF is required for the observed effects or whether it triggers the release of factors from activated endothelial cells or macrophages that then sustain the observed effects on hematopoietic cells in VEGF-treated mice. To investigate this issue, the following experiments were performed. Mice with control (PBS) and VEGF pumps were lethally irradiated and reconstituted with control BM cells. Five days later the histologic types of hematopoietic microcolonies in the spleens were analyzed. The first group was comprised of control mice with PBS pumps, the second group included mice after 28 days infusion of VEGF, and the third group included mice with 22 days of VEGF infusion. The rate of infusion was the same in all mice (50 ng/h). The ALZET pumps used in this study were functional only for about 28 days. Therefore, mice in the third group received VEGF for 5 to 6 days after reconstitution with BM cells, whereas mice in the second group did not receive VEGF after reconstitution. In mice of the third group (active VEGF infusion) a significantly higher number of megakaryocytes was found (125.6 ± 25.6 cells per/mm2v 4.2 ± 1.6 cells/mm2 in controls, P < .001), whereas in mice of the second group (no VEGF infusion after irradiation) this increase was much less pronounced (23.2 ± 4.8 cells/mm2,P < .05). Mice from the third group had a significantly higher percentage of macrophage/granulocyte (myeloid) colonies in the spleen (76.4% ± 5.1% v 25.2% ± 3.7% in control,P < .001). The changes in the percentage of myeloid colonies in mice of the second group was much smaller (34.6% ± 5.7%, P > .05). These data indicate that the observed effects required the presence of VEGF during progenitor differentiation and outgrowth.
VEGF affects progenitor cells during the first days after the start of the infusion.
In the next group of experiments we tried to establish the time course for the stimulation of myeloid progenitor cells in VEGF-treated mice. BM cells were isolated from mice 3, 5, 7, and 28 days after implantation of the pumps. BM cells were cultured for 14 days in semisolid methylcellulose medium supplemented with growth factors supporting growth of erythroid and myeloid colonies, but without VEGF. Three-day infusions with VEGF led to slightly elevated numbers of CFU-GM colonies. Five- and 7-day infusions resulted in dramatic increases in the numbers of CFU-GM, but not erythroid colonies (Fig 9). After 28 days of infusion the effect was much less pronounced. This implies that the numbers of committed progenitors of the granulocyte/macrophage lineage as detected in this assay are greatly increased after approximately 1 week of in vivo exposure to VEGF.
Effect of VEGF infusion on colony efficiency of BM cells. Mice were treated with infusion of VEGF (50 ng/h; ▪) or PBS (control; ) for 3, 5, 7, or 28 days. After that time, BM cells were isolated and cultured in triplicate in semisolid methylcellulose medium supplemented with recombinant cytokines supporting growth of erythroid and myeloid colonies (Methocult GF M3434; Stem Cell Technologies). BFU-E colonies were scored on day 8 and the remaining colonies were evaluated on day 12-13. Each experiment included two mice per group. The number of colonies was calculated per 105 cells. Mean ± SE of two independent experiments is shown.
) for 3, 5, 7, or 28 days. After that time, BM cells were isolated and cultured in triplicate in semisolid methylcellulose medium supplemented with recombinant cytokines supporting growth of erythroid and myeloid colonies (Methocult GF M3434; Stem Cell Technologies). BFU-E colonies were scored on day 8 and the remaining colonies were evaluated on day 12-13. Each experiment included two mice per group. The number of colonies was calculated per 105 cells. Mean ± SE of two independent experiments is shown.
Effect of VEGF infusion on colony efficiency of BM cells. Mice were treated with infusion of VEGF (50 ng/h; ▪) or PBS (control; ) for 3, 5, 7, or 28 days. After that time, BM cells were isolated and cultured in triplicate in semisolid methylcellulose medium supplemented with recombinant cytokines supporting growth of erythroid and myeloid colonies (Methocult GF M3434; Stem Cell Technologies). BFU-E colonies were scored on day 8 and the remaining colonies were evaluated on day 12-13. Each experiment included two mice per group. The number of colonies was calculated per 105 cells. Mean ± SE of two independent experiments is shown.
) for 3, 5, 7, or 28 days. After that time, BM cells were isolated and cultured in triplicate in semisolid methylcellulose medium supplemented with recombinant cytokines supporting growth of erythroid and myeloid colonies (Methocult GF M3434; Stem Cell Technologies). BFU-E colonies were scored on day 8 and the remaining colonies were evaluated on day 12-13. Each experiment included two mice per group. The number of colonies was calculated per 105 cells. Mean ± SE of two independent experiments is shown.
VEGF exerts different effects on early and late progenitors.
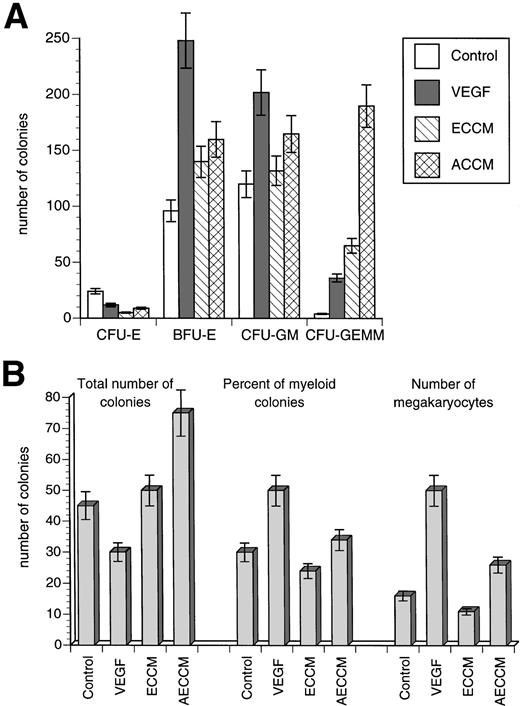
We compared the in vitro effect of VEGF on late, lineage-committed progenitor cells and on early progenitor and stem cells. BM cells were isolated from control healthy mice and were treated in vitro for 2 hours with either VEGF (100 ng/mL) or with conditioned medium (50% vol/vol) obtained from endothelial cells with or without VEGF exposure. Endothelial cells were seeded at 50% confluency and were cultured for 3 days to obtain endothelial cell conditioned medium (ECCM). In parallel, endothelial cells were stimulated with 10 ng/mL VEGF for 3 days (activated ECCM [AECCM]). In our preliminary in vitro experiments, this concentration of VEGF did not affect the growth of progenitor cells. Media from cell cultures were collected, filter-sterilized, and used in further experiments. Two groups of experiments were performed. In the first, cells were cultured in semisolid methylcellulose medium together with a cocktail of cytokines supporting the growth of myeloid and erythroid colonies. In the other group of experiments, cells were injected into lethally irradiated control syngeneic mice. The number and type of spleen colonies were analyzed 11 days later.
Treatment of BM cells with ECCM and AECCM in vitro dramatically increased the number of myeloid and mixed colonies in semisolid medium (CFU-GM and CFU-GEMM). The effect of recombinant VEGF was less pronounced, although it also increased the number of CFU-GM and CFU-GEMM colonies. Treatment of BM cells with VEGF alone also stimulated the growth of erythroid colonies (CFU-E, BFU-E) (Fig 10A).
Effect of VEGF treatment in vitro on progenitor cells. ECCM and AECCM were obtained as described in the text. BM cells were obtained from control mice and incubated for 2 hours with either 100 ng/mL VEGF or with 50% vol/vol ECCM or AECCM. After that time, cells were washed and used in further experiments. Each experiment included two mice per group. Mean ± SE of two independent experiments is shown. (A) Colonies in semisolid methylcellulose medium. Cells were cultured in triplicate in methylcellulose medium and the number of colonies per 105 BM cells were scored exactly as described in the legend to Fig 9. Cumulative results from all experiments are shown. Y-axis, the number of colonies per 105 cells. (B) Spleen colonies were scored based on morphological criteria. Cumulative results from all experiments are shown.
Effect of VEGF treatment in vitro on progenitor cells. ECCM and AECCM were obtained as described in the text. BM cells were obtained from control mice and incubated for 2 hours with either 100 ng/mL VEGF or with 50% vol/vol ECCM or AECCM. After that time, cells were washed and used in further experiments. Each experiment included two mice per group. Mean ± SE of two independent experiments is shown. (A) Colonies in semisolid methylcellulose medium. Cells were cultured in triplicate in methylcellulose medium and the number of colonies per 105 BM cells were scored exactly as described in the legend to Fig 9. Cumulative results from all experiments are shown. Y-axis, the number of colonies per 105 cells. (B) Spleen colonies were scored based on morphological criteria. Cumulative results from all experiments are shown.
Treatment of BM cells with ECCM and AECCM also increased the number of spleen colonies. However, treatment of BM cells with VEGF alone not only did not increase the total number of spleen colonies, but rather significantly reduced total colony formation. Differences were also observed in the histologic type of colonies generated in the presence of endothelial cell–derived factors and VEGF. Treatment of BM cells with AECCM had little effect on the type of spleen colonies, whereas VEGF treatment considerably increased the proportion of macrophage/granulocyte colonies in the spleen. This was also associated with a significant increase in the number of megakaryocytes (Fig 10B). These data show that short in vitro exposure to VEGF paralleled the effects of endothelial cell supernatent on lineage-commited progenitor cells (semisolid methylcellulose colonies) but had different effects on early progenitor and stem cells (spleen colonies) not observed with conditioned medium. These data support direct, rapid, and sustained effects of transient VEGF exposure on the early progenitors and stem cells.
Effect of continuous VEGF infusion on NF-κB activation.
We have previously reported that VEGF results in a block of NF-κB activation in hematopoietic progenitors in vitro.15 To investigate whether the same effect could be observed after in vivo treatment with VEGF, BM cells were isolated from mice at different times after the initiation of VEGF infusion. Granulocytes were removed from harvested BM after centrifugation on a lympholyte-M gradient. BM cells were then depleted for T and B cells, macrophages, and dendritic cells using monoclonal antibodies and complement. The remaining cells were then stimulated with tumor necrosis factor-α (TNF-α), and protein extracts made for EMSA as described in Materials and Methods to assess for induction of NF-κB DNA binding. VEGF infusion inhibited TNF-α–induced NF-κB activation by day 7 (Fig 11A). To investigate whether the same effect could be observed in tumor-bearing mice, mice were inoculated with 2 × 105 D459 cells. Mice were killed on day 18 and day 32 after tumor inoculation, when tumors reached 80 mm2 and 300 mm2 in area, respectively. The measured serum level of VEGF was 20 to 30 pg/mL on day 18 and 70 to 90 pg/mL on day 32. Similar inhibition of the ability of NF-κB to specifically bind DNA was found in BM progenitors from the tumor-bearing mice (Fig 11B). Thus, blockade of NF-κB activation in hematopoietic progenitor cells by VEGF or tumors in vivo is a relatively early event that precedes the observed morphologic changes in hematopoietic development.
(A) Effect of VEGF infusion on NF-κB binding to DNA. Mice were treated with VEGF or PBS for 7 or 28 days. BM cells were obtained and lin− progenitor cells were prepared as described in Materials and Methods. Cells were stimulated with 10 ng/mL murine TNF-, nucleoproteins were extracted, and EMSA was performed as described in Materials and Methods. Control, BM cells from mice treated with PBS for 7 days; VEGF, mice treated with VEGF (50 ng/h) for 7 or 28 days; M, binding of nucleoproteins from control TNF-–stimulated BM cells to mutant DNA sequence (negative control); 1, nonstimulated cells; 2, cells stimulated with 10 ng/mL TNF- for 10 minutes; 3, cells stimulated with TNF- for 20 minutes. Three experiments with the same results were performed. (B) Effect of tumor cells on NF-κB binding activity in BM cells. Mice were injected subcutaneously with 2 × 105 D459 tumor cells. Mice were killed on day 18 (tumor size, 80 mm2) or on day 32 (tumor size, 300 mm2) after tumor injection. Lin+ cells were removed and EMSA was performed exactly as described in the legend to Fig 10A. Control, tumor-free mice. 1, 2, 3: The same as in Fig 10A. Two independent experiments with the same results were performed.
(A) Effect of VEGF infusion on NF-κB binding to DNA. Mice were treated with VEGF or PBS for 7 or 28 days. BM cells were obtained and lin− progenitor cells were prepared as described in Materials and Methods. Cells were stimulated with 10 ng/mL murine TNF-, nucleoproteins were extracted, and EMSA was performed as described in Materials and Methods. Control, BM cells from mice treated with PBS for 7 days; VEGF, mice treated with VEGF (50 ng/h) for 7 or 28 days; M, binding of nucleoproteins from control TNF-–stimulated BM cells to mutant DNA sequence (negative control); 1, nonstimulated cells; 2, cells stimulated with 10 ng/mL TNF- for 10 minutes; 3, cells stimulated with TNF- for 20 minutes. Three experiments with the same results were performed. (B) Effect of tumor cells on NF-κB binding activity in BM cells. Mice were injected subcutaneously with 2 × 105 D459 tumor cells. Mice were killed on day 18 (tumor size, 80 mm2) or on day 32 (tumor size, 300 mm2) after tumor injection. Lin+ cells were removed and EMSA was performed exactly as described in the legend to Fig 10A. Control, tumor-free mice. 1, 2, 3: The same as in Fig 10A. Two independent experiments with the same results were performed.
DISCUSSION
Defective functional maturation of dendritic cells in cancer could be one of the important mechanisms which allows tumors to escape immune system control. We recently identified VEGF as one of the factors responsible for this defective DC maturation in vitro.8VEGF is 34- to 42-kD protein produced by almost all tumor cells and responsible for the formation of tumor neovasculature.30 We found that VEGF binds to hematopoietic progenitor cells through the VEGF-specific receptor Flt-1, and blocks activation of the transcription factor NF-κB in these cells.15 However, the direct effect of recombinant VEGF on DC maturation in vitro was rather weak and was seen only at concentrations of 50 to 100 ng/mL, more than 100 times the serum levels observed in cancer patients. This raised questions about the relevance of this in vitro finding to the situation in vivo. It was also unclear which cells were the targets of VEGF action. The CD34+ cell population used in the previous studies included lineage-committed progenitor cells with a small percentage of polypotent stem cells. The in vitro effects of VEGF were more pronounced on a population of CD34+ CD38− CD33− cells representing early progenitor cells (unpublished observations). This suggests that VEGF may specifically affect the early progenitors or stem cells. To better address the role of VEGF in hematologic effects observed in tumor-bearing animals, we studied the effects of continuous exposure to recombinant VEGF in otherwise healthy animals. Because VEGF has a short in vivo half-life and is almost undetectable within 50 minutes after administration, we used an in vivo model utilizing a continuous infusion of VEGF via an osmotic infusion pump. These pumps provide a continuous infusion for up to 28 days. We tested several infusion rates of VEGF for their ability to block DC function. Doses from 50 ng/h to 300 ng/h had equivalent effects on DC differentiation. The former dose resulted in a steady-state serum level of 120 to 160 pg/mL, which is within the range of VEGF concentrations reported in tumor-bearing mice and in patients with cancer.26-29 Therefore, this rate of VEGF infusion can be considered as pathologically relevant to VEGF concentrations observed in tumor-bearing hosts.
VEGF infusion inhibits DC differentiation in vivo.
Prolonged VEGF infusion led to considerable inhibition of DC development, which became more evident with longer exposure times. The fraction of DC was decreased more than fourfold in the spleen and twofold in lymph nodes, and the number of LC in the skin decreased twofold after 28 days of VEGF infusion. This decrease was accompanied by significant decreases in the functional activity of lymph node DC and profound inhibition of the function of splenic DC. These effects were observed at a dose of 50 ng/h with a total VEGF infusion of 33.6 μg over 28 days. It is interesting that splenic DC from VEGF-treated mice had an increased ability to take up FITC-dextran. This is likely to be a reflection of immature status, because mature DC usually lose this function concurrent with acquiring increased ability to present antigens.31 Two-week infusions, even at the highest tested dose (total VEGF amount, 100 μg), did not significantly decrease the numbers of DC in tissues. However, it did inhibit the function of splenic, but not lymph node DC, perhaps related to the rates of DC turnover in these organs. The turnover of DC in spleen is much higher than in lymph nodes and skin. The half-life of DC in the spleen is 1 to 2 weeks, but in lymph nodes and skin it is 3 to 4 weeks or longer.32-35 It may explain the differences between changes in spleen and lymph node cells. It also suggests that VEGF does not affect mature DC, but rather inhibits differentiation of cells de novo to replenish cells lost due to natural turnover.
Infusion of VEGF resulted in the appearance of immature myeloid cells.
Inhibition of DC differentiation by VEGF was associated with alterations in other cell lineages in the spleen and in lymph nodes. We observed a significant expansion of CD45+CD34− Sca-1− cells in spleen and lymph nodes. No significant expansion of Flk+ cells was found. These data ruled out the presence of increased numbers of endothelial cells in these sites. Detailed analysis of other surface molecules showed a dramatic expansion of Gr-1+ cells in the spleen and a less profound, but still significant, increase of this population in lymph nodes. No significant increase of mature granulocytes in the spleen was detected based on morphological criteria and myeloperoxidase staining. However, significantly increased numbers of granulocytes were observed in peripheral blood and in the lymph nodes. Gr-1 can also be expressed on immature macrophages, but the Gr-1+ cells in our study did not express Mac-1, a specific marker of macrophages. Thus, VEGF infusion resulted in an expansion of immature myeloid cells. Whether these cells represent early stages of macrophage differentiation or are granulocyte precursors, as well as their functional or inhibitory activity and their role in cancer, are currently under investigation. Otsuji et al36 recently described Gr-1+ macrophage-like cells in spleens from tumor-bearing mice. These cells were able to decrease expression of CD3ζ chain of TCR complex on T cells, one of the proposed mechanisms of defective T-cell function in cancer. Expansion of myeloid progenitors in the BM was evident as early as 5 days after the start of VEGF infusion, but was much less pronounced by day 28, possibly reflecting redistribution or exhaustion of the pool of BM myeloid progenitors after 4 weeks of continuous stimulation.
Two other interesting phenomena were observed during VEGF infusion. One is the dramatic expansion of megakaryocytes observed 7 days after the start of the infusion. By the end of the fourth week, the number of megakaryocytes in the spleen was increased more than 100-fold compared with a control PBS infusion. The other observation is the expansion of B cells, but not T cells, in lymph nodes seen only after 4 weeks of VEGF infusion. The total number of B cells was increased almost sixfold, whereas the number of T cells was slightly decreased. The mechanisms of these effects are not clear, but could result from direct effects of VEGF on lymphoid progenitors or be mediated by altered interactions with other cell populations or factors produced in vivo. These possibilities will be discussed in more detail below.
Possible direct role of VEGF in the observed changes in differentiation of hematopoietic cells.
The effect of continuous exposure to increased levels of VEGF in vivo is likely to be complex, involving many different factors. VEGF affects the function of several different cell types. For example, monocytes express the Flt-1 VEGF receptor and respond to VEGF by increased migration.37 However, this was achieved only at high VEGF concentrations, and the physiological relevance of this effect is unclear. The primary and probably the most important targets for VEGF in adult animals are endothelial cells. They express high levels of high-affinity receptors for VEGF and respond to relatively low VEGF concentrations in vitro (1 to 10 ng/mL). Endothelial cells, especially BM microvascular endothelial cells, play an important role in the proliferation and differentiation of hematopoietic cells. Endothelial cells produce several hematopoietic growth factors such as SCF, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-6 and IL-8,38-40 and probably others as well. Therefore, it is possible that endothelial cells could release growth factors in response to activation by VEGF that could affect the differentiation of hematopoietic cells. Rafii et al41 showed that endothelial cells can support proliferation and differentiation of myeloid and megakaryocytic progenitors. It is interesting that they observed expansion of megakaryocytes from CD34+ progenitors in vitro after 7 days of coculture with endothelial cells, suggesting that this process is rapid. Serum-free conditioned medium from endothelial cells also contains a factor with a molecular weight (MW) > 30 kD that enhances the in vitro colony cell formation of human hematopoietic progenitor cells.42 This effect was not abrogated by neutralizing anti-SCF receptor antibodies and had no apparent species-specific restrictions in its activity. It would be interesting to see whether this factor could be VEGF.
We investigated whether the continuous presence of VEGF was necessary or whether a short period of exposure would lead to persistent changes in the host that would then lead to the observed effects. Our experiments showed that the continuous presence of VEGF was necessary and ruled out the possibility that VEGF was just a trigger for lasting effects mediated by other factors. However, these data do not rule out the involvement of other factors induced by VEGF. They do indirectly support the previously reported observations that blockade of VEGF-specific receptor interaction may prevent the negative effects of VEGF on DC maturation8 and, thus, that blocking VEGF or VEGF receptors in vivo may improve DC function, and hence, immune responses in cancer.
To clarify the possible effects of endothelial cell–derived factors, we performed in vitro experiments with BM cells from control mice using recombinant VEGF and supernatants from VEGF-stimulated and control endothelial cells. Two different experimental systems were used: semisolid methylcellulose medium supplemented with recombinant cytokines, which predominantly supports growth of “late” lineage committed progenitors,43,44 and a spleen colony assay in irradiated mice, which are formed by predominantly “early” pluripotent progenitors and stem cells.45-47 Conditioned medium from activated endothelial cells (AECCM) and VEGF had similar effects on “late” progenitors. They both stimulated growth of myeloid and erythroid progenitors (Fig 10A), confirming the previously reported observation that VEGF enhanced colony formation by relatively mature subsets of human granulocyte-macrophage and erythroid progenitor cells.48 The preferential stimulation of mixed colonies by AECCM may be due to the presence of other growth factors in conditioned medium. However, the effects of endothelial cell–derived factors and VEGF were different on “early” progenitors and stem cells. Whereas AECCM increased the total number of these colonies with little effect on their histological type, VEGF had an inhibitory effect on the total number of colonies, but increased the proportion of myeloid colonies and the number of megakaryocytes. Thus, VEGF and endothelial cell–derived factors had similar effects on relatively late progenitors, but significantly differed in their effects on multilineage progenitors and stem cells. These data confirm the results of Broxmeyer et al,48 who demonstrated that VEGF inhibited colony formation by less mature subsets of granulocyte-macrophage, erythroid, and multipotential progenitor cellls. Interestingly, these investigators also showed direct effects of VEGF on flow-sorted CD34+ cells, decreasing the chance that recombinant VEGF might be acting by stimulating the production of factors from other cells present in the BM.
In a very recent study, Nakayama et al49showed that VEGF significantly enhanced the production of CD34+ progeny cells from mouse embryonic stem cells. These CD34+ were enriched for myeloid, but not erythroid, colony-forming cells.49 When cultured on stromal cells in the presence of IL-2 and IL-7, these CD34+ cells developed B220+, CD19+ B lymphocytes, and CD3− cytotoxic lymphocytes, but not CD3+T cells. A shift of the T/B ratio toward B cells was observed in our study (Fig 5). Thus, the lineage redistribution that we observed with continuous VEGF infusion may be explained by direct effects of VEGF on early progenitors. The greater sensitivity of earlier progenitors to VEGF could be the basis for the effectiveness of vastly lower concentrations in vivo than were needed in vitro.
Role of NF-κB transcription factor in the effects mediated by VEGF.
What could be the possible molecular mechanisms of these VEGF effects on hematopoietic progenitor cells? We have previously shown that VEGF inhibited the activation of the transcription factor NF-κB in hematopoietic progenitor cells in vitro. Blocking NF-κB in vitro with a dominant negative IκBα prevented DC differentiation.15 NF-κB regulates the transcription of many genes involved in immune responses including those for cytokines and growth factors.50,51 NF-κB is present as an inactive complex in the cytoplasm of many cells bound to members of the IκB family of inhibitory proteins. Activation of NF-κB involves phosphorylation, dissociation, and degradation of IκB followed by release and nuclear translocation of NF-κB. This activation can be mediated by a variety of stimuli including bacterial lipopolysaccharide, phorbol myristate acetate (PMA), and TNF-α. Authentic NF-κB is composed of 50- and 65-kD subunits, which bind to a 10-bp motif in the promoter of responsive genes. Several subunits of NF-κB have been identified: p50, p52, p65 (RelA), cRel, and RelB. These subunits form both homodimeric and heterodimeric complexes and differentially regulate gene expression. Several studies have recently shown that RelB, a component of NF-κB, is required for the development of DC.52-54 Therefore, we hypothesized that NF-κB might be the transcription factor responsible for the observed effects of VEGF in vivo. The studies reported here support this hypothesis. NF-κB activation was blocked in BM cells as early as 7 days after the start of VEGF infusion. This well preceded any morphological changes. The same inhibitory effect was detected in tumor-bearing mice.
There are intriguing data in the literature on the impact of alterations in individual members of the NF-κB family on hematopoiesis. It has been reported that targeted disruption of RelB leads to myeloid hyperplasia, splenomegaly due to extramedullary hematopoiesis, and a reduced population of dendritic cells, but did not result in gross impairment of T and B cells.55 These findings are similar to those observed in our study with infusion of VEGF. It would be interesting to determine what role, if any, RelB plays in the effects mediated by VEGF. Several groups have reported abnormal T-lymphocyte development induced by targeted inhibition of NF-κB in T cells. In these studies, the T-cell lineage was targeted to express a trans-dominant form of IκBα that constitutively represses the activity of multiple NF-κB/Rel proteins. Transgenic cells expressing this inhibitor exhibited a significant proliferative defect, which was not reversed by the addition of exogenous IL-2. In addition to deregulated T-cell growth and survival, transgene expression impaired the development of normal T-cell populations.56 In transgenic mice it resulted in a reduction in peripheral T lymphocytes.57 Despite the severe defects in T-cell development, mice lacking either p50 or p52 proteins did not have significant defects in B-cell development. Only in double knockout mice was a significant defect in B-cell development observed.58,59 Introduction of p50/p65-deficient fetal liver cells into lethally irradiated hosts resulted in a severe deficit of fetal liver–derived lymphocytes and their immediate precursors but an overabundance of fetal liver-derived granulocytes. Simultaneous transplantation of wild-type BM cells rescued the production of p50/p65-deficient lymphocytes. These results suggest that NF-κB mediates the development or survival of an early lymphocyte precursor.60 Taken together, these data are consistent with our hypothesis that VEGF may affect hematopoiesis through effects on NF-κB, and perhaps specifically on RelB. This may explain the activation of myelopoiesis by VEGF and its effects on T- and B-cell production as well as those on dendritic cells. The effects of VEGF on different members of NF-κB family as well as on the function of B and T lymphocytes deserve further investigation.
In conclusion, in this report we have for the first time described the effect of continuous in vivo overexposure to VEGF on hematopoiesis. We showed here that VEGF, likely in combination with other factors, not only induced severe inhibition of DC differentiation, but also affected other lineages of hematopoietic cells. These effects resemble the situation observed in tumor-bearing hosts, and occur at levels of VEGF seen in tumor-bearing animals, or more than 100-fold less than required in vitro. These findings may not only help improve our understanding of the mechanisms of tumor-associated immune nonresponsiveness, but also identify a new role that VEGF plays in pathological as well as physiological hematopoiesis. These findings additionally suggest that blocking VEGF action may improve the function of the immune system in cancer.
ACKNOWLEDGMENT
We thank Genentech Inc for providing us with recombinant VEGF. We thank Dr M. Koury for a critical review of this manuscript and for the helpful comments.
Supported by National Institutes of Health Grant No. CA76321 to D.P.C., and by pilot project funding to D.G. from the Vanderbilt Cancer Center Core Grant No. CA68485 and ACS Institutional Grant No. IN 25-37. Experiments were performed in part through use of the VUMC Cell Imaging Resource (supported by Grants. No. CA68485 and DK20593).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Dmitry Gabrilovich, MD, PhD, The Vanderbilt Cancer Center, Vanderbilt University School of Medicine, 648 MRB II, Nashville, TN 37232-6838; e-mail:dmitry.gabrilovich@mcmail.vanderbilt.edu.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal