Abstract
Recent studies have demonstrated that band 3 carries antigens of the Diego blood group system and have elucidated the molecular basis of several previously unassigned low incidence and high incidence antigens. Because the available serological data suggested that band 3 may carry additional low incidence blood group antigens, we screened band 3 genomic DNA encoding the membrane domain of band 3 for single-strand conformational polymorphisms. We found that the putative first ectoplasmic loop of band 3 carries blood group antigen ELO, 432 Arg→Trp; the third putative loop harbors antigens Vga (Van Vugt), 555 Tyr→His, BOW 561 Pro→Ser, Wu (Wulfsberg), 565 Gly→Ala, and Bpa (Bishop), 569 Asn→Lys; and the putative fourth ectoplasmic loop carries antigens Hga (Hughes), 656 Arg→Cys, and Moa (Moen), 656 Arg→His. We studied erythrocytes from carriers of five of these blood group antigens. We found similar levels of reticulocyte mRNA corresponding to the two band 3 gene alleles, normal content and glycosylation of band 3 in the red blood cell membrane, and normal band 3-mediated sulfate influx into red blood cells, suggesting that the mutations do not have major effect on band 3 structure and function. In addition to elucidating the molecular basis of seven low incidence blood group antigens, these results help to create a more accurate structural model of band 3.
BAND 3 (anion exchanger 1 [AE1]) is the most abundant integral protein of the red blood cell (RBC) membrane, being present in more than 1 million copies per RBC. It consists of two structurally and functionally largely independent domains. The N-terminal cytoplasmic domain of band 3 links the membrane to the underlying spectrin-based membrane skeleton and interacts with several glycolytic enzymes, hemoglobin, and hemichromes. The main function of the C-terminal membrane domain is to mediate exchange of chloride and bicarbonate anions across the plasma membrane. Current structural models predict that the membrane domain consists of 12 to 14 transmembrane helices connected by ectoplasmic and endoplasmic loops.1-4 The longest, fourth loop of band 3 is N-glycosylated1,4 and the carbohydrate chain carries more than half of the RBC ABO blood group epitopes.5
Mutations of band 3 protein have been implicated in the pathogenesis of Southeast Asian ovalocytosis,6,7 hereditary spherocytosis,8-11 congenital acanthocytosis,12and, recently, distal renal tubular acidosis.13,14 An amino acid substitution in the cytoplasmic domain of band 3 underlies the abnormal electrophoretic mobility of a polymorphic band 3 variant known as band 3 Memphis.15,16 Yet another variant of band 3, known as band 3 Memphis II, displays, in addition to the abnormal electrophoretic mobility, an increased binding of the anion flux inhibitor dihydrodiisothiocyanatodisulfonate, disodium salt (H2DIDS).17 Spring et al18 reported that the Memphis II variant of band 3 carries the Dia blood group antigen.
Dia is a low incidence blood group antigen in Caucasians that is antithetical to Dib.19 Prevalence of Dia is much higher in American Indians, reaching up to 54% in some groups of South American Indians.20 The underlying polymorphism is a single amino acid substitution in position 854, with proline of the wild-type band 3 corresponding to the Dibantigen and leucine to the Dia antigen.21,22Dia and Dib became the first fully characterized antigens of the Diego blood group system assigned to the band 3 protein.21 Subsequently, Bruce et al23and our group24 have mapped the low incidence blood group antigen Wra to the C-terminal end of the fourth ectoplasmic loop. Glutamic acid 658 from the wild-type band 3 sequence underlies the high incidence antigen, Wrb, whereas the low incidence antigen, Wra, has lysine in the same position.
Recently, we and others have shown25,26 that three additional low incidence antigens, Rba,27WARR,28 and Wda,29 are associated with different single point mutations on band 3 and therefore belong to the Diego system. The amino acid changes associated with these three antigens are, respectively, 548Pro→Leu,30,31552Thr→Ile,32,33 and 557Val→Met.30,31,34,35 Although only one subject has been studied for another low incidence antigen, Tra, the experimental evidence strongly suggests that it is also located on the erythroid band 3 protein, with the underlying polymorphism being 551Lys→Asn.31
Discovery of the molecular basis of these antigens yielded the first insight into the Diego blood group system as well as valuable information on the position and size of the external loops of band 3. In addition, in the case of Wrb, it helped to characterize the site of the band 3-glycophorin A interaction, because the Wrb antigen requires the presence of both these proteins for its expression.24,36,37 These results also suggested band 3 might carry additional low incidence antigens. We therefore reviewed the list of remaining low incidence antigens that had serological characteristics consistent with the possibility of their being carried by band 3 and that we had in liquid nitrogen storage. We found that antigens Bpa (Bishop),38 Wu (Wulfsberg),39 Moa (Moen),40Vga (Van Vugt),41 Hga(Hughes),42 BOW,43 and ELO44fulfilled these criteria and proceeded with characterization of their molecular basis.
MATERIALS AND METHODS
Subjects.
Samples were obtained through the Serum, Cell and Rare Fluid (SCARF) exchange program or as gifts from numerous colleagues. Initial testing was performed on DNA isolated from blood samples stored in liquid nitrogen; additional testing was performed on freshly drawn blood samples shipped on ice to Boston. The subjects were heterozygotes for the studied blood group antigens.
DNA preparation.
DNA was isolated from blood samples (∼150 μL) that had been stored in liquid nitrogen or that had been obtained as fresh samples using the QIAamp Blood Kit (QIAGEN Inc, Chatsworth, CA). The manufacturer’s procedure for DNA isolation from whole blood was followed. The eluted DNA was used directly for polymerase chain reaction (PCR) amplification.
Single-strand conformational polymorphism (SSCP) analysis and sequencing of band 3 genomic DNA.
SSCP screening was performed according to Orita et al,45 46with minor modifications. Exons encoding the membrane domain of band 3 were PCR-amplified using pairs of intronic primers flanking the exons (Table 1; 35 cycles of 1 minute at 94°C and 1 minute at 60°C). To each 10 μL PCR reaction, 2.5 μCi32P-dATP (3,000 μCi/mmol; ICN, Costa Mesa, CA) was added. The PCR products were diluted in formamide loading buffer (86% formamide, 10% glycerol, 20 mmol/L EDTA, 0.25% bromophenol blue, 0.25% xylene cyanol), heat-denatured by boiling for 5 minutes, and quickly cooled on ice. Samples were electrophoresed for 16 hours at room temperature in a nondenaturing polyacrylamide mutation detection enhancement (MDE) gel (FMC BioProducts, Rockland, ME). Briefly, 25 mL MDE gel solution was mixed with 69 mL deionized water, 6 mL 10× TBE, 0.4 mL 10% ammonium persulfate, and 40 μL TEMED, and electrophoresis was performed at 8 W in the presence and at 4 W in the absence of 10% glycerol. The gel was exposed to Kodak XAR-5 film (Eastman Kodak, Rochester, NY) overnight at −80°C using intensifying screens. The band 3 gene exons displaying the SSCP were directly sequenced using the Sequenase version 2.0 DNA Sequencing Kit (Amersham, Arlington Heights, IL). We limited our SSCP screening to exons 11 to 20 of the band 3 gene, because these 10 exons encode the whole membrane domain of band 3 and mutations causing altered immunogenicity of band 3 are to be expected only in this portion of band 3.
Intronic Primers Used for PCR Amplification of Exons Encoding the Membrane Domain of Band 3
| Exon . | Sense Primer (5′ to 3′) . | Antisense Primer (5′ to 3′) . | ||
|---|---|---|---|---|
| 11 | 11A | TCCTCCAGCTACTCCCTCTGA | 11B | CAGAGGGTCAGAGGCAAGAGT |
| 12 | 12A | CCTGGAAATGATCTCCTGACCT | 12B | ACCATGCATCAGGCAGGTGGT |
| 13 | 13A | CCTTGCCCCAAGACCCTTTGA | 13B | CTTATACACAACCTCCCGTGTG |
| 14 | 14A | CCAAGCCAAAGCTGGGAGAGA | 14B | GGGCTGGGATAGGGCAGTGT |
| 15 | 15A | GGGGAGTGACTGGGCACTGA | 15B | ATGAGGACCTGGGGGGTATCA |
| 16 | 16A | CCATCCTCCTGCCCTTGGCA | 16B | GCCAGGGAAAGGTCTCTGCC |
| 17 | 17A | GGAGAACCCTGCTTACCCCTC | 17B | GAGGATGGTGAAGACGCGACC |
| 18 | 18A | AACCTGGGCTGAGAGTGTGCG | 18B | TCTACCACCCCAGGCTGGGC |
| 19 | 19A | GGGGTGTGATAGGCACTGACC | 19B | CCCTCGCATGCTCCCAGCTC |
| 20 | 20A | CTCTTCTGGCCCCAGGGTCT | 20B | TGCTCCAGGAGGATCCTGAGT |
| Exon . | Sense Primer (5′ to 3′) . | Antisense Primer (5′ to 3′) . | ||
|---|---|---|---|---|
| 11 | 11A | TCCTCCAGCTACTCCCTCTGA | 11B | CAGAGGGTCAGAGGCAAGAGT |
| 12 | 12A | CCTGGAAATGATCTCCTGACCT | 12B | ACCATGCATCAGGCAGGTGGT |
| 13 | 13A | CCTTGCCCCAAGACCCTTTGA | 13B | CTTATACACAACCTCCCGTGTG |
| 14 | 14A | CCAAGCCAAAGCTGGGAGAGA | 14B | GGGCTGGGATAGGGCAGTGT |
| 15 | 15A | GGGGAGTGACTGGGCACTGA | 15B | ATGAGGACCTGGGGGGTATCA |
| 16 | 16A | CCATCCTCCTGCCCTTGGCA | 16B | GCCAGGGAAAGGTCTCTGCC |
| 17 | 17A | GGAGAACCCTGCTTACCCCTC | 17B | GAGGATGGTGAAGACGCGACC |
| 18 | 18A | AACCTGGGCTGAGAGTGTGCG | 18B | TCTACCACCCCAGGCTGGGC |
| 19 | 19A | GGGGTGTGATAGGCACTGACC | 19B | CCCTCGCATGCTCCCAGCTC |
| 20 | 20A | CTCTTCTGGCCCCAGGGTCT | 20B | TGCTCCAGGAGGATCCTGAGT |
Sequence comparisons and structural predictions.
All 12 currently known amino acid sequences of human,1,4mouse,2 rat,47 chicken,48,49 and trout50 erythroid band 3 proteins (AE1) and of the related anion exchangers AE2 from human,51,52 mouse,53rat,48 and rabbit54 and AE3 from human,55 mouse,56 and rat48 were retrieved from Genbank and aligned using the program CLUSTAL (PCGene; Intelligenetics, Mountain View, CA). Predictions of the number and position of band 3 transmembrane helices were made using programs RAOARGOS, HELIXMEM, and SOAP (PCGene) based on the reported human AE1 cDNA sequence.1 4 Based on these predictions, sequence comparisons, and available data on band 3 modifications by enzymes and chemicals with known cleavage and binding sites, we created a model of band 3 with 14 transmembrane helices.
Detection of band 3 mRNA alleles in reticulocyte RNA.
Total reticulocyte RNA was isolated by ammonium chloride lysis57 and reverse transcribed using random primers. cDNA was PCR-amplified using primers flanking the mutations, and the PCR product was digested with the appropriate restriction endonuclease. The digested PCR product was visualized by electrophoresis in agarose gels stained by ethidium bromide and the amount of DNA of individual bands was quantitated by densitometric scanning using the Eagle Eye II system (Stratagene, La Jolla, CA) and the ONE-Dscan 1.0 program (Scanalytics, Billerica, MA).
Sulfate fluxes and di-isothiocyano-dihydrostilbene disulphonate (DIDS) titration curves.
Cells were washed three times at 4°C in 140 mmol/L NaCl, 10 mmol/L Na phosphate, pH 7.4 (PBS), and three times in 84 mmol/L trisodium citrate, 1 mmol/L EGTA, pH 6.5, and subjected to assays of unidirectional disodium 35S-sulfate (ICN, Costa Mesa, CA) uptake in the absence or in the presence of increasing concentrations of the anion transport inhibitor DIDS (Molecular Probes, Eugene, OR), as described.10 Each flux study in RBCs carrying the studied antigens was performed in parallel using RBCs from unrelated subjects not carrying the antigens. The dependence of the sulfate influx on the increasing concentrations of DIDS was obtained using the linear least squares fit.
Enzyme treatment of intact RBCs.
One volume of washed RBCs was treated with 4 vol of α-chymotrypsin (5 mg/mL) for 1 hour at 37°C. Enzyme-treated cells were washed three times with PBS. Both treated and untreated antigen-positive RBCs were tested in parallel with the appropriate antisera.
Quantitation of erythrocyte membrane proteins.
Freshly drawn blood anticoagulated in acid citrate/dextrose was shipped on ice to Boston. Within 48 hours of phlebotomy, erythrocyte ghosts were prepared using the method of Dodge et al,58 with minor modifications described in Jarolim et al.8 Erythrocyte membrane proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by densitometry as described,9 and the relative amounts of RBC membrane proteins were expressed as ratios of integrated densitometric peaks of individuals’ proteins to the peak of band 3. The biochemical findings were correlated with the clinical data and the results were statistically evaluated.
RESULTS
SSCP are detected in three exons.
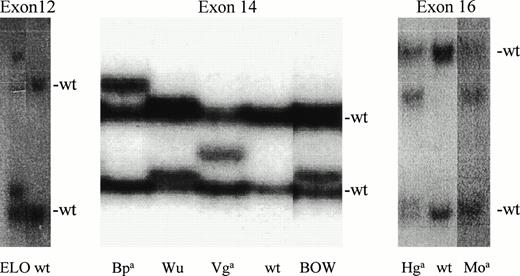
We detected SSCP polymorphisms in exon 12 in the carrier of the ELO antigen; in exon 14 for Vga, BOW, Wu, and Bpa; and in exon 16 for Hga and Moa(Fig 1).
Detection of seven SSCPs. SSCP screening detected polymorphisms in exon 12 in genomic DNA from the ELO heterozygote, four polymorphisms in exon 14, and two polymorphisms in exon 16. Two normal bands (n) visible in wild-type (wt) homozygotes correspond to complementary strands of PCR-amplified DNA. Heterozygosity is reflected by the presence of an additional 1 to 2 bands.
Detection of seven SSCPs. SSCP screening detected polymorphisms in exon 12 in genomic DNA from the ELO heterozygote, four polymorphisms in exon 14, and two polymorphisms in exon 16. Two normal bands (n) visible in wild-type (wt) homozygotes correspond to complementary strands of PCR-amplified DNA. Heterozygosity is reflected by the presence of an additional 1 to 2 bands.
Sequence analysis of genomic DNA shows seven amino acid substitutions.
We PCR-amplified and directly sequenced exons containing the SSCPs. The detected mutations are summarized in Table2. We have verified the presence of these mutations in additional unrelated carriers of these seven blood group antigens with the exception of Vga, for which only related individuals were available. All seven mutations either create or abrogate restriction sites (Table 2). In six cases, we used PCR amplification followed by restriction digestion with an appropriate restriction endonuclease to confirm the presence of the mutation in additional carriers of the blood group antigen. Because endonuclease Tth 2 is not commercially available, we directly sequenced genomic DNA from the additional Bp(a+) subject.
Summary of Detected Band 3 Mutations
| Blood Group . | No. of Subjects . | Codon . | Triplet . | Amino Acid . | Band 3 Loop . | Conserved . | Restriction Site Abrogated . | Restriction Site Created . |
|---|---|---|---|---|---|---|---|---|
| ELO | 2 | 432 | CGG → TGG | Arg → Trp | 1 | 4/5, 4/12 | Msp I, Nci I | BstNI |
| Vga | 2* | 555 | TAC → CAC | Tyr → His | 3 | N/A, N/A† | Dra III | |
| BOW | 2 | 561 | CCC → TCC | Pro → Ser | 3 | 3/5, 10/12 | Ban I | BstEII |
| Wu | 6 | 565 | GGC → GCC | Gly → Ala | 3 | 3/5, N/A† | Apa I | |
| Bpa | 2 | 569 | AAC → AAA | Asn → Lys | 3 | 5/5, 12/12 | Tth 2‡ | |
| Hga | 4 | 656 | CGT → TGT | Arg → Cys | 4 | 1/5, 1/12 | Cac8I | |
| Moa | 2 | 656 | CGT → CAT | Arg → His | 4 | 3/5, 3/12 | Bsm I |
| Blood Group . | No. of Subjects . | Codon . | Triplet . | Amino Acid . | Band 3 Loop . | Conserved . | Restriction Site Abrogated . | Restriction Site Created . |
|---|---|---|---|---|---|---|---|---|
| ELO | 2 | 432 | CGG → TGG | Arg → Trp | 1 | 4/5, 4/12 | Msp I, Nci I | BstNI |
| Vga | 2* | 555 | TAC → CAC | Tyr → His | 3 | N/A, N/A† | Dra III | |
| BOW | 2 | 561 | CCC → TCC | Pro → Ser | 3 | 3/5, 10/12 | Ban I | BstEII |
| Wu | 6 | 565 | GGC → GCC | Gly → Ala | 3 | 3/5, N/A† | Apa I | |
| Bpa | 2 | 569 | AAC → AAA | Asn → Lys | 3 | 5/5, 12/12 | Tth 2‡ | |
| Hga | 4 | 656 | CGT → TGT | Arg → Cys | 4 | 1/5, 1/12 | Cac8I | |
| Moa | 2 | 656 | CGT → CAT | Arg → His | 4 | 3/5, 3/12 | Bsm I |
Both subjects from the same family.
Because of deletions and insertion in band 3 homologues from evolutionarily older species, the degree of conservation cannot be established in this region of putative third ectoplasmic loop.
Restriction endonuclease Tth 2 is not commercially available.
Position of the mutated amino acids in band 3.
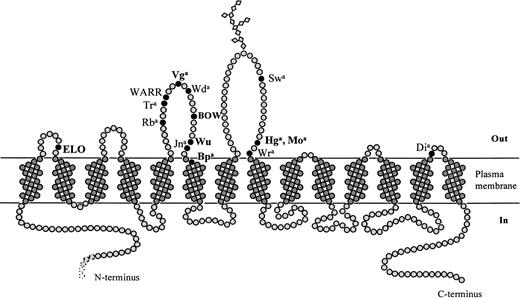
Figure 2 schematically depicts the membrane domain of band 3. The seven newly characterized blood group antigens are shown in bold. Previously characterized blood group antigens residing on band 3 are also shown. According to this scheme, six of seven mutated amino acids are located in the putative first, third, and fourth ectoplasmic loops of band 3, whereas asparagine 569, which is mutated to lysine in Bp(a+) subjects, is located at the external end of the sixth transmembrane segment. Proline 561, mutated to serine in the BOW-positive subject, is also relatively highly conserved; however, the degree of evolutionary conservation in the region of the third external loop shown in Table 2 changes to some extent with variations in the alignment parameters.
Antigens of the Diego blood group system. Schematic representation of the membrane domain of band 3 based on the structural predictions described in the Materials and Methods and Results. Positions of all antigens of the Diego blood group system are indicated; the seven antigens described in this report are shown in bold.
Antigens of the Diego blood group system. Schematic representation of the membrane domain of band 3 based on the structural predictions described in the Materials and Methods and Results. Positions of all antigens of the Diego blood group system are indicated; the seven antigens described in this report are shown in bold.
Evolutionary conservation of mutated amino acids.
We aligned the five known AE1 amino acid sequences of the erythroid band 3 homologues as well as all 12 available sequences from the AE gene family and evaluated the conservation of individual amino acids. The results show (Table 2) that most of the mutated amino acids are not conserved in evolution. The only exception again appears to be the Bpa antigen (569 Asn→Lys) that is conserved in all 12 aligned amino acid sequences. Consequently, mutation of Asn 569 would be most likely to affect band 3 structure and anion exchange function.
Effects of enzyme treatment on RBC agglutinability.
We have digested intact RBCs from the carriers of all seven studied blood group antigens by trypsin, α-chymotrypsin, pronase, and ficin. The results of testing untreated and enzyme-treated antigen-positive RBCs are shown in Table 3. At least two sera containing antibodies to the seven low incidence antigens were tested on two independent experiments. Whereas the results for Vga, BOW, Wu, Hga, and Moa are expected, the results with ELO and Bpa were not and will be discussed later.
Agglutination of Normal and Enzyme-Treated Antigen-Positive Cells
| . | Untreated Cells . | Treated Cells . | |||
|---|---|---|---|---|---|
| Trypsin . | α-Chymotrypsin . | Pronase . | Ficin . | ||
| ELO | 2+ | 2+ | 2+/0 | 2+/0 | 2+ |
| Vga | 3+ | 3+ | 0 | 0 | 3+ |
| BOW | 2+ | 2+ | 0 | 0 | 2+ |
| Wu | 1+ | 1+ | 0 | 0 | 1+ |
| Bpa | 3+ | 0 | 0 | 0 | 0 |
| Hga | 3+ | 3+ | 3+ | 3+ | 3+ |
| Moa | 1+ | 1+ | 1+ | 1+ | 1+ |
| . | Untreated Cells . | Treated Cells . | |||
|---|---|---|---|---|---|
| Trypsin . | α-Chymotrypsin . | Pronase . | Ficin . | ||
| ELO | 2+ | 2+ | 2+/0 | 2+/0 | 2+ |
| Vga | 3+ | 3+ | 0 | 0 | 3+ |
| BOW | 2+ | 2+ | 0 | 0 | 2+ |
| Wu | 1+ | 1+ | 0 | 0 | 1+ |
| Bpa | 3+ | 0 | 0 | 0 | 0 |
| Hga | 3+ | 3+ | 3+ | 3+ | 3+ |
| Moa | 1+ | 1+ | 1+ | 1+ | 1+ |
Similar quantities of normal and mutant mRNA alleles are detected in reticulocyte RNA.
We isolated and reverse-transcribed total reticulocyte RNA and amplified the cDNA as described. The PCR products were digested with the appropriate restriction endonuclease and electrophoresed in an ethidium bromide-stained gel. Densitometric scanning of the gel allowed us to estimate the relative content of mRNA corresponding to the two band 3 alleles of the heterozygous subjects. Using this semiquantitative technique, we have not detected significant differences between the content of the two band 3 cDNA alleles, suggesting that the mutations affect neither the transcription of the band 3 gene alleles nor the subsequent RNA processing (data not shown).
The mutations do not affect band 3 expression and function.
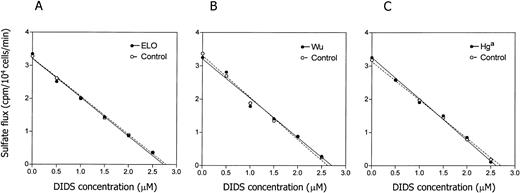
SDS-PAGE electrophoresis showed a normal electrophoretic pattern of band 3 protein in the carriers of the blood group antigens, including similar profiles of band 3 glycosylation (not shown). Subsequent densitometric scanning detected normal content of band 3 and other RBC membrane proteins. Because band 3 is the principal anion exchange protein of the RBC membrane, we compared anion fluxes in erythrocytes from carriers of the studied blood group antigens with those in normal RBCs. We did not detect significant differences between heterozygotes for the seven studied antigens and control subjects. Results of the DIDS titration of sulfate influx in the cells expressing the ELO, Wu, and Hga antigens are shown in Fig 3.
Band 3-mediated influx of radiolabeled sulfate. Influx of radiolabeled sulfate into control cells and cells from carriers of the seven low incidence blood group antigens was measured as described. No differences between the control wild-type cells and cells expressing the blood group polymorphisms were detected. Results are shown for the ELO, Wu, and Hga antigens.
Band 3-mediated influx of radiolabeled sulfate. Influx of radiolabeled sulfate into control cells and cells from carriers of the seven low incidence blood group antigens was measured as described. No differences between the control wild-type cells and cells expressing the blood group polymorphisms were detected. Results are shown for the ELO, Wu, and Hga antigens.
DISCUSSION
As of 1995, 37 low incidence antigens were recognized as being discrete genetic characteristics unassigned to a particular blood group system.59 Many of the antibodies to these low incidence antigens occur together in multispecific sera.60 Often these same sera contain antibodies to glycophorin A determinants or to Wra, whose antithetical antigen Wrb has been shown to result from the interaction between glycophorin A and band 3.23,24 36 Thus, we undertook a concerted effort to identify band 3 polymorphisms recognized by these multispecific sera.
This work has led to the identification of seven new band 3 mutations. All substitutions were studied in more than one subject and the molecular basis of some of them was evaluated by enzyme treatment of intact, antigen-carrying erythrocytes. Based on these results, the International Society for Blood Transfusion (ISBT)/Working Party on Blood Group Terminology assigned the antigens to the Diego blood group as 010008 (ELO), 010009 (Wu), 010010 (Bpa), 010011 (Moa), 010012 (Hga), 010013 (Vga), and 010015 (BOW).
The clinical significance of the seven characterized antigens and their corresponding antibodies is unclear. The antigens do not represent a major problem in blood transfusion, because the majority of donors will lack the antigen. With the exception of ELO,61 62 they have not been reported in association with a hemolytic disease of the newborn.
Because it is not clear whether amino acids in the immediate vicinity of the mutated amino acid residues are sufficient to form the epitope of the blood group antigen or whether the epitope depends on the conformation of other portions of band 3, we treated RBCs with enzymes known to modify external loops of band 3. α-Chymotrypsin cleaves band 3 at tyrosines 553 and 555 of the third ectoplasmic loop and, quite predictably, α-chymotrypsin treatment abrogated agglutination of the Vga-, Wu-, and BOW-positive cells with the corresponding antibodies. The agglutination of Hga- and Moa-positive cells was not affected, because their epitopes are not located in the third loop. The reactivity of both examples of anti-ELO was unaffected by trypsin or ficin treatment of antigen-positive RBCs. However, whereas one anti-ELO was unaffected by α-chymotrypsin or pronase, the other was nonreactive with RBCs treated with either of these enzymes. Although this has been observed previously,63 the reason has not been determined. Perhaps some anti-ELO antibodies recognize an epitope formed by the interaction of loop 1 of band 3 with another membrane component that is α-chymotrypsin or pronase sensitive (such as the third loop of band 3). Unexpectedly, neither example of anti-Bpa agglutinated antigen-positive RBCs that had been treated with any of the enzymes. Again, it is possible that the epitope recognized by anti-Bpa requires an interaction of the third loop of band 3 with another, enzyme-sensitive component.
Comparison of the 12 known AE1, AE2, and AE3 sequences predicts a relatively low degree of conservation for the ectoplasmic loops compared with the transmembrane segments. Accordingly, five mutations occur in poorly conserved amino acids, whereas one, asparagine 569, that is mutated to Lys in the Bp(a+) subjects is conserved in all 12 AE homologues. The high degree of evolutionary conservation of asparagine 569 and its position at the beginning of the sixth transmembrane segment in the band 3 model (Fig 2) suggested that its substitution by positively charged lysine may have major structural and functional consequences. However, we have observed neither morphological abnormalities of the Bp(a+) RBCs nor differences between DIDS titration of sulfate influx in the Bp(a+) and control RBCs. Proline 561, mutated in BOW, is conserved in AE1 and AE2; however, only 3 of 5 AE1 sequences contain proline in the corresponding position. With the exception of BOW, we have studied DIDS-inhibitable sulfate influx in fresh erythrocytes from carriers of the other band 3 mutations and found no effect on sulfate influx. Based on these results, we propose that the first, third, and fourth ectoplasmic loops are not involved in the regulation of band 3-mediated anion exchange.
The main contributions of this work, besides clarifying the molecular basis of seven blood group antigens, are twofold. First, the results strongly support extracellular localization of the mutated amino acids. Because available serological data suggest that additional low incidence blood group antigens may be carried by band 3, ongoing characterization of such antigens may further improve the structural model of band 3.
Second, some of the antigens are located in the regions that have been implicated in the adhesion of modified RBCs to vascular endothelium.64-71 Band 3 was hypothesized to function as a receptor during invasion of human erythrocytes by Plasmodium falciparum.64 Naturally occurring anti-band 3 autoantibodies were found to recognize modified band 3 protein on the surface of Plasmodium falciparum-infected erythrocytes.65 Cytoadherence-related neoantigens onPlasmodium falciparum-infected human erythrocytes, resulting from the exposure of normally cryptic regions of the band 3 protein,66 were placed into the third ectoplasmic loop of band 3.67,68 The antibodies in sera of individuals living in a malaria-endemic region recognize peptide motifs from the extracellular loops of band 3,69 and the immune response to these band 3 neoantigens is associated with low parasite density.70 Monoclonal antibodies that react with human band 3 residues 542-555 from the third external loop recognize different band 3 conformations in uninfected and Plasmodium falciparum-infected erythrocytes.71 The cytoadherence of Plasmodium falciparum could be blocked both with synthetic peptides corresponding to motifs from the third ectoplasmic loop of band 3.67 Erythrocytes from carriers of low incidence blood group antigens in ectoplasmic loops of band 3 may serve as a model for evaluation of the sequence requirements for adhesion of Plasmodium falciparum-infected erythrocytes to the vascular endothelium.
Kay72 assigned the senescent RBC antigen to the third ectoplasmic loop, specifically amino acids 538-554. Erythrocytes carrying polymorphisms in the external loops of band 3 will again be useful for evaluation of the role of band 3 in erythrocyte cell aging. It is interesting to note that multiple antibodies to these low prevalence antigens are often found concomitantly in sera that contain autoreactive erythrocyte antibodies as well as in sera from individuals who have not received any prior RBC stimulus.
Finally, in a recent communication, Thevenin et al73reported that synthetic peptides derived from the second and third ectoplasmic regions of band 3 completely inhibited adherence of sickle cells to an endothelial monolayer in a static assay. These results again suggested that the third external loop of band 3 plays a role in the sequence-specific adhesion of modified RBCs to the vascular endothelium.
The majority of the data on the adhesion of parasitized and sickled erythrocytes have been obtained from in vitro studies using antibodies and synthetic peptides. Both these experimental approaches are prone to artifacts. Large antibody molecules may interfere with numerous interactions upon binding to the RBC surface. Inhibition of interactions by synthetic peptides is also frequently nonspecific, and the peptides may mimic other yet unknown antigens with similar or identical amino acid sequences. Erythrocytes from donors with substitutions in the ectoplasmic loops of band 3 represent therefore a valuable system for testing of the role of this portion of band 3 in erythrocyte aging, in cytoadherence of malarial parasites, or in adhesion of parasitized and sickled RBCs to the endothelium.
ACKNOWLEDGMENT
The authors thank the many colleagues who, over many years, sent us samples of RBCs expressing low incidence antigens. We also thank the following people who recently responded to requests for blood samples from selected donors: Ranjan Malde from the National Blood Service (London and the South East), London, UK; Graham Rowe from the National Blood Service (Wales), Cardiff, Wales; Judy Martin from the American Red Cross (Badger Region), Madison, WI; and Marilyn Moulds from Gamma Biologicals, Inc (Houston, TX). We thank Dr Jiri Zavadil. Milena Pohlova from the Institute of Hematology and Blood Transfusion (Prague, Czech Republic) for help with DNA sequencing and for preparation of the figures.
Supported in part by a grant from the National Blood Foundation (P.J.), by Grant No. 4118-3 from the Grant Agency of the Ministry of Health, Czech Republic (P.J.), and by a National Institutes of Health Specialized Center of Research (SCOR) grant in Transfusion and Medicine (HL 54459; M.E.R.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to P. Jarolim, MD, PhD, Department of Pathology, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis St, Boston, MA 02111; e-mail: pjarolim@rics.bwh.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal