Abstract
In recent studies, the sequence of Kaposi's sarcoma-associated herpes virus (KSHV) or human herpes virus-8 (HHV-8) was detected in dendritic cells (DC) of patients with multiple myeloma (MM). A concern was raised whether there is an causal association between the viral infection and development of these tumors. In the present study, we have examined DC generated from blood adherent cells from 8 Swedish MM patients at different clinical stages and 2 patients with monoclonal gammopathy of undetermined significance. In addition, 6 myeloma cell lines and bone marrow cells from 2 MM patients were also studied. By polymerase chain reaction (PCR), including nested PCR, no virus DNA was demonstrable in the patients' DC or in myeloma cell lines or fresh bone marrow cells. Moreover, no antibody against KSHV was found in the serum of these 10 patients. Thus, our results indicate that blood-derived DC of MM patients in Sweden usually are not infected with KSHV/HHV-8. This study also suggests that KSHV/HHV-8 is not regularly associated with MM and consequently does not play a primary role in the pathogenesis of these tumors.
THE NEWLY DISCUSSED herpes virus, Kaposi's sarcoma-associated herpes virus (KSHV) or human herpes virus-8 (HHV-8), has been shown to be associated with all forms of Kaposi's sarcoma (KS),1,2 with primary effusion lymphoma (PEL) or body-cavity-based-lymphoma (BCBL) as well as with some cases of Castleman's disease.3 Recently, it was claimed that KSHV/HHV-8 was also associated with multiple myeloma (MM), particularly the stromal dendritic cells (DC) of the bone marrow (BM)4,5and circulating DC or monocytes.6
A concern was raised whether the virus is regularly associated with MM and of pathogenic importance in the development of these tumors, or whether it was just a reflection of the epidemic or endemic prevalence of the virus in the local populations. In view of ongoing clinical trials of immunotherapy in MM patients with peripheral blood-derived DC, it is of crucial importance to examine whether these cells are infected with KSHV/HHV-8. In this study, we have examined DC generated from peripheral blood adherent cells of 8 patients with MM (2 in stage I and 6 in stage II-III) and 2 patients with monoclonal gammopathy of undetermined significance (MGUS). The polymerase chain reaction (PCR), including nested PCR, and serological studies for the presence of virus and/or antibodies, respectively, were used. In addition, 6 commonly used myeloma cell lines derived from Swedish patients and samples of bone marrow cells from 2 MM patients were also tested.
MATERIALS AND METHODS
Samples.
Blood samples from 8 patients with MM and 2 patients with MGUS were obtained. The main characteristics of the patients are shown in Table 1. In addition, 6 commonly used myeloma cell lines derived from Swedish patients, eg, U-266, U-266-1970, U-266-1984, U-1996, and U-1958,7 8 and samples of bone marrow cells from 2 MM patients were also included.
Characteristics of the Patients, Serum Monoclonal Ig, and Detection of Virus (KSHV/HHV-8) DNA and Antibodies
| Patient No. . | Age (yr)/ Sex . | Diagnosis and Clinical Stage . | Monoclonal Ig . | Monoclonal Ig Concentration (g/L) . | PCR* (KSHV/ HHV8) . | Serological Study-151 . |
|---|---|---|---|---|---|---|
| 1 | 66/M | MGUS | IgG1κ | 20 | — | — |
| 2 | 50/F | MGUS | IgG4λ | 19 | — | — |
| 3 | 68/F | MM, IA | IgG1κ | 30 | — | — |
| 4 | 54/M | MM, IA | IgG1κ | 25 | — | — |
| 5 | 36/M | MM, IIA | IgG1λ | 41 | — | — |
| 6 | 76/M | MM, IIA | IgAκ | 26 | — | — |
| 7 | 68/M | MM IIA | IgAκ | 38 | — | — |
| 8 | 51/F | MM, IIIA | IgG | 58 | — | — |
| 9 | 54/M | MM, IIIA | Nonsecretory | — | — | — |
| 10 | 62/F | MM, IIIA | IgAκ | 25 | — | — |
| Patient No. . | Age (yr)/ Sex . | Diagnosis and Clinical Stage . | Monoclonal Ig . | Monoclonal Ig Concentration (g/L) . | PCR* (KSHV/ HHV8) . | Serological Study-151 . |
|---|---|---|---|---|---|---|
| 1 | 66/M | MGUS | IgG1κ | 20 | — | — |
| 2 | 50/F | MGUS | IgG4λ | 19 | — | — |
| 3 | 68/F | MM, IA | IgG1κ | 30 | — | — |
| 4 | 54/M | MM, IA | IgG1κ | 25 | — | — |
| 5 | 36/M | MM, IIA | IgG1λ | 41 | — | — |
| 6 | 76/M | MM, IIA | IgAκ | 26 | — | — |
| 7 | 68/M | MM IIA | IgAκ | 38 | — | — |
| 8 | 51/F | MM, IIIA | IgG | 58 | — | — |
| 9 | 54/M | MM, IIIA | Nonsecretory | — | — | — |
| 10 | 62/F | MM, IIIA | IgAκ | 25 | — | — |
Culture of DC from peripheral blood.
Immature DC were generated from the adherent cells of peripheral blood mononuclear cells (PBMC)9 in medium supplemented with fetal calf serum. Briefly, PBMC were plated in 24-well tissue culture plates (Nunc, Nunclon, Roskilde, Denmark) at a density of 7.5 × 106 cells/well and allowed to adhere. After 2 hours at 37°C, the nonadherent cells were removed and the plates were washed twice with phosphate-buffered saline (PBS). The complete cell culture medium supplemented with 20 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Leucomax; Sandoz, Basel, Switzerland) and 10 ng/mL interleukin-4 (IL-4; Genzyme Corp, Cambridge, MA) was added and the adherent cells were cultured for 7 days without change of the medium. After 7 days of culture, 40% to 70% of the cells appeared as loosely adherent clusters or isolated, floating cells with typical dendritic morphology (data not shown). Cells were then collected from the plates and washed free of cytokines. The percentage of DC (large cells) and the expression of their surface markers were analyzed by flow cytometry (fluorescence-activated cell sorter [FACS] analysis). The capacity of the cultured cells to present antigens and to induce T-cell stimulation was also evaluated.
PCR and nested PCR.
PCR was performed with 100 ng DNA for each specimen. DNA from BCBL cells and PBMC from healthy individuals served as positive and negative controls. A total of 30 cycles of PCR amplification was performed on all samples with primers KS-1,21 at 55°C. For the nested PCR, 30 cycles of a single-round PCR amplification were performed with primer pairs KS-4,5 at 60°C, followed by 25 cycles of amplification with primers KS-1,2 at 55°C, as previously described.1,2 All DNA were checked for performance by β-globin PCR as described.10
Detection of serum antibodies against KSHV/HHV-8.
Cytospins of BCBL cells stimulated for virus replication with tetradecanoylphorbol acetate (TPA; Sigma, St Louis, MO) for 3 days11 were incubated with patients' serum diluted 1/10 to 1/100 and bound antibodies were assayed with peroxidase-labbelled antihuman Ig (DAKO A/S, Glostrup, Denmark). Serum from acquired immunodeficiency syndrome (AIDS)-KS patients was used as the positive controls.
RESULTS
DC generated from adherent cells of PBMC.
Based on light scatter properties, 2 cell populations appeared: a large-cell population (40% to 70%) and a lymphocyte population (10% to 20%) containing mostly CD3+ cells.12 Most of the large cells expressed high levels of CD4, CD13, CD33, CD40, CD86, and HLA-DR, and moderate levels of CD1A and CD80. CD14 and CD83 were low or negative (Table2). These large cells fulfill thus the phenotypic characteristics of immature DC.9
Phenotypic Properties of the Large-Cell Population Obtained in Culture With GM-CSF and IL-4 for 7 Days
| Surface Markers . | Cultured Cells . |
|---|---|
| CD1A | 38.2 ± 13.2* |
| CD3 | 0.6 ± 0.5 |
| CD4 | 81.0 ± 12.1 |
| CD13 | 97.4 ± 2.4 |
| CD14 | 14.0 ± 9.7 |
| CD33 | 97.1 ± 0.8 |
| CD40 | 85.4 ± 5.6 |
| CD80 | 42.6 ± 14.8 |
| CD83 | 8.6 ± 6.9 |
| CD86 | 79.4 ± 11.9 |
| HLA-DR | 95.2 ± 1.8 |
| Surface Markers . | Cultured Cells . |
|---|---|
| CD1A | 38.2 ± 13.2* |
| CD3 | 0.6 ± 0.5 |
| CD4 | 81.0 ± 12.1 |
| CD13 | 97.4 ± 2.4 |
| CD14 | 14.0 ± 9.7 |
| CD33 | 97.1 ± 0.8 |
| CD40 | 85.4 ± 5.6 |
| CD80 | 42.6 ± 14.8 |
| CD83 | 8.6 ± 6.9 |
| CD86 | 79.4 ± 11.9 |
| HLA-DR | 95.2 ± 1.8 |
*Mean ± SD of 4 experiments.
The functional properties of cultured cells were evaluated by allogeneic MLR and by proliferation assay for the presentation of recall antigens purified protein derivative (PPD) and tetanus toxoid (TT). Allogeneic or autologous T cells (1 × 105cells) were cocultured with 1 × 104 cultured cells or autologous monocytes for 6 days (MLR) or 3 days in the presence of the antigens. The culture cells, as compared with monocytes, were much more efficient in inducing alloreactive T-cell activation (cpm × 10−3: 44.5 ± 18.9 v 9.8 ± 8.3; mean ± SD of 4 experiments). Antigen-specific T-cell stimulation induced by cultured cells was fourfold higher than that induced by monocytes (PPD-induced: 14.8 ± 5.2 v 3.6 ± 1.6; TT-induced: 12.2 ± 4.5 v 3.6 ± 1.8), indicating that functional DC were generated.
PCR for KSHV/HHV-8.
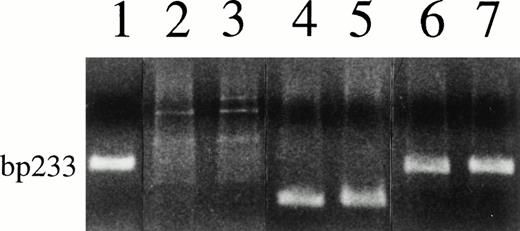
The results are summarized in Table 1. No virus sequence was demonstrable by single or nested PCR in any of the patients' cell preparations. Figure 1 shows the representative negative results on DC derived from 2 MM patients. The 233-bp PCR product was detected in the positive control BCBL cells and also in the patients' samples mixed with BCBL cell DNA, indicating that there was no inhibition of patients' DNA preparation on viral amplification. The presence of functional DNA in the patients' preparations was confirmed by β-globin PCR (Fig 1).
Negative PCR test results for KSHV/HHV-8 on cell DNA from 2 myeloma patients (lanes 2 and 3) and a clear KSHV/HHV-8 band in BCBL cells (lane 1); positive PCR for β-globin of the patients' cell DNA (lanes 4 and 5); and no effects on viral amplification of patients' DNA mixed with BCBL cell DNA (lanes 6 and 7).
Negative PCR test results for KSHV/HHV-8 on cell DNA from 2 myeloma patients (lanes 2 and 3) and a clear KSHV/HHV-8 band in BCBL cells (lane 1); positive PCR for β-globin of the patients' cell DNA (lanes 4 and 5); and no effects on viral amplification of patients' DNA mixed with BCBL cell DNA (lanes 6 and 7).
In none of the myeloma cell lines, the fresh MM biopsy cells or PBMC from healthy individuals was the KSHV sequence found (data not shown).
Serological study.
No antibodies to nuclear or cytoplasmic viral antigens were demonstrated by the immunofluorescence assay (IFA) in any of the patients' sera (Table 1), as compared with a high titer (≥1/200) in the control KS patients' sera (data not shown).
DISCUSSION AND CONCLUSION
The possible association of KSHV and MM is indeed still an open question. Although additional data from the same group5,6and from another group13 seemed to confirm the original findings, preliminary observation from other groups showed no association between KSHV and MM.14-19 However, these negative data were obtained from serological tests14-16,18,19 and PCR amplification of fresh BMMC or PBMC.16,17 Thus, these negative results are considered by Rettig et al20 not necessarily contradictory to their original findings.
Our results indicate that peripheral blood-derived DC of MM patients in Sweden are in general not infected with KSHV/HHV-8 and may thus be safe with regard to risk of transmission in clinical practice. Also, the myeloma cell lines and the Ficoll-isolated cells from the bone marrow of 2 MM patients were negative for KSHV/HHV-8. Although this study can not rule out the possibility that the bone marrow stromal cells might be infected,4 this seems unlikely, because no antibodies against KSHV/HHV-8 could be detected in the serum of the patients. These negative results are supported by recent studies from Europe and United States.14-16,18,19 We therefore suggest that KSHV/HHV-8 is not regularly associated with MM and consequently does not play a primary pathogenic role for the development of these tumors, at least not in Swedish and other reported European patients. The reported association of KSHV/HHV-8 with some myelomas4-6may therefore reflect the epidemic or endemic prevalence of the virus in local populations without being an obligatory pathogenic factor in MM. However, in view of the ongoing discussion,4 13-20donors and patients receiving bone marrow transplants or bone marrow-derived DC preparations should be tested rigorously for KSHV/HHV-8 infection.
ACKNOWLEDGMENT
The technical assistance of Joseph Lawrence and secretarial assistance of A. Popescu-Greaca are acknowledged.
Supported by the Swedish Cancer Society, the Cancer Society in Stockholm, the Swedish Society of Medicine, the Karolinska Institute's Foundation for Research, and the Concerted Action “Pathogenesis in AIDS Kaposi's Sarcoma.”
Address reprint requests to Qing Yi, MD, PhD, Immunological Research Laboratory, CMM, Department of Medicine, Karolinska Hospital, S-171 76 Stockholm, Sweden; e-mail: Qing.Yi@cmm.ki.se.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.
© 1998 by the American Society of Hematology.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal