Abstract
We examined signaling by erythropoietin in highly purified human colony forming unit-erythroid cells, generated in vitro from CD34+ cells. We found that erythropoietin induces tyrosine phosphorylation of Jak2, STAT5A, and STAT5B. Tyrosine phosphorylation of Jak2 reaches a peak around 10 minutes after stimulation and is maximum at 5 U/mL of erythropoietin. Tyrosine phosphorylation of STAT5 is accompanied by the translocation of activated STAT5 to the nucleus as shown by electrophoretic mobility shift assay (EMSA) using 32Pi-labeled STAT5 binding site in the β-casein promoter. Tyrosine phosphorylation STAT1 or STAT3 was not detected in human erythroid precursors after stimulation with erythropoietin. Crkl, an SH2/SH3 adapter protein, becomes coimmunoprecipitated specifically with STAT5 from erythropoietin-stimulated erythroid cells; although it was shown to become associated with c-Cbl in the studies using cell lines. Thus, human erythroid precursors can be expanded in vitro in sufficient numbers and purity to allow its usage in signal transduction studies. This report sets a basis for further studies on signaling in primary cultured human erythroid precursors, which in turn contribute to our better understanding in the differentiation processes of erythrocytes and their precursors.
ERYTHROPOIETIN is a glycoprotein hormone that is essential for normal erythropoiesis.1-4 In vitro, erythropoietin is absolutely required for the survival, proliferation, and differentiation of erythroid precursors, although a recent study showed that interleukin-6 (IL-6) and its soluble form of the receptor may also have a similar effect on the erythroid precursors in the presence of stem cell factor (SCF).5 The effect of erythropoietin is exerted through binding to the specific receptors on the surface of responding cells, leading to dimerization of the receptors and activation of Jak2 tyrosine kinase.6 Whether Jak2 is preassociated with the erythropoietin receptor before the ligand binding or not is a controversial issue.7,8 A recent report suggests that the association is a multistep process, partially regulated through phosphorylation of the receptor on tyrosine and serine residues.9 It was reported by many that activation of Jak2 is accompanied by tyrosine phosphorylation of numerous proteins including Jak2 itself, STAT proteins, Shc, Vav, and the erythropoietin receptor, although tyrosine kinases other than Jak2 may also phosphorylate these proteins.4,6-16 Colony-forming unit-erythroid (CFU-E) comprises only 0.5% or less of all cells in normal bone marrow.1 2 Accordingly, most of the studies cited above have used murine factor–dependent cell lines genetically engineered to express erythropoietin receptors or murine and human leukemic cell lines responding to erythropoietin. Although such cells would undergo differentiation in response to erythropoietin, the signaling processes in them could be affected by cellular transformation.
MATERIALS AND METHODS
Reagents.
HEPES, sodium dodecyl sulfate (SDS), 2-mercaptoethanol (2-ME), sodium orthovanadate, bovine serum albumin (BSA), DNase, chicken egg albumin, Iscove's Modified Dulbecco's Medium (IMDM), propidium iodide (PI), protein A-Sepharose, protein G-Sephalose, isopropyl β-D-thiogalactopyranoside (IPTG), Triton X-100, and Tris (hydroxymethyl) aminomethane (Tris) were purchased from Sigma (St Louis, MO). Polyvinylidene difluoride (PVDF) membranes (pore size, 0.45 μm) were from Millipore Corporation (Bedford, MA). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) molecular standards and 32Pi were from Amersham (Arlington Heights, IL) or BioRad (Richmond, CA). Double stranded Poly (dI-dC) was from Pharmacia Biotech (Milwaukee, WI). Enhanced chemiluminescence (ECL) reagents including secondary antibodies were purchased from Amersham. Insulin (porcine sodium, activity United States Pharmacopoeia [USP] U/mg) was purchased from Calbiochem and Behring Diagnostics, La Jolla, CA). Antiphosphotyrosine murine monoclonal antibody (4G10) was used as described.17-19 STAT3, Crkl and Jak2 antisera, and anti-STAT1 and glutathione S-transferase (GST) monoclonal antibodies were from Santa Cruz (Santa Cruz, CA). An anti-STAT5 monoclonal antibody was from Transduction Laboratories (Lexington, KY). Nitroblue tetrazolium chloride (NBT) and 5-bromo-4 chloro-3-indolyl phosphate p-toluidine salt (BCIP) were from GIBCO BRL (Gaithersburg, MD). For EMSA, an anti-STAT5 antiserum (a kind gift from Dr Hiroshi Wakao, University of Tokyo, Tokyo, Japan), described previously, was used.20 STAT5A and STAT5B antisera were from R&D systems (Minneapolis, MN). Recombinant erythropoietin, IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), and SCF were kindly donated from Kirin Brewery Co Ltd (Tokyo, Japan). Erythropoietin was also kindly donated from Chugai Pharmaceutical Co Ltd (Tokyo, Japan). Vitamin B 12 and folic acid were from Sankyo Pharmaceutical Co (Tokyo, Japan) and Takeda Pharmaceutical Co (Osaka, Japan), respectively. Human IL-3 (108 chronic myelogenous leukemia U/mg) was from Amgen Biologicals (Thousand Oaks, CA). Dynabeads M 450 coated with goat antimouse IgG were from Dynal Inc (Great Neck, NY). Fetal calf serum (FCS), penicillin, and streptomycin were from Flow Laboratories Inc (McLean, VA).
Ex vivo generation of erythroid progenitor cells.
Recombinant human granulocyte colony-stimulating factor (G-CSF; Chugai Pharmaceutical Co and Kyohwa Hakkoh Pharmaceutical Co, Tokyo, Japan) was administered to healthy adult volunteers (who had previously signed consent forms approved by the Hokkaido University School of Medicine and the Hokkaido Red Cross Blood Center Committee for the Protection of Human Subjects), as described elsewhere.21 The mobilized peripheral blood (PB) CD34+ cells were isolated with immunomagnetic beads, as described in detail elsewhere.22,23 The cells were then cryopreserved and stored until use in a tank with liquid nitrogen. The frozen PB CD34+ cells were thawed, suspended in IMDM containing 30% heat inactivated and 100 U/mL DNase, and were centrifuged at 400g for 5 minutes at 4°C. The cells were washed two times with IMDM containing 20% FCS, and resuspended in IMDM containing 0.3% deionized bovine serum albumin (BSA). The cells were then cultured in liquid phase, as described elsewhere with minor modification.24 In brief, the cells ranging from 0.5 × 104 to 2.0 × 104 cells/mL were suspended in a mixture containing 20% FCS, 10% heat-inactivated pooled human AB serum, 1% BSA, 10 μg/mL insulin, vitamin B 12 at 10 μg/mL, and folic acid at 15 μg/mL, with recombinant human IL-3 at 100 U/mL, recombinant human SCF at 100 ng/mL, and erythropoietin (180,000 U/mg) at 4 U/mL, in the presence of 5 × 10−5 mol/mL 2-ME, penicillin at 50 U/mL and streptomycin at 50 U/mL, and IMDM in a 50 mL polystyrene flask (Corning Costar Corp, Cambridge, MA). After incubation for 8 days at 37°C in a 5% CO2/95% atmosphere, the cells were collected, washed twice with IMDM containing 0.3% BSA, and were factor starved in IMDM containing 20% FCS for 12 hours (day-8 cells).25-27
Semisolid culture of progenitors.
Day-8 cells were incubated in triplicate at a concentration of 500 cells/mL in flat-bottomed, 48-well, tissue culture plates (Linbro, Flow Laboratories) with 0.25 mL serum-free fibrin clots, as described with erythropoietin at 2 U/mL with or without SCF at 100 ng/mL and IL-3 at 100 U/mL.25 After 7 days of incubation at 37°C in a 5% CO2/95% atmosphere, the clots were fixed and stained with benzidine-hematoxylin.26 Erythroid colony forming cells (ECFC) were defined as cells that gave rise to colonies of 2 to 49 hemoglobinized cells after 7 days of culture of day-8 cells.27
Flow cytometry.
Phenotyping of the purified cells and day-8 cells was analyzed by fluorescence-activated cell sorting vantage (Becton Dickinson, Mountain View, CA) run by Lysis II, as reported previously.28 In brief, the cells were washed twice with staining medium (SM; phosphate buffered saline [PBS] supplemented with 0.05% NaN3 and 3% FCS). After counting the number of cells, 1 × 106cells were incubated with HPCA-2 (8G12, fluorescein isothiocyanate-conjugated [FITC] or phycoerythrin-conjugated [PE], Becton Dickinson), glycophorin A (Gly A FITC; DAKO Japan Co, Kyoto, Japan), My7 (CD13 PE; Coulter Immunology, Hialeah, FL), and CD117 (c-kit PE; Immunotech, Marseilles, France) for 20 minutes on ice. The cells were washed with SM twice and resuspended in 1 mL of SM containing 5 μg/mL of PI. Cell aggregates and dead cells were excluded using forward and side scatter and PI staining, respectively, and 10,000 events were analyzed.
Immunoprecipitation, gel electrophoresis, and Western blotting.
After starvation, day-8 cells were stimulated with erythropoietin. Cells were lysed by the addition of an equal amount of lysis buffer (15 mmol/L HEPES, 150 mmol/L NaCl, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), 10 mmol/L EGTA, 1 mmol/L sodium orthovanadate, 0.8 μg/mL leupeptin, 2% Triton X-100 (vol/wt), pH 7.4). After 20 minutes on ice, the lysates were centrifuged at 10,000g (at 4°C) for 20 minutes. The supernatant was removed and precleared with preimmune serum and protein A-Sepharose or protein G-Sephalose (40 μL of 50% slurry) for 1 hour. Antisera were then added and incubated for 2 to 3 hours on ice. Protein A-Sepharose (40 μL of 50% slurry) was added and incubated for 1 hour. The immune complexes were washed with 1 mL of cold washing buffer (15 mmol/L HEPES, 150 mmol/L NaCl, 1 mmol/L PMSF, 10 mmol/L EGTA, 1 mmol/L sodium orthovanadate, 0.8 μg/mL leupeptin, 1% Triton X-100 (vol/wt), pH 7.4) three times and then resuspended in Laemmli's sample buffer (10% glycerol, 1% SDS, 5% 2-ME, 50 mmol/L Tris-HCl (pH 6.8),29and 0.002% bromophenol blue),24 with 10 mmol/L EGTA and 1 mmol/L sodium orthovanadate. After boiling at 95°C for 5 minutes, one-dimensional electrophoresis was performed on SDS 10% or 7.5 to 15% polyacrylamide gels as described.30 Separated proteins were electrophoretically transferred from the gel onto PVDF membranes or nitrocellulose in a buffer containing Tris (25 mmol/L), glycine (192 mmol/L) and 20% methanol at 0.2 amps for 12 hours at room temperature. To block residual protein binding sites, membranes were incubated in TBST (Tris-buffered saline [TBS]; 10 mmol/L Tris, 150 mmol/L NaCl, pH 7.6 with 0.1% Tween 20) with 10% chicken egg albumin. The blots were washed with TBST and incubated overnight with primary antibodies at a final concentration of 1.0 μg/mL in TBST. The primary antibody was removed and the blots were washed four times in TBST and incubated with horseradish peroxidase–conjugated or alkaline phosphatase–conjugated second antibodies diluted 1:3000 in TBST. Blots were then washed four times in TBST. Antibody reactions were detected with NBT/BCIP or chemiluminescence according to the manufacturer's instructions.
EMSA.
Nuclear extracts (40 μL, containing 10 to 20 μg protein) were prepared from day-8 cells (2 × 107 cells) by lysis followed by high salt extraction as described previously.20Two microliters of nuclear extracts were mixed with 20 μL of binding buffer [10 mmol/L Tris-HCl, 50 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L 1,4 dithiothreitol, 0.1% NP-40, 5% glycerol, 1 mg/mL bovine serum albumin, and 2 mg/mL poly(dI-dC), pH 7.5] containing 50,000 cpm end-labeled bovine β-casein promoter probe (5′-AGATTCTAGGAATTCAAATC-3′). The mixture was incubated for 30 minutes at room temperature. Complexes were separated on 5% nondenaturing polyacrylamide gels in 0.25 × Tris borate EDTA buffer and detected by autoradiography.
TF-1 cell.
Human IL-3 and GM-CSF–dependent TF-1 cell line was kindly provided by Dr Hisamaru Hirai (University of Tokyo, Tokyo, Japan).30Cells were maintained in IMDM containing 10% fetal calf serum (FCS) and 1 ng/mL human GM-CSF. Before stimulation with GM-CSF or IL-3, cells were incubated in IMDM containing 10% FCS without exogenous IL-3 or GM-CSF for 18 hours. After two washes, they were incubated in PBS (pH 7.4) at 37°C.
GST binding assays.
The GST-SH2 domain of Crkl fusion protein (GST-Crkl-SH2) was generated as previously described.30 31 The GST-fusion construct was transformed into Escherichia coli DH-5α and protein expression was induced by 0.5 mmol/L IPTG to exponentially growing cells. The GST-Crkl-SH2 was isolated from sonicated bacterial lysates using glutathione sepharose beads. A total of 2.5 μg of GST-fusion proteins were incubated with 50 μL of glutathione sepharose beads in bacterial lysis buffer (150 mmol/L NaCl, 16 mmol/L Na2HPO4, 4 mmol/L NaH2PO4, [pH 7.3] containing 10 μg of aprotinin, 1 mmol/L orthovanadate, 1 mmol/L PMSF, and 0.1% β-mercaptoethanol). The beads were washed three times with PBS and were incubated with cell lysates for 3 hours on ice. Proteins were separated by SDS-PAGE and transferred onto PVDF membranes for immunoblot analysis.
RESULTS
The purified fraction from mobilized PB contained 96.7% ± 1.6% (n = 3, mean ± SD) CD34+ cells. Cultivation of PB CD34+ cells for 8 days in serum-containing medium with erythropoietin, IL-3, and SCF resulted in a 120 ± 49-fold expansion of the total cell number (day-8 cells, n = 8) with a viability of 96% ± 3%. A total of 71% ± 13% cells expressed the specific erythroid marker Gly A, whereas CD34 and myelomonocytic marker CD13 were almost absent, as shown in Table 1. In morphologic analysis by May-Grunwald-Giemsa staining, day-8 cells predominantly consisted of immature erythroid cells with a purity of 92% ± 6%,27 whereas most of the remaining cells showed a basophilic/eosinophilic-like feature. The maturation level of erythroid progenitor cells closely associates with colony stimulating factor (CSF) requirements for erythroid development.24 When CSF requirements of day-8 cells were analyzed in serum-free medium, the ECFC required erythropoietin alone but not IL-3 and SCF for erythroid development, which indicates that ECFC in day-8 cells predominantly consists of mature erythroid progenitor cells. The erythroid progenitor cells that gave rise to aggregates with 8 to 49 erythroblasts and that were equivalent to CFU-E, consisted 78.8% ± 2.5%, whereas the rest of ECFC gave rise to aggregates with 2 to 7 erythroblasts.
Characterization of Expanded Cells
| Expansion rate | 119.9-fold ± 48.6-fold |
| Viability | 96.4% ± 2.7% |
| Purity of erythroid cells | 91.4% ± 5.9% |
| Purity of ECFC | |
| CSF combination | |
| EP | 40.5% ± 4.3% |
| EP + IL-3 | 41.1% ± 3.3% |
| EP + IL-3 + SCF | 42.5% ± 4.0% |
| Surface Marker | |
| Gly A | 79.5% ± 6.1% |
| CD34 | 2.6% ± 1.0% |
| CD117 | 33.0% ± 15.6% |
| CD13 | 3.7% ± 1.1% |
| Expansion rate | 119.9-fold ± 48.6-fold |
| Viability | 96.4% ± 2.7% |
| Purity of erythroid cells | 91.4% ± 5.9% |
| Purity of ECFC | |
| CSF combination | |
| EP | 40.5% ± 4.3% |
| EP + IL-3 | 41.1% ± 3.3% |
| EP + IL-3 + SCF | 42.5% ± 4.0% |
| Surface Marker | |
| Gly A | 79.5% ± 6.1% |
| CD34 | 2.6% ± 1.0% |
| CD117 | 33.0% ± 15.6% |
| CD13 | 3.7% ± 1.1% |
Values are mean ± standard deviation of eight independent experiments.
Abbreviations: ECFC, erythroid colony-forming cells; CSF, colony-stimulating factor; EP, erythropoietin; IL-3, interleukin-3; SCF, stem cell factor.
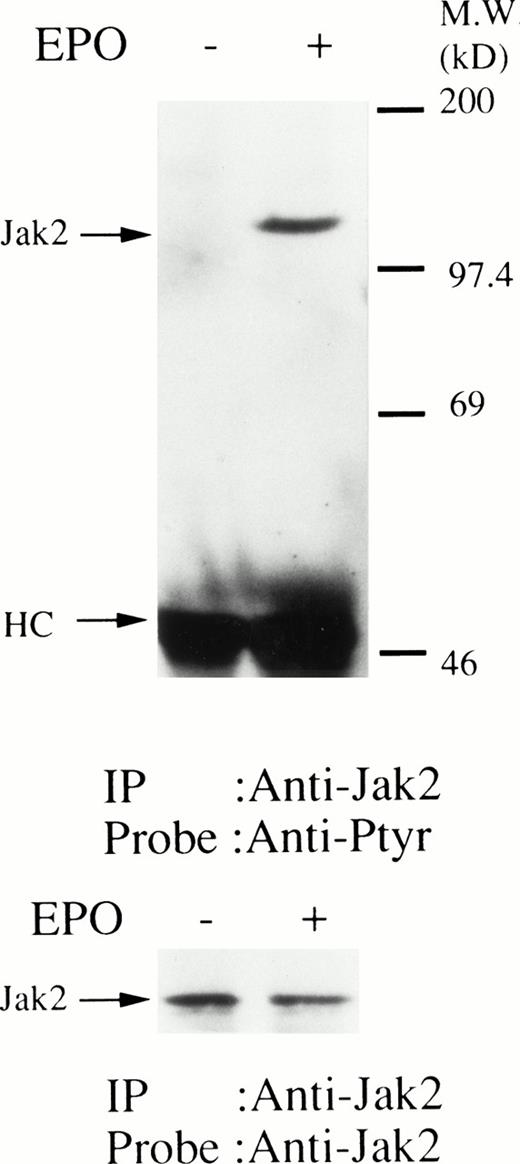
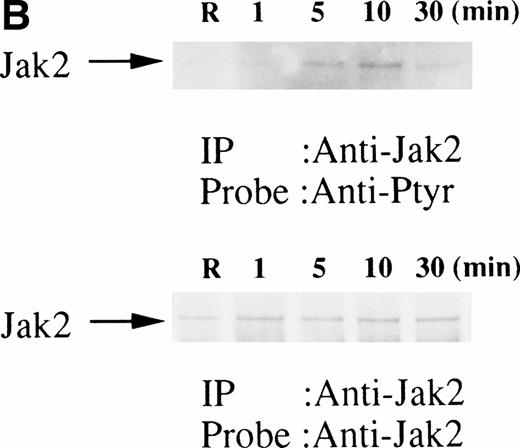
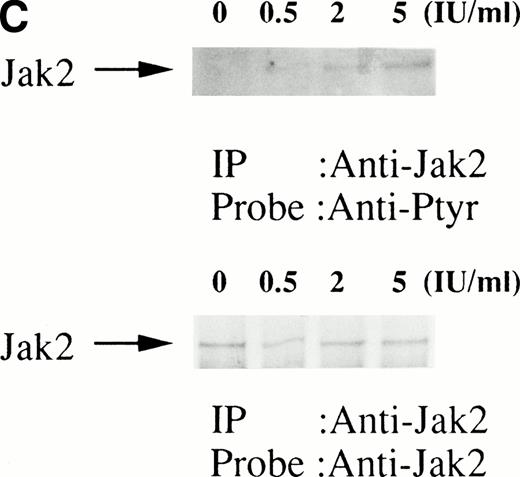
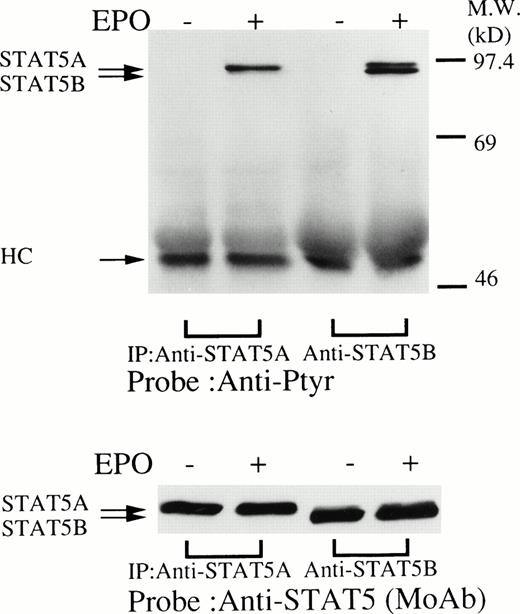
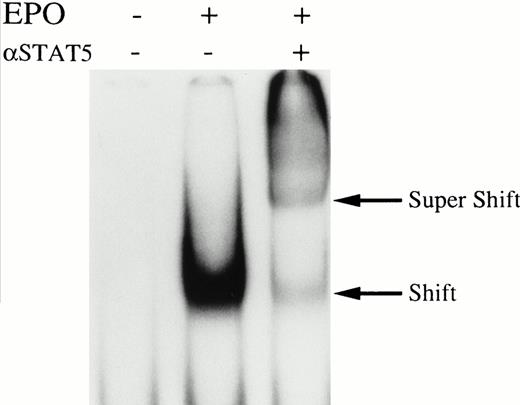
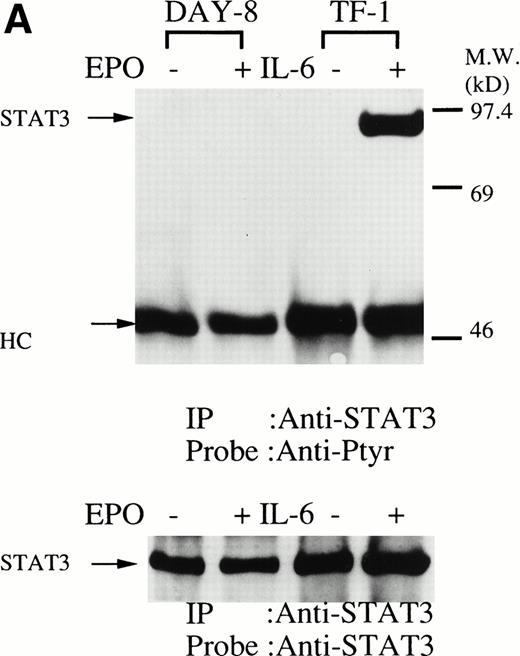
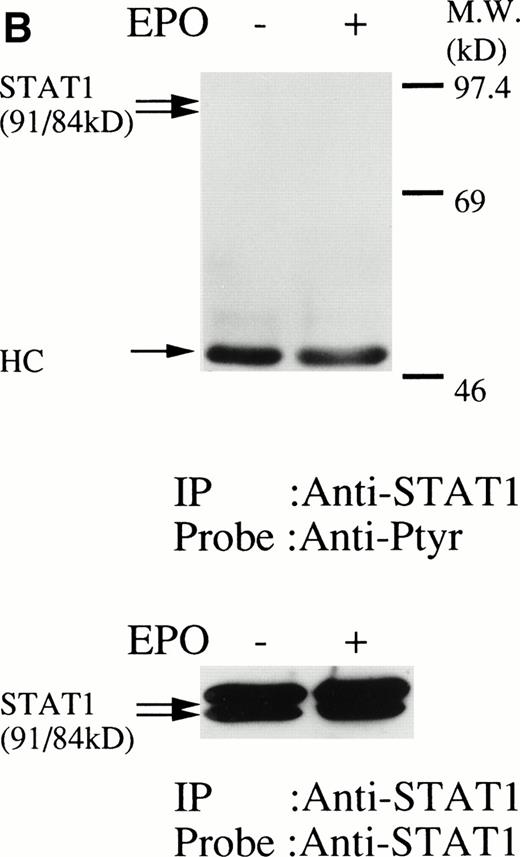
We then set out to examine some early signaling events in these human erythroid precursor cells. When Jak2 was immunoprecipitated from lysates of erythroid cells before and after stimulation with erythropoietin (10 U/mL), we saw the inducible tyrosine phosphorylation of Jak2 (Fig 1A, upper panel). Jak2 was equally immunoprecipitated before and after stimulation (Fig 1A, lower panel). Time course experiments of Jak2 tyrosine phosphorylation showed tyrosine phosphorylation within 1 minute that reached a peak within approximately 10 minutes after addition of erythropoietin and then decreased (10 U/mL; Fig 1B, upper panel). Erythropoietin induced protein tyrosine phosphorylation in erythroid cells in a dose-dependent manner (0.5 to 5 U/mL; Fig 1C, upper panel). Next, we examined whether STAT5A and B become tyrosine phosphorylated in erythroid cells stimulated with erythropoietin (10 U/mL, for 10 minutes). A STAT5 monoclonal antibody recognizes both STAT5A and STAT5B (Fig 2, lower panel). The latter moved faster than STAT5A in the gel. Both STAT5A and STAT5B became tyrosine phosphorylated (Fig 2, upper panel). The thin tyrosine phosphorylated band, which moved slower than STAT5B, may be STAT5A, because STAT5B antisera are weakly cross reactive with STAT5A according to the manufacturers' instructions. After tyrosine phosphorylation, STAT5 dimerizes and translocates to the nucleus where it activates or represses transcription.12,13 We next examined whether treatment of the erythroid cells with erythropoietin (10 U/mL, for 20 minutes) resulted in the binding of STAT5 to a phosphorylated probe from the β-casein promoter by EMSA (Fig3). Nuclear extracts were prepared from the erythroid cells before and after stimulation with erythropoietin (10 U/mL, for 20 minutes). Erythropoietin induced the electrophoretic shift of the end-labeled probe in the erythroid cells (Fig 3, shift). STAT5 antisera supershifted the DNA-protein complex and bound to the labeled probe (Fig 3, super shift), indicating that STAT5 is activated by erythropoietin. In contrast, neither STAT3 nor STAT1 was tyrosine phosphorylated in the erythroid cells stimulated by erythropoietin (Fig 4, upper panels; 10 U/mL, for 10 minutes), whereas IL-6–induced tyrosine phosphorylation of STAT3 in TF-1 was readily detected under the same experimental conditions.11,12 32 Needless to say, we cannot rule out slight erythropoietin-induced tyrosine phosphorylation of STAT1 and STAT3 under the threshold of our detection system.
(A, upper panel) Tyrosine phosphorylation of Jak2 in human erythroid cells stimulated by erythropoietin (10 U/mL). Day-8 cells were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100 before and after exposure to erythropoietin (10 U/mL). Jak2 was immunoprecipitated with specific Jak2 antisera. Immune complexes were resuspended in SDS sample buffer. Tyrosine phosphorylation of Jak2 was detected by 4G10 as described in the Materials and Methods. Bands were visualized by chemiluminescence. (A, lower panel) The same nylon membrane used in A was stripped of the antibody and reprobed for Jak2. Bands were visualized by NBT/BCIP. Lanes are the same as in (A). (B, upper and lower panels) The time course of tyrosine phosphorylation of Jak2. Tyrosine phosphorylation of Jak2 was detected as described in (A). Lane 1, resting erythroid cells. Lanes 2 to 5, 1 minute, 5 minutes, 10 minutes, and 30 minutes after exposure to erythropoietin (10 U/mL). (B, upper and lower panels) The time course of tyrosine phosphorylation of Jak2. Day-8 cells were stimulated with erythropoietin (10 U/mL) for the indicated time. Tyrosine phosphorylation of Jak2 was detected as described in (A). (C, upper and lower panels) The dose response of tyrosine phosphorylation of Jak2. Day-8 cells were stimulated with different concentrations of erythropoietin for 10 minutes. Tyrosine phosphorylation of Jak2 was detected as described in (A). {/ANNT;4224n;;0n;0n}A
(A, upper panel) Tyrosine phosphorylation of Jak2 in human erythroid cells stimulated by erythropoietin (10 U/mL). Day-8 cells were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100 before and after exposure to erythropoietin (10 U/mL). Jak2 was immunoprecipitated with specific Jak2 antisera. Immune complexes were resuspended in SDS sample buffer. Tyrosine phosphorylation of Jak2 was detected by 4G10 as described in the Materials and Methods. Bands were visualized by chemiluminescence. (A, lower panel) The same nylon membrane used in A was stripped of the antibody and reprobed for Jak2. Bands were visualized by NBT/BCIP. Lanes are the same as in (A). (B, upper and lower panels) The time course of tyrosine phosphorylation of Jak2. Tyrosine phosphorylation of Jak2 was detected as described in (A). Lane 1, resting erythroid cells. Lanes 2 to 5, 1 minute, 5 minutes, 10 minutes, and 30 minutes after exposure to erythropoietin (10 U/mL). (B, upper and lower panels) The time course of tyrosine phosphorylation of Jak2. Day-8 cells were stimulated with erythropoietin (10 U/mL) for the indicated time. Tyrosine phosphorylation of Jak2 was detected as described in (A). (C, upper and lower panels) The dose response of tyrosine phosphorylation of Jak2. Day-8 cells were stimulated with different concentrations of erythropoietin for 10 minutes. Tyrosine phosphorylation of Jak2 was detected as described in (A). {/ANNT;4224n;;0n;0n}A
(upper panel) Tyrosine phosphorylation of STAT5 in the erythroid day-8 cells stimulated by erythropoietin (10 U/mL) for 10 minutes. The erythroid cells were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100 before and after exposure to erythropoietin (10 U/mL). STAT5 was immunoprecipitated with STAT5A or STAT5B antisera as indicated. Immune complexes were resuspended in SDS sample buffer. Tyrosine phosphorylation of STAT5 was detected by 4G10 as described in Fig 1A. Bands were visualized by chemiluminescence. (lower panel) The same nylon membrane was stripped of the antibody and reprobed for STAT5 with an anti-STAT5 monoclonal antibody. Bands were visualized by chemiluminescence.
(upper panel) Tyrosine phosphorylation of STAT5 in the erythroid day-8 cells stimulated by erythropoietin (10 U/mL) for 10 minutes. The erythroid cells were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100 before and after exposure to erythropoietin (10 U/mL). STAT5 was immunoprecipitated with STAT5A or STAT5B antisera as indicated. Immune complexes were resuspended in SDS sample buffer. Tyrosine phosphorylation of STAT5 was detected by 4G10 as described in Fig 1A. Bands were visualized by chemiluminescence. (lower panel) The same nylon membrane was stripped of the antibody and reprobed for STAT5 with an anti-STAT5 monoclonal antibody. Bands were visualized by chemiluminescence.
Erythropoietin activates STAT5 in erythroid cells. Growth factor-deprived day-8 cells were stimulated with 10 U/mL erythropoietin and nuclear extracts were prepared. Nuclear extracts were subject to EMSA by using a β-casein probe. Lane 1, nuclear extracts from unstimulated cells; lane 2, nuclear extracts from erythropoietin-stimulated cells; lane 3, the same as in lane 2, except the nuclear extracts were incubated with STAT5 antisera before EMSA. Shift indicates probe complex in the absence of the antibodies. Super Shift indicates the supershifted band in the presence of STAT5 antisera.
Erythropoietin activates STAT5 in erythroid cells. Growth factor-deprived day-8 cells were stimulated with 10 U/mL erythropoietin and nuclear extracts were prepared. Nuclear extracts were subject to EMSA by using a β-casein probe. Lane 1, nuclear extracts from unstimulated cells; lane 2, nuclear extracts from erythropoietin-stimulated cells; lane 3, the same as in lane 2, except the nuclear extracts were incubated with STAT5 antisera before EMSA. Shift indicates probe complex in the absence of the antibodies. Super Shift indicates the supershifted band in the presence of STAT5 antisera.
(A, upper panel) Tyrosine phosphorylation of STAT3 was not tyrosine phosphorylated in the erythroid cells stimulated with erythropoietin for 10 minutes. The erythroid cells were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100 before and after exposure to erythropoietin (10 U/mL). STAT3 was immunoprecipitated with specific anti-STAT3 antisera. As a positive control, TF-1 cells were stimulated with IL-6 (100 ng/mL) for 10 minutes. Immune complexes were resuspended in SDS sample buffer. Tyrosine phosphorylation of STAT3 was detected by 4G10 as described in Fig 1A. Bands were visualized by chemiluminescence. Lane 1, resting erythroid cells; lane 2, 10 minutes after exposure to erythropoietin (10 U/mL); lane 3, resting TF-1 cells, lane 4, 10 minutes after exposure to IL-6 (100 ng/mL). (lower panel) The same nylon membrane was stripped of the antibody and reprobed for STAT3. Bands were visualized by chemiluminescence. Lanes are the same as in upper panel. (B) Anti-STAT1 was used instead of STAT3 antisera.
(A, upper panel) Tyrosine phosphorylation of STAT3 was not tyrosine phosphorylated in the erythroid cells stimulated with erythropoietin for 10 minutes. The erythroid cells were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100 before and after exposure to erythropoietin (10 U/mL). STAT3 was immunoprecipitated with specific anti-STAT3 antisera. As a positive control, TF-1 cells were stimulated with IL-6 (100 ng/mL) for 10 minutes. Immune complexes were resuspended in SDS sample buffer. Tyrosine phosphorylation of STAT3 was detected by 4G10 as described in Fig 1A. Bands were visualized by chemiluminescence. Lane 1, resting erythroid cells; lane 2, 10 minutes after exposure to erythropoietin (10 U/mL); lane 3, resting TF-1 cells, lane 4, 10 minutes after exposure to IL-6 (100 ng/mL). (lower panel) The same nylon membrane was stripped of the antibody and reprobed for STAT3. Bands were visualized by chemiluminescence. Lanes are the same as in upper panel. (B) Anti-STAT1 was used instead of STAT3 antisera.
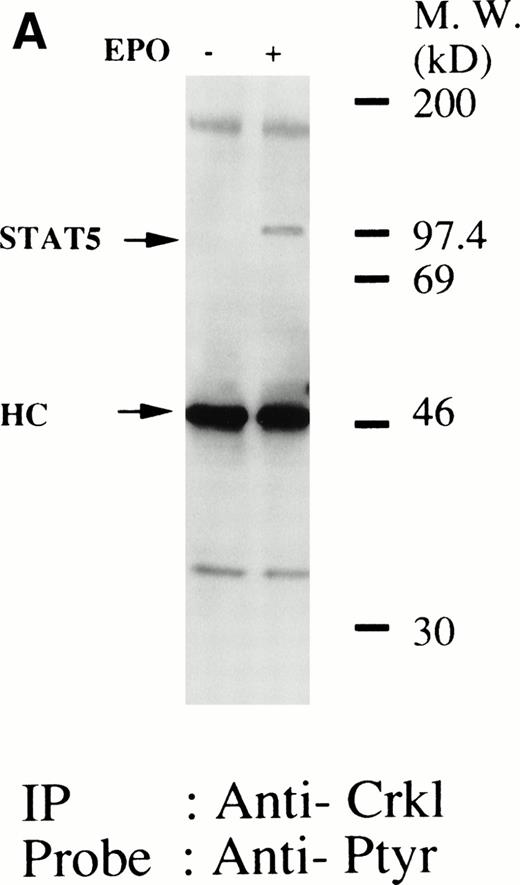
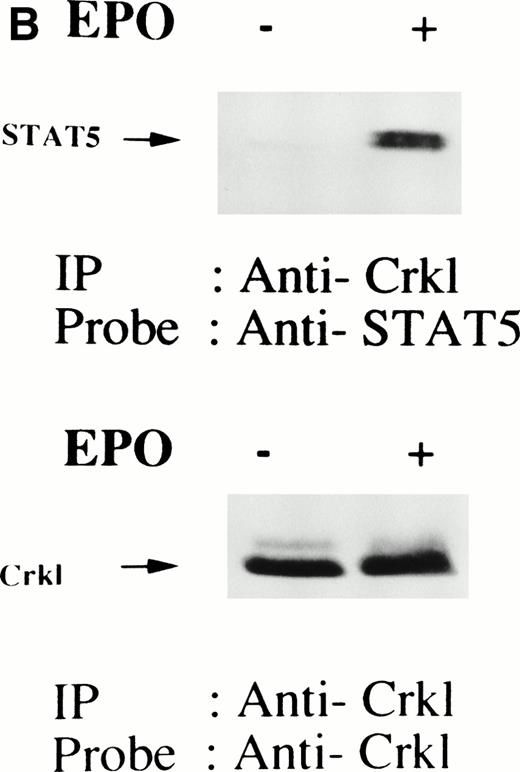
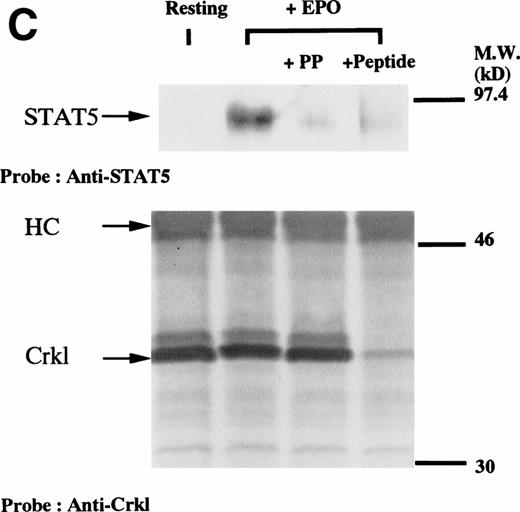
We have recently observed that Crkl, a 39-kD SH2/SH3 adapter protein, becomes associated with STAT5 in human platelets after stimulation by thrombopoietin (submitted). Given the similarity between thrombopoietin and erythropoietin signal transductions, we examined phosphotyrosine immunoblots of the Crkl immunoprecipitates from erythropoietin-treated erythroids, which show the presence of a protein with a molecular weight of approximately 95 to 100 kD (Fig 5A). This protein was reactive with an anti-STAT5 monoclonal antibody (Fig 5B, upper panel). Before and after stimulation the same amount of Crkl was immunoprecipitated from erythroid cells (Fig 5B, lower panel). To further confirm the specificity of Crkl-STAT5 interaction, the effects of phenylphosphate and Crkl immunizing peptide were examined. More STAT5 was coimmunoprecipitated from erythropoietin-stimulated cells than starved (resting) cells (Fig 5C, upper panel). Phenylphosphate (20 mmol/L), which is known to disrupt phosphotyrosine-dependent protein interactions and was added after the lysis of erythropoietin-stimulated cells, inhibited the coimmunoprecipitation of STAT5 with Crkl (Fig 5C, upper panel) but not immunoprecipitation of Crkl (lower panel). Crkl immunizing peptide inhibited immunoprecipitation of Crkl and STAT5, suggesting that the Crkl antisera is specific.
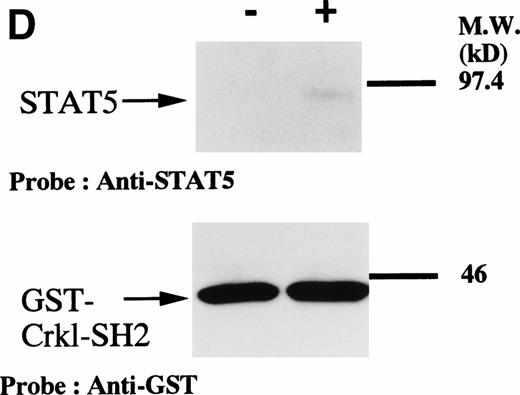
(A and B) Crkl immunoprecipitates from erythropoietin-stimulated erythroid cells contain a tyrosine phosphorylated 95- to 100-kD protein, which is also recognized by a STAT5 monoclonal antibody. Erythroid cells were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100 before and after exposure to eythropoietin (10 U/mL for 10 minutes). Crkl was immunoprecipitated with specific Crkl antisera. The Crkl immunoprecipitates were divided into two. Tyrosine phosphorylated proteins (A), STAT5 (B, upper panel), and Crkl (B, lower panel) in the Crkl immunoprecipitates were detected as described in Fig 1. (C) Crkl-STAT5 coimmunoprecipitation was inhibited by phenylphosphate (PP, 20 mmol/L) or Crkl immunizing peptide (20 μg/mL). Erythroid cells were divided equally to four samples. Three samples were incubated wtih eythropoietin (10 U/mL for 10 minutes). Crkl was immunoprecipitated with specific Crkl antisera. STAT5 (upper panel) and Crkl (lower panel) in the Crkl immunoprecipitates were detected as described in (A) and (B). (D) Bacterially expressed the SH2 domain of Crkl (GST-Crkl-SH2) binds to STAT5 from erythropoietin-stimulated erythroid cells. Lysates from starved (−) erythroid cells or cells stimulated with 10 U/mL of erythropoietin for 10 minutes (+) were analyzed for binding to GST-Crkl-SH2. Bound proteins were separated by SDS-PAGE, transferred to PVDF membranes and immunoblotted with STAT5 (upper panel) or GST (lower panel) monoclonal antibodies.
(A and B) Crkl immunoprecipitates from erythropoietin-stimulated erythroid cells contain a tyrosine phosphorylated 95- to 100-kD protein, which is also recognized by a STAT5 monoclonal antibody. Erythroid cells were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100 before and after exposure to eythropoietin (10 U/mL for 10 minutes). Crkl was immunoprecipitated with specific Crkl antisera. The Crkl immunoprecipitates were divided into two. Tyrosine phosphorylated proteins (A), STAT5 (B, upper panel), and Crkl (B, lower panel) in the Crkl immunoprecipitates were detected as described in Fig 1. (C) Crkl-STAT5 coimmunoprecipitation was inhibited by phenylphosphate (PP, 20 mmol/L) or Crkl immunizing peptide (20 μg/mL). Erythroid cells were divided equally to four samples. Three samples were incubated wtih eythropoietin (10 U/mL for 10 minutes). Crkl was immunoprecipitated with specific Crkl antisera. STAT5 (upper panel) and Crkl (lower panel) in the Crkl immunoprecipitates were detected as described in (A) and (B). (D) Bacterially expressed the SH2 domain of Crkl (GST-Crkl-SH2) binds to STAT5 from erythropoietin-stimulated erythroid cells. Lysates from starved (−) erythroid cells or cells stimulated with 10 U/mL of erythropoietin for 10 minutes (+) were analyzed for binding to GST-Crkl-SH2. Bound proteins were separated by SDS-PAGE, transferred to PVDF membranes and immunoblotted with STAT5 (upper panel) or GST (lower panel) monoclonal antibodies.
Because phenyl phosphate inhibited the coimmunoprecipitation of STAT5 with Crkl and we only see tyrosine phosphorylation of STAT5 in erythroid cells, we suspected that the SH2 domain of Crkl may be responsible for the binding of Crkl to the phosphorylated STAT5. Accordingly, we examined the binding of STAT5 to bacterially expressed GST-Crkl-SH2 protein (Fig 5D). Lysates from starved (−) erythroid cells or cells stimulated with 10 U/mL of erythropoietin for 10 minutes (+) were incubated with GST-Crkl-ST2. The SH2 domain showed only a trace amount of binding to STAT5 from starved cells with a significant increase using lysates from erythroid cells.
DISCUSSION
In this study, we examined erythropoietin-signaling in human erythroid precursors expanded in vitro. This corroborates the recent study of Dai et al33 and indicates that these studies are feasible. Because these cells, unlike murine factor–dependent cell lines genetically engineered to express erythropoietin receptors or murine and human leukemic cell lines, regularly undergo full terminal differentiation and become reticulocytes, we believe that these studies are more likely to be reflective of in vivo situations, although the presence of the artifacts arising from in vitro culture still cannot be ruled out. When Drachman et al examined murine mature megakaryocytes and established cell lines for proximate signal transduction triggered by thrombopoietin, they found that there were significant differences in these cells, suggesting that the results of the studies using the established cell lines may not readily be extended to primary cells.34 These considerations led us to examine primary cultured human erythroid precursor cells for early signaling induced by erythropoietin.
We found that Jak2 became tyrosine phosphorylated in a time- and dose-dependent fashion in human erythroid cells, which was consistent with previous reports.6-15 Erythropoietin-induced tyrosine phosphorylation of both STAT5A and STAT5B in human erythroid cells is interesting, because only STAT5B became tyrosine phosphorylated in chicken erythroid cells.15 We did not detect any increase in tyrosine phosphorylation of STAT1 and STAT3 in erythropoietin-stimulated human erythroid cells. On the other hand, Penta and Sawyer14 found that erythropoietin-induced STAT1 tyrosine phosphorylation and its activation in murine erythroid cells. Further, in rat erythroid cells from fetal liver, STAT3 was tyrosine phosphorylated on stimulation with erythropoietin.16 Thus, these and our current data suggest the presence of species-specific differences in signaling by erythropoietin, although it is formally possible that different sensitivities used in detection of STAT5 activation may have contributed to the differences.
Further, our data suggest that erythropoietin and thrombopoietin may induce tyrosine phosphorylation and activation of different sets of STATs in human primary cells in spite of their similarity in amino acid sequence,1-3,35,36 although such differences may be obscured in the studies that used established cell lines.8-11,36 We have previously reported that thrombopoietin induces tyrosine phosphorylation of STAT3 and STAT5 in thrombopoietin-stimulated human platelets.18 Drachman et al reported that the same set of STATs was tyrosine phosphorylated and activated in thrombopoietin-stimulated murine megakaryocytes.34 Thus, it is possible that the differences in activated STATs by erythropoietin and thrombopoietin may be important in the lineage-specific developments of both erythrocytic and megakaryocytic precursors.
The differences between the studies that used primary erythroids and those that used cell lines are not limited to STAT proteins. Barber et al have shown that Crkl becomes tyrosine phosphorylated and associated with c-Cbl on stimulation by erythropoietin.37 However, we found that in primary erythroids Crkl becomes associated with a 95-kD tyrosine phosphorylated protein, which has been identified as STAT5. This coimmunoprecipitation of Crkl and STAT5 is not likely caused by the cross reactivity of Crkl antisera with STAT5 because of the following three reasons (Fig 5): (1) Crkl antisera coimmunoprecipitated STAT5 mostly after stimulation of cells with erythropoietin, suggesting that, if this is a cross reactivity, the Crkl antisera must recognize only the activated form of STAT5. (2) Crkl immunoprecipitation and STAT5 coimunoprecipitation were inhibited by Crkl immunizing peptides whose amino acid sequence is not found in STAT5. (3) Phenylphosphate inhibited coimmunoprecipitation of STAT5 without impairing the immunoprecipitation of Crkl, suggesting that Crkl-STAT5 interaction may be dependent on phosphotyrosine on STAT5, because we did not detect tyrosine phosphorylation of Crkl. The last supposition was supported by the results of in vitro binding of GST-Crkl-SH2 to STAT5 from stimulated erythroid cells (Fig 5D), suggesting that STAT5 could be binding to Crkl through its phosphotyrosine-dependent interaction with the SH2 domain of Crkl. However, STAT5 is thought to dimerize when tyrosine phosphorylated through its SH2 domain binding to the tyrosine phosphate of another STAT protein.38,39 Possible explanations for this finding include the fact that dimerization of STAT5 is required for binding to Crkl or that erythropoietin treatment results in an altered subcellular localization of Crkl or STAT5 that allows these proteins to interact. Alternatively, another protein could be involved in mediating the complex formation. Although more studies are necessary, our study indicates that activated STAT5 complex is not simply made of dimerized STAT5 in human erythroid cells and that the complex may include Crkl and possible other proteins as well. Taken together, our data suggest that the results of the studies that used the primary erythroids may be quite different from those obtained from the experiments that were solely dependent on cell lines, giving a rationale for further examinations of the signal transduction of primary human erythroid precursors (Fig 5). Further, the importance of examinations of proximal signal transduction was supported by the recent reports, suggesting that abrogation of tyrosine phosphorylation may be related to the increased erythropoiesis in polycythemic patients.33 40
ACKNOWLEDGMENT
We thank Dr C.I. Civin for the generous gift of monoclonal antibodies, without which this work could not have been done. We are grateful to Drs Hiroshi Wakao and Atsushi Miyajima for helpful discussions. We thank Dr S.C. Clark, Dr Hiroshi Wakao, Genetics Institute, Kirin Brewery Co, and Chugai Pharmaceutical Co for the generous gifts of recombinant human growth factors; Dr Hisamaru Hirai for TF-1 cells; and Taisho Pharmaceutical Co for providing chymopapain.
A.O. and K.S. contributed equally to this work.
Supported in part by grants in aid from The Ministry of Education, Science, Sports and Culture of Japan (A.O., K.S., and Y.I.), a research grant for Idiopathic Disorders of Hematopoietic Organs Research Committee from the Ministry of Health and Welfare of Japan (K.S.), the Ryoichi Naito Foundation for Medical Research (A.O.), a research grant for the Development of Advanced Therapeutics from Hokkaido University School of medicine and the Sankyo Life Science Foundation (K.S.), and research grants for Life Sciences and Medicine, Keio University Medical Science Fund (A.O.).
Address reprint requests to Atsushi Oda, MD, PhD, Department of Internal Medicine, School of Medicine, Keio University, 35 Shinanomachi, Tokyo 160, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.
© 1998 by the American Society of Hematology.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal