Abstract
Iron-mediated carcinogenesis is thought to occur through the generation of oxygen radicals. Iron chelators are used in attempts to prevent the long term consequences of iron overload. In particular, 1,2-dimethyl-3-hydroxypyrid-4-one (L1), has shown promise as an effective chelator. Using an established hepatocellular model of iron overload, we studied the generation of iron-catalyzed oxidative DNA damage and the influence of iron chelators, including L1, on such damage. Iron loading of HepG2 cells was found to greatly exacerbate hydrogen peroxide–mediated DNA damage. Desferrithiocin was protective against iron/hydrogen peroxide–induced DNA damage; deferoxamine had no effect. In contrast, L1 exposure markedly potentiated hydrogen peroxide–mediated oxidative DNA damage in iron-loaded liver cells. However, when exposure to L1 was maintained during incubation with hydrogen peroxide, L1 exerted a protective effect. We interpret this as indicating that L1's potential toxicity is highly dependent on the L1:iron ratio. In vitro studies examining iron-mediated ascorbate oxidation in the presence of L1 showed that an L1:iron ratio must be at least 3 to 1 for L1 to inhibit the generation of free radicals; at lower concentrations of L1 increased oxygen radical generation occurs. In the clinical setting, such potentiation of iron-catalyzed oxidative DNA damage at low L1:iron ratios may lead to long-term toxicities that might preclude administration of L1 as an iron chelator. Whether this implication in fact extends to the in vivo situation will have to be verified in animal studies.
IRON IS THOUGHT to play a role in carcinogenesis through the generation of oxygen free radicals.1,2 In the hereditary iron overload condition of increased iron absorption, hemochromatosis, hepatic iron accumulation causes fibrosis, cirrhosis, and ultimately hepatocellular carcinoma in 25% to 35% of untreated homozygotes.3-6 The prevalence of neoplastic disease may be higher in β-thalassemia heterozygotes than in the general population.7 In addition, epidemiological studies have implicated increased body iron stores in the pathogenesis of lung and intestinal carcinomas.8-10 Iron, mainly in its non-protein-bound, low molecular weight form, causes cellular damage by participating in the generation of the hydroxyl radical,11thought to be the principal effector of oxidative DNA damage and mutagenesis.12
Iron chelators are presently used in attempts to prevent the long-term consequences of iron overload. Deferoxamine is the most widely used agent, but has significant disadvantages, including its high cost, parenteral method of administration, toxicity, and frequent noncompliance, leading to a search for a safe, orally active alternative.11 Recently, encouraging results have been reported using the orally active iron chelator 1,2-dimethyl-3-hydroxypyrid-4-one (L1). This agent is presently being studied in clinical trials and appears to be effective with an acceptable toxicity profile.13-16 Both deferoxamine and L1 have been shown to inhibit oxygen radical formation in vitro, but their use as therapeutic agents for this purpose has not yet been fully examined.17 18
Using an established hepatocellular model of iron overload, we studied the generation of iron-catalyzed oxidative DNA damage in the form of DNA single-strand breaks. In addition, we examined the influence of iron chelators, including L1, on such iron-mediated DNA damage.
MATERIALS AND METHODS
Cell culture.
The human hepatocellular line HepG2 (American Type Culture Collection, Rockville, MD) was grown in monolayer in Dulbecco's Modified Eagle Media supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin G, 100 μg/mL streptomycin, and 25 ng/mL amphotericin B, at 37°C in 5% CO2. As indicated below, in some experiments, cells were maintained for short periods in media without serum and rendered iron-free by the addition of a chelating resin, iminodiacetic acid (iron-free media). Except for the deliberate addition of iron, all buffers were similarly made iron-free, the resin being removed by filtration immediately before use.
Iron loading of HepG2 cells.
Nontransferrin-bound iron loading of HepG2 cells using ferric citrate was performed as previously described.19 20 Ferric citrate solution (final concentration of ferric iron 10 mmol/L; molar iron:citrate ratio 1:10) was freshly prepared for each experiment. Cells were exposed to 1 mmol/L ferric citrate in media for 20 hours. Cell viability was maintained under these conditions for at least this length of time. Both HepG2 cells in media alone and in media with iron-free citrate served as controls.
Exposure of iron-loaded HepG2 cells to hydrogen peroxide.
After exposure to ferric citrate, cells were washed twice with iron-free phosphate buffered saline (PBS) and exposed to 100 μM hydrogen peroxide in iron-free media for 30 minutes at 37°C. After hydrogen peroxide exposure, cells were washed twice and harvested by trypsinization. Viable cells were isolated by Nycoprep gradient centrifugation. Cell viability was assessed by trypan blue exclusion and was consistently greater than 90%. DNA single-strand breaks were then assessed as described below.
Exposure of iron-loaded HepG2 cells to iron chelators.
For some experiments, iron-loaded HepG2 cells were incubated with various iron chelators before hydrogen peroxide exposure. For this, cells were washed with iron-free PBS and then incubated for 1 hour with one of the following iron chelators in iron-free media: deferoxamine mesylate, desferrithiocin, or L1. Concentrations used are indicated in the result section and figure legends. After exposure to a chelator, cells were again washed with iron-free PBS before exposure to hydrogen peroxide. In experiments in which L1 exposure was maintained, incubation with hydrogen peroxide occurred in iron-free media containing 1 mmol/L L1.
Measurement of DNA single-strand breaks by alkaline elution.
Alkaline elution was performed as described,21,22 with some modifications.23 Briefly, HepG2 cells in exponential growth phase were labeled with [3H]thymidine (35 Ci/mmol, 1μCi/mL, 1 μmol/L in tissue culture medium) for 24 hours and then exposed to oxidant stress with or without iron chelators as described above. Ice-cold suspensions of 2 × 105 radiolabeled cells were then lysed on 2.0 μm polycarbonate filters using lysis solution (25 mmol/L Na2−EDTA, 2% sodium dodecyl sulfate (SDS), 40 mmol/L glycine, pH 9.6, 0.85 mg/mL proteinase K). Single-strand breaks were assessed by alkaline elution of DNA from the filters using elution buffer (20 mmol/L Na2−EDTA, 54 mmol/L tetraethylammonium hydroxide, pH 12.3). The elution rate was 0.03 mL/minute with fractions collected at 30 minute intervals. Radioactivity in each fraction and that remaining on the filter was determined by liquid scintillation counting (Beckman LS 5000TD, Palo Alto, CA). All studies were performed in dim light to prevent ultraviolet light–induced DNA damage. To ensure that measured DNA damage was not caused by available intracellular iron during cell lysis or DNA elution, additional control experiments were performed. The addition of 10 μmol/L ferrous iron to the cell lysis and DNA elution buffers did not increase DNA strand breaks, indicating little oxygen radical generation under the conditions of cell lysis and DNA elution (4°C; pH 12.3). Furthermore, the addition of deferoxamine during cell lysis and DNA elution did not result in decreased DNA damage. Results are reported as the mean ± standard error of the mean of three experiments performed in duplicate unless otherwise stated.
Ascorbate oxidation by iron: Effect of iron chelators.
Fresh solutions of 5 mmol/L phosphate buffer (pH 7.4) containing 100 μmol/L sodium ascorbate, 30 μmol/L ferric iron, and increasing concentrations of L1 (0 to 180 μmol/L) or deferoxamine (0 to 90 μmol/L) were incubated for 40 minutes at room temperature. The rate of ascorbate consumption was measured by absorbance spectroscopy (Beckman DU 70 spectrophotometer) at 265 nm at 10 and 40 minutes. A 0.1 decrease in absorbance has been determined to be equivalent to 70 μmol/L of oxidized ascorbate.24
Statistics.
For alkaline elution experiments, unless otherwise stated, all values are presented as the mean ± standard error of the mean of a given number of independent experiments. The data were evaluated using the unpaired Student's t-test: P values less than .05 were considered to be statistically significant. For ascorbate oxidation experiments, a single representative experiment is shown, with points indicating the mean ± standard deviation of 9 replicates.
Materials.
The following materials were purchased from Sigma (St Louis, MO): deferoxamine mesylate, sodium ascorbate, ferric iron, hypoxanthine, 2-deoxy-D-ribose, xanthine oxidase, thiobarbituric acid, and trichloroacetic acid. Hydrogen peroxide and proteinase K were obtained from Fischer Biotech (Pittsburgh, PA). Nycoprep was purchased from GIBCO BRL (Gaithersburg, MD),3H-thymidine from ICN Corp (Costa Mesa, CA), and the 2 μmol/L polycarbonate filters from Costar (Cambridge, MA). Desferrithiocin was a gift from I. Schraufstätter (Research Institute of Scripps Clinic, La Jolla, CA) and L1 was kindly provided by Drs N. Olivieri (Hospital for Sick Children, Toronto, Canada) and R. McClelland (University of Toronto, Toronto, Canada). The L1 was synthesized as described by Kontoghiorghes and Sheppard25 and was greater than 98% pure.26
RESULTS
DNA damage in iron-loaded HepG2 cells.
Oxidative DNA damage after iron loading of HepG2 cells with subsequent hydrogen peroxide exposure is shown in Fig1. Iron loading alone did not lead to increased DNA single-strand breaks compared with control cells exposed to media. Even in the absence of iron loading, hydrogen peroxide caused a significant degree of strand cleavage, with approximately 50% of the total cellular DNA eluting through the filter. When iron-loaded cells were exposed to hydrogen peroxide, oxidative DNA damage was greatly exacerbated (85% DNA eluting through the filter). No strand cleavage was observed in control cells exposed to citrate buffer alone (data not shown).
Generation of DNA single-strand breaks in iron-loaded HepG2 cells exposed to hydrogen peroxide. Iron-loaded cells were exposed to 100 μmol/L hydrogen peroxide for 30 minutes. Cells were harvested and DNA single-strand breaks were quantified by alkaline elution. Shown are DNA strand breaks in control cells exposed to media alone (□), iron-loaded cells (•), hydrogen peroxide–exposed cells, and iron-loaded cells exposed to hydrogen peroxide. The results are the mean ± SEM of three experiments performed in duplicate (*P< .03 compared with hydrogen peroxide exposed cells).
Generation of DNA single-strand breaks in iron-loaded HepG2 cells exposed to hydrogen peroxide. Iron-loaded cells were exposed to 100 μmol/L hydrogen peroxide for 30 minutes. Cells were harvested and DNA single-strand breaks were quantified by alkaline elution. Shown are DNA strand breaks in control cells exposed to media alone (□), iron-loaded cells (•), hydrogen peroxide–exposed cells, and iron-loaded cells exposed to hydrogen peroxide. The results are the mean ± SEM of three experiments performed in duplicate (*P< .03 compared with hydrogen peroxide exposed cells).
Influence of iron chelators on oxidative DNA damage in iron-loaded HepG2 cells.
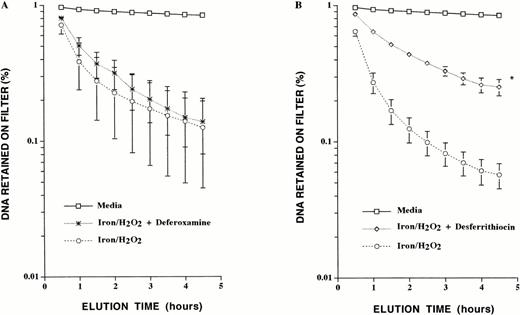
The influence of the iron chelators deferoxamine (which penetrates cells poorly), and of desferrithiocin and L1 (both of which are readily cell permeable) on hydrogen peroxide–mediated DNA damage in iron-loaded HepG2 cells was examined. Deferoxamine, desferrithiocin, and L1 form hexadentate, tridentate, and bidentate ligands with iron, respectively.27 Hence, to compare these chelators at equipotent iron-chelating concentrations, the following concentrations were used: deferoxamine, 0.33 mmol/L; desferrithiocin, 0.67 mmol/L; and L1, 1 mmol/L. Iron-loaded HepG2 cells were incubated with each chelator for 1 hour, then washed in iron-free PBS before exposure to hydrogen peroxide. Determination of DNA single-strand breaks by alkaline elution was then performed. Although deferoxamine failed to protect cells from iron/hydrogen peroxide–mediated oxidative DNA damage, desferrithiocin did have a protective effect, with a dramatic decrease in the amount of DNA single strand breaks generated (Fig 2).
Effect of deferoxamine and desferrithiocin on iron/hydrogen peroxide–mediated DNA single-strand breaks. (A) Iron-loaded HepG2 cells were exposed to 0.33 mmol/L deferoxamine for 1 hour before exposure to 100 μmol/L hydrogen peroxide for 30 minutes. (B) Iron-loaded HepG2 cells were incubated with 0.67 mmol/L desferrithiocin before exposure to hydrogen peroxide. Shown are DNA strand breaks in control cells exposed to media alone, iron-loaded cells exposed to hydrogen peroxide, iron-loaded cells exposed to deferoxamine before exposure to hydrogen peroxide, and iron-loaded cells exposed to desferrithiocin before exposure to hydrogen peroxide. The results are the mean ± SEM of three experiments performed in duplicate (*P < .03 compared with iron-loaded cells exposed to hydrogen peroxide).
Effect of deferoxamine and desferrithiocin on iron/hydrogen peroxide–mediated DNA single-strand breaks. (A) Iron-loaded HepG2 cells were exposed to 0.33 mmol/L deferoxamine for 1 hour before exposure to 100 μmol/L hydrogen peroxide for 30 minutes. (B) Iron-loaded HepG2 cells were incubated with 0.67 mmol/L desferrithiocin before exposure to hydrogen peroxide. Shown are DNA strand breaks in control cells exposed to media alone, iron-loaded cells exposed to hydrogen peroxide, iron-loaded cells exposed to deferoxamine before exposure to hydrogen peroxide, and iron-loaded cells exposed to desferrithiocin before exposure to hydrogen peroxide. The results are the mean ± SEM of three experiments performed in duplicate (*P < .03 compared with iron-loaded cells exposed to hydrogen peroxide).
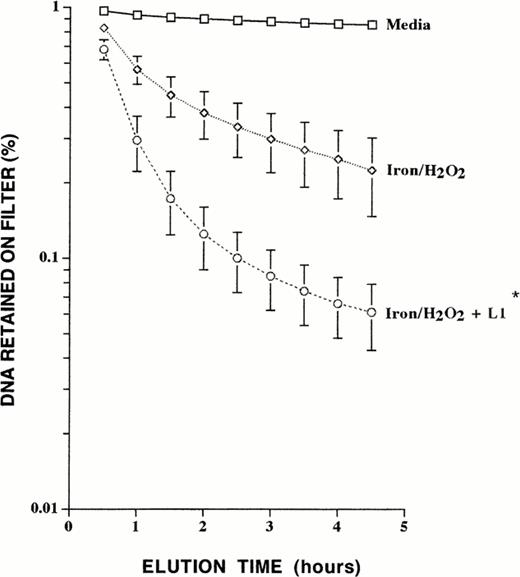
In contrast to desferrithiocin and deferoxamine, L1 markedly potentiated hydrogen peroxide–mediated oxidative DNA damage in iron-loaded liver cells (Fig 3). Exposure of control cells to L1 alone yielded no damage, and exposure to L1 followed by hydrogen peroxide caused slightly increased damage compared with exposure to hydrogen peroxide alone (data not shown). The effects of 1 mmol/L and 10 mmol/L L1 on hydrogen peroxide–induced DNA damage were then compared. The alkaline elution profiles obtained showed no differences in the DNA-damage potentiating effect at either concentration, with 94% of the total DNA eluting through the filter at 1 mmol/L L1 and 95% at 10 mmol/L L1. In contrast to this potentiation of damage after sequential exposure of iron-loaded cells to L1 and hydrogen peroxide (with cell washing in between), when L1 exposure was maintained during incubation with hydrogen peroxide, L1 did protect against oxidative DNA damage (Fig 4). This suggests that the ability of L1 to influence iron-catalyzed oxygen radical generation is highly dependent on the effective intracellular L1:iron ratio. Furthermore, inclusion of desferrithiocin during incubation with L1 partially reversed its DNA-damage potentiating effect (Fig 5).
Effect of L1 on iron/hydrogen peroxide–induced DNA single-strand breaks. Iron-loaded HepG2 cells were incubated in 1 mmol/L L1 for 1 hour, followed by exposure to 100 μmol/L hydrogen peroxide for 30 minutes. Shown are DNA strand breaks in control cells exposed to media alone, iron-loaded cells exposed to hydrogen peroxide, and iron-loaded cells exposed to L1 before exposure to hydrogen peroxide. The results are the mean ± SEM of four experiments performed in duplicate (*P < .03 compared with iron-loaded cells exposed to hydrogen peroxide).
Effect of L1 on iron/hydrogen peroxide–induced DNA single-strand breaks. Iron-loaded HepG2 cells were incubated in 1 mmol/L L1 for 1 hour, followed by exposure to 100 μmol/L hydrogen peroxide for 30 minutes. Shown are DNA strand breaks in control cells exposed to media alone, iron-loaded cells exposed to hydrogen peroxide, and iron-loaded cells exposed to L1 before exposure to hydrogen peroxide. The results are the mean ± SEM of four experiments performed in duplicate (*P < .03 compared with iron-loaded cells exposed to hydrogen peroxide).
Effect of sustained L1 exposure on iron/hydrogen peroxide–mediated DNA single-strand breaks. Iron-loaded liver cells were incubated in 1 mmol/L L1 for 1 hour, followed by exposure to 100 μmol/L hydrogen peroxide for 30 minutes during which exposure to 1 mmol/L L1 was maintained. Illustrated is a representative experiment performed in duplicate and the values obtained were averaged. Shown are DNA single-strand breaks in control cells exposed to media alone, iron-loaded cells exposed to hydrogen peroxide, and iron-loaded cells exposed to hydrogen peroxide in the presence of L1.
Effect of sustained L1 exposure on iron/hydrogen peroxide–mediated DNA single-strand breaks. Iron-loaded liver cells were incubated in 1 mmol/L L1 for 1 hour, followed by exposure to 100 μmol/L hydrogen peroxide for 30 minutes during which exposure to 1 mmol/L L1 was maintained. Illustrated is a representative experiment performed in duplicate and the values obtained were averaged. Shown are DNA single-strand breaks in control cells exposed to media alone, iron-loaded cells exposed to hydrogen peroxide, and iron-loaded cells exposed to hydrogen peroxide in the presence of L1.
Combined effect of desferrithiocin and L1 on iron/hydrogen peroxide–mediated DNA single-strand breaks. Iron-loaded liver cells were simultaneously exposed to 1 mmol/L L1 and 0.67 mmol/L desferrithiocin for 1 hour, followed by incubation with 100 μmol/L hydrogen peroxide for 30 minutes in the absence of either chelator. Illustrated is a representative experiment performed in duplicate and the values obtained were averaged. Shown are DNA single-strand breaks in control cells exposed to media alone, iron-loaded cells exposed to hydrogen peroxide, iron-loaded cells exposed to desferrithiocin followed by hydrogen peroxide, iron-loaded cells exposed to L1 followed by hydrogen peroxide, and iron-loaded cells exposed to both desferrithiocin and L1 followed by hydrogen peroxide.
Combined effect of desferrithiocin and L1 on iron/hydrogen peroxide–mediated DNA single-strand breaks. Iron-loaded liver cells were simultaneously exposed to 1 mmol/L L1 and 0.67 mmol/L desferrithiocin for 1 hour, followed by incubation with 100 μmol/L hydrogen peroxide for 30 minutes in the absence of either chelator. Illustrated is a representative experiment performed in duplicate and the values obtained were averaged. Shown are DNA single-strand breaks in control cells exposed to media alone, iron-loaded cells exposed to hydrogen peroxide, iron-loaded cells exposed to desferrithiocin followed by hydrogen peroxide, iron-loaded cells exposed to L1 followed by hydrogen peroxide, and iron-loaded cells exposed to both desferrithiocin and L1 followed by hydrogen peroxide.
Iron-mediated ascorbate oxidation in the presence of increasing concentrations of iron chelator.
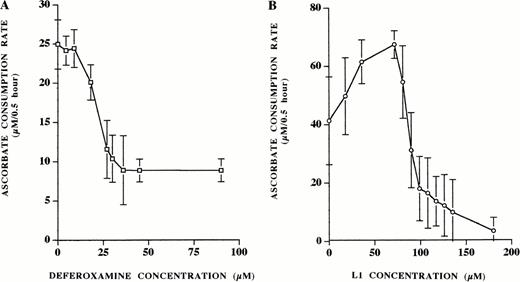
The measurement of ascorbate oxidation to compare the influence of different iron chelators on iron-dependent free radical damage has previously been described.24 We used this method to examine the influence of the L1:iron ratio on oxygen radical generation in vitro and compared this to the effect of deferoxamine. Even at low concentrations, addition of deferoxamine to the reaction solution caused a decrease in ascorbate consumption (Fig 6A). Ascorbate consumption continued to decrease in a dose-dependent manner until a plateau was reached at 30 μmol/L deferoxamine (equimolar concentration with iron). In contrast, L1 showed a bimodal effect on ascorbate oxidation (Fig 6B). Initially, at low L1 concentration, augmentation of ascorbate oxidation was observed. The initial rate of oxidation increased from 42 μmol/L/0.5 hour to 70 μmol/L/0.5 hour at 70 μmol/L L1. A further increase in L1 concentration resulted in decreased ascorbate consumption, dropping below the baseline oxidation level only at 90 μmol/L L1 (L1:iron ratio 3:1).
Ascorbate oxidation by iron: effect of deferoxamine and L1. Increasing concentrations of chelator were added to fresh solutions of phosphate buffer containing 100 μmol/L sodium ascorbate and 30 μmol/L ferric ion. The rate of ascorbate consumption was measured by absorbance spectroscopy at 265 nm at 10 and 40 minutes. (A) Ascorbate oxidation in response to increasing concentrations of deferoxamine (0 to 90 μmol/L). (B) Ascorbate oxidation in response to increasing concentrations of L1 (0 to 180 μmol/L). Shown is a single representative experiment with points indicating the mean ± the standard deviation of nine replicates.
Ascorbate oxidation by iron: effect of deferoxamine and L1. Increasing concentrations of chelator were added to fresh solutions of phosphate buffer containing 100 μmol/L sodium ascorbate and 30 μmol/L ferric ion. The rate of ascorbate consumption was measured by absorbance spectroscopy at 265 nm at 10 and 40 minutes. (A) Ascorbate oxidation in response to increasing concentrations of deferoxamine (0 to 90 μmol/L). (B) Ascorbate oxidation in response to increasing concentrations of L1 (0 to 180 μmol/L). Shown is a single representative experiment with points indicating the mean ± the standard deviation of nine replicates.
DISCUSSION
Current evidence suggests that transition metals, in particular iron, react with hydrogen peroxide in cell nuclei leading to oxygen radical generation and consequent DNA damage.28-30 The iron-catalyzed generation of oxygen radicals is believed to mediate its mutagenic and carcinogenic effects.31 In situations of iron overload, as occurs in thalassemia and hemochromatosis, iron chelators have been shown to prevent and reverse iron-mediated tissue damage.32 In addition, in vitro data suggest that iron chelators can inhibit iron-catalyzed oxygen radical formation.17 18 This has led us to postulate that administration of iron chelators may decrease the risk of carcinogenesis in hemochromatosis. However, our results suggest that the iron chelator L1 may, under some circumstances, sensitize cells to iron-induced DNA damage.
Iron-mediated oxidative DNA damage has previously been studied in iron-loaded hepatic nuclei33 and in murine hybridoma cells.34 The use of the human hepatocellular line HepG2 for the study of iron-catalyzed DNA damage may be more physiologically relevant.35 Effective iron-loading of HepG2 cells using55Fe complexed with citrate has been previously shown.19 Iron citrate was used because citrate is one of the main physiologic chelators comprising nontransferrin-bound iron. In iron overload states, elevated plasma levels of nontransferrin-bound iron are thought to be an important cause of hepatic iron loading.36 Furthermore, studies using this methodology have shown that cellular iron accumulation occurred at concentrations (400 μg/100 mg protein) similar to those found in hepatic tissue from patients with hemochromatosis.3,37 For the present studies, we did not quantitate bulk iron uptake by HepG2 cells, nor potential chelator-induced bulk iron removal, because such measurements would show nothing about the iron compartment that is relevant to DNA damage, ie, nuclear iron, which cannot be accurately quantitated. The marked sensitization of iron-loaded cells to hydrogen peroxide-mediated oxidative DNA damage that we observed however, implies effective iron loading resulting in an increased amount of available iron for participation in the Fenton reaction. It is likely that iron-catalyzed oxidative DNA damage fueled by lower levels of endogenous hydrogen peroxide occurs over time in vivo, as shown by animal data showing evidence of increasing oxidative DNA damage with aging.38
Iron chelators have been shown to inhibit the production of oxygen radical species resulting in decreased lipid peroxidation and oxidative DNA damage both in vitro and in vivo.11,28,39,40The iron chelators used in the current study included deferoxamine, desferrithiocin, and L1. All three chelators have high iron affinity and specificity. Deferoxamine's failure to affect oxidative DNA damage in this model probably relates to its poor cellular permeability. Indeed, cellular uptake of deferoxamine and subsequent release of intracellular iron has been shown to occur more slowly than for hydroxypyridione chelators.41,42 In contrast, desferrithiocin, which readily penetrates cells,27 does inhibit iron-mediated DNA strand cleavage, as expected.
The paradoxical potentiation of iron-mediated DNA damage by L1 cannot be explained by a direct toxic effect of the chelator, because exposure of HepG2 cells to L1 alone yielded no damage. Rather, L1's effect might be explained by the stoichiometry of its interaction with iron. L1 forms a bidentate ligand with iron as follows:
The log cumulative association constants for these reactions are 15, 27, and 36 respectively.43 Low concentrations of L1 may in fact facilitate iron-mediated oxygen radical generation.11,44 We hypothesized that this is due to a greater facility of [FeL]2+ or [FeL2]+ as compared with free or protein-bound iron to participate in the Fenton reaction, thereby leading to increased hydroxyl radical production.27Thus, after exposure to 1 mmol/L L1, the resultant intracellular L1 concentration during hydrogen peroxide exposure may not be high enough to fully chelate iron as [FeL3], thereby allowing increased DNA damage. We postulated that increasing the extracellular L1 concentration to 10 mmol/L would result in an increased intracellular L1:iron ratio, with increased [FeL3] formation and decreased iron-catalyzed oxidative DNA damage. Instead, we found that exposure to 10 mmol/L L1 caused similar potentiation of iron-mediated DNA damage as 1 mmol/L L1. This failure of increased L1 concentration to reduce iron-mediated oxidative DNA damage may be due to L1's relatively high cellular permeability.45 46 After exposure to both L1 concentrations, the iron-loaded HepG2 cells had been washed in iron-free PBS before exposure to hydrogen peroxide. During washing and hydrogen peroxide exposure, noniron-bound L1 could rapidly diffuse down its concentration gradient out of cells, resulting in lower intracellular levels of L1 that would lead to potentiation of iron-catalyzed oxidative DNA damage. Thus, increasing the initial concentration of L1 from 1 to 10 mmol/L would not necessarily result in an increased intracellular concentration of L1 during hydrogen peroxide exposure when oxidative DNA damage is produced.
When experimental conditions were modified to maintain L1 exposure during incubation with hydrogen peroxide, thereby maintaining the intracellular L1 concentration, a dramatic reduction in iron-catalyzed DNA damage ensued. This supports our hypothesis that potentiation of iron-mediated DNA damage by L1 is dependent on the intracellular L1:iron ratio. The protective effect against DNA damage of continuous incubation with L1 also argues against the possibility that potentiation of oxidative DNA damage is due to an inhibitory effect on DNA repair. Furthermore, addition of a second chelator, desferrithiocin, partially reversed L1's DNA damaging effect. This is probably mediated by competitive inhibition between the two chelators and supports our hypothesis that L1's toxic effect is due to its iron chelating properties.
The in vitro observation of a bimodal pattern of ascorbate oxidation by iron in the presence of increasing concentrations of L1 supports the concept that L1's ability to potentiate or to inhibit iron-catalyzed oxygen free radical formation in vivo is highly dependent on the L1:iron ratio. It appears that this ratio must be at least 3:1 for L1 to inhibit the generation of free radicals; at lower concentrations of L1 increased oxygen radical generation occurs.
L1 has been developed as a potential alternative to deferoxamine in the treatment of iron overload, to be used on a widespread clinical scale in patients with iron overload conditions such as thalassemia and hemochromatosis. Initial clinical experience with L1 has shown it to be an effective chelator, inducing increased urinary iron excretion, decreased ferritin levels and decreased hepatic iron.15,16However, the potentiation of iron-catalyzed oxidative DNA damage at low L1:iron ratios that we observed in these in vitro studies of HepG2 cells has important potential clinical implications. L1 is given orally on a daily basis with an elimination half-life of approximately 90 minutes.47 The maximum plasma concentration of L1 after a single oral dose (50 mg/kg) in patients with iron overload is approximately 300 μmol/L.47 This plasma concentration is lower than that used experimentally (1 mmol/L). As iron-catalyzed oxidative DNA damage in vitro occurs at low L1:iron ratios, one might expect that at pharmacologically achieved levels of L1 in vivo, iron-mediated DNA damage would be even more likely to occur. The main toxicities of L1 recognized at this time include severe neutropenia, agranulocytosis, arthropathy, gastrointestinal intolerance, and liver enzyme abnormalities.11,48-50 The mechanism of these toxicities is not known, though acceleration of hydroxyl radical formation by incompletely complexed L1:iron has been proposed.11,49,51 Controlled animal studies have shown that iron-overloaded gerbils administered the hydroxypyridione CP94 (1,2-diethyl-3-hydroxypyrid-4-one) developed cardiac and hepatic fibrosis.52 There have been no reports of an increased incidence of malignancy in patients receiving L1. However, the long latent period required for the development of human malignancy does not preclude such an effect. Notwithstanding these implications, our findings in this limited in vitro study will have to be corroborated in vivo by appropriate animal studies to determine if they are clinically relevant.
Supported in part by National Institute of Health grants CA65021 (H.E.), HL30160, and HL37528 (R.P.H.).
Address Correspondence to Helen Enright, MD, The Adelaide and Meath Hospital, Iallaght, Dublin 24, Ireland.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.
© 1998 by the American Society of Hematology.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal