Abstract
We analyzed the effect of CD40 triggering on the fludarabine-induced apoptosis of B chronic lymphocytic leukemia (B-CLL) cells. Peripheral blood samples obtained from 15 patients were incubated with fludarabine in the absence or the presence of the anti-CD40 monoclonal antibody (MoAb) G28-5. In 12 patients a significant proportion of apoptotic cells, ranging from 22% to 38% (mean ± SE: 28.5 ± 1.6), were detected after 3 days of culture. In 9 of these samples, the addition of G28-5 reduced apoptosis by at least 30.1% and by 57.1% ± 7.8% on average (P = .0077). Because the CD40 antigen activates NF-κB/Rel transcription factors in B cells, and NF-κB/Rel complexes can inhibit cell apoptosis, we investigated whether the antiapoptotic effect of G28-5, in our system, could be related to modulation of NF-κB/Rel activity. As expected, B-CLL cells displayed significant levels of nuclear NF-κB/Rel activity; p50, RelA, and c-Rel components of the NF-κB/Rel protein family were identified in these complexes. After exposure to fludarabine, NF-κB/Rel complexes were decreased in the nuclei. The addition of G28-5 upregulated the NF-κB/Rel levels. To determine the involvement of NF-κB/Rel activity in the G28-5–mediated inhibition of apoptosis, we blocked the transcription factor with a decoy oligonucleotide, corresponding to the NF-κB/Rel consensus sequence. Cells incubated with the anti-CD40 MoAb in the presence of the decoy oligonucleotide but not a control oligonucleotide displayed a complete impairment of the G28-5 antiapoptotic effect, indicating that NF-κB/Rel activity was required for the inhibition of apoptosis. These results suggest that CD40 triggering in vivo could counteract the apoptotic effect of fludarabine on B-CLL cells and that its neutralization, or the use of NF-κB/Rel inhibitors, could improve the therapeutic effect of fludarabine.
© 1998 by The American Society of Hematology.
THE ABILITY TO induce apoptosis is a relevant property of several chemotherapeutic drugs.1Particularly, neoplasias that have a low growth fraction, such as chronic lymphocytic leukemia (CLL), might be more vulnerable to apoptotic stimuli than to agents that attack dividing cells. In these pathologies, the extent of drug-induced apoptosis correlates with the response to chemotherapy in vivo.2 Mechanisms that affect cell sensitivity to apoptosis, including mutations and/or altered expression of apoptosis-regulating genes such as p53,3,4 Rb,5 and bcl-2,6 are believed to play roles in CLL neoplastic expansion and resistance to chemotherapy. Furthermore, physiological modifiers of cell apoptosis—interferon (IFN)-α7 and -γ,8interleukin-4 (IL-4),9 basic fibroblast growth factor,10 and IL-1311—can protect CLL cells from death. The analysis of the complex arrays of factors that influence CLL apoptosis is needed for the understanding of the disease pathogenesis and the improvement of therapies.
Among the intracellular modulators of apoptosis, there are NF-κB/Rel transcription factors. These are dimers of proteins (p50/p105 or NF-κB1, p52/p100 or NF-κB2, p65 or RelA, c-Rel and RelB) that have approximately 300 aminoacid Rel regions. The NF-κB/Rel complexes are either found in cell nuclei or retained in the cytoplasm by inhibitors of the IκB (α-ε) family; these latter are proteolyzed on cell stimulation by a number of agents, allowing NF-κB/Rel dimers to reach the nucleus. The NF-κB/Rel factors control the expression of a wide range of genes, such as those encoding immunoglobulins, cytokines, chemokines, interferons, major histocompatibility complex proteins, growth factors, and cell adhesion molecules.12 Furthermore, the apoptotic response of some normal and neoplastic cell types to tumor necrosis factor (TNF) or daunorubicin13-16 can be downregulated by the NF-κB/Rel nuclear activity. In some cells, including B lymphocytes, the NF-κB/Rel complexes stimulate the expression of the zinc finger protein-coding gene A20 that inhibits apoptosis17; other apoptosis-suppressing, NF-κB/Rel–regulated genes will presumably be identified.
The induction of NF-κB/Rel activities by apoptogenic agents such as TNF-α is believed to constitute a pathway by which the apoptotic stimulus limits or terminates its own effect.13-16Independent mechanisms that result in NF-κB/Rel stimulation might be expected to inhibit apoptosis as well. In B cells, a possible link between NF-κB/Rel induction and the rescue from apoptosis might be recognized in the activity of CD40 antigen. This is a 45- to 50-kD transmembrane glycoprotein18-20 that belongs to the TNF receptor superfamily.21 The interaction between CD40 and its ligand CD40-L (CD154), expressed on activated T cells,19-23 can inhibit the normal and neoplastic B-cell apoptosis induced by engagement of the B-cell receptor (BcR) or serum deprivation.24-26 Because of its antiapoptotic properties, the CD40 molecule is believed to exert profound influences on the growth of B lymphocytes and to direct the T-lymphocyte–mediated rescue of germinal center B cells.19,20,27 Furthermore, the CD40 molecule appears functional in B-lineage acute lymphoblastic leukemia cells that can be stimulated to proliferate and maturate via CD40 triggering.28 In mouse spleen,29 human tonsilla, Daudi cells,30 and immature B cells,31 the triggering of CD40 induces the nuclear activity of NF-κB/Rel complexes. Although CD40 antigen interacts with the NF-κB/Rel intracellular inducer TRAF2,32 the NF-κB/Rel activation was found to be independent of the association with this molecule in B cells.33
We investigated whether CD40 antigen could influence the apoptosis of B-CLL cells induced by fludarabine, and we also investigated the involvement of NF-κB/Rel factors in this regulation. The aim of this work was to contribute to the understanding of the mechanisms that regulate B-CLL apoptosis, and to the improvement of therapies active on slowly dividing malignancies.
MATERIALS AND METHODS
Patients.
We studied 15 patients affected by B-cell CLL. Fourteen patients had a CD5+ CD23+ phenotype, whereas 1 patient was CD5+ CD23−. These patients had a clinical Rai stage34 of 0 to 4 and had not received any therapy for at least 3 years before blood collection.
Cells.
Peripheral blood mononuclear cells were isolated from the heparinized blood of healthy donors by differential centrifugation through a Ficoll-Hypaque density gradient (ICN Flow, Opera, Italy) at 400g for 30 minutes. Cells were washed with phosphate-buffered saline (PBS) and resuspended in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (FCS; ICN Flow).
Fludarabine, monoclonal antibodies (MoAbs), and oligonucleotides.
Fludarabine (9-β-D-arabinosyl-2-fluoroadenine-monophosphate) was obtained from Schering (SpA, Milan, Italy). The anti-CD40 MoAb-producing hybridoma (G28-5) was obtained from American Type Culture Collection. An anti-HLA-A1 MoAb, HO5, kindly provided by Dr Soldano Ferrone (New York Medical College, NY), was used as control. The phosphorothioate oligonucleotides, corresponding to the κB consensus sequence (5′-CCTTGAAGGGATTTCCCTCC-3′) or its mutated (“scrambled”) form (5′-CCTTGAATTTATTTAAATCC-3′)35,36were purchased from Primm Srl (Milan, Italy).
Analysis of apoptosis.
Apoptosis was measured by flow cytometry as described.37Briefly, the cells (5 × 105) were washed in PBS and resuspended in 1 mL of a solution containing 0.1% sodium citrate, 0.1% Triton X-100, and 50 μg/mL propidium iodide (Sigma Chemical Co, Gallarate, Italy). After incubation at 4°C for 30 minutes in the dark, cell nuclei were analyzed with a Becton Dickinson FACScan (Sunnyvale, CA) flow-cytometer using the Lysis 1 program. Cellular debris was excluded from analysis by raising the forward scatter threshold, and the DNA content of the nuclei was registered on a logarithmic scale. The percentage of cells in the hypodiploid region was calculated.
Immunofluorescence.
The cells (5 × 105/100 μL) were incubated with CD19 fluorescein isothiocyanate (FITC) or CD69 phycoerythrin (PE)-conjugated antibodies (Becton Dickinson) in 10% FCS/RPMI 1640 medium. After a 30-minute incubation at 4°C, the cells were washed in PBS and analyzed with the FACScan.
Nuclear extracts.
Cell nuclear extracts were prepared38 from 20 × 106 cells by cell pellet homogenization in two volumes of 10 mmol/L Hepes, pH 7.9; 10 mmol/L KCl; 1.5 mmol/L MgCl2; 1 mmol/L EDTA; 0.5 mmol/L dithiothreitol (DTT); 0.5 mmol/L phenylmethylsulfonyl fluoride; and 10% glycerol. Nuclei were centrifuged at 1,000g for 5 minutes, and washed and resuspended in two volumes of the above specified solution. KCl (3 mol/L) was added to reach 0.39 mol/L KCl. Nuclei were extracted at 4°C for 1 hour and centrifuged at 10,000g for 30 minutes. The supernatants were clarified by centrifugation and stored at −80°C. Protein concentration was determined by using the Bradford method.
Electrophoretic mobility shift assays (EMSAs).
The double-stranded κB oligonucleotide (Promega, Madison, WI) corresponded to the IL-2 receptor α NF-κB consensus sequence 5′-CAACGGCAGGGGAATCTCCCTCTCCTT-3′.39 The SP1 oligonucleotide was purchased from CEINGE (Naples, Italy). The oligonucleotides were end-labeled with [γ-32P] ATP (Amersham International Plc, Milan, Italy) using a polynucleotide kinase (Boehringer Mannheim, Milan, Italy) to a specific activity of 2 to 5 × 104 cpm/mL. The AP1 oligonucleotide was purchased from Promega. EMSAs were performed as described.40 Briefly, end-labeled DNA fragments (2 × 104) cpm were incubated at room temperature for 20 minutes with 5 μg of nuclear protein in the presence of 1 μg poly(dI-dC) in 20 μL of a buffer consisting of 10 mmol/L Tris-HCl, pH 7.5; 50 mmol/L NaCl; 1 mmol/L EDTA; 1 mmol/L DTT; and 5% glycerol. Protein-DNA complexes were separated from a free probe on a 5.5% polyacrilamide gel and run in 0.25× Tris borate buffer at 200 V for 3 hours at room temperature. The gels were dried and exposed to X-ray film (Kodak AR, Milan, Italy). The intensity of the bands, expressed as integrated optical density (O.D.), was measured by using NIH Image 1.61 for PowerMacintosh. The values were normalized by comparison with SP1 complex as an internal control.
Anti-p50, -p65 (Rel A), and -c Rel antibodies.
The rabbit antibodies against the C-terminal peptide (529-551) of human p65 and against the N-terminal peptide (1-21) of p105/p50 were kindly provided by Dr Warner C. Greene (Gladstone Institute of Virology and Immunology, San Francisco, CA). The rabbit antibodies against the C-terminal peptide (537-587) of c Rel were a kind gift of Dr Nancy Rice (Frederick Cancer Research and Development Center, Frederick, MD).
Statistical analysis.
Wilcoxon's test was used to perform statistical analysis of the results.
RESULTS
Effect of CD40 triggering on the fludarabine-induced apoptosis of B-CLL.
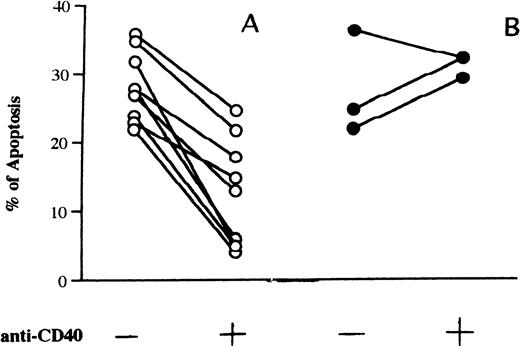
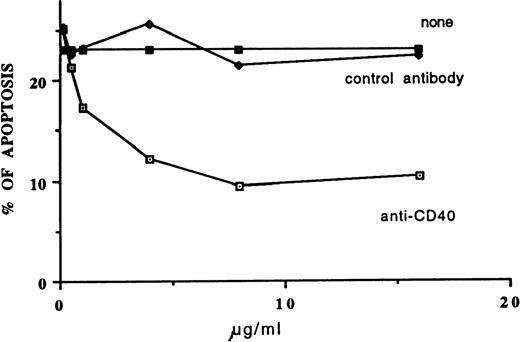
We incubated the cells of 15 patients with fludarabine (0.8 μg/mL) in the presence or absence of MoAb G28-5 and measured the percentage of apoptotic elements after 3 days of culture. In 3 samples very low levels of apoptosis (<10%) were detected in the presence of the drug. Twelve patients displayed apoptosis, with values ranging from 22% to 38% (mean ± SE: 28.5% ± 1.6%). In 9 of these samples (Fig 1A), the addition of MoAb G28-5 to the cultures reduced apoptosis by at least 30.1% and by 57.1% ± 7.8% on average. The inhibition of apoptosis was significant (P = .0077), because the apoptosis percentages were reduced from 28.3% ± 1.6% to 12.5% ± 2.6% in the presence of the anti-CD40 MoAb. In the remaining 3 cases, CD40 triggering did not reduce or slightly (≤10%) reduced the fludarabine-induced apoptosis (Fig 1B). A dose-response curve of the MoAb G28-5 effect is shown in Fig 2.
(A) Inhibition of fludarabine-induced apoptosis by the anti-CD40 MoAb G28-5. (B) Samples with no response or only low response to anti-CD40 MoAb. Cells (1 × 106/mL) from patients with B-CLL were incubated in 10% FCS-RPMI 1640 medium with fludarabine (0.8 μg/mL) and in the absence or presence of MoAb G28-5 (ascites, 1:1000). After 3 days of cell culture the percentages of apoptosis were calculated. Any experimental point has been performed in duplicate; standard deviations of the mean apoptosis values were less than 10%. The levels of apoptosis in cells incubated without fludarabine, either in the presence or the absence of anti-CD40 MoAb, were less than 10%.
(A) Inhibition of fludarabine-induced apoptosis by the anti-CD40 MoAb G28-5. (B) Samples with no response or only low response to anti-CD40 MoAb. Cells (1 × 106/mL) from patients with B-CLL were incubated in 10% FCS-RPMI 1640 medium with fludarabine (0.8 μg/mL) and in the absence or presence of MoAb G28-5 (ascites, 1:1000). After 3 days of cell culture the percentages of apoptosis were calculated. Any experimental point has been performed in duplicate; standard deviations of the mean apoptosis values were less than 10%. The levels of apoptosis in cells incubated without fludarabine, either in the presence or the absence of anti-CD40 MoAb, were less than 10%.
Dose-response curve of MoAb G28-5 antiapoptotic effect. B-CLL cells (1 × 106/mL) were incubated in 10% FCS-RPMI 1640 medium with fludarabine (0.8 μg/mL) in the presence of the indicated concentrations of MoAb G28-5 or control (HO5) antibody. After 3 days of cell culture, the apoptosis percentages were calculated.
Dose-response curve of MoAb G28-5 antiapoptotic effect. B-CLL cells (1 × 106/mL) were incubated in 10% FCS-RPMI 1640 medium with fludarabine (0.8 μg/mL) in the presence of the indicated concentrations of MoAb G28-5 or control (HO5) antibody. After 3 days of cell culture, the apoptosis percentages were calculated.
Analysis of NF-κB/Rel activity in B-CLL cells.
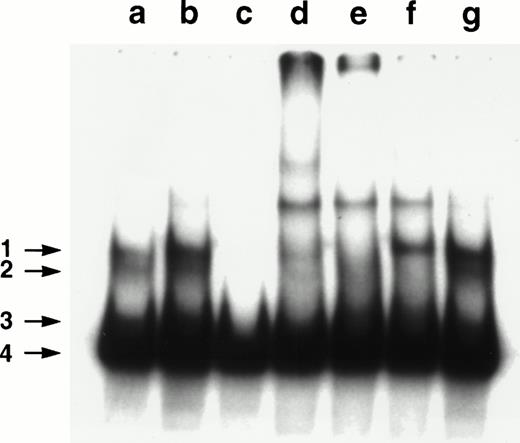
In cells obtained from 13 of the 15 patients studied, we could analyze the nuclear levels of NF-κB/Rel complexes by EMSA. Twelve of the 13 samples showed a migration pattern in which four bands could be recognized. A representative result is shown in Fig3. The bands indicated by arrows 1 through 3 (a) appeared to correspond to specific NF-κB/Rel complexes because they were not affected by the incubation of the extract with unlabeled AP1 oligo, whereas (b) disappeared in the presence of a competing, unlabeled κB oligonucleotide (c). Band 4 was probably caused by the not specific binding of the labeled oligonucleotide to an abundant protein present in the nuclear extracts.41 To analyze the composition of the NF-κB/Rel complexes, the extract was incubated in the presence of rabbit anti-p50 (d), -RelA (e), –c-Rel (f) or preimmune (g) serum. The band indicated by arrow 1 was supershifted by both anti-p50 and -Rel A antibodies, whereas the band indicated by arrow 2 was supershifted by both anti-p50 and –c-Rel and band 3 only by anti-p50. Therefore, p50/RelA, p50/c-Rel, and p50/p50 dimers were likely to be present in the complexes corresponding to, respectively, bands 1, 2, and 3.
Analyses of NF-κB/Rel nuclear complexes in B-CLL cells. A nuclear extract was obtained from B-CLL cells and incubated with a32P-labeled κB oligonucleotide, in the absence (a) or presence of a 50× molar excess of unlabeled AP1 oligonucleotide (b), a 50× molar excess of unlabeled κB oligonucleotide (c), anti-p50 (d), -RelA (e), –c-Rel (f), or preimmune (g) serum.
Analyses of NF-κB/Rel nuclear complexes in B-CLL cells. A nuclear extract was obtained from B-CLL cells and incubated with a32P-labeled κB oligonucleotide, in the absence (a) or presence of a 50× molar excess of unlabeled AP1 oligonucleotide (b), a 50× molar excess of unlabeled κB oligonucleotide (c), anti-p50 (d), -RelA (e), –c-Rel (f), or preimmune (g) serum.
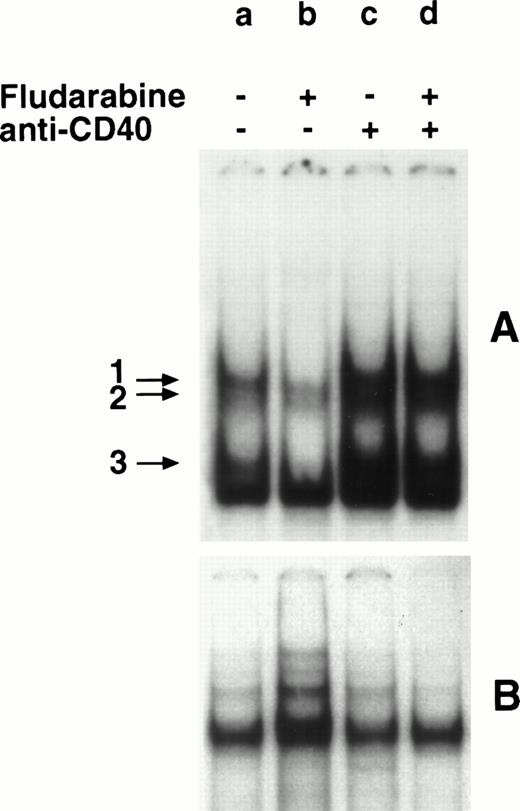
In 9 of the 12 samples, fludarabine induced both apoptosis and a marked reduction of NF-κB/Rel levels; in the remaining 3 samples, the drug induced very low levels (<10%) of apoptosis and apparently did not modulate the NF-κB/Rel levels. Of the 9 fludarabine-responsive samples, 2 could not be rescued from drug-induced apoptosis by the anti-CD40 MoAb; consistently, the MoAb did not upregulate the NF-κB/Rel activity. The remaining 7 samples displayed both inhibition of apoptosis and induction of NF-κB/Rel complexes by MoAb G28-5. A representative result is shown in Fig 4. Compared with cells incubated in control medium (A, a), cells incubated with fludarabine (A, b) displayed a reduction in the intensities of the bands, corresponding to NF-κB/Rel-DNA complexes (arrows 1 through 3). The intensities of these bands were markedly higher in cells incubated with MoAb G28-5, either alone (A, c) or with fludarabine (A, d).
Effect of fludarabine and anti-CD40 MoAb on NF-κB/Rel nuclear complexes. B-CLL cells (1 × 106/mL) were incubated in 10% FCS-RPMI 1640 medium, in the absence (a) or presence of fludarabine (0.8 μg/mL) (b), MoAb G28-5 (10 μg/mL) (c), or MoAb G28-5 + fludarabine (d). After 20 hours, nuclear extracts were obtained and incubated with a 32P-labeled κB (A) or SP-1 (B) oligonucleotide. The DNA-protein complexes were analyzed by EMSA. The SP1-normalized integrated O.D. (×10−2) (see Materials and Methods) of the bands 1 + 2 were: a, 1.1; b, 0.2; c, 3.9; d, 2.7.
Effect of fludarabine and anti-CD40 MoAb on NF-κB/Rel nuclear complexes. B-CLL cells (1 × 106/mL) were incubated in 10% FCS-RPMI 1640 medium, in the absence (a) or presence of fludarabine (0.8 μg/mL) (b), MoAb G28-5 (10 μg/mL) (c), or MoAb G28-5 + fludarabine (d). After 20 hours, nuclear extracts were obtained and incubated with a 32P-labeled κB (A) or SP-1 (B) oligonucleotide. The DNA-protein complexes were analyzed by EMSA. The SP1-normalized integrated O.D. (×10−2) (see Materials and Methods) of the bands 1 + 2 were: a, 1.1; b, 0.2; c, 3.9; d, 2.7.
Effect of NF-κB/Rel inhibition on the antiapoptotic activity of MoAb G28-5.
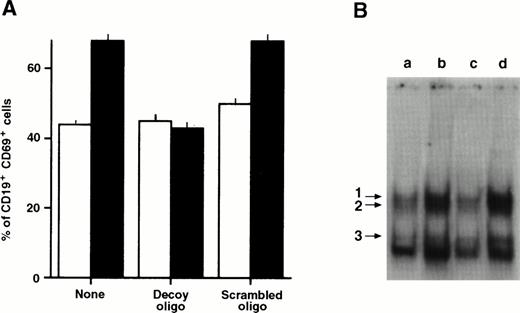
To determine whether the NF-κB/Rel activity was required for the antiapoptotic effect of the MoAb G28-5, we incubated the cells with a decoy oligonucleotide, corresponding to the κB consensus sequence.35 The oligonucleotide subtracts NF-κB/Rel complexes, resulting in a decrease of their nuclear levels and impairment of target genes expression.35,36,42,43 We verified that in cells triggered via CD40, the increase of the NF-κB/Rel–controlled CD69 antigen44 was inhibited by the decoy, but not by a mutated form (“scrambled”) oligonucleotide (Fig 5A). Furthermore, the levels of nuclear NF-κB/Rel complexes, stimulated by CD40 (Fig 5B, lane b), were reduced in cultures incubated with the decoy (lane c) but not the scrambled (lane d) oligonucleotide. In five different experiments, we compared the effects of the MoAb G28-5 on the apoptotic responses of the cells incubated with fludarabine in the absence or presence of decoy or scrambled oligonucleotides. The results are shown in Fig6. The presence of the decoy oligonucleotide slightly modified the apoptosis percentages of cells incubated with or without fludarabine. Therefore, the inhibition of NF-κB/Rel complexes was not sufficient to induce or significantly enhance cell apoptosis. On the other hand, the rescuing effect of MoAb G28-5 was specifically abolished by cell incubation with the decoy oligonucleotide.
Effect of a κB decoy oligonucleotide on CD69 expression (A) and the levels of NF-κB/Rel nuclear complexes (B) in CD40-stimulated B-CLL cells. (A) B-CLL cells (1 × 106/mL) were stimulated with anti-CD40 MoAb (10 μg/mL) in the absence or the presence of the 5-μmol/L κB decoy or scrambled oligonucleotide. After 3 days the cells were collected, washed, incubated with CD69 PE and CD19 FITC, and analyzed by FACScan. The proportion of the CD19+ cells in the different cultures was 74.8 ± 2.4. (□), Medium; (▪), anti-CD40 MoAb. (B) B-CLL cells (1 × 106/mL) were incubated in 10% FCS-RPMI 1640 medium (a), and with anti-CD40 MoAb (10 μg/mL) in the absence (b) or presence of the 5-μmol/L κB decoy (c) or scrambled (d) oligonucleotide. After 20 hours, nuclear extracts were obtained and incubated with the 32P-labeled κB oligonucleotide. The DNA-protein complexes were analyzed by EMSA.
Effect of a κB decoy oligonucleotide on CD69 expression (A) and the levels of NF-κB/Rel nuclear complexes (B) in CD40-stimulated B-CLL cells. (A) B-CLL cells (1 × 106/mL) were stimulated with anti-CD40 MoAb (10 μg/mL) in the absence or the presence of the 5-μmol/L κB decoy or scrambled oligonucleotide. After 3 days the cells were collected, washed, incubated with CD69 PE and CD19 FITC, and analyzed by FACScan. The proportion of the CD19+ cells in the different cultures was 74.8 ± 2.4. (□), Medium; (▪), anti-CD40 MoAb. (B) B-CLL cells (1 × 106/mL) were incubated in 10% FCS-RPMI 1640 medium (a), and with anti-CD40 MoAb (10 μg/mL) in the absence (b) or presence of the 5-μmol/L κB decoy (c) or scrambled (d) oligonucleotide. After 20 hours, nuclear extracts were obtained and incubated with the 32P-labeled κB oligonucleotide. The DNA-protein complexes were analyzed by EMSA.
Effect of the κB decoy oligonucleotide on the levels of B-CLL cell apoptosis. B-CLL cells (1 × 106/mL) were incubated in 10% FCS-RPMI 1640 medium, in the presence of the indicated reagents. After a 3-day incubation, cell apoptosis was analyzed. (▧), RPMI; (▪), decoy 5 μmol/L; (□), scrambled 5 μmol/L.
Effect of the κB decoy oligonucleotide on the levels of B-CLL cell apoptosis. B-CLL cells (1 × 106/mL) were incubated in 10% FCS-RPMI 1640 medium, in the presence of the indicated reagents. After a 3-day incubation, cell apoptosis was analyzed. (▧), RPMI; (▪), decoy 5 μmol/L; (□), scrambled 5 μmol/L.
DISCUSSION
These results show that CD40 antigen modulates the fludarabine-induced apoptosis in B-CLL lymphocytes. The CD40 effects were analyzed in unfractionated mononucleate cell populations, which, when cultured, displayed a low proportion of apoptotic elements (<10%), compared with the spontaneous apoptosis percentages observed in purified B leukemic cells.11,45-48 However, in three cases, in which the 15%, 41%, and 73% of spontaneous apoptosis were detected after 5 days of culture, the presence of anti-CD40 MoAb did not reduce such percentages, in accord with results reported by other authors49 (results not shown). On the other hand, in fludarabine-treated cultures, the CD40 triggering rescued the cells from apoptosis. Therefore, in addition to inhibiting the spontaneous or BcR-induced apoptosis of normal B cells and B-cell lines,18,19 24-27 CD40 appears to regulate also the chemotherapeutic drug-induced apoptosis in cells from patients with B-CLL.
A constitutive activity of NF-κB/Rel activity was found in B-CLL. The complexes comprised p50 homodimers, p50/c-Rel, and p50-RelA heterodimers. This pattern is similar to those described in immature and mature B-cell lines.12,50 Cell incubation with fludarabine resulted in the disappearance of the nuclear NF-κB/Rel activity. This might rely on a reduced production of NF-κB/Rel proteins, due to the fludarabine-caused hampering of RNA synthesis. Alternatively, the drop in NF-κB/Rel nuclear activity might be induced during the apoptosis program—consistently, a decline in NF-κB/Rel nuclear levels had been observed during the BcR-induced apoptosis of murine B cells.51 However, although being part of the fludarabine activity, the downmodulation of NF-κB/Rel nuclear factors is not likely to be sufficient for the triggering of apoptosis. Indeed, the block of NF-κB/Rel complexes, obtained by incubating the cells with the decoy oligonucleotide alone, did not result in inducing apoptosis.
On CD40 triggering, the nuclear levels of NF-κB/Rel complexes were increased. When the NF-κB/Rel complexes were blocked by the presence of a decoy oligonucleotide, the antiapoptotic activity of CD40 was also abrogated. This strongly argues for a role of NF-κB/Rel complexes in the antiapoptotic activity of CD40. Whether A2051and/or other NF-κB/Rel–controlled genes induced by CD40 triggering are responsible for the antiapoptotic mechanism remains to be determined.
These results suggest that the CD40-mediated survival pathway, triggered by CD40-L–expressing T lymphocytes,19-23 could counteract the therapeutic effect of fludarabine, and possibly other apoptogenic drugs in vivo. Furthermore, the CD40-mediated regulation of fludarabine-induced apoptosis in B-CLL cells can represent a model of NF-κB/Rel–controlled response to chemotherapy in a lymphoid tumor. This example supports the possible use of NF-κB/Rel inhibitors in antitumour therapies.52 Reagents, like anti–CD40-L antibodies, that neutralize NF-κB/Rel–stimulating molecules of the cell's environment could as well potentiate the therapeutic effect of the drug.
Finally, the question arises whether the CD40-mediated antiapoptotic pathway, active in B-CLL cells, can contribute to the prolonged life span and accumulation of these leukemic cells53 as a part of the pathogenetic mechanism of the disease. The role of CD40/CD40-L system in cell resistance to apoptotic drugs should, as well, be investigated.
ACKNOWLEDGMENT
We thank Dr Antonella De Pascale (Schering SpA, Milan, Italy) and Dr Felicetto Ferrara (Cardarelli Hospital, Naples, Italy) for providing us with the fludarabine. We also thank Carmine Del Gaudio for his excellent technical help.
Supported by AIRC, FSN 96, and Regione Campania.
M.C.T. and S.V. equally contributed to this work.
Address reprint requests to M. Caterina Turco, MD, Dipartimento di Biochimica e Biotecnologie Mediche, University of Napoli, Federico II, Via S Pansini, 5, 80131 Napoli, Italia; e-mail:turco@dbbm.unina.it.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal